Changes in the Skin Microbiome Following Dermatological Procedures: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
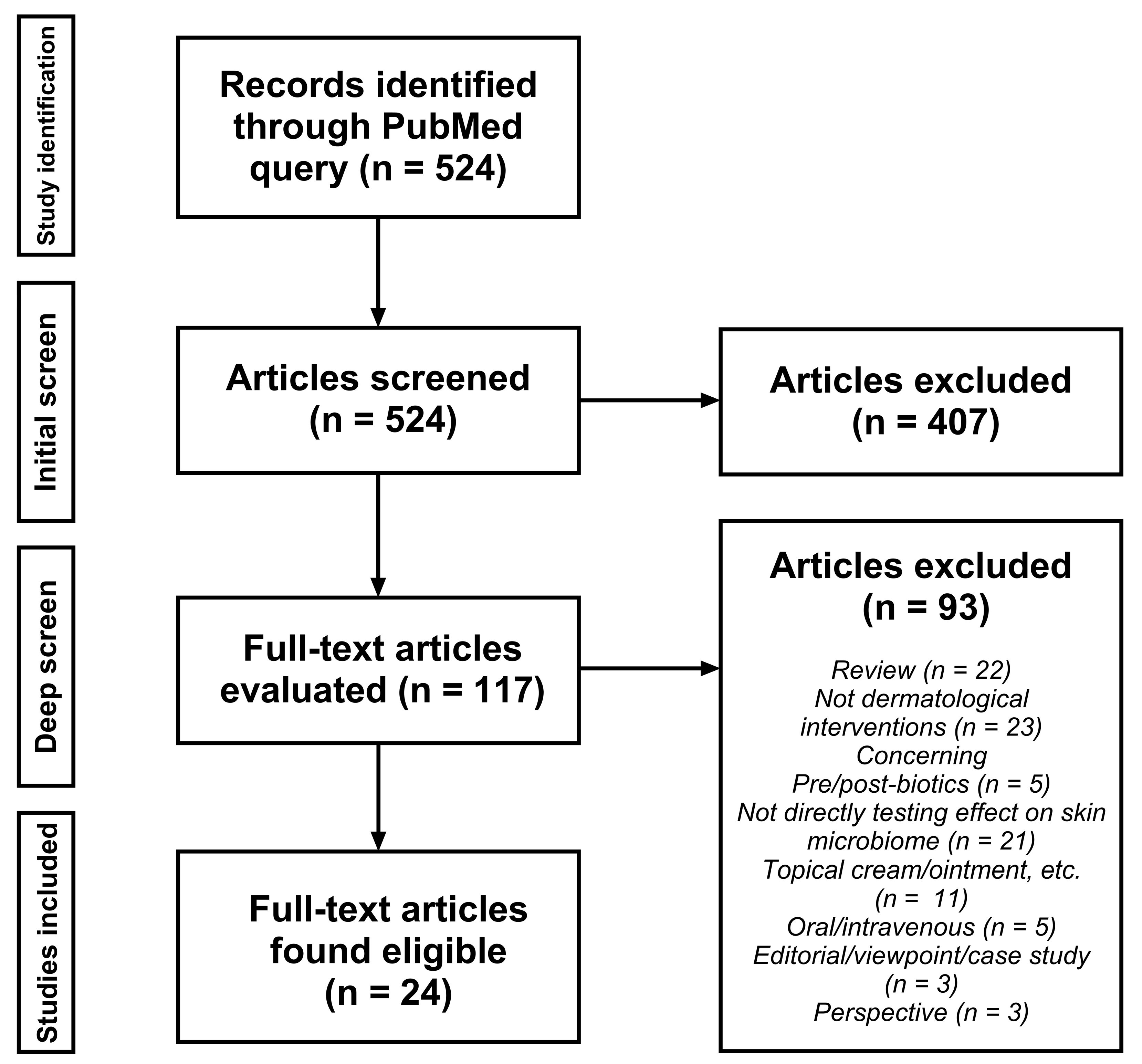
2.2. Study Identification and Selection
2.3. Data Management
3. Results
3.1. Phototherapy Significantly Alters the Skin Microbiome
| Author | Year | Dermatologic Procedure | Study Size (n) | Study Length | Condition | Species | Outcomes |
|---|---|---|---|---|---|---|---|
| Burns et al. [53] | 2019 | UV Phototherapy | 6 | Short-Term (24 h) | None | Human | There was an increase in Cyanobacteria, along with a decrease in Lactobacillaceae and Pseudomonadaceae. |
| Kwong et al. [41] | 2019 | UV Phototherapy | 18 (13 were subjected to UV) | Long-Term (9 weeks) | Atopic Dermatitis | Human | Microbial diversity in lesional skin increased after treatment. The Staphylococcus aureus proportion decreased with treatment. |
| Lossius et al. [42] | 2021 | UV Phototherapy | 16 | Long-Term (6–8 weeks) | Atopic Dermatitis | Human | Lesional AD skin microbiota showed higher diversity after 6–8 weeks of treatment, while NLS and nose/throat microbiota remained unchanged. No significant changes in microbiota were observed after only three NB-UVB treatments. |
| Hooper et al. [43] | 2022 | UV Phototherapy | 40 (25 exposed, 15 control) | Long-Term (~6.2 months) | Cutaneous Lymphoma | Human | Microbial diversity increased in NB-UVB responders. The relative abundance of S. aureus and Staphylococcus lugdunensis was reduced post-treatment. Higher levels of Staphylococcus capitis and Staphylococcus warneri were recorded in responder lesional skin before NB-UVB. Decreased S. aureus and increased S. capitis, Staphylococcus hominis, Staphylococcus pettenkoferi, and S. warneri levels were found in responder skin post-treatment. Staphylococcus species abundance is more similar between non-responders and non-NB-UVB patients than between responders and non-NB-UVB patients. |
| Assarsson et al. [51] | 2018 | UV Phototherapy | 26 | Long-Term (10.5 weeks) | Chronic Plaque Psoriasis | Human | Increased relative abundance of Clostridium and decreased relative abundance of Pseudomonas occurred in both lesional and non-lesional skin, along with increased Megasphaera in non-lesional skin. |
| Dotterud et al. [52] | 2008 | UV Phototherapy | 40 (20 dermatitis, 20 control) | Long-Term (6 weeks) | Atopic Dermatitis | Human | S. aureus counts in lesional skin showed a non-significant decrease after 4 weeks of treatment, with a slight increase observed after a 2-week follow-up. Similar trends were observed in non-lesional skin and the forehead. |
| Wang et al. [61] | 2012 | UV Phototherapy | 5 human, 5 mice | Short-Term (1 day) | None | Human and Mouse | Reduced porphyrin production occurred in human facial bacteria and in Cutibacterium acnes-inoculated mouse ears. |
| Yuan et al. [44] | 2020 | UV Phototherapy | 60 | N/A | Vitiligo | Human | The NB group showed significantly higher diversity indices compared to NF, while the NF and DB groups did not differ significantly. Staphylococcus, Bacillus, and Prevotella were enriched in DF compared to DB, while Propionibacterium showed the opposite trend. |
| Park et al. [40] | 2021 | UV Phototherapy | 20 (10 with atopic dermatitis, 10 without) | Long-Term (2 months) | Atopic Dermatitis | Dog | Phototherapy altered the skin microbiome in dogs with AD, increasing Actinobacteria and Cyanobacteria and decreasing Staphylococcus pseudintermedius. Higher alpha diversity occurred after treatment. |
| Kurosaki et al. [54] | 2020 | UV Phototherapy | 22 (11 lesional, 11 non-lesional) | Long-Term (2 months) | Atopic Dermatitis | Human | An increase in Cyanobacteria and a decrease in Bacteroidetes occurred in lesional skin. A significant reduction in the abundance of S. aureus was also found at the species level in lesional skin. |
| Liu et al. [48] | 2021 | Light Therapy | 39 (20 acne, 19 healthy) | Long-Term (3 months) | Acne | Human | There was a significant increase in the relative abundance of Staphylococcus epidermidis, while C. acnes decreased. |
| Muñoz Declara et al. [49] | 2024 | Laser Therapy (905 nm, 808 nm) | 20 | Short-Term (6 days) | Atopic Dermatitis | Dog | No significant alterations in microbiome composition or diversity were observed, but a decrease in the relative abundance of S. pseudintermedius was noted in the treated areas of some dogs. |
| Park et al. [58] | 2023 | Laser Therapy (755 nm) | 21 | Long-Term (3 months) | Rosacea | Human | There was a decrease in the relative abundance of Cutibacterium, Streptococcus, Clostridium, Bacteroides, and Lactobacillus. An increase in the relative abundance of Staphylococcus, Neisseriaceae, Corynebacterium, Anaerococcus, and Lawsonella also occurred. There was a decrease in alpha diversity after treatment. |
| Rupel et al. [59] | 2019 | Laser Therapy (445 nm) | 15 (8 treatment, 7 control) | Short-Term (single application) | None | Mouse | Blue laser light decreased Pseudomonas aeruginosa both in vitro and in vivo. This inhibited biofilm formation. |
| Guo et al. [45] | 2022 | ALA Photodynamic Therapy | 26 (18 with acne, 8 without) | Long-Term (3 weeks) | Acne | Human | Reduced alpha diversity occurred after treatment. There was no statistically significant difference observed among different groups for C. acnes at the genus level. There was an increase in the abundance of Pseudomonas, Gordonia, Leptotrichia, and Mycobacterium, restoring them to healthy levels. |
| Yang et al. [46] | 2021 | ALA Photodynamic Therapy | 5 | Long-Term (2 months) | Acne | Human | PDT inhibited C. acnes in the follicular microbiome. Bacillus and Lactococcus increased post-PDT. ALA-PDT increased microbiome diversity and made the follicular microbiome more like the epidermal microbiome taxonomically and functionally. |
| Tao et al. [47] | 2021 | ALA Photodynamic Therapy | 11 | Long-Term (6 weeks) | Acne | Human | There was a notable decrease in the relative abundance of C. acnes, whereas Pseudomonas fluorescens significantly increased. No effect on S. epidermidis was found. Additionally, ALA-PDT was correlated with heightened microbiota diversity and reductions in the relative abundance of functional genes related to energy metabolism and DNA replication. |
3.2. Various Dermatological Procedures Affect the Skin Microbiome
| Author | Year | Dermatologic Procedure | Study Size (n) | Study Length | Condition | Species | Outcomes |
|---|---|---|---|---|---|---|---|
| Shao et al. [63] | 2023 | Chemical Peel | 28 | Long-Term (2 months) | Acne | Human | Staphylococcus and Propionibacterium proportions tended to decrease. |
| Bhardwaj et al. [64] | 2024 | Chemical Peel | 9 | Short-Term (single application, 20 min) | Hyperpigmentation | Human | Non-significant raise in Shannon’s diversity index, a mathematical measure of species diversity within a community [70]. Beta diversity remained constant. No change in the abundance of Staphylococcus epidermidis. Reduction in Cutibacterium acnes. Decreased Porphyrin. |
| Janssens-Böcker et al. [65] | 2024 | Mask | 28 | Long-Term (4 weeks) | None | Human | Shannon’s diversity index significantly increased from baseline, but showed no difference compared to untreated areas. The genus Staphylococcus, as well as S. epidermidis, specifically decreased significantly over time, but not compared to untreated areas. No significant changes were observed for Corynebacterium, Pseudomonas, or C. acnes between time points or compared to untreated areas. |
| Frommherz et al. [66] | 2022 | Electrotherapy (HF therapy) | N/A | N/A | Acne | N/A | Bacterial species decreased, including Aerococcus viridans, Bacillus cereus, Aerococcus urinaeequi, Staphylococcus lugdunensis, Staphylococcus haemolyticus, Micrococcus yunnanensis, Micrococcus luteus, and Mycobacterium species. Dermatophytes decreased in colony count post-HF treatment, including Trichophyton benhamiae, Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton violaceum, and Microsporum canis. |
| Xu et al. [67] | 2023 | Piercing | 28 | Long-Term (2 weeks) | None | Human | Decrease in the relative frequency of C. acnes and a significant increase in the relative frequency of S. epidermidis in the piercing microbiome. |
| Yilmaz et al. [68] | 2023 | Micropigmentation | 125 (35 corneal tattoos, 40 corneal leukoma, 50 healthy) | Unknown | Corneal Leukoma | Human | No significant difference between native and tattooed eyes. |
| Verbanic et al. [69] | 2020 | Debridement | 20 | Short-Term (immediately after debridement) | Chronic Wound | Human | Sharp debridement did not directly alter the wound microbiome compared to the original wound surface. However, aerobes and facultative anaerobes, particularly the genus Enterobacter, were significantly associated with wounds that did not heal within 6 months. |
4. Discussion and Conclusions
5. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kong, H.H. Skin Microbiome: Genomics-Based Insights into the Diversity and Role of Skin Microbes. Trends Mol. Med. 2011, 17, 320–328. [Google Scholar] [CrossRef]
- Ying, S.; Zeng, D.-N.; Chi, L.; Tan, Y.; Galzote, C.; Cardona, C.; Lax, S.; Gilbert, J.; Quan, Z.-X. The Influence of Age and Gender on Skin-Associated Microbial Communities in Urban and Rural Human Populations. PLoS ONE 2015, 10, e0141842. [Google Scholar] [CrossRef]
- Dimitriu, P.A.; Iker, B.; Malik, K.; Leung, H.; Mohn, W.W.; Hillebrand, G.G. New Insights into the Intrinsic and Extrinsic Factors That Shape the Human Skin Microbiome. mBio 2019, 10, e00839-19. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Fischbach, M.A. What Lives on Our Skin: Ecology, Genomics and Therapeutic Opportunities of the Skin Microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Segre, J.A. Skin Microbiome: Looking Back to Move Forward. J. Investig. Dermatol. 2012, 132, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Chandra, S.H.; Srinivas, R.; Dawson, T.L.; Common, J.E. Cutaneous Malassezia: Commensal, Pathogen, or Protector? Front. Cell. Infect. Microbiol. 2021, 10, 614446. [Google Scholar] [CrossRef]
- Schommer, N.N.; Gallo, R.L. Structure and Function of the Human Skin Microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Foley, R.; Kelly, P.; Gatault, S.; Powell, F. Demodex: A Skin Resident in Man and His Best Friend. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 62–72. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Claesen, J.; Spagnolo, J.B.; Ramos, S.F.; Kurita, K.L.; Byrd, A.L.; Aksenov, A.A.; Melnik, A.V.; Wong, W.R.; Wang, S.; Hernandez, R.D.; et al. A Cutibacterium Acnes Antibiotic Modulates Human Skin Microbiota Composition in Hair Follicles. Sci. Transl. Med. 2020, 12, eaay5445. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between Skin Microbiota and Immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Roesner, L.M.; Werfel, T.; Heratizadeh, A. The Adaptive Immune System in Atopic Dermatitis and Implications on Therapy. Expert Rev. Clin. Immunol. 2016, 12, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Morohashi, M. Pathogenesis of Acne. Med. Electron Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hunt, R.L.; Villaruz, A.E.; Fisher, E.L.; Liu, R.; Liu, Q.; Cheung, G.Y.C.; Li, M.; Otto, M. Commensal Staphylococcus Epidermidis Contributes to Skin Barrier Homeostasis by Generating Protective Ceramides. Cell Host Microbe 2022, 30, 301–313.e9. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; McBain, A.J.; O’Neill, C.A. Strain-Dependent Augmentation of Tight-Junction Barrier Function in Human Primary Epidermal Keratinocytes by Lactobacillus and Bifidobacterium Lysates. Appl. Environ. Microbiol. 2013, 79, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the Skin and the Role of Biofilms in Infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Swaney, M.H.; Nelsen, A.; Sandstrom, S.; Kalan, L.R. Sweat and Sebum Preferences of the Human Skin Microbiota. Microbiol. Spectr. 2023, 11, e04180-22. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.C.; Kalan, L.R. The Dynamic Balance of the Skin Microbiome across the Lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef]
- Youn, S.H.; Choi, C.W.; Choi, J.W.; Youn, S.W. The Skin Surface pH and Its Different Influence on the Development of Acne Lesion According to Gender and Age. Skin Res. Technol. 2013, 19, 131–136. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.-M. Staphylococcus Epidermidis in the Human Skin Microbiome Mediates Fermentation to Inhibit the Growth of Propionibacterium Acnes: Implications of Probiotics in Acne Vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Nodake, Y.; Matsumoto, S.; Miura, R.; Honda, H.; Ishibashi, G.; Matsumoto, S.; Dekio, I.; Sakakibara, R. Pilot Study on Novel Skin Care Method by Augmentation with Staphylococcus epidermidis, an Autologous Skin Microbe—A Blinded Randomized Clinical Trial. J. Dermatol. Sci. 2015, 79, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Ertürk Bergdahl, G.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common Skin Bacteria Protect Their Host from Oxidative Stress through Secreted Antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and Its Interplay with the Environment. Am. J. Clin. Dermatol. 2020, 21, 4–11. [Google Scholar] [CrossRef]
- Sanders, D.; Grunden, A.; Dunn, R.R. A Review of Clothing Microbiology: The History of Clothing and the Role of Microbes in Textiles. Biol. Lett. 2021, 17, 20200700. [Google Scholar] [CrossRef] [PubMed]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef]
- Park, J.; Schwardt, N.H.; Jo, J.-H.; Zhang, Z.; Pillai, V.; Phang, S.; Brady, S.M.; Portillo, J.A.; MacGibeny, M.A.; Liang, H.; et al. Shifts in the Skin Bacterial and Fungal Communities of Healthy Children Transitioning through Puberty. J. Investig. Dermatol. 2022, 142, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.M.; Nolan, Z.T.; Banerjee, K.; Paine, A.R.; Cong, Z.; Gettle, S.L.; Longenecker, A.L.; Zhan, X.; Agak, G.W.; Nelson, A.M. Evolution of the Facial Skin Microbiome during Puberty in Normal and Acne Skin. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Tomczak, H.; Lodyga, M.; Czajkowski, R.; Żaba, R.; Adamski, Z. The Microbiome of the Human Skin and Its Variability in Psoriasis and Atopic Dermatitis. Adv. Dermatol. Allergol. Dermatol. Alergol. 2021, 38, 205–209. [Google Scholar] [CrossRef]
- Gardiner, M.; Vicaretti, M.; Sparks, J.; Bansal, S.; Bush, S.; Liu, M.; Darling, A.; Harry, E.; Burke, C.M. A Longitudinal Study of the Diabetic Skin and Wound Microbiome. PeerJ 2017, 5, e3543. [Google Scholar] [CrossRef]
- Reiss, Z.; Rob, F.; Kolar, M.; Schierova, D.; Kreisinger, J.; Jackova, Z.; Roubalova, R.; Coufal, S.; Mihula, M.; Thon, T.; et al. Skin Microbiota Signature Distinguishes IBD Patients and Reflects Skin Adverse Events during Anti-TNF Therapy. Front. Cell. Infect. Microbiol. 2023, 12, 1064537. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Powell, E.J.; Ellis, J.R. Scoping Review Protocol: Changes in Skin Microbiome Post-Dermatological Interventions. 2024. Available online: https://www.protocols.io/view/scoping-review-protocol-changes-in-skin-microbiome-261ge53owg47/v2 (accessed on 16 May 2024).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- American Medical Association: CPT—Current Procedural Terminology. Available online: https://www.ama-assn.org/amaone/cpt-current-procedural-terminology (accessed on 17 May 2024).
- Dol, J.; Tutelman, P.R.; Chambers, C.T.; Barwick, M.; Drake, E.K.; Parker, J.A.; Parker, R.; Benchimol, E.I.; George, R.B.; Witteman, H.O. Health Researchers’ Use of Social Media: Scoping Review. J. Med. Internet Res. 2019, 21, e13687. [Google Scholar] [CrossRef]
- Rathod, D.G.; Muneer, H.; Masood, S. Phototherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Park, J.-Y.; Kim, S.-M.; Kim, J.-H. Efficacy of Phototherapy with 308-Nm Excimer Light for Skin Microbiome Dysbiosis and Skin Barrier Dysfunction in Canine Atopic Dermatitis. Front. Vet. Sci. 2021, 8, 762961. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, J.Y.; Shin, J.-W.; Huh, C.-H.; Park, K.-C.; Du, M.-H.; Yoon, S.; Na, J.-I. Changes in Lesional and Non-Lesional Skin Microbiome During Treatment of Atopic Dermatitis. Acta Derm. Venereol. 2019, 99, 284–290. [Google Scholar] [CrossRef]
- Lossius, A.H.; Sundnes, O.; Ingham, A.C.; Edslev, S.M.; Bjørnholt, J.V.; Lilje, B.; Bradley, M.; Asad, S.; Haraldsen, G.; Skytt-Andersen, P.; et al. Shifts in the Skin Microbiota after UVB Treatment in Adult Atopic Dermatitis. Dermatology 2022, 238, 109–120. [Google Scholar] [CrossRef]
- Hooper, M.J.; Enriquez, G.L.; Veon, F.L.; LeWitt, T.M.; Sweeney, D.; Green, S.J.; Seed, P.C.; Choi, J.; Guitart, J.; Burns, M.B.; et al. Narrowband Ultraviolet B Response in Cutaneous T-Cell Lymphoma Is Characterized by Increased Bacterial Diversity and Reduced Staphylococcus aureus and Staphylococcus lugdunensis. Front. Immunol. 2022, 13, 1022093. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Meng, D.; Wu, L.; Wang, X.; Zhang, D.; Luo, Z.; Pang, Y.; Liu, G. The Impact of NBUVB on Microbial Community Profiling in the Lesional Skin of Vitiligo Subjects. Microb. Pathog. 2020, 140, 103943. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, M.; Yuan, Y.; Yuan, M.; Chen, Y.; Yu, H.; Liu, R.; Ruan, Z.; Xie, Q.; Jiao, X.; et al. Photodynamic Therapy Treats Acne by Altering the Composition of the Skin Microbiota. Skin Res. Technol. 2022, 29, e13269. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, S.; Zeng, R.; Zheng, H.; Ge, Y. Modulation of Skin Microbiome in Acne Patients by Aminolevulinic Acid-Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 36, 102556. [Google Scholar] [CrossRef]
- Tao, S.; Wang, Z.; Quan, C.; Ge, Y.; Qian, Q. The Effects of ALA-PDT on Microbiota in Pilosebaceous Units of Patients with Severe Acne: A Metagenomic Study. Photodiagnosis Photodyn. Ther. 2021, 33, 102050. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zhou, L.; Chen, L.; Chen, X.; Xiong, X.; Deng, Y. The Effect of Intense Pulsed Light on the Skin Microbiota and Epidermal Barrier in Patients with Mild to Moderate Acne Vulgaris. Lasers Surg. Med. 2021, 53, 1348–1355. [Google Scholar] [CrossRef]
- Muñoz Declara, S.; D’Alessandro, A.; Gori, A.; Cerasuolo, B.; Renzi, S.; Berlanda, M.; Zini, E.; Monici, M.; Cavalieri, D.; Zanna, G. Evaluation of the Impact of Near-Infrared Multiwavelength Locked System Laser Therapy on Skin Microbiome in Atopic Dogs. Animals 2024, 14, 906. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. Beneficial Effects of UV Radiation Other than via Vitamin D Production. Dermato-Endocrinology 2012, 4, 109–117. [Google Scholar] [CrossRef]
- Assarsson, M.; Duvetorp, A.; Dienus, O.; Söderman, J.; Seifert, O. Significant Changes in the Skin Microbiome in Patients with Chronic Plaque Psoriasis after Treatment with Narrowband Ultraviolet B. Acta Derm. Venereol. 2018, 98, 428–436. [Google Scholar] [CrossRef]
- Dotterud, L.K.; Wilsgaard, T.; Vorland, L.H.; Falk, E.S. The Effect of UVB Radiation on Skin Microbiota in Patients with Atopic Dermatitis and Healthy Controls. Int. J. Circumpolar Health 2008, 67, 254–260. [Google Scholar] [CrossRef]
- Burns, E.M.; Ahmed, H.; Isedeh, P.N.; Kohli, I.; Van Der Pol, W.; Shaheen, A.; Muzaffar, A.F.; Al-Sadek, C.; Foy, T.M.; Abdelgawwad, M.S.; et al. Ultraviolet Radiation, Both UVA and UVB, Influences the Composition of the Skin Microbiome. Exp. Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Tsurumachi, M.; Kamata, Y.; Tominaga, M.; Suga, Y.; Takamori, K. Effects of 308 Nm Excimer Light Treatment on the Skin Microbiome of Atopic Dermatitis Patients. Photodermatol. Photoimmunol. Photomed. 2020, 36, 185–191. [Google Scholar] [CrossRef]
- Glass, G.E. Photobiomodulation: A Review of the Molecular Evidence for Low Level Light Therapy. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1050–1060. [Google Scholar] [CrossRef]
- Biener, G.; Masson-Meyers, D.S.; Bumah, V.V.; Hussey, G.; Stoneman, M.R.; Enwemeka, C.S.; Raicu, V. Blue/Violet Laser Inactivates Methicillin-Resistant Staphylococcus aureus by Altering Its Transmembrane Potential. J. Photochem. Photobiol. B 2017, 170, 118–124. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Bumah, V.V.; Biener, G.; Raicu, V.; Enwemeka, C.S. The Relative Antimicrobial Effect of Blue 405 Nm LED and Blue 405 Nm Laser on Methicillin-Resistant Staphylococcus aureus In Vitro. Lasers Med. Sci. 2015, 30, 2265–2271. [Google Scholar] [CrossRef]
- Park, S.; Jang, H.; Seong, S.H.; Kim, J.Y.; Lee, E.J.; Bae, Y.J.; Ahn, Y.J.; Kim, J.; Oh, S.H. The Effects of Long-Pulsed Alexandrite Laser Therapy on Facial Redness and Skin Microbiota Compositions in Rosacea: A Prospective, Multicentre, Single-Arm Clinical Trial. Photodermatol. Photoimmunol. Photomed. 2024, 40. [Google Scholar] [CrossRef]
- Rupel, K.; Zupin, L.; Ottaviani, G.; Bertani, I.; Martinelli, V.; Porrelli, D.; Vodret, S.; Vuerich, R.; Passos da Silva, D.; Bussani, R.; et al. Blue Laser Light Inhibits Biofilm Formation In Vitro and In Vivo by Inducing Oxidative Stress. npj Biofilms Microbiomes 2019, 5, 29. [Google Scholar] [CrossRef]
- Shi, L.; Liu, P.; Liu, J.; Yang, Y.; Chen, Q.; Zhang, Y.; Zhang, H.; Wang, X. Application of 5-aminolevulinic Acid-photodynamic Therapy in Common Skin Diseases. Transl. Biophotonics 2020, 2, e201900028. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Shu, M.; Jiang, Y.; Gallo, R.L.; Liu, Y.-T.; Huang, C.-M. The Response of Human Skin Commensal Bacteria as a Reflection of UV Radiation: UV-B Decreases Porphyrin Production. PLoS ONE 2012, 7, e47798. [Google Scholar] [CrossRef]
- Poorian, B.; Keyhan, S.O.; Chavoshinejad, M. Chemical Peeling. In Integrated Procedures in Facial Cosmetic Surgery; Keyhan, S.O., Fattahi, T., Bagheri, S.C., Bohluli, B., Amirzade-Iranaq, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 413–420. ISBN 978-3-030-46993-1. [Google Scholar]
- Shao, X.; Chen, Y.; Zhang, L.; Zhang, Y.; Ariyawati, A.; Chen, T.; Chen, J.; Liu, L.; Pu, Y.; Li, Y.; et al. Effect of 30% Supramolecular Salicylic Acid Peel on Skin Microbiota and Inflammation in Patients with Moderate-to-Severe Acne Vulgaris. Dermatol. Ther. 2023, 13, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Handler, M.Z.; Mao, J.; Azadegan, C.; Panda, P.K.; Breunig, H.G.; Wenskus, I.; Diaz, I.; König, K. A Novel Professional-Use Synergistic Peel Technology to Reduce Visible Hyperpigmentation on Face: Clinical Evidence and Mechanistic Understanding by Computational Biology and Optical Biopsy. Exp. Dermatol. 2024, 33, e15069. [Google Scholar] [CrossRef]
- Janssens-Böcker, C.; Wiesweg, K.; Doberenz, C. Native Collagen Sheet Mask Improves Skin Health and Appearance: A Comprehensive Clinical Evaluation. J. Cosmet. Dermatol. 2024, 23, 1685–1702. [Google Scholar] [CrossRef] [PubMed]
- Frommherz, L.; Reinholz, M.; Gürtler, A.; Stadler, P.-C.; Kaemmerer, T.; French, L.; Clanner-Engelshofen, B.M. High-Frequency Devices Effect in Vitro: Promissing Approach in the Treatment of Acne Vulgaris? An. Bras. Dermatol. 2022, 97, 729–734. [Google Scholar] [CrossRef]
- Xu, C.C.Y.; Lemoine, J.; Albert, A.; Whirter, É.M.; Barrett, R.D.H. Community Assembly of the Human Piercing Microbiome. Proc. R. Soc. B Biol. Sci. 2023, 290, 20231174. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.F.; Sarmıs, A.; Mutlu, M.A.; Oguz, H. Does Corneal Tattooing Affect the Conjunctival Microbiota? Cutan. Ocul. Toxicol. 2024, 43, 46–51. [Google Scholar] [CrossRef]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial Predictors of Healing and Short-Term Effect of Debridement on the Microbiome of Chronic Wounds. NPJ Biofilms Microbiomes 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Witkin, E.M. Radiation-Induced Mutations and Their Repair. Science 1966, 152, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Epe, B.; Hegler, J.; Wild, D. Singlet Oxygen as an Ultimately Reactive Species in Salmonella Typhimurium DNA Damage Induced by Methylene Blue/Visible Light. Carcinogenesis 1989, 10, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Lubart, R.; Lipovski, A.; Nitzan, Y.; Friedmann, H. A Possible Mechanism for the Bactericidal Effect of Visible Light. Laser Ther. 2011, 20, 17–22. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Visible Light-Induced Killing of Bacteria as a Function of Wavelength: Implication for Wound Healing. Lasers Surg. Med. 2010, 42, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hoenes, K.; Bauer, R.; Spellerberg, B.; Hessling, M. Microbial Photoinactivation by Visible Light Results in Limited Loss of Membrane Integrity. Antibiotics 2021, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Maclean, M.; Grant, M.H.; Ramakrishnan, P.; MacGregor, S.J.; Anderson, J.G. The Effects of 405 Nm Light on Bacterial Membrane Integrity Determined by Salt and Bile Tolerance Assays, Leakage of UV-Absorbing Material and SYTOX Green Labelling. Microbiology 2016, 162, 1680–1688. [Google Scholar] [CrossRef]
- Wan, M.T.; Lin, J.Y. Current Evidence and Applications of Photodynamic Therapy in Dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, A.; Matuschka, K. A Controlled Trial to Determine the Efficacy of Red and Near-Infrared Light Treatment in Patient Satisfaction, Reduction of Fine Lines, Wrinkles, Skin Roughness, and Intradermal Collagen Density Increase. Photomed. Laser Surg. 2014, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ablon, G. Phototherapy with Light Emitting Diodes. J. Clin. Aesthetic Dermatol. 2018, 11, 21–27. [Google Scholar]
- Kim, S.; Kim, J.; Lim, W.; Jeon, S.; Kim, O.; Koh, J.-T.; Kim, C.-S.; Choi, H.; Kim, O. In Vitro Bactericidal Effects of 625, 525, and 425 Nm Wavelength (Red, Green, and Blue) Light-Emitting Diode Irradiation. Photomed. Laser Surg. 2013, 31, 554–562. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Baker, T.L.; Bumah, V.V. The Role of UV and Blue Light in Photo-Eradication of Microorganisms. J. Photochem. Photobiol. 2021, 8, 100064. [Google Scholar] [CrossRef]
- Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods 2020, 9, 1895. [Google Scholar] [CrossRef]
- Galo, I.D.C.; Prado, R.P.; Santos, W.G.D. Blue and Red Light Photoemitters as Approach to Inhibit Staphylococcus aureus and Pseudomonas aeruginosa Growth. Braz. J. Biol. 2021, 82, e231742. [Google Scholar] [CrossRef]
- Mancini, S.; Cuomo, R.; Poggialini, M.; D’Aniello, C.; Botta, G. Autolytic Debridement and Management of Bacterial Load with an Occlusive Hydroactive Deressing Impregnated with Polyhexamethylene Biguanide. Acta Bio Medica Atenei Parm. 2017, 88, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, R.; Zhang, Q.; He, S.; Wang, Y. Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia coli and Staphylococcus aureus. Int. J. Environ. Res. Public Health 2022, 19, 12761. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J.M.; Scholz, C.F.P.; Enghild, J.; Rohde, H.; Kilian, M.; Thürmer, A.; Brzuszkiewicz, E.; Lomholt, H.B.; Brüggemann, H. Antagonism between Staphylococcus Epidermidis and Propionibacterium Acnes and Its Genomic Basis. BMC Genom. 2016, 17, 152. [Google Scholar] [CrossRef] [PubMed]
- Francuzik, W.; Franke, K.; Schumann, R.R.; Heine, G.; Worm, M. Propionibacterium Acnes Abundance Correlates Inversely with Staphylococcus aureus: Data from Atopic Dermatitis Skin Microbiome. Acta Derm. Venereol. 2018, 98, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.A.; Wylie, T.N.; Gula, H.; Hogan, P.G.; Boyle, M.G.; Muenks, C.E.; Sullivan, M.L.; Burnham, C.-A.D.; Wylie, K.M. Longitudinal Dynamics of Skin Bacterial Communities in the Context of Staphylococcus aureus Decolonization. Microbiol. Spectr. 2022, 10, e02672-21. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, A.J.; Meisel, J.S.; Horwinski, J.; Zheng, Q.; Bradley, C.W.; Grice, E.A. Antiseptic Agents Elicit Short-Term, Personalized and Body Site-Specific Shifts in Resident Skin Bacterial Communities. J. Investig. Dermatol. 2018, 138, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, A.J.; Meisel, J.S.; Horwinski, J.; Zheng, Q.; Grice, E.A. Topical Antimicrobial Treatments Can Elicit Shifts to Resident Skin Bacterial Communities and Reduce Colonization by Staphylococcus aureus Competitors. Antimicrob. Agents Chemother. 2017, 61, e00774-17. [Google Scholar] [CrossRef]
- Wongpiyabovorn, J.; Soonthornchai, W.; Wilantho, A.; Palasuk, M.; Payungporn, S.; Sodsai, P.; Poomipak, W.; Weschawalit, S.; Ruchusatsawat, K.; Baillie, G.S.; et al. Effect of Tacrolimus on Skin Microbiome in Atopic Dermatitis. Allergy 2019, 74, 1400–1406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellis, J.R.; Powell, E.J.; Tomasovic, L.M.; Marcheskie, R.L.; Girish, V.; Warman, A.; Sivaloganathan, D. Changes in the Skin Microbiome Following Dermatological Procedures: A Scoping Review. Appl. Microbiol. 2024, 4, 972-985. https://doi.org/10.3390/applmicrobiol4020066
Ellis JR, Powell EJ, Tomasovic LM, Marcheskie RL, Girish V, Warman A, Sivaloganathan D. Changes in the Skin Microbiome Following Dermatological Procedures: A Scoping Review. Applied Microbiology. 2024; 4(2):972-985. https://doi.org/10.3390/applmicrobiol4020066
Chicago/Turabian StyleEllis, Jeremy R., Eron J. Powell, Luke M. Tomasovic, Rachel L. Marcheskie, Vishruth Girish, Anmol Warman, and Darshan Sivaloganathan. 2024. "Changes in the Skin Microbiome Following Dermatological Procedures: A Scoping Review" Applied Microbiology 4, no. 2: 972-985. https://doi.org/10.3390/applmicrobiol4020066
APA StyleEllis, J. R., Powell, E. J., Tomasovic, L. M., Marcheskie, R. L., Girish, V., Warman, A., & Sivaloganathan, D. (2024). Changes in the Skin Microbiome Following Dermatological Procedures: A Scoping Review. Applied Microbiology, 4(2), 972-985. https://doi.org/10.3390/applmicrobiol4020066






