Abstract
The Bacillus megaterium LVN01 species native to Colombia has demonstrated the ability to metabolize different coproducts or industrial waste (such as fique juice, cane molasses, and residual glycerol) and accumulate polyhydroxybutyrate (PHB), giving it potential in the bioplastics industry. In this research, the potential of liquid digestate as a carbon source for the production of PHA polymers in fermentation processes with this bacterial strain was evaluated. Favorably, it was found that B. megaterium utilizes the nutrients from this residual substrate to multiply appropriately and efficiently synthesize poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). Bench-scale aerobic batch fermentation, under the operational conditions of this research [volume: 3 L; temperature: 30.8 °C; agitation: 400 rpm; pH: 7.0 ± 0.2; dissolved oxygen: 100% saturation; antifoam: 10% (v/v)], generated maximum values of dry cell weight (DCW) (0.56 g cell L−1) at 60 h, while the maximum PHBV yield (360 mg PHBV L−1) occurred at 16 h, which is very favorable for sustainable degradable bioplastics production. Additionally, GC–MS and NMR analyses confirmed that the PHBV copolymer synthesized by B. megaterium is made up of the monomers 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV). Furthermore, the thermal properties determined by TGA (Tonset = 283.1 °C; Tendset = 296.98 °C; Td = 290.114 °C) and DSC (Tm = °C 155.7 °C; ΔHf = 19.80 J g−1; Xcr = 18.17%) indicate that it is a thermally stable biopolymer with low percentages of crystallinity, providing flexibility that facilitates molding, adaptation, and application in various industrial sectors.
1. Introduction
Industrial development has brought great benefits to humanity, but many of its processes and products have also negatively impacted natural ecosystems, affecting human health and all other forms of life. Among the most critical cases is the production and use of conventional plastics. These not only deplete non-renewable fossil resources but also represent one of the primary sources of environmental contamination due to their resistance to degradation, which leads to the massive accumulation of these contaminants in various ecosystems [1].
As has been reported [2], the production of plastics increased from 1.5 metric tons in 1950 to 250 metric tons in 2010 and to 400.3 metric tons in 2022. The emergence of circular plastics, which entails a second use of the existing ones, has achieved a slight decrease in global production, close to 10% [2]. Plastics represent a large fraction of the waste discarded in terrestrial and aquatic ecosystem, where microplastics and plastic islands have been found [3,4]. According to reports from 2019, plastic pollution generated 1.8 billion tons of greenhouse gas emissions, which is equivalent to 3.4% of the total emissions generated worldwide [5].
Faced with this environmental crisis, the production of bioplastics, or the green polymers of the future, is becoming established as an alternative with which to replace petroplastics [6]. These polyesters are classified into three types [1]: polylactic acid (PLA), polybutylene succinate (PBS), and polyhydroxyalkanoates (PHAs). The latter are the only ones that are synthesized and catabolized naturally by bacteria, and which are, therefore, natural chemical compounds, which confers greater added value to their industrial production, with the estimate that in 2023, their global market will reach 57.8 million dollars. If government regulations and policies continue to reduce single-use plastics, it is possible that by 2028, the PHA market will have an increase of close to 50%, represented by 98.5 million dollars [7].
PHAs are secondary metabolites of a wide variety of bacteria, fungi, and some plants, which produce and accumulate them as an energy reserve when external conditions make their survival difficult [8]. These biopolymers hold significant potential to replace conventional petrochemical-derived plastics as they possess similar physicochemical characteristics while also demonstrating superior properties in terms of thermal processability and biocompatibility. This renders them highly suitable for the fabrication of biomedical, dental, and electronic devices, as well as for utilization across sectors, including construction, automotive, packaging, and agriculture [3,8]. Due to PHA’s biodegradable and biocompatible properties, its applications extend to the production of medical and dental devices. Moreover, tests are being developed to stimulate tissue regeneration and microsphere production for drug administration [9].
Although a variety of microorganisms (more than 300 species) have been used to obtain a wide range of biopolymers, only a few bacteria have been extensively investigated for the manufacture of bioplastics due to their higher efficiency and production rates. These include Bacillus megaterium, Bacillus cereus, Bacillus subtilis, Pseudomona aeruginosa, P. putida, Halomonas sp., Pseudomona fluorescens, P. oleovorans, R. eutropha, and Cupriavidus necator [10]. Particularly, the bacterium Bacillus megaterium is an excellent biological machine for producing PHAs because it has a robust expression system and a cell wall deficient in lipopolysaccharides, which facilitates the release of the biopolymer synthesized intracellularly by the bacteria [11,12].
Structurally, PHAs are classified according to the composition of their monomers into (a) short-chain (SCL) PHAs, made up of monomers with 3 to 5 carbons, such as poly-3 hydroxyvalerate [P3HV] and poly-3-hydroxybutyrate [P3HB]; (b) medium-chain (MCL) PHAs, with monomers of 6 to 14 carbons, such as the copolymer 3-poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) known as [3 3(HB-co-HV] or [p(3HB-co-3HV] or PHBV; and (c) mixed-chain (MCM), which combine the previous two and therefore consist of monomers between 3 and 14 carbons, as is the case of poly (3HB co-3HV-co-3HHx). From the industrial point of view, PHB and the copolymer PHBV [9] stand out. The length of the carbon chain defines the fragile nature of those with a short or flexible chain for medium-chain examples [13].
To a large extent, the chain length is influenced by the type of substrate used to induce the production of PHAs of microbial origin. Substrates with a high carbohydrate content mainly stimulate the production of SCL PHAs (short-chain polyhydroxyalkanoate) polymers, while a high fatty acid content stimulates those of MCM PHAs. The most investigated SCL PHAs are those formed by P3HB. To improve the mechanical properties of P3HB and expand its applications, the production of heteropolymers such as PHBV has been investigated, which, due to the presence of the 3HV monomer in its structure, presents better flexibility characteristics, improving the properties of PHB [9].
To induce the production of copolymers in bacteria, carbon substrates that include mixtures of pure volatile fatty acids (VFAs), or materials or wastewater with high VFA content, are used. Bacteria of the genus Bacillus synthesize copolymers of PHBV in crops supplemented with a mixture of VFA from the controlled hydrolysis of pea peels, apple pomace, onion peels, and potato peels [14]. The authors found that Bacillus cereus EGU43, cultured on pea hull hydrolysates, accumulated the copolymer of PHBV with a 3HV content of 1% w/w.
The co-culture of Pseudomona sp. ST2 and Bacillus sp. CS8 in culture media with acetic and propionic acids as a carbon source, supplemented with glucose, produces up to 35% PHBV copolymer [15]; when a mixture of glucose and propionic acid is used, individual strains produce more PHAs than when glucose is used as the sole carbon source. With Alcaligenes eutrophus NCIMB 11599, grown in valeric acid or propionic acid, PHBV is obtained with better yields in the first case. Ralstonia eutropha KCTC 2658, grown in a mixture of acetic, propionic, and butyric acid in a ratio of 1:2:2 [16], can achieve a PHBV production of 50% of the DCW. Cupriavidus necator, grown in wastewater effluent rich in acetic, propionic, and butyric acid, without adding any exogenous substrate, produces a substantial amount of PHBV copolymer (55% of the DCW) [17]. Recently, Ferre-Guell and Winterburn [18] obtained high concentrations of PHBV copolymer by growing Haloferax mediterranei in a mixture of butyrate and valerate acids with the addition of surfactants (Tween 80) to increase the bioavailability of the substrate, with a productivity of 10.2 mg/L·h in batch fermentation [18]. Such approaches can potentially reduce the production costs of PHAs and offer environmental benefits through waste reuse.
Now, although the global production capacity of bio-based PHA has expanded every year, the industrial production of PHA has been limited due to its production costs (around USD 4000–15,000/metric tons), which significantly exceed the costs of polymers derived from fossil fuels (around USD 1000–1500/metric tons) [3]. The raw material (substrate) represents about 50% of the total production costs [19]; therefore, the profitability of PHA production is largely defined by the costs of raw materials, especially carbon source substrates. This is associated with the fact that the accumulation of PHA occurs under aerobic conditions, so a large part of the substrate is used in microbial intracellular respiration with the formation of CO2 and water-soluble secondary metabolites. Only part of the carbon source, much less than half, is directed to cellular biomass growth and PHA accumulation [19].
In addition to the above, industrial PHA production processes are based on fermentation technology in which they use expensive substrates (purified sugars, edible vegetable oils, among others) that, in addition, are part of the family basket. Therefore, its use puts the technology in competition with the food industry and makes the total production of PHAs more expensive. Therefore, there is an urgent need to use carbon source substrates that do not affect food security, are economical and affordable, and are sustainable for the sustainable synthesis of PHAs [3,20].
Low-value substrates have been evaluated as carbon sources for the production of PHAs coming from different productive sectors: the dairy industry (cheese/whey, dairy wastewater), oil and paper factories, agricultural crops (oil effluents palm/soybean/fruit), and animal waste (chicken/cow manure) [3]. However, they are substrates that require prior treatments to be used as microbial culture media, which entails additional costs. For this reason, attention has been directed to using substrates that come from a first benefit, such as the generation of biofuels, specifically volatile fatty acids (VFA), i.e., intermediate metabolites in the anaerobic digestion (AD) of residual biomass that are used for the production biogas [21]. In this sense, researchers have directed their attention to strengthening VFA production as a collateral product of biogas production [22]. Colombian researchers reported an interesting work in which they take advantage of fishing waste for this purpose, with which digestate rich in VFA is also obtained [23].
Bacillus megaterium LVN01, native to Colombia, has shown the ability to produce PHAs from residual glycerol from the biodiesel industry [24], carob fruits [25], residues from fique processing (fique juice) [12], and frying oil. In this research, B. megaterium LVN1 showed the ability to synthesize PHBV from liquid digestate, a residual substrate from the anaerobic digestion of fish visors.
In this context, the PROBIOM (production, structure, and application of biomolecules) research group of the Universidad Nacional de Colombia contributed its research experience to the production of PHAs from different substrates (glycerol, fique juice, carob pulp, frying oil) to give a second use to the waste from the fishing industry through its participation in the royalty project “Strengthening of artisanal fishing activity in the Colombian Nariño Pacific towards a sustainable use of the resource. Tumaco”. Within the project, the first alternative use for fishing waste has been to produce biogas via the Environmental Prospective group of the Universidad Nacional de Colombia, Palmira headquarters. PROBIOM uses the digestate from this first phase as the main substrate of B. megaterium LVN01 for the production of PHA.
In Colombia, artisanal fishing supplies around 12,000 tons of fish/year to the national market, i.e., 8% of the total capture fishery in the country. It is estimated that 45% of the total catch by artisanal fishing becomes waste [23,26], which produces serious economic losses for this sector and environmental problems associated with the inadequate disposal of waste. In Colombia, waste fishing boats are thrown directly into the ocean or taken to land [23].
Participation in the project “Strengthening artisanal fishing activity in the Colombian Nariño Pacific towards sustainable use of the resource. Tumaco”, which, among its objectives, has “Increasing the use of fishing surpluses and waste in the Pacific of Nariño”, provided a platform for said initiative. Within the aforementioned objective, the initial focus is on the production of biogas from fishing waste via the Environmental Prospective group of the Universidad Nacional de Colombia, Palmira headquarters. The authors of this research (the PROBIOM group) use the digestate, a coproduct of the initial stage, as the main substrate for the growth of B. megaterium LVN01 and the microbial synthesis of PHA while exploring a new waste stream, not previously reported, and conferring a potential value to fishing waste.
2. Materials and Methods
2.1. Ethics Statement
The results of this research are articulated according to the Agreement of Access to Genetic Resources and Derivatives No. 159, signed by the Ministry of Environment and Sustainable Development and the Universidad Nacional de Colombia, Resolution 0004, 2 January 2018, under the project called “Production and characterization of polyhydroxyalkaloates synthesized by native strains from organic waste.
2.2. Study Area and Bacterial Strain
B. megaterium LVN01 (Code GenBank: QJGY00000000.1) was isolated by the PROBIOM group from soil samples collected in the municipality of Guarne (06°16′50″ N and 75°26′37″ W, department of Antioquia, Colombia) and initially characterized using molecular (16S rDNA sequence similarity), morphological, and biochemical techniques [12]. Bacterial cultures were stored at −4 °C in Luria Bertani broth (LB broth, MERCK, Darmstadt, Germany) with 20% glycerol (v/v).
2.3. Carbon Source
A liquid residue from the biogas pilot plant, which operates with residual biomass from artisanal fishing waste, was utilized as a substrate for fermentations. The plant is situated in the city of San Andrés de Tumaco on the premises of the Universidad Nacional de Colombia (at Tumaco, department of Nariño, Colombia). The samples were provided by the Environmental Prospective research group of the same university as part of the project “Strengthening Artisanal Fishing Activity in the Colombian Pacific of Nariño, Towards a Sustainable Use of the Resource”.
This liquid residue, known as digestate, is a valuable source of VFA. For preservation, it was stored frozen at −4 °C, and before its use in the process, it underwent filtration through thick paper (pore size 20 μm) to remove sediments larger than 20 μm. This step facilitated microbial fermentation activity in the medium enriched with this substrate and prevented obstruction of the equipment sensors where the process was conducted.
The bromatological characterization of the digestate revealed the presence of several nutrients, including VFAs (59.04 ± 0.9 g/L), with significant concentrations of acetic acid (24.49 ± 1.82 g/L), butyric acid (11.81 ± 0.02 g/L), and isobutyric acid (9.36 ± 0.02 g/L). This composition represents a potential carbon source for B. megaterium, which is of interest to this research (Table S1).
2.4. Culture Media
Nutritive agar (Merck, Microbiological Grade, Darmstadt, Germany) was composed of 5.0 × 10−3 g/L pluripeptone, 3.0 × 10−3 g/L meat extract, 8.0 × 10−3 g/L sodium chloride, and 15.0 × 10−3 g/L agar, with a final pH: 7.3 ± 0.2. Luria Bertani broth (LB broth, MERCK, Darmstadt, Germany) consisted of 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl, with the pH adjusted to 7.0, using approximately 0.2 mL of NaOH (5 N).
Minimum medium salts (MMS) were supplemented with KH2PO4 (1.5 g/L), Na2HPO4 (3.6 g/L), (NH4)2SO4 (1.0 g/L), MgSO4·7H2O (0.2 g/L), yeast extract (0.1 g/L), residual glycerol (20 g/L) as a carbon source, and 1.0 mL/L trace element (TE) solution consisting of FeSO4·7H2O (10 g/L), ZnSO4·7H2O (2.25 g/L), CuSO4·5H2O (1.0 g/L), MgSO4·5H2O (0.5 g/L), CaCl2·2H2O (2.0 g/L), NaB4O7·10H2O (0.23 g/L), (NH4)6Mo7O24 (0.1 g/L), and 10 mL HCl (35%), with a final pH of 7.3 ± 0.2.
2.5. Activation of Bacterial Strain and Inoculum Preparation
2.5.1. Activation of B. megaterium LVN01
A total of 10 µL of the previously preserved cells were cultured in LB broth with 20% glycerol on nutrient agar plates at 37 °C for 24 h. The presence and purity of the microorganism were determined via Gram staining and spore staining with malachite green. PHA accumulation capacity was verified via Nile blue staining, described later in the paper.
2.5.2. Inoculum Preparation
A colony of B. megaterium LVN01 was resuspended in 10 mL of LB broth and incubated for 24 h in an orbital shaker (Heidolph Unimax 1010 with Inkubator 1000, Heidolph instruments, Schwabach, Germany) at 37 °C and 200 rpm. Subsequently, 1 mL of the activated microorganism was diluted in 50 mL of LB culture medium. The cultures were incubated in an orbital shaker (37 °C and 200 rpm) for 6 h or until the solution in each flask reached an optical density at 600 nm (OD600) greater than 1.0 unit. Finally, for the inoculum, a new fermentation was conducted taking 100 mL of the pre-inoculum and 200 mL of MMS medium. The culture was incubated in an orbital shaker at 37 °C and 200 rpm for approximately 24 h, until the estimated OD600 readings were between 0.1 and 0.3 units.
2.5.3. Characterization of B. megaterium and Visualization of the Accumulated PHA
The Gram staining protocol [27] was used to identify the morphology of the bacterial cells of B. megaterium LVN01. The stained slide was allowed to air dry and observed with a microscope (Leica DM500, Leica microsystems, Wetzlar, Germany) (Figure S1). Spore staining was then developed using the Shaeffer–Fulton methodology [28,29]; the slide was covered with safranin for 2 min, washed, allowed to dry, and observed under the microscope (Figure S2). Polyhydroxyalkanoate (PHA) accumulation was first visualized with Nile red [30], selecting colonies that showed red-orange fluorescence under UV light (340 nm), potentially classified as PHA producers (Figure S3a). Subsequently, to confirm the presence of PHA, the selected colonies were stained with Nile blue following the methodology of Ostle and Holt (1982) [31] and observed under a fluorescence microscope at 450 nm (Nikon Eclipse 80i, Tokyo, Japan). This process allowed for the visualization of the biopolymer granules (Figure S3b).
2.6. Aerobic Fermentative Processes
The bioprocesses were carried out in a 7 L bioreactor with a stirred tank (Applikon, equipped with two Rushton-type propellers and an EZ2 biocontroller, series 2310110012, Delf, Netherlands), operated in batch mode. The initial working volume was 3 L (diluted liquid digestate, MMS, and inoculum in a ratio of 80:10:10) (Figure S4). Temperature was set at 30.8 °C, stirring speed at 400 rpm, and time at 60 h. To 300 mL of MMS, 300 mL of the B. megaterium inoculum was added, followed by 2.4 L of the digestate or carbon source. Under these conditions, the initial OD600 of the medium was recorded between 0.1 and 0.3 units. The fermentation medium was maintained at pH 7.0 ± 0.2 (adjusted using HCl (MERCK, Darmstadt, Germany) or NaOH (MERCK, Darmstadt, Germany, 1 M, as required), and the dissolved oxygen was set at 100% saturation by injecting several pulses of extra dry industrial oxygen during fermentation (1 vvm), through a 0.22 μm vent filter, with an oxygen flow of 3 L/min to maintain aerobic conditions. Drops of a 10% (v/v) antifoam solution of silicone (Antioqueña from chemicals, food grade, Colombia) were added at the beginning of each batch and when necessary.
In this research, optimal operational parameters established by Gómez et al. [24] were applied for PHA production, using B. megaterium LVN01 and residual glycerol as a carbon source, in a 5 L bioreactor operated in batch mode with an effective volume of 3 L. The proportion of digestate (carbon source) used was derived from a previous laboratory-scale study, which showed the highest values of PSC (27.1 mg) and PHA (14.8 mg) at 36 h during batch fermentations in 500 mL flasks under orbital shaking (T: 30 °C, 200 rpm), pH: 7.0, and an MMS–digestate ratio of 20:80.
Fermentation kinetics were monitored for 60 h, with samples (20 mL) taken in triplicate every two hours. Bacterial growth was measured spectrophotometrically (OD600), followed by determination of dry cell weight (DCW) and PHA-type biopolymer extraction.
Each sample was centrifuged at 10,000× g for 10 min at 4 °C (Centrifuge Heraeus Megafuge 16R, Thermo Scientific, Kalkberg, Germany). The bacterial pellets were washed twice consecutively with 20 mL of deionized water. Finally, the granules were resuspended in 700 µL of deionized water and frozen at − 4 °C until subsequent lyophilization (at −50 °C, 0.01 mBar for 24 h). The collected bacterial biomass was dried at 60 °C (Binder class 2.0 oven, Germany) until it reached a constant weight, and it was reported as DCW (grams of cells per liter of culture medium).
2.7. Extraction and Purification of PHA
The biopolymer was isolated from the freeze-dried biomass following the methodology of Gómez et al. [24]. The dry biomass was treated with a mixture of 10% (v/v) sodium hypochlorite (NaClO, Químicos JM, Medellín, Colombia) and chloroform (CHCl3) (MERCK, 99 %, Darmstadt, Germany) in a 1:1.5 ratio. The sample was homogenized by gentle and rapid shaking (vortex) and incubated at 40 °C and 200 rpm for 3 h. Subsequently, it was centrifuged (10,000× g, 10 min, 4 °C) to separate the phases. The organic phase containing the PHA was filtered through thick filter paper. The filtrate was dried at room temperature (27 °C) in a biosafety cabinet (BIOBASE, model BBS-DDC, series BBS11V1805175D, Shandong, Chinese, air speed 0.3–0.5 m/s), and its dry mass was measured using a scale (Shimakzu, AUW22OD series, Kyoto, Japan with a working range from 220 g to 1 mg).
For PHA purification, the procedure of Gómez et al. [24] was followed, with some modifications: 500 µL of cold (0–4 °C) 70% v/v methanol (J.T. Baker, G.R, Phillipsburg, NJ, USA) was added to each tube containing the dry filtrate to precipitate the particles. The solution was refrigerated at 4 °C for 12 h and then centrifuged at 8000× g and 4 °C for 10 min. The solvent was evaporated at room temperature (27 °C) in a gas extraction hood to recover the PHA.
Next, the biopolymer (PHA) was consecutively washed with n-hexane (MERCK, G.R, Darmstadt, Germany), acetone (MERCK, G.R, Darmstadt, Germany), and diethyl ether (Mallinckrodt, 99.9%, Saint Louis, MO, USA). Each solvent (250 μL) was added dropwise to the dry PHA sample, followed by centrifugation (8000× g, 4 °C, 10 min). The supernatant was discarded, and the precipitate was air-dried at room temperature (27 °C) for 12 h to remove any remaining solvent. This washing process was repeated 2-to-3 times until an almost-white PHA was obtained to ensure its purity.
To calculate the PHA ratio based on the amount of accumulated dry weight, expressed as a percentage, the following equation was used:
2.8. PHA Characterization
2.8.1. Gas Chromatography–Mass Spectrometry (GC–MS/SIM)
The analysis of the samples was conducted according to guidelines ISO 12966-2:2017 [32] with some modifications. Poly (3-hydroxybutyric acid) (Sigma-Aldrich, Lot: 09217BD-337, St. Louis, MO, USA) was used as a reference standard. The chromatographic analysis was performed on a GC AT 6890 Series Plus (AT, Palo Alto, CA, USA) coupled with a mass selective detector (AT, MSD 5973) operated in SIM mode. The injection was performed in splitless mode (Viny = 2 μL). The column used in the analysis was 60 m × 0.25 mm × 0.25 μm DB-5MS 5%-Ph-PDMS.
2.8.2. Nuclear Magnetic Resonance Spectroscopy (1H-NMR and 13C-NMR)
A 600 MHz NMR spectrometer was used (Bruker, Avance III HD, nitrogen TCI cryoprobe, Topspin Software v3.6.5, 5 mm tube, Billerica, MA, USA). The sample was previously prepared by dissolving 30 mg of biopolymer in deuterated chloroform (CDCl3), and 700 μL tetramethyl silane (TMS) was used as an internal control.
2.9. Thermal Properties of PHA
2.9.1. Differential Scanning Calorimetry
A Q2000 calorimeter (TA INSTRUMENTS, New Castle, DE, USA) was used in this analysis for differential scanning calorimetry (DSC). An exactly 5.72 mg sample (PHA) was used for the analysis. The test was conducted with a flow of nitrogen as a purge gas of 50 mL/min; then, the equipment was parameterized for the material. There was a stabilization period at a temperature of 25 °C, with heating up to 200 °C at a speed of 10 °C/min.
2.9.2. Thermogravimetric Analysis
For thermogravimetric analysis (TGA) a TGA/SDTA851e (Mettler Toledo, OH, USA) thermogravimetric analyzer was used, operating under a nitrogen atmosphere with a flow rate of 40 mL/min. A 10 mg polyhydroxybutyric acid (PHB) sample was subjected to a temperature range between 30 °C and 800 °C at a rate of 10 °C/min.
2.10. Analysis and Presentation of Results
All tests conducted in this research were conducted in triplicate. The results are presented as median ± standard deviation in both tables and graphs. Sigma Plot version 10.0 software (Systat Software, Inc; Chicago, IL; USA) was used to design the graphs, and the statistical analysis was developed in STATGRAPHICS Centurion version XVI.II (Statgraphics Technologies, Inc.; The Plains, VA, USA).
3. Results
3.1. Fermentation Kinetics
The lag phase of the culture of B. megaterium (LVN01) in digestate extended to nearly 40 h, with its exponential phase occurring between 40 and 66 h (Figure 1). The system was sampled up to 60 h based on previous laboratory-scale experiments under the same operational parameters (Figure S5). Samples were evaluated in triplicate to ensure the accuracy of the results. However, due to the dispersion of the data, it was determined that calculating the median of each experimental unit would provide a more representative measure of the average values:
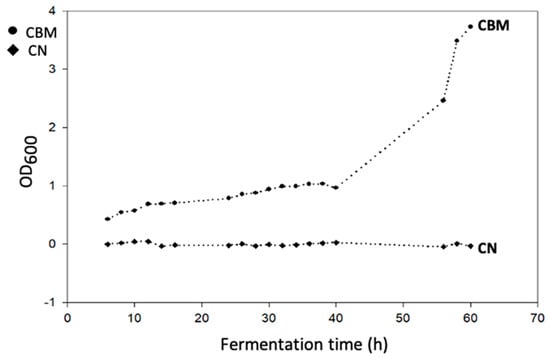
Figure 1.
Growth curve of B. megaterium LVN01 in Biogas digestate (CBM, circles); negative control: medium with digestate (CN, diamonds); operational conditions: 3L, 400 rpm, 30.8 °C, pH 7.
3.2. Poly(3-Hydroxybutyrate)-Co-(3-Hydroxyvalerate) (PHBV) Recovery
The recovery of the biopolymer is essential to assess the success of the bioprocess. Under the parameters of this study, the highest amounts of PHA were detected at 16 h and 24 h, with values of 10.8 mg and 10.3 mg, respectively (Figure 2).
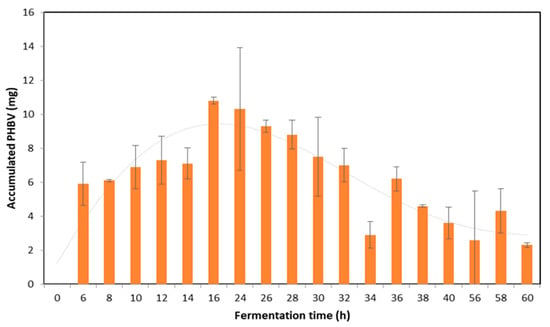
Figure 2.
PHBV accumulated by B. megaterium LVN01 using biogas digestate as a function of fermentation time expressed in hours (h). (V: 3 L; t: 60 h, 400 rpm; T: 30.8 °C, pH 7.0). The dotted line shows the accumulation trend of PHBV during fermentation.
The optimal performance of B. megaterium LVN01 in the digestate occurred at 16 h, with a PHB yield of 2.7 g PHB g cell−1 (270%) and a DCW of 0.133 g L−1 (Figure 3). The maximum values of DCW = 0.563 g L−, reached at 60 h in the fermentation process of B. megaterium with digestate (Figure 3), contrast with the maximum production yield of PHA (360 mg PHBV L−1) observed at 16 h of the process. An inverse trend can be observed between biomass and PHBV production (Figure 4).
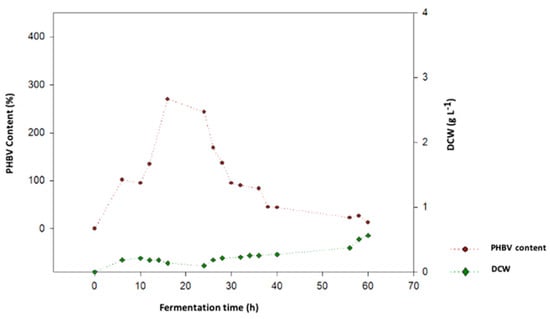
Figure 3.
Cell growth in terms of DCW of B. megaterium LVN01 cultivated in biogas digestate vs. PHBV production (V: 3 L; t: 60 h, 400 rpm; T: 30.8 °C, pH 7.0).
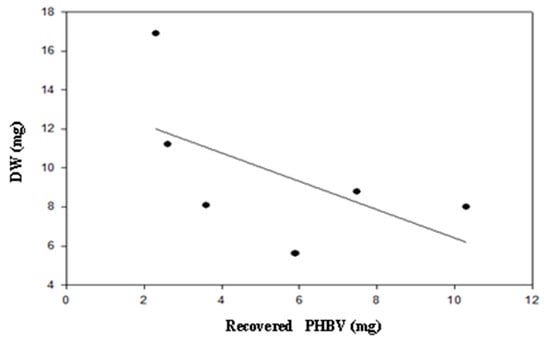
Figure 4.
Dry weight (DW) of B. megaterium LVN01 in digestate from the Biogas plant, San Andrés de Tumaco (V: 3 L; t: 60 h, 400 rpm; T: 30.8 °C, pH 7.0).
3.3. PHBV Characterization
3.3.1. Gas Chromatography with Mass Selective Detector (GC–MS)
The structure of PHBV was initially determined via derivatization (methanolysis), followed by GC–MS analysis. The chromatogram (Figure 5) of the methyl esters derived from the biopolymer extract showed two main signals with retention times of 10.9 min and 14.6 min. Furthermore, it revealed a signal at 14.1 min, albeit with very low intensity (Figure S6). Another low-intensity signal at 13.8 min requires additional mass analysis.
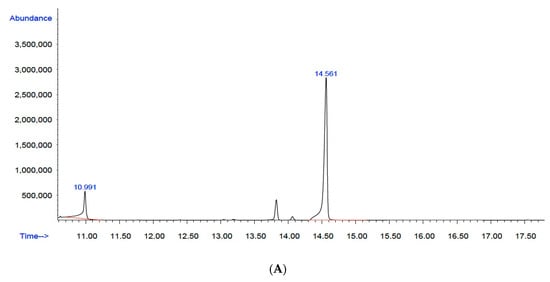
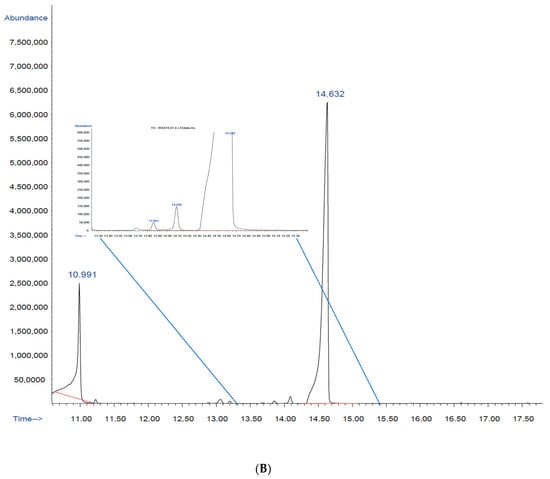
Figure 5.
Gas chromatography–mass spectrometry (GC–MS): (A) chromatogram of the commercial polymer poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (Part N° 403121, Sigma–Aldrich, Lot: MKBL7384V); (B) chromatogram of the copolymer biosynthesized by B. megaterium LVN01 from biogas digestate. The enlargement shows the values of the three peaks, from left to right: 13.861; 14.095 and 14.634.
Furthermore, another peak is observed at 10.991 min, which represents the internal standard used in the analysis, i.e., benzoic acid ester. The presence of this internal standard is essential to guarantee the precision of the quantification and the consistency of the results.
3.3.2. Nuclear Magnetic Resonance (NMR)
To reconfirm the chemical structure of the PHA inferred from the GC–MS analysis, NMR spectroscopy (1H-NMR and 13C-NMR) was used. The analysis results showed the copolymer, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), to be constituted by the monomers 3HB and 3HV (Figure 6), which is in full agreement with the results of the GC–MS analysis.
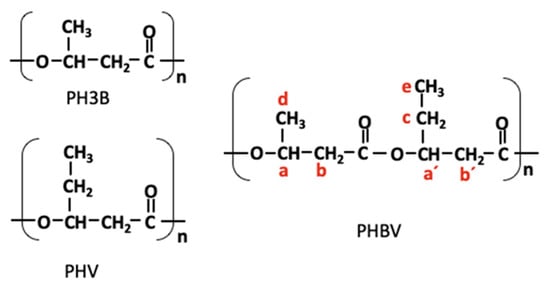
Figure 6.
Chemical structure of PHBV copolymer. PH3B: poly (3-hydroxybutyric acid); PHV: poly (3-hydroxyvaleric acid); PHBV: poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid); n: repetition of monomeric units; a: CH; b: CH2; c: CH2; d: CH3; e: CH3; a’: CH; b’: CH2.
The 1H-NMR spectrum (Figure 7) showed characteristic signals of the PHBV compound (Table 1). Some shifts are typical of the two monomers (3HB and 3HV). However, those that mark the difference between the two subunits appear at δ 1.73–1.78 ppm, δ 1.38 ppm, and δ 1.00 ppm.
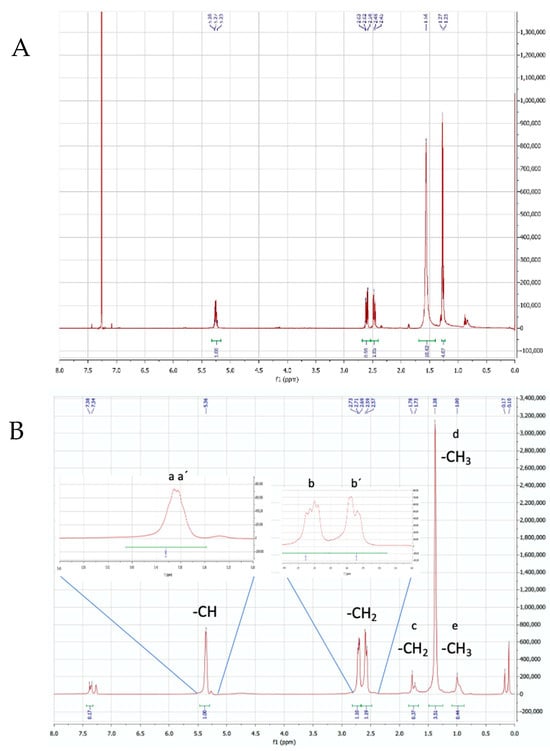
Figure 7.
1H-NMR spectrum of PHBV synthesized by B. megaterium from biogas digestate (batch fermentation), showing the chemical shift of the characteristic functional groups: (A) commercial standard (Figure S7(A1–A3)); (B) synthesized by Bacillus megaterium (Figure S7(B1,B2)).

Table 1.
Characterization of protons 1H-NMR.
The 13C-NMR spectrum (Figure 8) shows the chemical shifts of the signals corresponding to the different types of carbon atoms present in the structure of the biopolymer obtained through the fermentation of B. megaterium LVN01. By comparing these signals (C=O δ 169.18 ppm; CH δ 67.63 ppm; CH2 δ 40.80 ppm; and CH3 δ 19.77 ppm) with those of the commercial PHBV spectrum and those reported by other authors for PHB [24,33], it can be inferred that B. megaterium LVN01 accumulates mostly PHB, which has significant implications in various biotechnological and environmental applications.
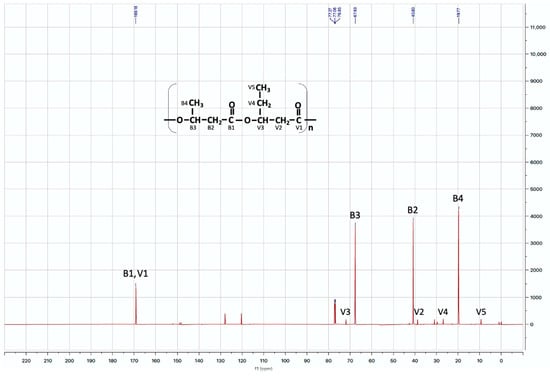
Figure 8.
13C-NMR spectrum, corresponding to the PHBV sample synthesized by B. megaterium LVN01 in liquid digestate.
3.4. Determination of Thermal Properties
The thermal properties of PHBV produced by B. megaterium were analyzed using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) under a nitrogen atmosphere.
The thermal stability of the obtained biopolymer was determined through thermogravimetric analysis. Based on the thermogravimetric (TG) and derivative (DTG) curves (Figure 9), it is inferred that the obtained PHBV exhibits a single stage of decomposition under a continuous and uniform process. The TG curve profile (Figure 9A) indicates that it is a thermally stable copolymer in the temperature range of 35.0–254.540 °C, reaching a Td 1% of 266.2 °C (the temperature at which a weight loss of 1% occurs) at 23.58 min. It continues with minor mass losses over time, until reaching a Td 10% of 280.9 °C (t = 25.08 min). Subsequently, the material begins to degrade, with an initial decomposition temperature Td (Tonset) of 283.1 °C and a final decomposition temperature (Tendset) of 296.98 °C. In this temperature range, and in just 2 min, the compound loses about 72% of its weight, with a maximum Td of 290.114 °C, at which the maximum decomposition rate is reached (Figure 9B). In the Tendset, a weight loss of 86% is observed, and approximately 90% of the mass is lost in the temperature range of 253.376 °C to 308.084 °C.
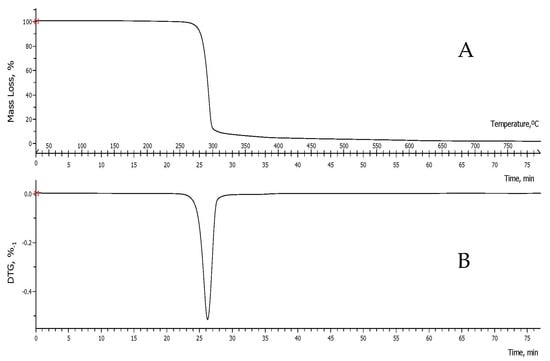
Figure 9.
Curves of the thermogram (TG) (A) and its derivative (DTG) (B) of the PHBV sample synthesized by B. megaterium LVN01 from biogas digestate.
Comparing these results with those of Gómez et al. (2020), who reported a Td of 266.2 °C and a weight loss of 97.7% in the temperature range of 230 °C to 300 °C for PHB synthesized by B. megaterium using residual glycerol as a substrate [24], it can be concluded that B. megaterium adjusts its biochemical battery to metabolize biogas digestate, producing a PHA with better physical properties than that synthesized by the bacteria when using residual glycerol. In this context, the thermal stability of the material is particularly important, which is associated with the Td, PHAs with low melting temperatures, and/or higher thermal stability values that are more desirable [34]. The marked difference between the Td values recorded for the PHA synthesized by B megaterium with respect to biogas digestate (Td = 290.114 °C) and residual glycerol (Td = 266.2 °C) allows us to deduce the presence of two different chemical structures, which complements the results of GC–MS and NMR.
In any case, the Td (290.114 °C) of the PHBV under study is above the maximum Td values reported in the literature for PHB: 220 °C [34], 252 °C [35], and 280 °C [36], among others. On the other hand, despite exceeding them, it is closer to the Td values reported for PHBV: 279.236 °C [34], 285.9 °C [37], and 286 °C [37], among others.
The DSC analysis of the biopolymer synthesized by B. megaterium LVN01 from the biogas digestate revealed three exothermic thermal events (Figure 10). The two most significant have melting temperatures of Tm1 = 143.01 °C and Tm2 = °C 155.7 °C and fusion enthalpies of (ΔHf) = 1.541 J g−1 and (ΔHf) = 19.80 J g−1. These results agree with those of other studies; for example, Abbasi et al. [38] obtained PHBV via the fermentation of dairy manure with mixed microbial consortia (MMC), with 3HV contents between 16% and 24%, and reported that some of the extracted PHBV showed two melting temperatures (Tm); the lowest Tm1 ranged between 126.1 °C and 159.7 °C, and the highest Tm2 varied between 152.1 °C and 170.1 °C.
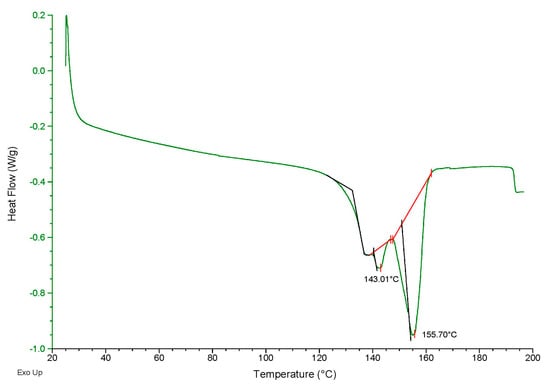
Figure 10.
DSC thermogram of the PHBV sample synthesized by B. megaterium LVN01 from biogas digestate (Green curve). The red line shows the temperature changes between each thermal event. The black line corresponds to the glass transition.
The authors Abbasi et al. [39] indicate that the presence of isomorphism phenomena can lead to the presence of two fusion peaks [38,39,40]. As PHBV has a semi-crystalline structure, crystals with higher HV content will have a higher amorphous phase ratio and will therefore melt first during heating (Tm1). In crystals with lower HV content, the crystallinity ratio is higher, so the crystals will melt at a higher temperature (Tm2) [39,41].
The degree of crystallinity Xcr (%) was calculated from the values recorded in the calorimetric curve (DSC) using equation:
where:
- ΔHf: Enthalpy of fusion of PHB produced by B. megaterium LVN01;
- ΔHf: Enthalpy of fusion of pure PHB, equivalent to 109 J g−1 [42].
The calculations associated with the most significant event showed an Xcr = 18.17% for the PHBV obtained under the operational parameters of this research, which is lower than that obtained for the biopolymer (PHB) synthesized by the same species (B. megaterium LVN01) from residual glycerol, reaching Xcr = 35.7% [24]. Other authors have reported Xcr values for PHB in the range of 30% to 60% [43,44,45].
4. Discussion
Maximizing the production of PHA-type biopolymers through the microbial biofermentation of different substrates has become a crucial field of research in the face of the growing demand for sustainable alternatives for the production of plastics. This bioindustry seeks environmentally friendly and economically viable processes that can compete with the petroleum-derived plastics industry [46]. The main problem associated with the commercial disposal of bioplastics is the high production costs, to which raw materials significantly contribute [20,46,47,48]. Given this, researchers have turned their attention to the search for economic and sustainable substrates, especially residual biomass, that make an industrial process viable.
In this research, taking advantage of the ability of B. megaterium LVN01 to metabolize different substrates through different pathways [12,24,49,50,51,52] was considered important in the evaluation of biogas digestate, a byproduct of the anaerobic digestion of the waste products of artisanal fishing. It has not been explored before; or, at least, it has yet not been reported in the same sense as it is in this research.
Our results demonstrate that biogas digestate, a substrate of residual origin rich in volatile fatty acids, is an excellent substrate for the growth of B. megaterium and the production of PHA-type bioplastics. The dynamics of cell growth (Figure 1), under the operational conditions of this work, show the advantage of biogas digestate over residual glycerol, another substrate evaluated under similar conditions. As has been observed, the adaptive phase of B. megaterium (LVN01) in the digestate lasts 40 h, almost double the time reported (24 h) for the same microbial strain in residual glycerol [50]. Typically, once the late stationary phase or exponential phase is reached, the microbial metabolism of B. megaterium begins to accumulate PHA [53]. However, the accumulation response can vary depending on the type of substrate [54]; when the substrate is digestate, the efficiency of PHBV accumulation increases (360 mg PHBV L−1 in 16 h) in earlier phases. This can be attributed to the stress conditions to which it is subjected [54], considering that digestate is a fairly complex substrate. The optimal performance of B. megaterium (LVN01) in digestate occurred at 16 h. In contrast, Gómez et al. showed that B. megaterium (LVN01) in residual glycerol needs 48 h to reach a yield of 137.5 g PHB L−1 with a DCW of 58.1 mg PHB gcell−1; after this time, these parameters decrease drastically [49].
It has been highlighted that in the digestate, the DCW values are higher, and the bacterial cells accumulate a greater amount of PHBV per gram of biomass in short periods of time. The excellent storage capacity of the biopolymer is particularly evident after the first 24 h of incubation, and it remains at relatively high levels until 32 h (Figure 2). In agreement with what has been reported by other authors, it is inferred that the first stage of fermentation is a period of initial adaptation in which the bacteria multiply and increase their biomass before beginning to synthesize and accumulate PHA efficiently [53,55,56,57].
The results support the idea that microbial kinetics are strongly influenced by the chemical nature of the carbon source. Unlike residual glycerol, the digestate is a complex mixture of organic compounds that includes fatty acids and other byproducts of anaerobic digestion of organic material. This complexity could mean that B. megaterium (LVN01) requires a longer time to adapt to the medium and assimilate the available nutrients to grow efficiently, thus explaining its extended growth kinetics. Additionally, in the early stages of fermentation, rapid bacterial growth (higher DCW) is observed before the bacteria prioritize PHBV synthesis. This inverse relationship (Figure 4) between biomass and PHB production follows the trend reported by other authors for B. megaterium from other substrates [11,24,50,58].
In conclusion, the lag phase (adaptive phase) plays a crucial role in fermentation, being important for the microorganism to activate its metabolism and make the most of the nutrients in the culture medium. In complex substrates such as digestate, B. megaterium requires long acclimatization times before it achieves optimal growth, while it can multiply rapidly in simple substrates. This information is essential to designing and optimizing fermentation processes since the microbial adaptation time with respect to the substrate can significantly affect the productivity and efficiency of the process [21]. In this research, B. megaterium was able to synthesize and accumulate PHBV during the first hours of the bioprocess (16 h–32 h), using biogas digestate as a carbon source, which is promising for efficient industrial production. Meanwhile, it could reduce the dependence on oil, a non-renewable resource, and on expensive and less sustainable carbon sources such as agricultural crops [59,60,61,62,63].
The chemical characterization of the biopolymer synthesized by B. megaterium from biogas digestate was conducted using gas chromatography with a mass selective detector (GC–MS) and nuclear magnetic resonance spectroscopy (1H-NMR and 13C NMR).
The results obtained in this research demonstrate the ability of B. megaterium to produce PHBV from different substrates through fermentation processes. The chromatogram (GC–MS) of the polymeric extract (Figure 5) was compared with that of the commercial PHBV standard. In both cases, two main peaks were observed with retention times (RT) of 10.9 min and 14.6 min, identified via the GC–MS database as 2-butenoic acid methyl ester and the methyl ester of 3-hydroxybutyric acid, respectively, which are characteristic of the 3HB monomer.
The lower intensity peak that appeared at 14.1 min is attributed to the methyl ester of 2-pentanoic acid, typical of the 3HV monomer, identified by the team’s data system with a quality of 97% (Figure S6). These are formed by methanolysis of 3HB, resulting in C4 esters, and 3HV, resulting in C4 and C5 esters [33]. 2-butenoic, 3-hydroxybutyric, and 2-pentanoic acids are also byproducts of the thermal degradation of PHBV [64]. The information obtained via GC–MS supports the predominant presence of polyhydroxybutyric acid and, in lower proportions, polyhydroxyvaleric acid in the extracts of B. megaterium.
Nuclear magnetic resonance spectroscopy allowed us to elucidate the chemical structure of the biopolymer synthesized by B. megaterium LVN01 under batch-type aerobic fermentation with carbon source biogas digestate. The 1H-NMR (Figure 7) and 13C-NMR (Figure 8) spectra of the purified PHBV sample and the pure standard were similar, showing seven typical signals of the poly(3-hydroxybutyrate-co-3 hydroxyvalerate) copolymer between 0.75 ppm and 5.50 ppm, which are associated with the chemical shifts (δ) of the 3HB (3-hydroxybutyrate) and 3HV (3-hydroxyvalrate) subunits of the copolymer PHBV.
The H-NMR signals detect the presence of characteristic functional groups of both monomeric fractions (3HB and 3HV), i.e., δ = 5.356–5.363 ppm (a and a’), concerning the protons of the methine group (-CH), and δ = 2.57–2.59 ppm and δ = 2.69–2.73 ppm (b and b’), corresponding to the protons of the methylene group (CH2). Particularly, the 3HB subunit exhibits a δ = 1.25–1.26 ppm, belonging to the methyl group (CH3) (c). On the other hand, 3HV gives rise to shifts at δ = 1.73–1.78 ppm (d) and δ = 1.0 ppm (e), which allow us to infer the resonance of the methylene (d) and methyl protons (e) in the side chain (ethyl). These last displacements mark the difference between the monomers (HV and HB) and confirm the presence of PHBV. The findings of this research coincide with those reported by other authors for the PHBV copolymer [39,65,66,67,68,69,70].
The results of 13C-NMR analysis are consistent with those of 1H- NMR (Figure 7). The 13C-NMR spectrum presents signals identifying the non-equivalent carbon atoms in each monomer. For HB, carbon shifts were observed at 19.65 ppm, 40.68 ppm, and 67.52 ppm, corresponding to the methyl (–CH3), methylene (–CH2–), and ester (–O–CH–) groups, respectively. Particularly for HV, the signals of the two methylene groups (–CH2–) were observed at 26.74 ppm and 30.82 ppm. In this monomer, the peak corresponding to the ester group (–O–CH–) shifted to 71.90 ppm. Finally, a resonance of 169.14 ppm of the carbon atoms of the carbonyl group (–C–) is presented for both monomers (3-HB and 3-HV). These data coincide with those reported by other researchers [38,39,56,69] and allow us to determine the presence of the PHBV copolymer in the polymeric extract of B. megaterium.
The chemical characterization of the B. megaterium biopolymer is consistent with the characteristics derived from the analysis of the thermal properties of the biopolymer using TGA (Figure 9) and DSC (Figure 10). The values of the thermal properties (Tm = 153 °C and Td = 290.14 °C) of PHBV produced by B. megaterium from biogas digestate are within the range of the values measured by Wang et al. [71], who analyzed three PHAs synthesized by Ralstonia eutropha from levulinic acid. The researchers found melting temperatures (Tm) of 101.93 °C, 150.18 °C, and 172.05 °C, as well as thermal decomposition temperatures (Td) of 284.4 °C, 298.3 °C, and 263.4 °C, for PHB with 0%, 0.16%, and 53% HV, respectively [62]. However, the percentage of crystallinity (% Xcr) = 18 of the PHBV under study differed markedly from those reported for PHB with 0% (% Xcr = 61.44%), 0.16% (% Xcr = 50.34%), and HV 53% (% Xcr = 51.92%). Nonetheless, regarding this parameter, the results of this research are also comparable with those of Abbasi et al., who, for a series of PHBV produced by fermentation, reported Xcr values via DSC between 16.6% and 29% [39]. In their research, the PHBV with the lowest 3HV content (0.16) recorded the highest Xcr (29%). From their study, Abbasi et al. [39] and other researchers [71] concluded that the degree of crystallinity and the melting point of the biopolymer decrease with the HV content in the polymer, potentially resulting in an improvement in the ductility and flexibility of the polymer.
The biopolymer produced in this research presents thermodynamic properties that differentiate it from the PHB structure and bring it closer to the PHBV line. In this context, it is relevant to note that according to the literature, when a copolymer such as PHBV is produced, it has a lower melting point and greater flexibility compared to the PHB homopolymer [72]. The lower crystallinity and lower melting point of PHBV from B. megaterium suggest an appreciable percentage of HV with a more flexible structural order. This aligns with the characterization of short-chain biopolymers, which are known to have lower crystallinity and lower melting points, classifying them as elastomers and providing them with an elongation capacity at break greater than 100% [73,74].
In this study, B. megaterium LVN01 demonstrated its efficient ability to produce PHBV from biogas digestate, a substrate rich in volatile fatty acids, in just a 16 to 24 h fermentation period. This is promising for the industrial production of biopolymers since PHBV not only has appropriate physical characteristics for use in different industrial fields but also has a high degree of biodegradability, which makes it very attractive in the pharmaceutical industry of biopolymers.
The physical characteristics of PHBV from B. megaterium are intrinsically related to the biodegradation properties and ease of decomposition of PHAs. These degradation processes are influenced by the specific chemical composition of the polymer and the surrounding environmental conditions. The degradation of objects made with PHAs has been reported to vary widely, from a few months in soils with stable temperatures to a couple of years in marine environments. It should be noted that certain types of PHAs, such as poly(3-hydroxybutyrate-co-3-hydroxyvalerate), are notably affected in their crystallinity, which modulates their degradation rates [75].
The potential of the aerobic fermentation process with the B. megaterium system using biogas digestate is strengthened by the use of an economical substrate for the production of PHA-type biopolymers, specifically PHBV. The main advantages of using digestate lie in its availability and low cost, considering that it is a coproduct of the biogas production process from residual biomass from the artisanal fishing industry in Tumaco, Colombia. In addition to the economic advantage, there are positive environmental and social impacts: first, digestates represent alternatives to petroleum-derived plastics, which generate significant pollution during their manufacture and consume a non-renewable resource; second, fishing waste (fish viscera) is utilized to produce biogas, and then, from the residual effluent (digestate rich in volatile fatty acids), PHA-type biopolymers are produced. With this approach, pollution from fishing waste, often discarded into the sea, is avoided. All this is conducted in a biorefinery environment, contributing to increasing the added value of the artisanal fishing production chain in communities living in vulnerable conditions, as well as the circular bioeconomy of biogas production.
Regarding the sustainability of the substrate, it is important to mention that artisanal fishing in the Colombian Pacific generates an abundant amount of waste, representing a valuable source of renewable organic matter for the efficient production of PHBV through fermentation with B. megaterium. Thus, the cost of raw materials for large-scale PHBV production could be significantly reduced, with important effects in terms of economics and environmental sustainability.
5. Conclusions
Liquid digestate, a byproduct of biogas production through the anaerobic digestion of residual biomass from the artisanal fishing industry, is a renewable secondary substrate suitable for cultivating the native Colombian bacteria B. megaterium LVN01 and synthesizing PHA-type biopolymers, specifically the copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV).
Under the operational conditions of this research, the B. megaterium digestate system produces appreciable quantities of PHBV in just 16 h, with PHBV productivities of 360 mg/L·h, which adds further value to the fishing residue (fish viscera). Within the framework of the circular economy, this could strengthen the biogas bioindustry and provide added value to the artisanal fishing production chain in Colombia. Analyses conducted on the PHBV copolymer demonstrate that it is a thermostable, flexible polymer. Combined with its biodegradable nature, this suggests its potential usefulness in a wide variety of industrial applications. However, further research is required to define the percentages of participation of the HV monomer in the PHBV polymeric structure, as well as complementary research to establish optimal bioprocess operations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/applmicrobiol4030072/s1: Figure S1: Gram staining of Bacillus megaterium incubated in Luria Bertani broth for 24 h; Figure S2: Observation of stained spores from wild-type Bacillus megaterium samples grown on nutrient agar under stress conditions (44 °C) at 100× magnification; Figure S3: Observation under fluorescence microscope (λ = 510–560 nm) of growth of wild-type Bacillus megaterium adapted to digestate from the homogeneous tank from the biogas plant at Tumaco. A. Growth on Nile Red agar B. Nile Blue staining; Figure S4: Laboratory-scale experiments for the growth of B. megaterium in digestate under the following conditions: pH 7.0, Temperature: 30.8 °C, agitation 200 rpm, and three digestate: MMS ratio conditions 100:0, 80:20, 50:50; Figure S5: Growth curve at laboratory scale of B. megaterium LVN01 in Biogas digestate, operational conditions: 10 mL, 200 rpm, 30.8 °C, pH 7.0; Figure S6: Reconstructed ion current mass spectrum for PHBV obtained from fermentation of Bacillus megaterium LVN01 in digestate; Table S1: Waste liquid digestate composition reported by the “Environmental Prospective” research group of the Universidad Nacional de Colombia—Palmira headquarters.
Author Contributions
Conceptualization, A.L.M.M., M.Y.-P., K.A.C.C. and P.E.Z.M.; data curation, A.L.M.M., M.Y.-P., K.A.C.C. and P.E.Z.M.; lab, A.L.M.M., M.Y.-P., K.A.C.C. and P.E.Z.M.; methodology, A.L.M.M., M.Y.-P., K.A.C.C. and P.E.Z.M.; project administration, A.L.M.M., M.Y.-P. and K.A.C.C.; resources, A.L.M.M. and M.Y.-P.; supervision, A.L.M.M. and M.Y.-P.; validation, A.L.M.M., M.Y.-P., K.A.C.C. and P.E.Z.M.; visualization, A.L.M.M., M.Y.-P. and K.A.C.C.; writing—original draft, A.L.M.M., M.Y.-P. and K.A.C.C.; writing—review and editing, A.L.M.M., M.Y.-P., K.A.C.C. and P.E.Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
Universidad Nacional de Colombia: Strengthening artisanal fishing activity in the Colombian Nariñense Pacific towards a sustainable use of the resource. Tumaco, Code Hermes: 49521, BPIN: 2020000100068, General System of Royalties (SGR).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors express their gratitude to the General Royalties System for their financial support through project BPIN 2020000100068. Additionally, they thank the directors of the Laboratory of Chemical Analysis and Environmental Processes, the Laboratory of Natural Venoms, and the Laboratory of Biological Processes at the Universidad Nacional de Colombia, Medellin campus, for their collaboration and support with physical infrastructure, equipment, and, in some cases, essential reagents for conducting the experimental assays. Thanks are also due to the Environmental Research Laboratory at the Palmira campus of the Universidad Nacional de Colombia, specifically to Luz Stella Cadavid Rodríguez for providing the digestate samples for this research, and to Elena Stashenko, director of the Chromatography and Mass Spectrometry Laboratory at the Universidad Industrial de Santander, for her support with the analysis of the PHA samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jaso Sánchez, M.A. El surgimiento de los bioplásticos: Un estudio de nichos tecnológicos. Acta Univ. 2020, 30, 1–24. [Google Scholar]
- Plastics Europe, Enabling a Sustainable Future. Plastics—The Fast Facts. Infografía. 2023. Available online: https://plasticseurope.org/es/plastics-europe-publica-plastics-the-fast-facts-2023/ (accessed on 20 March 2024).
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bh Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar]
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.A.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H.; et al. Recent advances in sustainable plastic upcycling and biopolymers. Biotechnol. J. 2020, 15, 1900489. [Google Scholar] [CrossRef] [PubMed]
- Organización de las Naciones Unidas. Todo lo que necesitas saber sobre la contaminación por plásticos. ONU programa para el medio ambiente. Available online: https://www.unep.org/es/noticias-y-reportajes/reportajes/todo-lo-que-necesitas-saber-sobre-la-contaminacion-por-plasticos (accessed on 25 April 2023).
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Madhumitha Jaganmohan. Available online: https://www.statista.com/aboutus/our-research-commitment/2234/madhumitha-jaganmohan (accessed on 20 March 2024).
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Kaniuk, Ł.; Stachewicz, U. Development and advantages of biodegradable PHA polymers based on electrospun PHBV fibers for tissue engineering and other biomedical applications. ACS Biomater. Sci. Eng. 2021, 7, 5339–5362. [Google Scholar] [CrossRef]
- Koller, M. Production of polyhydroxyalkanoate (PHA) biopolyesters by extremophiles. MOJ Polym. Sci. 2017, 1, 1–19. [Google Scholar] [CrossRef]
- Cal, A.J.; Kibblewhite, R.E.; Sikkema, W.D.; Torres, L.F.; Hart-Cooper, W.M.; Orts, W.J.; Lee, C.C. Production of polyhydroxyalkanoate copolymers containing 4-hydroxybutyrate in engineered Bacillus megaterium. Int. J. Biol. Macromol. 2021, 168, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Moreno, S.A.; Marín Montoya, M.A.; Mora Martínez, A.L.; Yepes Pérez, M.D.S. Identification of polyhydroxyalkanoate-producing bacteria in soils contaminated with fique wastes. Rev. Colomb. De Biotecnol. 2012, 14, 89–100. [Google Scholar]
- Dhangdhariya, J.H.; Dubey, S.; Trivedi, H.B.; Pancha, I.; Bhatt, J.K.; Dave, B.P.; Mishra, S. Polyhydroxyalkanoate from marine Bacillus megaterium using CSMCRI’s Dry Sea Mix as a novel growth medium. Int. J. Biol. Macromol. 2015, 76, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ray, S.; Kalia, V.C. Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour. Technol. 2016, 200, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Jamil, N. Polyhydroxyalkanoates (PHA) production in bacterial co-culture using glucose and volatile fatty acids as carbon source. J. Basic Microbiol. 2018, 58, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Sawant, S.S.; Kim, B.S. Production of polyhydroxyalkanoates by Ralstonia eutropha from volatile fatty acids. Korean J. Chem. Eng. 2013, 30, 2223–2227. [Google Scholar] [CrossRef]
- Martinez, G.A.; Bertin, L.; Scoma, A.; Rebecchi, S.; Braunegg, G.; Fava, F. Production of polyhydroxyalkanoates from dephenolised and fermented olive mill wastewaters by employing a pure culture of Cupriavidus necator. Biochem. Eng. J. 2015, 97, 92–100. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Increased production of polyhydroxyalkanoates with controllable composition and consistent material properties by fed-batch fermentation. Biochem. Eng. J. 2019, 141, 35–42. [Google Scholar] [CrossRef]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Pandey, J.P.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef]
- Kleerebezem, R.; Joosse, B.; Rozendal, R.; Van Loosdrecht, M.C. Anaerobic digestion without biogas? Rev. Environ. Sci. Bio/Technol. 2015, 14, 787–801. [Google Scholar] [CrossRef]
- Cadavid-Rodríguez, L.S.; Castro-López, V.E.; Placido, J. Evaluation of optimal fermentation conditions for volatile fatty acids production from artisanal fish waste. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Gómez-Cardozo, J.R.; Velasco-Bucheli, R.; Marín-Pareja, N.; Ruíz-Villadiego, O.S.; Correa-Londoño, G.A.; Mora-Martínez, A.L. Fed-batch production and characterization of polyhydroxybutyrate by Bacillus megaterium LVN01 from residual glycerol. Dyna 2020, 87, 111–120. [Google Scholar] [CrossRef]
- Salazar, A. Parámetros Operacionales Óptimos Para La Producción de Bioplásticos a Partir de Fuentes Renovables Azucaradas; Universidad Nacional de Colombia: Medellín, Colombia, 2011. [Google Scholar]
- Rai, A.K.; Swapna, H.C.; Bhaskar, N.; Halami, P.M.; Sachindra, N.M. Effect of fermentation ensilaging on recovery of oil from fresh water fish viscera. Enzym. Microb. Technol. 2010, 46, 9–13. [Google Scholar] [CrossRef]
- López-Jácome, L.E.; Hernández-Durán, M.; Colín-Castro, C.A.; Ortega-Peña, S.; Cerón-González, G.; Franco-Cendejas, R. Las tinciones básicas en el laboratorio de microbiología. Investig. En Discapac. 2014, 3, 10–18. [Google Scholar]
- Tinción de Esporas: Fundamento, Técnicas y Usos. Lifeder. Available online: https://www.lifeder.com/tincion-de-esporas/ (accessed on 18 January 2024).
- Pérez, R.; Juárez, M.; Rodríguez, L.P. Manual de Laboratorio de Técnicas Microbiológicas; Departamento de Ciencias Básicas Academia de Microbiología. Instituto Politécnico Nacional: Pereira, Colombia, 2011. [Google Scholar]
- Spiekermann, P.; Rehm, B.H.; Kalscheuer, R.; Baumeister, D.; Steinbüchel, A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ostle, A.G.; Holt, J.G. Nile blue A as a fluorescent stain for poly-beta-hydroxybutyrate. Appl. Environ. Microbiol. 1982, 44, 238–241. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- Pradhan, S.; Dikshit, P.K.; Moholkar, V.S. Production, characterization, and applications of biodegradable polymer: Polyhydroxyalkanoates. In Advances in Sustainable Polymers: Synthesis, Fabrication and Characterization; Springer: Singapore, 2020; pp. 51–94. [Google Scholar]
- Liu, J.; Zhao, Y.; Diao, M.; Wang, W.; Hua, W.; Wu, S.; Chen, P.; Ruan, R.; Cheng, Y. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) production by Rhodospirillum rubrum using a two-step culture strategy. J. Chem. 2019, 2019, 8369179. [Google Scholar] [CrossRef]
- Jakić, M.; Vrandečić, N.S.; Erceg, M. Thermal degradation of poly (3-hydroxybutyrate)/poly (ethylene oxide) blends: Thermogravimetric and kinetic analysis. Eur. Polym. J. 2016, 81, 376–385. [Google Scholar] [CrossRef]
- Vahabi, H.; Michely, L.; Moradkhani, G.; Akbari, V.; Cochez, M.; Vagner, C.; Renard, E.; Saeb, M.R.; Langlois, V. Thermal stability and flammability behavior of poly (3-hydroxybutyrate)(PHB) based composites. Materials 2019, 12, 2239. [Google Scholar] [CrossRef]
- Thiré, R.M.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef]
- Abbasi, M.; Coats, E.R.; McDonald, A.G. Green solvent extraction and properties characterization of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) biosynthesized by mixed microbial consortia fed fermented dairy manure. Bioresour. Technol. Rep. 2022, 28, 101065. [Google Scholar] [CrossRef]
- Abbasi, M.; Pokhrel, D.; Coats, E.R.; Guho, N.M.; McDonald, A.G. Effect of 3-hydroxyvalerate content on thermal, mechanical, and rheological properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymers produced from fermented dairy manure. Polymers 2022, 14, 4140. [Google Scholar] [CrossRef]
- Guho, N.M.; Pokhrel, D.; Abbasi, M.; McDonald, A.G.; Alfaro, M.; Brinkman, C.K.; Coats, E.R. Pilot-scale production of poly-3-hydroxybutyrate-co-3-hydroxyvalerate from fermented dairy manure: Process performance, polymer characterization, and scale-up implications. Bioresour. Technol. Rep. 2020, 12, 100588. [Google Scholar] [CrossRef]
- Souza Junior, O.F.; Staffa, L.H.; Costa, L.C.; Chinelatto, M.A. Thermal and Rheological Behavior of Binary Blends of Poly (hydroxybutyrate-co-hydroxyvalerate) and Poly (ethylene-co-vinyl acetate) with Different Vinyl Acetate Content. Macromol. Symp. 2019, 383, 1800020. [Google Scholar] [CrossRef]
- Chan, C.H.; Kummerlöwe, C.; Kammer, H.W. Crystallization and melting behavior of poly (3-hydroxybutyrate)-based blends. Macromol. Chem. Phys. 2004, 205, 664–675. [Google Scholar] [CrossRef]
- Bora, L.; Das, R.; Gohain, D. A novel melt stable and high tensile strength biopolymer (polyhydroxyalkanoates) from Bacillus megaterium (MTCC10086) and its characterization. J. Basic Microbiol. 2014, 54, 1012–1016. [Google Scholar] [CrossRef]
- Castillo, D. Efecto del gen fadH1 en la Producción de PHA Contenido Monómeros Insaturados por Pseudomona Putida; Pontificia Universidad Javeriana: Bogotá, Colombia, 2008. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef] [PubMed]
- Kosseva, M.R.; Rusbandi, E. Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Murphy, R.; Narayan, R.; Davies, G. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Gómez Cardozo, J.R.; Velasco-Bucheli, R.; Del Cerro, C.; Mata, I.D.L.; Mora Martínez, A.L. Engineering of Bacillus megaterium for improving PHA production from glycerol. Asia Pac. J. Mol. Biol. Biotechnol. 2019, 27, 64–72. [Google Scholar]
- Gómez Cardozo, J.R.; Mora Martínez, A.L.; Yepes Pérez, M.; Correa Londoño, G.A. Production and characterization of polyhydroxyalkanoates and native microorganisms synthesized from fatty waste. Int. J. Polym. Sci. 2016, 2016, 6541718. [Google Scholar] [CrossRef]
- Salazar, A.; Yepes, M.; Correa, G.; Mora, A. Polyhydroxyalkanoate production from unexplored sugar substrates. Dyna 2014, 81, 73–77. [Google Scholar] [CrossRef]
- Cardona, A.C.; Mora, A.L.; Marín, M. Identificación molecular de bacterias productoras de polihidroxialcanoatos en subproductos de lácteos y caña de azúcar. Rev. Fac. Nac. De Agron. Medellín 2013, 66, 7129–7140. [Google Scholar]
- Salgaonkar, B.B.; Mani, K.; Braganca, J.M. Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J. Appl. Microbiol. 2013, 114, 1347–1356. [Google Scholar] [CrossRef]
- Alkotaini, B.; Sathiyamoorthi, E.; Kim, B.S. Potential of Bacillus megaterium for production of polyhydroxyalkanoates using the red algae Gelidium amansii. Biotechnol. Bioprocess Eng. 2015, 20, 856–860. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent advances in their synthesis and applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, X.R.; Guo, Y. Synthetic biology of microbes synthesizing polyhydroxyalkanoates (PHA). Synth. Syst. Biotechnol. 2016, 1, 236–242. [Google Scholar] [CrossRef]
- Tian, P.; Shang, L.; Ren, H.; Mi, Y.; Fan, D.; Jiang, M. Biosynthesis of polyhydroxyalkanoates: Current research and development. Afr. J. Biotechnol. 2009, 8, 709–714. [Google Scholar]
- Cavalheiro, J.M.; Raposo, R.S.; De Almeida, M.C.M.; Cesário, M.T.; Sevrin, C.; Grandfils, C.; Da Fonseca, M. Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef]
- Polyhydroxyalkanoate (PHA) Market by Type (Short Chain Length, Medium Chain Lenth), Production Methods (Sugar Fermentation, Vegetable Oil Fermentation), Application (Packing & Food Services Biomedical) and Region-Global Forecast to 2028. Available online: https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html?gad_source=1&gclid=Cj0KCQjwhtWvBhD9ARIsAOP0GogViHNhj3GJBVD8HaHD2hI_z9qSSB0dUmUTmNE-rvudbLClu9nmLCIaAgzzEALw_wcB (accessed on 15 May 2024).
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Lin, K.Y.; Tsang, D.C.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Abd El-malek, F.; Khairy, H.; Farag, A.; Omar, S. The sustainability of microbial bioplastics, production and applications. Int. J. Biol. Macromol. 2020, 157, 319–328. [Google Scholar] [CrossRef]
- Loizidou, M.; Moustakas, K.; Rehan, M.; Nizami, A.S.; Tabatabaei, M. New developments in sustainable waste-to-energy systems. Renew. Sustain. Energy Rev. 2021, 151, 111581. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Xiang, H.; Wen, X.; Miu, X.; Li, Y.; Zhou, Z.; Zhu, M. Thermal depolymerization mechanisms of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. Mater. Int. 2016, 26, 58–64. [Google Scholar] [CrossRef]
- Miranda, D.A.; Marín, K.; Sundman, O.; Hedenström, M.; Quillaguaman, J.; Gorzsás, A.; Brosrom, M.; Carlborg, M.; Lundvist, J.; Romero-Soto LJonsson, L.J.; et al. Production and characterization of poly (3-hydroxybutyrate) from Halomonas boliviensis LC1 cultivated in hydrolysates of quinoa stalks. Fermentation 2023, 9, 556. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P.; Tarafdar, A.; Agarwal, A.; Chaudhuri, S.K.; Sindhu, R. An environmentally sustainable green process for the utilization of damaged wheat grains for poly-3-hydroxybutyrate production. Environ. Technol. Innov. 2021, 21, 101271. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative structure-property characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) s films under hydrolytic and enzymatic degradation: Finding a transition point in 3-hydroxyvalerate content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef]
- Mohapatra, S.; Pattnaik, S.; Maity, S.; Sharma, S.; Akhtar, J.; Pati, S.; Samantaray, D.P.; Varma, A. Comparative analysis of PHAs production by Bacillus megaterium OUAT 016 under submerged and solid-state fermentation. Saudi J. Biol. Sci. 2020, 27, 1242–1250. [Google Scholar] [CrossRef]
- Han, J.; Hou, J.; Zhang, F.; Ai, G.; Li, M.; Cai, S.; Liu, H.; Wang, L.; Wang, Z.; Zhang, S.; et al. Multiple propionyl coenzyme A-supplying pathways for production of the bioplastic poly (3-hydroxybutyrate-co-3-hydroxyvalerate) in Haloferax mediterranei. Appl. Environ. Microbiol. 2013, 79, 2922–2931. [Google Scholar] [CrossRef]
- Cheng, H.N.; Biswas, A.; Vermillion, K.; Melendez-Rodriguez, B.; Lagaron, J.M. NMR analysis and triad sequence distributions of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Polym. Test. 2020, 90, 106754. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and thermal properties of PHBV produced from levulinic acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef] [PubMed]
- Forni, D.; Bee, G.; Kreuzer, M.; Wenk, C. Novel biodegradable plastics in sheep nutrition-Effects of NaOH pretreatment of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) on in vivo digestibility and on in vitro disappearance (Rusitec). J. Anim. Phys. Anim. Nutrit. 1999, 81, 41–50. [Google Scholar] [CrossRef]
- Elles Montero, A.E.; García Echeverry, C.A. Mezcla Sinérgica Entre Polihidroxibutirato (PHB) y Caucho Natural (Látex) para Obtener un Copolímero. Bachelor’s Thesis, Universidad de Cartagena, Cartagena, Colombia, 2012. [Google Scholar]
- Barham, P.; Keller, A.; Otun, E.; Holmes, P. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Getzabeth, M. Producción de Poli-Hidroxialcanoatos por Bacterias del Género Bacillus de Origen Marino. Master’s Thesis, Centro de Investigaciones Biológicas, Madrid, Spain, 2008. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).