Production of Functional Vinegar Enriched with γ-Aminobutyric Acid through Serial Co-Fermentation of Lactic Acid and Acetic Acid Bacteria Using Rice Wine Lees
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starter Cultures
2.3. GABA and Vinegar Production
2.4. Moisture Content Assay
2.5. Alcohol Quantification
2.6. Reducing Sugars Assay
2.7. Measurement of Viable Bacterial Counts
2.8. pH and Titratable Acidity
2.9. Qualitative Analysis of GABA
2.10. Quantitative Analysis of the Free Amino Acid Content
2.11. Measurement of Organic Acids
2.12. Quantitative Analysis of Sodium
2.13. Determination of Storage Stability of GABA
2.14. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Composition of Rice Wine Lees
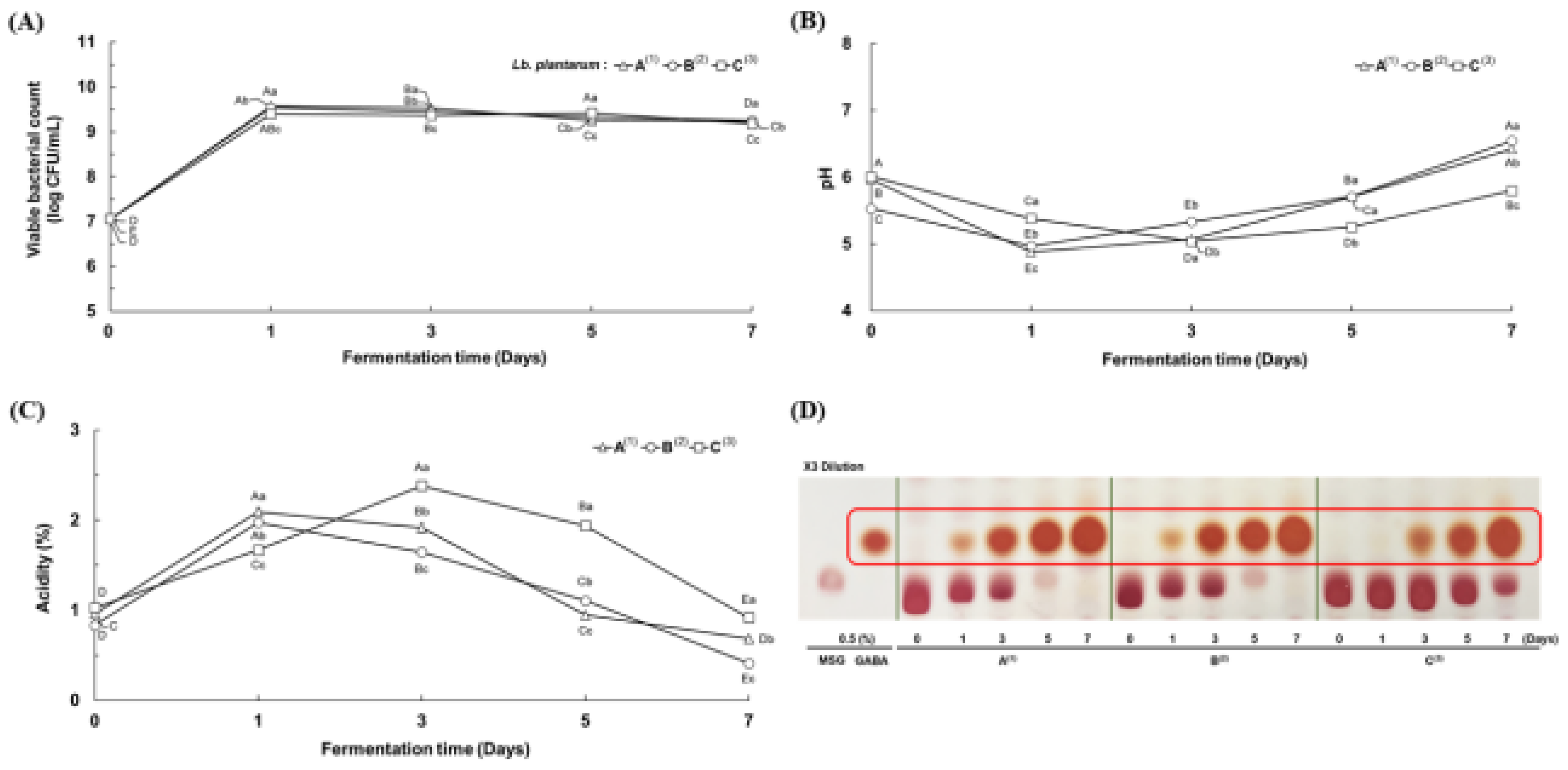
3.2. Physicochemical Properties of the First LAB Fermented Rice Wine Lees
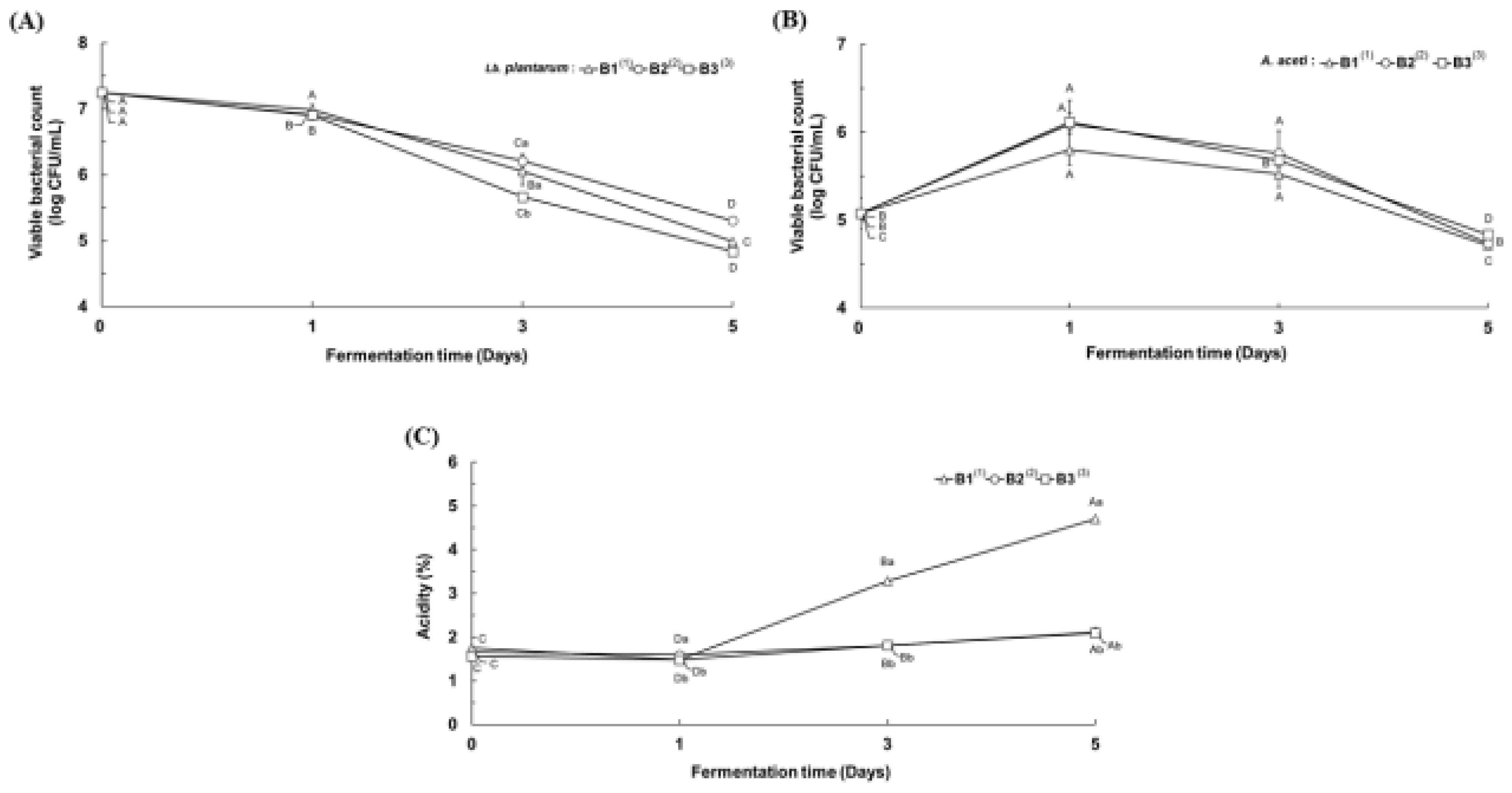
3.3. Physicochemical Properties of the Second Serial Co-Fermented Rice Wine Lees
3.4. Quantitative Properties of the Serial Co-Fermented Vinegar Product
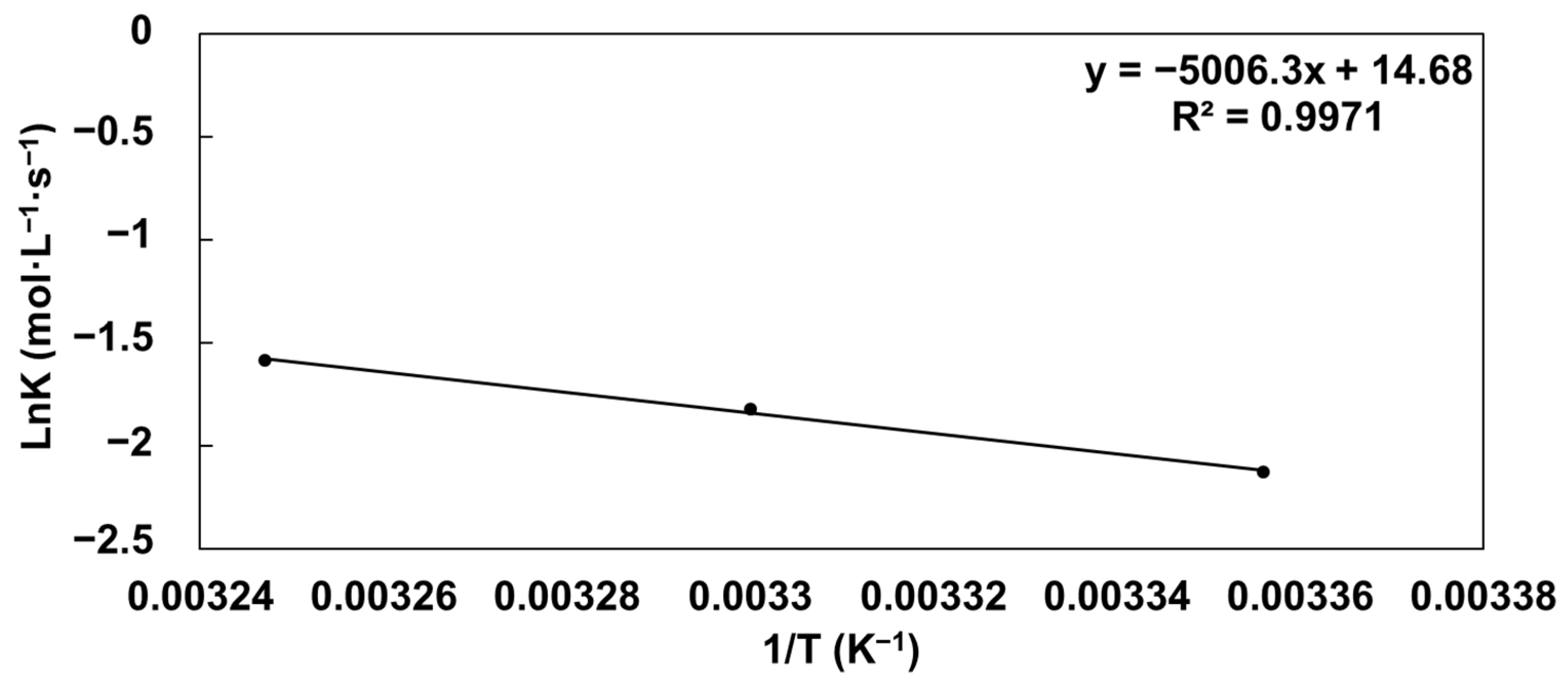
3.5. Evaluation of GABA Stability in Vinegar
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, D.-C.; In, M.-J. Preparation and characteristics of yogurt added with Korean rice wine lees powder. J. Appl. Biol. Chem. 2016, 59, 345–349. [Google Scholar] [CrossRef]
- Kwon, K.-J.; Kim, J.-S.; Chung, C.-W. Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Bacillus sp. EMK-5020 using Makgeolli lees enzymatic hydrolysate and propionic acid as carbon sources. J. Life Sci. 2022, 32, 510–522. [Google Scholar] [CrossRef]
- Kim, M.-S.; Shin, W.-C.; Sohn, H.-Y. Application of the lees of domestic traditional wine and its useful biological activity. J. Life Sci. 2015, 25, 1072–1079. [Google Scholar] [CrossRef]
- Shufang, T.; Weizhu, Z.; Fang, F.; Jingwen, Z.; Guocheng, D. The microbiome of Chinese rice wine (Huangjiu). Curr. Res. Food Sci. 2022, 5, 325–335. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Mrid, R.B.; Fal, S.; Arroussi, H.E.; Peng, W.; Tabatabaei, M.; et al. Progress in valorisation of agriculture, aquaculture and shellfish biomass into biochemicals and biomaterials towards sustainable bioeconomy. Chemosphere 2022, 291, 133036. [Google Scholar] [CrossRef]
- Moon, Y.-K.; Lee, K.-J.; Kim, G.-Y.; Song, C.-B.; Jeon, Y.-J.; Heo, M.-S. Characteristics of citrus by-product ferment using probiotics as starter. Microbiol. Biotechnol. Lett. 2006, 34, 158–165. [Google Scholar]
- Woo, S.-M.; Kim, T.-Y.; Yeo, S.-H.; Kim, S.-B.; Kim, M.-H.; Woo, S.-C.; Jeong, Y.-J. Effect of α-amylase treatment of brown rice alcohol fermentation by-product. J. Korean Soc. Food Preserv. 2007, 14, 617–623. [Google Scholar]
- Chen, G.; Zheng, F.; Lin, B.; Yang, Y.; Fang, X.; Verma, K.; Yang, L.F. Vinegar: A potential source of healthy and functional food with special reference to sugarcane vinegar. Front. Nutr. 2023, 10, 1145862. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-E. Market trends and research status of fermented vinegars. Food Ind. Nutr. 2020, 25, 50–57. [Google Scholar]
- Yi, M.-R.; Kang, C.-H.; Bu, H.-J. Acetic acid fermentation properties and antioxidant activity of lemongrass vinegar. Korean J. Food Preserv. 2017, 24, 680–687. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Iftikhar, A.; Lopez, F. Antioxidant effect of traditional and new vinegars on functional oil/vinegar dressing-based formulations. Eur. Food Res. Technol. 2022, 248, 1573–1582. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A. Milestone Review: Metabolic dynamics of glutamate and GABA mediated neurotransmission—The essential roles of astrocytes. J. Neurochem. 2023, 166, 109–137. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 26, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of fermentation for r-aminobutyric acid (GABA) production by yeast Kluyveromyces marxianus C21 in okara (soybean residue). Bioprocess Biosyst. Eng. 2022, 4, 1111–1123. [Google Scholar] [CrossRef]
- Nakatani, Y.; Fukaya, T.; Kishino, S.; Ogawa, J. Production of GABA-enriched tomato juice by Lactiplantibacillus plantarum KB1253. J. Biosci. Bioeng. 2022, 134, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.H.; Kim, M.S.; Kim, G.H.; Yoon, S.S. Probiotic properties and optimization of gamma-aminobutyric acid production by Lactiplantibacillus plantarum FBT215. J. Microbiol. Biotechnol. 2022, 32, 783. [Google Scholar] [CrossRef] [PubMed]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate decarboxylase from lactic acid bacteria-A key enzyme in GABA synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Pino, A.; Russo, N.; Solieri, L.; Sola, L.; Caggia, C.; Randazzo, C.L. Microbial consortia involved in traditional Sicilian sourdough: Characterization of lactic acid bacteria and yeast populations. Microorganisms 2022, 10, 283. [Google Scholar] [CrossRef]
- Cai, L.; Wang, W.; Tong, J.; Fang, L.; He, X.; Xue, Q.; Li, Y. Changes of bioactive substances in lactic acid bacteria and yeasts fermented kiwifruit extract during the fermentation. LWT 2022, 164, 113629. [Google Scholar] [CrossRef]
- Lee, S.-P.; Yoon, W.-K.; Lee, J.-H.; Garcia, C.V. Fermented milk product enriched with γ-PGA, peptides and GABA by novel co-fermentation with Bacillus subtilis and Lactiplantibacillus plantarum. Fermentation 2022, 8, 404. [Google Scholar] [CrossRef]
- Ko, M.-J.; Nam, H.-H.; Chung, M.-S. Optimization of drying conditions for the conversion of 6-gingerol to 6-shogaol under subcritical water extraction from ginger. Korean J. Food Sci. Technol. 2019, 51, 447–451. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Khota, W.; Panyakaew, P.; Kesorn, P.; Gunun, P.; Suwannasing, R.; Kimprasit, T.; Premsak, P.; Ketinum, K.; Anusorn, C.; Suwit, T.; et al. In vitro rumen fermentation of coconut, sugar palm, and durian peel silages, prepared with selected additives. Fermentation 2023, 9, 567. [Google Scholar] [CrossRef]
- Entani, E.; Ohmori, S.; Masai, H.; Suzuki, K.I. Acetobacter polyoxogenes sp. nov., a new species of an acetic acid bacterium useful for producing vinegar with high acidity. J. Gen. Appl. Microbiol. 1985, 31, 475–490. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Garcia, C.V.; Song, Y.C.; Lee, S.P. GABA-enriched water dropwort produced by co-fermentation with Leuconostoc mesenteroides SM and Lactobacillus plantarum K154. LWT 2016, 73, 233–238. [Google Scholar] [CrossRef]
- Lim, J.-S.; Park, Y.-H.; Jo, B.-S.; Kim, J.-E.; Lee, S.-P. Increased production of γ-aminobutyric acid using the extract of Phellinus linteus fruiting body by Lactiplantibacillus plantarum KS2020. Korean J. Food Preserv. 2022, 29, 472–481. [Google Scholar] [CrossRef]
- Kang, H.-M.; Kim, H.-J.; Park, E.-J.; Chae, J.-W.; Seo, D.-W.; Lee, S.-P. Comparison of mineral and ash contents in commercial beverages. Korean J. Food Preserv. 2021, 28, 758–770. [Google Scholar] [CrossRef]
- Kim, S.-M.; Cho, W.-K. Effects of Takju (Korean turbid rice wine) lees on the serum glucose levels in streptozotocin-induced diabetic rats. J. Korean Soc. Food Cult. 2006, 21, 638–643. [Google Scholar] [CrossRef]
- Park, K.-Y.; Park, S.-J.; Jun, H.-K. Effects of commercial salts on the growth of Kimchi-related microorganisms. Res. Bull. Kimchi Sci. Technol. 2001, 7, 31–38. [Google Scholar]
- Kwon, S.-Y.; Lee, S.-P. Enrichment of gamma-aminobutyric (GABA) in old antler extract fermented by Lactiplantibacillus plantarum. Korean J. Food Sci. Technol. 2018, 50, 37–43. [Google Scholar] [CrossRef]
- Hur, S.-S. Production of fermented Saccharina japonica extract with enhanced GABA content. Korean Soc. Appl. Sci. Technol. 2022, 39, 517–526. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Chen, L.; Lu, F.; Lu, Z. Biosynthesis of γ-aminobutyric acid by a recombinant Bacillus subtilis strain expressing the glutamate decarboxylase gene derived from Streptococcus salivarius ssp. thermophilus Y2. Process Biochem. 2014, 49, 1851–1857. [Google Scholar] [CrossRef]
- Hahn, Y.-S. Effect of ethanol and/or organic acids on the growth of Lactiplantibacillus plantarum, Leuconostoc mesenteroides, Kluyveromyces marxianus identified from mul-kimchi. J. East Asian Soc. Diet. Life 2003, 13, 425–432. [Google Scholar]
- Kim, W.-J. Understanding of killed lactic acid bacteria as a probiotics. Korean J. Community Pharm. 2018, 4, 115–122. [Google Scholar]
- Ghosh, S.; Chakraborty, R.; Chatterjee, A.; Raychaudhuri, U. Optimization of media components for the production of palm vinegar using response surface methodology. J. Inst. Brew. 2014, 120, 550–558. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyama, H.; Adachi, O. Respiratory chains and bioenergetics of acetic acid bacteria. Adv. Microb. Physiol. 1994, 36, 247–301. [Google Scholar] [CrossRef]
- Mas, A.; Torija, M.J.; García-Parrilla, M.D.C.; Troncoso, A.M. Acetic acid bacteria and the production and quality of wine vinegar. Sci. World J. 2014, 2014, 394671. [Google Scholar] [CrossRef]
- Sokollek, S.J.; Hertel, C.; Hammes, W.P. Cultivation and preservation of vinegar bacteria. J. Biotechnol. 1998, 60, 195–206. [Google Scholar] [CrossRef]
- Park, H.-K. Studies on the stability of multivitamin solutions. Korean Soc. Appl. Biol. Chem. 2000, 43, 39–45. [Google Scholar]
- Khan, W.; Bhatt, P.C.; Panda, B.P. Degradation kinetics of gamma amino butyric acid in Monascus-fermented rice. J. Food Qual. 2015, 38, 123–129. [Google Scholar] [CrossRef]
- Le, P.H.; Le, T.T.; Raes, K. Effects of pH and heat treatment on the stability of γ-aminobutyric acid (GABA) in germinated soymilk. J. Food Process. Preserv. 2020, 44, e14301. [Google Scholar] [CrossRef]
- Koh, W.Y.; Lim, X.X.; Teoh, E.S.W.; Kobun, R.; Rasti, B. The effects of gamma-aminobuytric acid (GABA) enrichment on nutritional, physical, shelf-life, and sensorial properties of dark chocolate. Foods 2023, 12, 213. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Content |
|---|---|
| Soluble solid content (%) | 30.74 ± 0.23 (1) |
| Alcohol content (%) | 7.26 ± 0.12 |
| pH | 3.96 ± 0.02 |
| Acidity (%) | 0.78 ± 0.10 |
| Reducing sugar content (mg/mL) | 51.22 ± 0.18 |
| First Fermented Rice Wine Lees Content | Alcohol Content (%) | Sodium (Na) Content (mg/g) | |
|---|---|---|---|
| 0 Day | 5 Days | 5 Days | |
| 40% | 5.1 ± 0.0 (1) | 0.0 ± 0.0 | 5.83 ± 0.04 |
| 45% | 5.4 ± 0.0 | 1.8 ± 0.2 | 7.68 ± 0.15 |
| 50% | 5.6 ± 0.0 | 2.5 ± 0.1 | 8.03 ± 0.06 |
| (A) | ||
| Condition | Glutamic Acid (mg/g) | GABA (mg/g) |
| First LAB fermentation (10% MSG) | 5.08 ± 0.21 (1) | 65.49 ± 3.41 |
| Second AAB fermentation (first fermented product: 40%) | 3.61 ± 0.22 | 22.61 ± 0.61 |
| (B) | ||
| Organic Acid (mg/g) | First Fermented Rice Wine Lees (Addition 10% MSG) | |
| Before AAB Fermentation | AfterAAB Fermentation | |
| Acetic acid | 10.42 ± 0.18 (1) | 52.21 ± 5.53 |
| Lactic acid | 14.11 ± 0.10 | 4.81 ± 0.16 |
| Malic acid | 1.04 ± 0.06 | 0.00 ± 0.00 |
| Oxalic acid | 0.02 ± 0.00 | 0.00 ± 0.01 |
| Citric acid | 0.00 ± 0.00 | 0.00 ± 0.00 |
| °C (K) | R2 | 1/T (K−1) | K | lnK | |
|---|---|---|---|---|---|
| 25 (298) | y = −0.1189x + 2.4238 | 0.9078 | 0.003356 | 0.1189 | −2.129472475 |
| 30 (303) | y = −0.1614x + 2.6486 | 0.7409 | 0.003300 | 0.1614 | −1.823869523 |
| 35 (308) | y = −0.2051x + 2.6629 | 0.7585 | 0.003247 | 0.2051 | −1.584257614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-H.; Kwon, M.-J.; Shin, D.-M.; Lee, S.-P. Production of Functional Vinegar Enriched with γ-Aminobutyric Acid through Serial Co-Fermentation of Lactic Acid and Acetic Acid Bacteria Using Rice Wine Lees. Appl. Microbiol. 2024, 4, 1203-1214. https://doi.org/10.3390/applmicrobiol4030082
Park Y-H, Kwon M-J, Shin D-M, Lee S-P. Production of Functional Vinegar Enriched with γ-Aminobutyric Acid through Serial Co-Fermentation of Lactic Acid and Acetic Acid Bacteria Using Rice Wine Lees. Applied Microbiology. 2024; 4(3):1203-1214. https://doi.org/10.3390/applmicrobiol4030082
Chicago/Turabian StylePark, Yun-Ho, Min-Jeong Kwon, Dong-Min Shin, and Sam-Pin Lee. 2024. "Production of Functional Vinegar Enriched with γ-Aminobutyric Acid through Serial Co-Fermentation of Lactic Acid and Acetic Acid Bacteria Using Rice Wine Lees" Applied Microbiology 4, no. 3: 1203-1214. https://doi.org/10.3390/applmicrobiol4030082
APA StylePark, Y.-H., Kwon, M.-J., Shin, D.-M., & Lee, S.-P. (2024). Production of Functional Vinegar Enriched with γ-Aminobutyric Acid through Serial Co-Fermentation of Lactic Acid and Acetic Acid Bacteria Using Rice Wine Lees. Applied Microbiology, 4(3), 1203-1214. https://doi.org/10.3390/applmicrobiol4030082






