Abstract
Pasteurized whey concentrate is used as a base for the production of ingredients for various food products. Whey concentrate (30% dry matter) was used to assess the thermal inactivation of Salmonella (S.) enterica serovar Senftenberg 775W (DSM 10062) and Escherichia (E.) coli AW1.7 (DSM 108612) strains in a pilot-scale pasteurizer mimicking industrial heat processing. These strains, chosen for their exceptional heat resistance, represent the most challenging scenario for pasteurization within the context of S. enterica and E. coli. Heat resistance was tested at temperatures of 56, 60, 64, 68, and 72 °C at an average holding time of 17.5 s. These exceptionally heat-resistant strains showed a relatively low reduction in numbers of between 0 and 4.2 log10 CFU/mL at lower inactivation temperatures of ≤68 °C. A reduction of at least 5 log10 CFU/mL, as required for adequate heat processing, was achieved for both species after heating at 72 °C for 17.5 s. This study shows that whey concentrate should not lead to contamination of food ingredients and can be considered safe after pasteurization at 72 °C for at least 17.5 s with respect to the pathogens tested.
1. Introduction
The pasteurization of milk has long been established to be of high relevance to public health, as it aims to provide a very high margin of safety regarding highly heat-resistant and particularly dangerous pathogens such as Coxiella (C.) burnetii or Mycobacterium tuberculosis [1,2,3,4]. According to the FDA’s grade A pasteurized milk ordinance [5], milk in the United States should be pasteurized at 72 °C for 15 s (high temperature–short time, HTST). This is similar to pasteurization in Europe, where HTST pasteurization of milk is performed under the same conditions, i.e., at least 72 °C for 15 s, according to regulation 853/2004 of the European Union, which follows the Codex Alimentarius recommendations [6]. This temperature–time regime was deduced from the thermal resistance of C. burnetii, which serves as the reference for most heat-resistant, non-sporulating pathogens likely to be present in milk. Pasteurization is thus designed to achieve at least a 5 log10 reduction of these heat-resistant microorganisms in whole milk (4% fat content) [4,7]. Pasteurization is, therefore, not designed to sterilize milk and the microbiological quality of the pasteurized milk is governed by various factors, such as the initial microbiota of the raw milk, the processing conditions, as well as post-heat treatment contamination.
The well-established pasteurization regimes for milk minimize the risk of public health infections. The situation is different for concentrated dairy products, where there are no proven pasteurization requirements. In fact, there is a lack of scientific literature on the inactivation of microorganisms in liquid dairy media other than the most commonly studied milk, especially for concentrated and highly viscous products. Kornacki and Marth [8] reported on the inactivation of Salmonella (S.) enterica subsp. enterica serovar Senftenberg (S. senftenberg) in retentates from ultrafiltration or diafiltration of whole and skimmed milk. Heat resistance of this microorganism tended to be higher in whole milk concentrate (25.7% dry matter), whereas it was reduced in skim milk concentrate (24.7% dry matter), when both were compared to non-concentrated milk. The applied heating technology (screw cap test tubes submerged in a water bath), however, can hardly be compared with industrial continuous milk processing as used in this study. Dega et al. [9] observed increasing heat resistance of Escherichia (E.) coli and S. typhimurium in skim milk of different dry matter. Mean D-values for E. coli increased from 1.8 min in milk with 10% dry matter to 2.4 min in milk concentrate with 30% dry matter [9]. For S. typhimurium, the corresponding increase was from 1.4 to 2.5 min for these concentrations of dry matter, respectively.
In the US in 2018, some popular snack food products were recalled because they all contained one common ingredient, i.e., whey powder, which appeared to be potentially contaminated with Salmonella bacteria (https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ampi-recalls-limited-amount-dry-whey-powder-because-possible-health-risk#recall-announcement, accessed on 5 February 2024). Salmonella is a bacterial pathogen that can survive in low-moisture foods (water activity < 0.7) such as dried whey or chocolate [10,11]. For example, a recent trans-national outbreak of salmonellosis linked to chocolate products in nine EU countries and the United Kingdom was investigated by the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) [12], with S. typhimurium being the causative agent. The extent of Salmonella survival depends on several factors, including water activity, fat content, and the addition of solutes to the food matrix.
Accordingly, low water activity, high fat content, and the presence of sucrose were all shown to offer heat-protective effects [10,11,13]. Furthermore, low molecular weight milk components were found to protect S. senftenberg 775W against heat effects by way of a mechanism that appears to be divalent cation dependent [14]. Salmonella Senftenberg 775W has been used since the early 1940′s as an exceptionally heat-resistant strain for this purpose until the present day [15]. Escherichia coli strains, including Shiga toxin-producing E. coli (STEC) strains, can adulterate milk by fecal contamination during the milking process, as ruminants are an important reservoir of STEC strains [16]. In an investigation with E. coli strains isolated from raw milk cheeses, including STEC strains, Peng et al. [16] classified 7 out of 47 generic E. coli isolates as heat resistant (55 °C, 15 min). Dlusskaya et al. [17] described several E. coli isolates from a commercial slaughterhouse, of which E. coli strain AW1.7 (DSM 108612) showed extreme heat tolerance [17]. In E. coli AW1.7, a genomic island termed the locus of heat resistance (LHR) has been described as the genetic determinant of this great heat tolerance and has been found in several Enterobacteriaceae [18]. Analogous to the use of S. senftenberg 775W for the species Salmonella enterica in thermal inactivation experiments, E. coli strain AW1.7 is used as highly heat-resistant strain for E. coli.
In general, bacterial heat tolerance studies are difficult to compare because they may be based on different equipment, holding temperatures, test strains, growth conditions, and different heating matrices. The aim of this study was to determine the maximum heat resistance of two occasional milk-borne pathogens (S. enterica and E. coli) in a specific product (whey concentrate with 30% dry matter) by applying HTST treatment in a pilot plant, providing flow conditions, temperature profiles, and residence times comparable to those used in industrial practice. The strains used were selected based on reports of special heat resistance.
2. Materials and Methods
2.1. Bacterial Strains and Cultivation Conditions
The Salmonella Senftenberg and E. coli AW1.7 strains used in this study and relevant information about these are listed in Table 1. All strains were cultured on TSA (Tryptic Soy Agar; Oxoid Deutschland GmbH, Wesel, Germany) at 37 °C. For heat inactivation studies, the cultures were grown in 100 mL of TSB-YE (Trypticase Soy-Yeast Extract broth; Oxoid) incubated at 37 °C for 24 h on a shaker at 100 rpm. For both strains, an inoculation level of the whey concentrate of at least 5 × 105 CFU/mL was aimed for (see Supplementary Material Table S1 for cell counts).

Table 1.
Test organisms used for inactivation experiments during high temperature–short time treatment of whey concentrate (30% dry matter).
2.2. Whey Concentrate
Whey concentrate was provided by a local processing plant that utilizes whey concentrates obtained from various cheese factories for the production of food ingredients. At the plant, whey concentrates are heat treated upon receipt, for a minimum of 20–40 s at 73–75 °C. For this study, whey concentrate was delivered without heat treatment (β-lactoglobulin denaturation < 5%). The total mesophilic bacteria count was determined upon receipt prior to heat treatment experiments and was less than 5000 CFU/mL. Dry matter was adjusted to 30 g/100 g, consisting of 75% lactose, 13% total protein (no casein detected), and 0.8% residual fat. Physical properties were pre-determined as follows: water activity 0.92–0.93; density 1130 kg/m3 at 20 °C, 1120 kg/m3 at 50 °C, and 1115 kg/m3 at 70 °C; apparent shear viscosity (150 1/s) 4.5 mPa s at 20 °C, 2.6 mPa s at 50 °C, and 3.3 mPa s at 70 °C (progressive β-lactoglobulin denaturation).
2.3. Pilot Plant Pasteurization and Heat Inactivation Studies
Experiments were performed three times for each strain in biological replicates. This implies that each culture was grown separately and used in independent experiments. The pre-cooled (5 °C) whey concentrate samples (20 L) used for pilot plant pasteurization were prepared in the autoclaved storage tank (stainless steel can) of the pasteurization unit. To inoculate the can, 10 mL of the broth culture, grown as described above, was thoroughly pre-mixed with 100 mL of the whey concentrate in an Erlenmeyer flask. The suspension was then transferred to the whey concentrate in the can and mixed for 15 min with a magnetic stirrer (350 rpm). Subsequently, a 10 mL sample was taken directly from the can with a sterile pipette for determination of the initial count of the test strains. Stirring was continued throughout the heating experiment.
The pilot plant pasteurizer was constructed analogous to those used in processing plants performing high temperature–short time (HTST) treatment, consisting of a heating, holding, and cooling section with plate heat exchangers in water counterflow mode (Figure 1). It has been utilized previously for similar studies, and its design and operation have been described with detailed heating profiles [21,22].
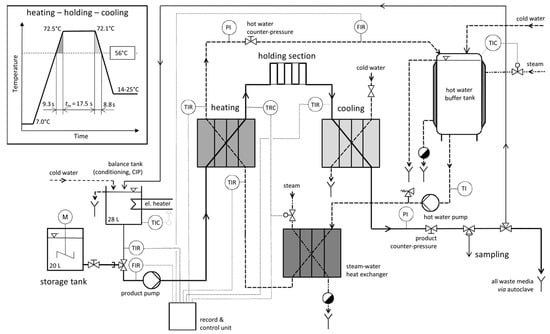
Figure 1.
Simplified process flow diagram of the pilot plant for high temperature–short time treatment of pathogens in concentrated whey at 25 L/h; inset plot of a temperature–time profile in the heating, holding, and cooling section based on a set temperature of 72.1 °C, average residence time given for media temperatures beyond 56 °C; sensor labels: P—pressure, T—temperature, F—flow, I—indicator, R—recorder, C—controller.
All experiments were set to a constant product flow rate of approx. 25 L/h, resulting in a Gaussian time distribution with a mean of tm = 17.5 s (tmin = 15.4 s, tmax = 19.7 s, Bo = 1.076). Actual mean holding times were between 17.2 and 18.2 s (Supplementary Material Table S1). Nominal heating temperatures were set to 72.0, 68.0, 64.0, 60.0, and 56.0 °C. During the experimental runs, temperatures of 71.7–72.5 °C, 68.1–68.4 °C, 64.1–64.3 °C, 60.2–60.4 °C, and 56.1–56.4 °C were recorded, respectively. The reference temperature for the thermal treatment was the temperature at the end of the holding section, which was approximately 0.3–0.4 K (°C) lower than the temperature at the outlet of the heat exchanger (Figure 1). The product and water flow rates were adjusted to be nearly equal at all times, resulting in linear temperature–time profiles of the whey concentrate in the plate heat exchangers. The inset plot in Figure 1 shows a temperature–time profile based on a run with 72.1 °C as the set temperature.
Samples were collected with a sterile one-way syringe (Sarstedt monovette, Nümbrecht, Germany) and needle through the reusable membrane of a self-sealing sampling valve (Janz-Präzisionstechnik GmbH, Malente, Germany) directly after the cooling section. Details of the cleaning and disinfection of the pilot plant pasteurizer are described elsewhere [21]. Briefly, prior to an experiment, tap water was heated to a temperature of 95 °C and circulated for 30 min. At the end of the experiment, an alkaline detergent (2% v/v NaOH) was circulated for 30 min at 80 °C, which was followed by rinsing with fresh water and circulation of an acidic cleaner (1% v/v H3PO4) for 20 min at 60 °C. Again, the pasteurizer was rinsed with fresh water and heated to 95 °C for 30 min. During the preparation of the storage tank (approx. 20 min), the pilot plant was operated with tap water, which was continuously discarded (no circulation) and autoclaved.
2.4. Detection of Survivors and Calculation of Reduction
The following selective agars were used for detection and counting of survivors: TBX (tryptone bile X-glucuronide agar; Oxoid) for E. coli and XLD (xylose lysine deoxycholate agar; Oxoid) for Salmonella. For XLD, S. senftenberg 775W showed a H2S weak to negative reaction. Initial counts in the whey concentrate and counts after heat treatment were determined by diluting samples in ¼ strength Ringer’s solution in a decimal dilution series and by spread plating 0.1 mL of appropriate dilutions in duplicate onto the respective selective agars. In addition, for heat-treated samples, 1 mL volumes were directly spread-plated (onto four plates in aliquots of 250 µL) and in portions of 0.1 mL (all undiluted) in duplicate onto each of the respective agar media. The analysis of 1 mL sample therefore resulted in a limit of quantification of 1 CFU/mL (0 log10). The number of colonies counted on a plate was set at 10–300 for diluted and 0–100 for undiluted samples. In parallel, 3 mL of the samples was used for enrichment in 10 mL of TSB-YE. The enrichment broth was streaked onto the agars after incubation at 37 °C for up to 72 ± 2 h using an inoculation loop. The analysis of 3 mL of sample therefore resulted in a limit of detection of 0.33 CFU/mL. The incubation conditions for agar were aerobic at 37 °C for 24 ± 2 h.
Log10 reductions were calculated by subtracting the log count of survivors from the log initial count. The complete dataset with absolute cell counts of all strains can be accessed in Supplementary Material Table S1. Positive results from enrichment samples, which were negative on direct plating, were considered to indicate at least the log10 reduction of the respective initial count.
To evaluate possible inhibitory effects of the selective agar compounds, the recovery rate was determined for the abovementioned selective agars and non-selective blood-agar (Columbia agar supplemented with 5% v/v defibrinated sheep blood; both Oxoid) in parallel. A preliminary comparison of the growth rates of the test strains on selective agars and (non-selective) blood agar showed equal growth on the respective agars. Therefore, a negative influence of the selective compounds present in the agars (TBX, XLD) used to determine viability in heat inactivation studies could be most likely excluded. However, a possible combination of growth inhibition due to selective compounds in addition to sublethal injury caused by the heat treatment remained possible and could not be rejected entirely.
2.5. Statistical Evaluation
All experiments were performed in triplicate as biological replicates. Replication in this context means that all strains were grown independently for each run prior to mixing. Descriptive statistics and statistical analysis of the log reduction data were performed using JMP (version 16.2; SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) pairwise comparisons were used to determine if there were significant differences between both organisms for each treatment temperature, as well as significant differences between the temperatures for each strain separately. A probability of p < 0.05 was used to determine statistical significance. The arithmetic mean value and standard deviation were calculated and are given in the text.
3. Results
Bacterial Inactivation by Temperature
At the lowest heating temperatures of 56 °C and 60 °C, only Salmonella showed minor reductions in cell count, namely by 0.3 ± 0.5 log10 CFU/mL and 0.6 ± 0.5 log10 CFU/mL (Table 2), respectively, which represented a significantly higher reduction at this temperature compared to the E. coli strain tested (p < 0.05).

Table 2.
Initial cell count and log10 reduction (CFU/mL) of heat-resistant Escherichia coli and Salmonella enterica test strains in whey concentrate (30% dry matter) due to high temperature–short time treatment with 17.5 s holding time; arithmetic mean and standard deviation (n ≥ 3); for absolute cell counts, see Supplementary Material Table S1.
At a heating temperature of 64 °C, the viable counts of S. senftenberg 775W were slightly reduced by 1.1 ± 0.5 log10 CFU/mL, which was not significantly higher than the slight reduction of 0.7 ± 0.5 log10 CFU/mL for E. coli (Figure 2).
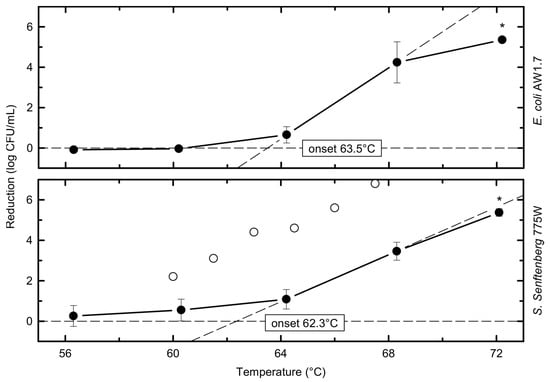
Figure 2.
Thermal reduction of individual strains as a function of temperature (closed circles) at a holding time of 17.5 s; literature data from whole raw milk by D’Aoust et al. (open circles) [23]; average points and standard deviations calculated from i ≥ 3, n ≥ 6; asterisks indicate data points with significant 5 log10 reduction (p < 0.05); lines connecting data points serve as a visual guide.
Starting at a heating temperature of 68 °C, the strains of both species showed significant decreases in cell count of 3.5–4.2 log10 CFU/mL. In addition, at 68 °C and 72 °C, there were no significant differences in reduction detectable between the two heat-resistant representatives of Salmonella and E. coli, in contrast to the lower temperatures. However, for both heat-resistant strains, the inactivation at 68 °C and 72 °C was significantly higher than the reduction during the 56 °C, 60 °C, and 64 °C treatments.
At the highest heating temperature of 72 °C, viable cells were reduced to undetectable levels or levels below 1 CFU/mL (only detectable after enrichment, which means 0 log10 CFU/mL). One exception with 2 CFU/mL survivors of S. senftenberg 775W occurred in one of the three replicates at 72 °C. From the plates plated with 1 mL that did not show colonies, one of the two enrichments showed growth and S. senftenberg was confirmed on XLD. For E. coli AW1.7, two of the three enrichments were positive for survivors with corresponding counts < 1 CFU/mL (limit of detection = 1 CFU in 3 mL). Thus, for S. senftenberg 775W and E. coli AW1.7, a reduction in cell numbers of 5.4 log10 CFU/mL was observed for a treatment of 72 °C for 17.5 s. When considering the maximum reduction at 72 °C, the initial bacterial count N0 was taken into account (Table 2).
4. Discussion
4.1. Inactivation of Highly Heat-Resistant Salmonella and E. coli
Thermal inactivation of bacterial contaminants depends on the food matrix, the bacterial strain, and the thermal processing technology. The characteristics of the food (e.g., moisture, water activity, sugar, fat content, and pH) can lead to very different results (D-values). As example, the D60-values of S. senftenberg 775W can vary depending on the matrix, from 0.12 in raw milk to 11.8 min in liquid egg yolks [15]. The present study showed a D60-value of 0.53 min in whey concentrate, which was higher than that determined in raw milk.
In a sucrose solution at an initial water activity (aw) of 0.98, the D-value (68.3 °C) of S. typhimurium was reportedly 0.12 min [24]. When the aw value was decreased to 0.83, the D-value was raised to 17.4 min [24]. As an extreme example, the D70-value of S. senftenberg 775W can increase up to 440 min in molten chocolate [24]. In this study, whey concentrate had a lactose content of 225 g/L and an aw value of 0.92–0.93. In a sucrose solution with aw = 0.94, a D68.3-value of 0.61 min was described for S. typhimurium [24], which was a far lower inactivation than the D68.3-value of 0.084 min in our study for whey concentrate.
On the other hand, the characteristics of the selected strains and grow conditions also lead to very different results (D-values exceeding that value up to 10–100 fold) [17,18,24]. While serotype does not reliably predict the thermal resistance in Salmonella and E. coli, there are large differences at the strain level. This finding has long been known for Salmonella, as various S. senftenberg strains showed D57-values of <1 min to 31 min [15]. For E. coli, the D60-values of strains within the species can vary between 0.11 min and 71 min [17,18]. In this study, we were unable to detect any quantifiable reduction of E. coli AW1.7 at 60 °C. Peace et al. previously described the inactivation of various pathogens in commercial pasteurization and also studied 29 strains of E. coli [3]. The E. coli strain with the highest heat resistance in that panel showed a D64-value of 0.27 min in UHT milk (AW1.7 was then still unknown), which was lower than the results of this study with a D64-value of 0.45 min for E. coli strain AW1.7 in whey concentrate. The growing conditions can also have an influence on the thermal resistance. If cells of S. Enteritidis were initial grown at 20 °C, a D56-value of 0.91 min was reported, while the same strain when grown at 44 °C exhibited a D56-value of 14.4 min [25]. For these reasons, modeling of new matrix–pathogen combinations without practical testing or transferring literature results to other foods is often inadequate.
Although heating at 72 °C for 17.5 s was determined sufficient for Salmonella and E. coli in whey concentrate to achieve the required safety of 5 log10 reduction, the very low number of survivors (positive enrichment) also showed that pasteurization conditions as used in this study are a minimal heating requirement for this product. It is currently unknown whether highly heat-resistant strains are selected by food production. However, due to the relatively recent identification of the genetic background (locus of heat resistance), monitoring is possible and recommended. Only then can changes be detected and, if necessary, adjustments made to the established thermal pasteurization process.
4.2. Impact of the Heating System and the Matrix Whey Concentrate
Comparison of heat inactivation data between different studies is difficult because they mostly differ in the matrix, heating equipment, and test strains used. In particular, the enhanced shear stress in continuous heating systems results in increased inactivation compared to batch heating systems mimicking a similar temperature–time profile [26]. In the case of Salmonella, however, a study by D’Aoust [27], who worked with S. senftenberg strain 775W and 20 other serovars and who used equipment similar to the pilot plant pasteurizer used in this study, is somewhat suitable for comparison. However, the authors operated their pilot plant with a minimum holding time of tmin = 16.2 s at a flow rate of 363 kg/h, thus resulting in a fully turbulent flow (Re = 10.500), whereas the pasteurizer in this study had a laminar flow (Re = 430). Therefore, its efficiency in terms of a steep heating profile was expected to be slightly higher than that of the pilot plant pasteurizer used in this study (Figure 1). In addition, whole raw milk was used in the study of D’Aoust et al. [26]. Whole raw milk has a dry matter content of 12–13%, whereas the whey concentrate (30% dry matter) used in our study was three to four times more viscous and, therefore, less efficient in heat transfer.
In the study by D’Aoust et al. [27], a mean reduction of 4.2 log10 CFU/mL was recorded at 60 °C for two cocktails of ten Salmonella serovars (each of non-human or human origin, including S. senftenberg 775W), while the reduction of S. senftenberg 775W in raw milk was only 2.2 log10 CFU/mL [26]. The reductions measured at 60 °C in our study were even lower, with 0.6 log10 CFU/mL for S. senftenberg 775W (Figure 2). At the higher heating temperature of 64.5 °C, the study by D’Aoust et al. [27] showed mean log10 reductions of approx. 7.9 log10 CFU/mL for the non-human isolate Salmonella cocktail and 4.6 log10 CFU/mL for S. senftenberg 775W. The human isolate mixture was not tested. In the present study, the reduction observed at this temperature was 1.1 log10 CFU/mL for S. senftenberg 775W (Table 2). Also, a higher inactivation (6.8 log10 CFU/mL) of S. senftenberg 775W was achieved at 67.5 °C by D’Aoust et al. [27] (the other Salmonella were not tested), whereas in our study the same strain was reduced by only 3.5 log10 CFU/mL at 68.3 °C. This generally lower inactivation in the present study may be due to the proposed higher efficacy of the plant utilized by D’Aoust et al. [27] in terms of heat transfer and a protective effect of the higher dry matter content of the whey concentrate used as matrix in our study. No studies on heat inactivation of E. coli could be found that used similar matrices or pasteurization equipment to that in our study.
The strains E. coli AW1.7 and S. senftenberg 775W are currently the most heat-tolerant strains known for E. coli [28] and Salmonella [15,19,26,28], respectively. This was the reason for selecting these strains for heat tolerance experiments in this study. Salmonella Senftenberg 775W has been known since the 1960′s to have exceptional heat resistance [15], as also shown in this study. Salmonella Senftenberg 775W was the most heat tolerant among approx. 300 Salmonella cultures representing 75 different serotypes. Similarly, E. coli AW1.7 has long been known to be an extremely heat-resistant strain of Escherichia coli and the genetic determinants associated with this heat resistance were found to be located on a genomic island encoding solute transport proteins and proteases [18]. Only in 2% of E. coli genomes was the locus of heat resistance (LHR) identified [18]. And heat resistance is not related to the virulence or phylogenetic groups of E. coli [18]. Nevertheless, such cases of strains with increased heat tolerance should be included in the consideration of thermal processes as a potential worst-case scenario to test or adjust thermal treatments of foods accordingly.
5. Conclusions
Overall, the results of this study clearly show that a 5 log10 reduction of both highly heat-tolerant strains of Salmonella and E. coli in whey concentrate could be achieved when heating at 72 °C for 17.5 s in a continuous pasteurization unit similar to industrial practice. This is a novel finding, as the heat tolerance of these pathogens in concentrated whey has not been described yet. Compared to the literature data for milk, whey concentrate appears to have a protective matrix effect on the pathogens studied. Nevertheless, the pasteurization parameters of 72 °C for 17.5 s are sufficient for whey concentrate, even for the most heat-resistant strains of Salmonella and E. coli. The important implication is that products made from whey concentrate, when used as ingredient in foods, should not lead to contamination with these bacterial pathogens, provided that the whey concentrate has been pasteurized at 72 °C for at least 17.5 s.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol4010036/s1. Table S1. Contains the data of the inactivation experiments (species; strain; temperature; flow rate; holding time; initial number; survival number; reductions).
Author Contributions
Conceptualization, G.F., M.S. and C.M.A.P.F.; methodology, G.F., S.N. and S.M.; software, G.F.; validation, G.F. and S.N.; formal analysis, G.F. and S.N.; investigation, G.F.; resources, C.M.A.P.F. and M.S.; data curation, G.F., S.N. and C.M.A.P.F.; writing—original draft preparation, G.F., S.N. and C.M.A.P.F.; writing—review and editing, G.F., S.N. and M.S.; visualization, G.F., S.N. and S.M.; supervision, C.M.A.P.F.; project administration, C.M.A.P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article or Supplementary Material and available upon request.
Acknowledgments
The authors would like to thank Svenja Feuerhahn, Maren Schmidt, and Marie-Kristin Schrader for their excellent technical assistance. We would also like to acknowledge the support and help of Philipp Hammer.
Conflicts of Interest
The authors declare no conflicts of interest. Meike Samtlebe was employed by the company wheyco GmbH. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be cons.
References
- Kells, H.R.; Lear, S.A. Thermal death time curve of Mycobacterium tuberculosis var bovis in artificially infected milk. Appl. Microbiol. 1960, 8, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.E. Bacterial Diseases—The Impact of Milk Processing to Reduce Risks; Bulletin of the International Dairy Federation; International Dairy Federation: Brussels, Belgium, 2002; pp. 20–25. [Google Scholar]
- Pearce, L.E.; Smythe, B.W.; Crawford, R.A.; Oakley, E.; Hathaway, S.C.; Shepherd, J.M. Pasteurization of milk: The heat inactivation kinetics of milk-borne dairy pathogens under commercial-type conditions of turbulent flow. J. Dairy Sci. 2012, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Microbiological considerations: Pasteurized milk. Int. J. Dairy Sci. 2015, 10, 206–218. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services/Public Health Service/Food and Drug Administration. Grade “A” Pasteurized Milk Ordinance, Including Provisions from the Grade “A” Condensed and Dry Milk Products and Condensed and Dry Whey: Supplement I to the Grade “A” Pasteurized Milk Ordinance. Available online: https://www.fda.gov/media/140394 (accessed on 9 March 2024).
- CAC/RCP 57–2004; Codex Alimentarius. Code of Hygienic Practice for Milk and Milk Products. Food and Agriculture Organization: Rome, Italy, 2004.
- Hudson, J.; Wong, T.; Lake, R. Pasteurisation of Dairy Products: Times, Temperatures and Evidence for Control of Pathogens. Available online: http://www.foodsafety.govt.nz/elibrary/industry/Pasteurisation_Dairy-Science_Research.pdf (accessed on 16 May 2023).
- Kornacki, J.L.; Marth, E.H. Thermal inactivation of Salmonella senftenberg and Micrococcus freudenreichii in retentates from ultrafiltered milks. LWT—Food Sci. Technol. 1993, 26, 21–27. [Google Scholar] [CrossRef]
- Dega, C.A.; Goepfert, J.M.; Amundson, C.H. Heat resistance of salmonellae in concentrated milk. Appl. Microbiol. 1972, 23, 415–420. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Kuile, B.H. Low-water activity foods: Increased concern as vehicles of foodborne pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef]
- Santillana Farakos, S.M.; Hicks, J.W.; Frank, J.F. Temperature resistance of Salmonella in low-water activity whey protein powder as influenced by salt content. J. Food Prot. 2014, 77, 631–634. [Google Scholar] [CrossRef]
- European centre for disease prevention and control, European food safety authority. Multi-country outbreak of monophasic Salmonella Typhimurium sequence type (ST) 34 linked to chocolate products—12 April 2022. oint ECDC-EFSA rapid outbreak assessment. EFS3 2022, 19, 7318E. [Google Scholar] [CrossRef]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef]
- Mañas, P.; Pagán, R.; Sala, F.J.; Condón, S. Low molecular weight milk whey components protect Salmonella senftenberg 775W against heat by a mechanism involving divalent cations. J. Appl. Microbiol. 2001, 91, 871–877. [Google Scholar] [CrossRef]
- Ng, H.; Bayne, H.G.; Garibaldi, J.A. Heat Resistance of Salmonella: The Uniqueness of Salmonella senftenberg 775W. Appl. Microbiol. 1969, 17, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Stephan, R.; Hummerjohann, J.; Blanco, J.; Zweifel, C. In vitro characterization of Shiga toxin-producing and generic Escherichia coli in respect of cheese-production relevant stresses. Arch. Leb. J. Food Saf. Food Qual. 2012, 63, 136–141. [Google Scholar] [CrossRef]
- Dlusskaya, E.A.; McMullen, L.M.; Gänzle, M.G. Characterization of an extremely heat-resistant Escherichia coli obtained from a beef processing facility. J. Appl. Microbiol. 2011, 110, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.G.; Zheng, J.; Garcia-Hernandez, R.; Ruan, L.; Gänzle, M.G.; McMullen, L.M. Genetic determinants of heat resistance in Escherichia coli. Front. Microbiol. 2015, 6, 932. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.V.; Harhay, G.P.; Bono, J.L.; Smith, T.P.L.; Harhay, D.M. Genome sequence of the thermotolerant foodborne pathogen Salmonella enterica Serovar Senftenberg ATCC 43845 and phylogenetic analysis of loci encoding increased protein quality control mechanisms. mSystems 2017, 2, e00190-16. [Google Scholar] [CrossRef] [PubMed]
- Guragain, M.; Bono, J.L.; Bosilevac, J.M. Complete closed genome sequence of the extremely heat-resistant strain Escherichia coli AW1.7. Microbiol. Resour. Announc. 2021, 10, e0050221. [Google Scholar] [CrossRef]
- Hammer, P.; Kiesner, C.; Walte, H.; Knappstein, K.; Teufel, P. Heat resistance of Mycobacterium avium ssp. paratuberculosis in raw milk tested in a pilot-plant pasteurizer. Kiel. Milchwirtsch. Forschungsberichte 2002, 54, 275–303. [Google Scholar]
- Peng, S.; Hummerjohann, J.; Stephan, R.; Hammer, P. Short communication: Heat resistance of Escherichia coli strains in raw milk at different subpasteurization conditions. J. Dairy Sci. 2013, 96, 3543–3546. [Google Scholar] [CrossRef]
- D’Aoust, J.-Y.; Emmons, D.B.; McKELLAR, R.; Timbers, G.E.; Todd, E.C.D.; Sewell, A.M.; Warburton, D.W. Thermal Inactivation of Salmonella Species in Fluid Milk 1. J. Food Prot. 1987, 50, 494–501. [Google Scholar] [CrossRef]
- Doyle, M.E.; Mazzotta, A.S. Review of studies on the thermal resistance of Salmonellae. J. Food Prot. 2000, 63, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.B.; Bradshaw, J.G.; Peeler, J.T. Thermal resistance of egg-associated epidemic strains of Salmonella enteritidis. J. Food Sci. 1991, 56, 391–393. [Google Scholar] [CrossRef]
- Stoeckel, M.; Abduh, S.B.M.; Atamer, Z.; Hinrichs, J. Inactivation of Bacillus spores in batch vs continuous heating systems at sterilisation temperatures. Int. J. Dairy Technol. 2014, 67, 334–341. [Google Scholar] [CrossRef]
- Liu, Y.; Gill, A.; McMullen, L.; Gänzle, M.G. Variation in heat and pressure resistance of verotoxigenic and nontoxigenic Escherichia coli. J. Food Prot. 2015, 78, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Solowey, M.; Sutton, R.R.; Calesnick, E.J. Heat resistance of Salmonella organisms isolated from spray-dried whole-egg powder. Food Technol. 1948, 2, 9–14. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).