Abstract
Instances of inflammatory bowel disease (IBD), a chronic inflammatory condition of the gastrointestinal tract, are rapidly increasing in western and newly industrialized countries. Exopolysaccharides (EPSs) are one of the strategies to enhance the gut microbiota and modulate the immune-inflammatory response deregulation in IBD patients. EPSs are produced by commensal bacteria such as Lactobacillus and Bifidobacterium. Additionally, Cyanobacteria species are promising sources of novel EPS and have potential pharmaceutical and therapeutic applications. The presence of uronic acids and sulphate groups in Cyanobacterial EPSs is an important factor that gives EPSs an anionic charge that is not seen in other prokaryotic species. This feature may impact their physico-chemical characteristics and biological properties. Additionally, Cyanobacterial EPSs have a wide range of biotechnological applications that include use as thickeners, stabilizers, and gelling agents in the food and pharmaceutical sectors. The present review focuses on the role of EPSs in IBD, with a special focus on EPSs derived from Cyanobacteria. This review also covers the biological properties of Cyanobacterial EPS in immuno-inflammatory responses and against pathogens as well as its role in biotechnological applications. Overall, Cyanobacterial EPSs have therapeutic potential against IBD due to their anti-inflammatory and immunoregulatory properties that can reduce inflammation and regulate the immune response and restore the gut microbiota of patients.
1. Introduction
The westernization of lifestyles and dietary habits over the past few decades has resulted in the spread of inflammatory bowel disease (IBD), which is a chronic inflammatory disease divided into two major types: Crohn’s disease and ulcerative colitis [1]. IBD is a multifactorial gut disorder involving immune dysfunction, genetic susceptibility, and environmental factors. The Western-style diet is characterized by a high caloric content combined with large amounts of fat and refined carbohydrates, and has been associated with a significant decrease in the biodiversity of gut microbiota [2]. In light of this issue, it is important to note that the consumption of processed foods in Western countries leads to changes in the gut microbiota [3,4]. This Western food is processed by hulling, heating, and adding preservatives, which all contribute to a reduction in beneficial microorganisms present in food. Furthermore, dietary additives such as emulsifiers, which are regularly used in processed foods, might also contribute to an increase in the risk of IBD [5]. It has also been found that high-fat diets are linked to dysbiosis, as well as a decrease in the abundance of Bacteroidetes and an increase in the abundance of Firmicutes and Proteobacteria in murine models [3,4].
Western diets rich in fat increase the population of bacteria producing lipopolysaccharides (LPSs), such as Enterobacteriaceae, while reducing the population of bacteria suppressing LPSs, such as Bifidobacterium. Furthermore, this diet alters the intestinal epithelial cells, leading to alterations in the intestinal barrier, including increased intestinal permeability and LPS translocation [6,7].
Different strategies targeted at the gut microbiota have emerged as potential therapeutic approaches [8]. Among them, probiotics have gained the greatest attention as a method to restore the gut microbiota biodiversity in IBD patients. Probiotics are live microorganisms that, when administered in adequate amounts, can confer a health benefit on the host [9]. They can be beneficial in restoring the gut microbiota’s composition, which in turn can lead to an improved digestion and absorption of nutrients, as well as improved immunity.
The major beneficial effects of probiotics have been observed to be derived from the metabolites and secreted compounds produced by live microbes rather than simply from the presence of these microorganisms themselves [10]. The compounds derived from probiotic compounds are known as postbiotics. In contrast to probiotics, postbiotics are not dependent on living microorganisms to induce health benefits [11]. The postbiotic components consist of short-chain fatty acids, exopolysaccharides, teichoic acids, vitamins, enzymes, peptides, and bacteriocins. These bioactive compounds appear to instill a wide range of health benefits, including improved immunity, reduced inflammation, and improved digestive health [12].
This review will focus on exopolysaccharides (EPSs) derived from commensal and probiotic bacteria, with a special focus on EPSs derived from Cyanobacteria. It will describe how EPSs’ composition from different strains of probiotic bacteria differs from that of Cyanobacteria. The factors that induce the production of EPSs from Cyanobacteria will also be discussed. This review will cover the biological properties of Cyanobacterial EPSs regarding immuno-inflammatory responses and against pathogens as well as their role in biotechnological applications. Finally, it will provide insights into the potential of EPS in food products.
2. Role of EPS Derived from Commensal and Probiotic Bacteria in IBD
Recently, more attention has been paid to EPSs produced by probiotic bacteria such as Lactobacillus, Lactococcus, Bifidobacterium, and Streptococcus for various applications, including medical applications. EPSs are released into the extracellular environment as capsules which help bacteria survive in extreme environmental conditions. Among their functions are protection from extreme temperature, UV-rays, aridity, salinity, unfavorable pH values, osmotic stress, phagocytosis, and chemical agents (antibiotics, heavy metals, and oxidants) [13,14,15]. EPSs refer to polysaccharides secreted or produced outside of bacteria during the bacterial growth phase.
These EPSs are identified as homopolysaccharides or heteropolysaccharides according to the composition of their monosaccharides. The major role of EPSs lies in the formation of complex structures by stimulating a variety of microbial interactions [16,17]. EPSs are produced during the growth process of many probiotic bacteria [18]. In some cases, EPSs are linked with the peptidoglycan via N-acetyl-muramic acid, while in other cases, they are more loosely linked and may exist as a slimy secretion.
Furthermore, they have many potential health benefits such as anti-inflammatory properties, pathogen growth-inhibitory properties, immunomodulatory activities, and the regulation of the intestinal barrier [19,20,21]. The immunological response can only be induced if the EPSs reach the colon intact. Numerous studies have shown that EPSs have beneficial biological effects in modulating immune responses. Their compositions are so diverse that they induce different immunomodulatory effects according to their composition.
Palik et al. showed that EPS from Bacillus subtilis represents a recently discovered probiotic-derived agent that provides protection against systemic Staphylococcus aureus infection by increasing the production of ROS as well as limiting the inflammatory activity of immune cells. Furthermore, the authors found that B. subtilis EPS is a powerful antibacterial agent [22].
IBD pathogenesis is well known to be associated with increased Th1 and Th17 cells [23]. Additionally, the abnormal and imbalanced function of Treg and Th2 cells occurs at the same time as the dysregulation of cytokine production, which is associated with the development of IBD.
Matsuzaki et al. showed that EPSs stimulated the production of a specific IgA in Peyer’s patch cells and influenced the Th1 and Th2 cell-mediated response in splenocytes [24] (Figure 1).
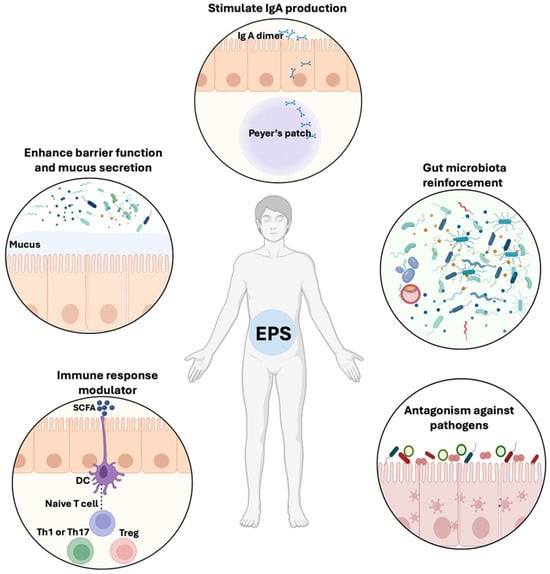
Figure 1.
Schematic representation of the beneficial effects of EPSs on gut homeostasis. EPSs from commensal bacteria including Lactobacillus and Bifidobacterium are involved in the reinforcement of the gut microbiota, the elimination of pathogenic microorganisms (like C. albicans and enterotoxigenic E. coli), and the modulation of immune response through SCFA production. EPSs increase the function of the intestinal barrier and mucus secretion, as well as IgA dimer production in Peyer’s Patches.
Chung et al. showed that EPSs derived from Bacillus subtilis restore intestinal barrier function by modulating tight junction-related proteins (claudin-1, claudin-2, and occludin) and epithelial-mesenchymal transition in DSS induced-colitis mice [17]. EPSs from Lactobacillus plantarum promoted colon mucosal healing and homeostasis, enhanced goblet cell differentiation, and increased Muc2 expression, which is the colon’s major gel-forming mucin [25] (Figure 1).
Additionally, they modulate the differentiation of T cells in the intestinal tract, and this may result in a relief of IBD symptoms [17]. In line with these observations, B-EPS promoted splenocyte proliferation in response to anti-CD3/CD28-primed splenocytes, confirming its ability to regulate T lymphocyte activation [26].
Gorska et al. analyzed the chemical structures and immunomodulatory properties of EPSs isolated from Lactobacilli isolated from mice with induced IBD (IBD “+”) and those obtained from healthy mice (IBD “−”). They found that EPSs produced from IBD “+” species are structurally different from those isolated from IBD “−”. Further, they observed that EPSs from L. animalis/murinus 148, L. animalis/murinus 116, and L. reuteri 115 (IBD “+”) were all immunologically silent. In addition, they found that different EPS structures leads to different immune responses by dendritic cells, suggesting that, under conditions of gut inflammation, changes in bacteria strains produce EPSs with specific motifs which are not present in lactobacilli IBD+ [27].
The EPSs isolated from L. casei SB27 significantly reduced the proliferation of the colon cancer cell HT-29 in vitro [28]. An EPS challenge activated the pro-apoptotic genes Bad and Bax by increasing mRNA expression, along with caspase-8 and caspase-3 [28]. The anti-biofilm activity of three distinct fragments of EPS isolated from the supernatant of L. fermentum has been demonstrated in vitro against Escherichia coli and Staphylococcus aureus, in addition to its anti-oxidant properties and ability to scavenge free radicals [29]. Additionally, EPSs derived from L. paracasei inhibit E. coli and S. aureus biofilms by acting against the quorum-sensing mechanism involved in biofilm formation [29]. Ksonzekova et al. showed that L. reuteri EPSs prevent the adhesion of enterotoxigenic E. coli to epithelial cells as well as the inflammatory response [30] (Figure 1).
Recent study has shown that EPSs isolated from L. buchneri can alleviate experimentally induced liver damage in mice [31]. The mice treated with EPSs also had similar levels of the cytokines TNF-α, IL-6, and IL-1β to the control group and significantly lower levels than the test group. In addition, EPSs increased the number of commensal bacteria and reduced the number of Helicobacteraceae, Enterobacteriaceae, and Lachnospiraceae [31] (Figure 2).
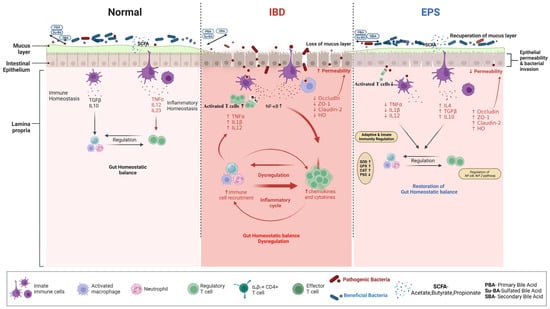
Figure 2.
Schematic representation illustrates the mechanisms of EPSs’ therapeutic effects on IBD. Immune tolerance maintains the gastrointestinal immune system physiological state, which ensures homeostatic balance. Inflammatory bowel disease (IBD) can cause gut dysbiosis and impaired intestinal barrier function, as well as excessive stimulation of the immune response. An imbalance in the gut microbiota population of the host contributes to leaky gut. This leads to the release of chemokines and cytokines that cause inflammation. An uncontrolled migration of several effector T cell subtypes disrupts the intestinal mucosa, effector, and regulatory T cells in IBD. In the presence of intestinal damage, pathogenic microorganisms are known to translocate from one area to another. Toxins, neuroactive substances, metabolites, and immunogenic peptides resulting from poor digestion can also pass through the leaky gut and cause an immune response and inflammation. The process of inflammation is triggered by several mechanisms, such as soluble inflammatory mediators like TNF-α, IL-1β, and IL-12, as well as cellular mediators like macrophages and cytotoxic T cells. All of these mediators work together to cause local tissue damage and to intensify the inflammation cascade that is already present. Additionally, the bile acid pool is changed under these conditions. After EPSs reach the colon and are metabolized by the gut microbiota, their effects on these receptors attenuate the production of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-12) and modulate macrophages’ and helper T cells’ polarization towards anti-inflammatory cytokines (IL-10, IL-4, TGF-β). In addition, the EPSs restore the altered gut microbiota community that contributes to the production of SCFAs, which in turn helps in the recovery of the tissues by strengthening the tight junction proteins (Occludin, ZO-1, HO, Claudin-2).
3. Cyanobacterial EPS
Cyanobacteria are a group of prokaryotic microorganisms that are among the oldest life forms on Earth [32]. The Cyanobacteria have the capability of producing energy through the process of photosynthesis, which is essential to the survival of other organisms in the water food chain [33]. Additionally, these microorganisms play a crucial role in nutrient cycles, such as the nitrogen cycle. They are involved in the process of converting atmospheric nitrogen into organic nitrogen. Furthermore, they provide a large amount of oxygen, which is essential for the survival of fish, plants, and mammals in the water food chain [34].
Cyanobacteria have been shown to synthesize biotechnological and pharmaceutical compounds with beneficial properties including anticancer, anti-inflammatory, and antibiotic ones. Cyanobacteria produce a variety of secondary metabolites since they synthesize both non-ribosomal peptides and polyketides [35]. A wide range of Cyanobacterial enzymes enable methylation, oxidation, and tailoring, contributing to a wide range of chemically diverse natural products [36].
Marine Cyanobacteria compounds are involved in a wide variety of biological interactions with bacteria, fungi, parasites, and invertebrates [37,38]. These interactions are responsible for a range of processes, such as nutrient cycling, bioremediation, and protection against predators. The beneficial effects of marine Cyanobacteria compounds on human tumor cell lines have been studied. Human lung cancer cells are highly sensitive to apratoxin D and symplocamide A produced by Symploca spp. [39]. It is believed that these compounds cause tumor cells to undergo apoptosis, or programmed cell death, thereby making them incapable of replicating [39].
Cyanobacteria have been identified as a significant source of organic compounds, including lipids, sugars, proteins, antioxidants, nucleic acids, and vitamins and minerals, that are crucial for life [40]. Cyanobacteria consist of highly concentrated bioactive substances, including polyunsaturated fatty acids, phycocyanin, carotenoids, peptides, and polysaccharides, and, especially, EPSs, which function as a barrier to protect them from external stresses [41].
The Cyanobacterial EPSs can be classified as released exo-polysaccharides (RPSs) or capsular exo-polysaccharides (CPSs). While RPSs are discharged into the extracellular environment, CPSs enclose their cells in the form of a mucilaginous layer [42].
From a chemical perspective, EPSs belong to the polysaccharide class, which is composed of high-molecular-weight biopolymers ranging from 10 to 1000 kDa [43]. To date, observed Cyanobacterial EPSs molecular masses have ranged from 80 to 2000 kDa [44]. The carbohydrate polymers are primarily composed of monosaccharide monomers, and they can be linked together by glycosidic bonds to form a whole structure [45] (the red caption in Figure 3A), but they may also contain non-saccharides like phosphate, lactate, acetate or glycerol [44]. Depending on the spatial orientation of this glycosidic bond, two anomers, α and β, can be obtained (Figure 3B). These anomers are stereoisomers with different specific rotations, and thus varied properties.
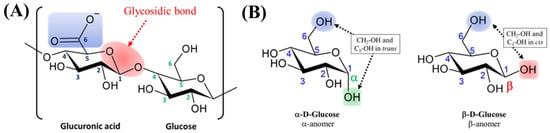
Figure 3.
(A) Structure of Glucuronic acid/Glucose disaccharide linked by a glycosidic bond in red; (B) geometry of the two anomers exemplified by Glucose.
EPSs’ physicochemical properties vary according to their source bacterial strain and are related to their inner chemical composition. The microorganism growth conditions affect the amount or type of heteropolysaccharide synthesis or secretion that occurs [46].
Around 75% of the Cyanobacterial EPSs described so far belong to the heteropolysaccharide class of biopolymers. In general, heteroglycans, such as hyaluronic acid or agarose, contain two or more different monosaccharide units. The compositions of Cyanobacterial EPSs consist of six or more monomers. To date, more than 13 different monosaccharides have been identified in Cyanobacterial EPSs: hexoses (glucose, galactose, mannose, and fructose), pentoses (ribose, arabinose, and xylose), deoxyhexoses (fucose, rhamnose, and methyl rhamnose), and uronic acids (glucuronic and galacturonic acids) [47]. Acetylated or amino-sugars have also been observed, such as N-acetyl glucosamine, 2,3-O-methyl rhamnose, 3-O-methyl rhamnose, 4-O-methyl rhamnose, and 3-O-methyl glucose [48]. Methyl, pyruvyl, and succinyl groups can be present, as well as sulfate groups [42]. Some reports also deal with the presence of polypeptides enriched with amino-acids (glycine, alanine, valine, leucine, isoleucine, and phenylalanine) [49] or proteins rich in aspartic or glutamic acids [50]. Thus, Cyanobacterial EPSs have displayed variable compositions, which explains their broad biological and physico-chemical range of applications [44].
To date, around 200 Cyanobacterial EPS chemical compositions have been elucidated. Collected reports on Cyanobacterial EPSs’ monomeric compositions underlined the presence of uronic acid units (Table 1). In addition to the fact that these uronic acid units are rarely found in EPSs produced by other types of bacteria, their presence is a crucial characteristic of Cyanobacterial EPSs. The EPSs produced by Cyanobacteria also contain sulphate groups. This is a feature that is unique to bacteria but which is also found in the EPSs produced by archaea and eukaryotes.
Uronic acids are a class of sugar acids bearing both carbonyl and carboxylic acid functional groups. The main representative of uronic acids is glucuronic acid, produced from the oxidation of the alcohol function in position 6 in glucose (Figure 3A). The pKa of the carboxylic acid moiety of glucuronic acid measured in a complex structure such as heparin ranged from 2.79 to 3.13 [51], which is similar to that of its monomeric form. In biological conditions, uronic acids are negatively charged.

Table 1.
Overview of monosaccharide compositions and their biological applications for different Cyanobacterial spp.
Table 1.
Overview of monosaccharide compositions and their biological applications for different Cyanobacterial spp.
| Cyanobacteria spp. | Structure | Monosaccharide Composition | Biological Application | Reference |
|---|---|---|---|---|
| Nostoc punctiforme | Pullulan-like polysaccharide | Glucose, Glucuronic Acid | Carbohydrate storage, desiccation tolerance | [52] |
| Calothrix marchica | Heteropolysaccharide | Xylose, Fructose, Rhamnose, and Glucuronic Acid | Biosorbent | [53] |
| Nostoc sp. HK-01 | Exopolysaccharide | Fucose, Rhamnose, Arabinose, Galactose, Glucose, Mannose, Xylose, Uronic Acid, Glucuronic Acid | Protect the cells against ion influx and water efflux. | [54] |
| Anabaena sp. PC-7120 | Exopolysaccharide | Fucose, Rhamnose, Arabinose, Galactose, Glucose, Glucuronic Acid | Protect the cells against ion influx and water efflux. | [55] |
| Anabaena laxa | Polysaccharide complex | Arabinose, Ribose, Rhamnose, Fucose, Xylose, Galactose, Galacturonic Acid | Stimulation on monocytes and granulocytes. | [56] |
| Nostoc commune | Sulfated Polysaccharide | Glucose, Galactose, Xylose | Wound-healing and anti-allergic cosmetics. | [57] |
| Gloeocapsagelatinosa | EPS | Arabinose, Fucose, Rhamnose, Xylose, Galactose, Glucose, Mannose, Glucoronic Acid, Galacturonic Acid | Enhanced Anti-oxidant, metal chelating and thermostable. | [58] |
| Leptolyngbya sp. | EPS | Mannose, Arabinose, Glucose, Rhamnose, Galacturonic Acid | Food and pharmaceutical applications. | [59] |
| Oscillatoria sancta | Exopolysaccharide | Mannose, Fuccose, Ribose, Arabinose | Biocontrol of plant-infecting fungal pathogens. | [60] |
| Arthrospira platensis strain MMG-9 | EPS | Fructose, Fucose, Galactose, Glucose (+), Mannose, Rhamnose, Ribose, Xylose, Uronic Acid | - | [61] |
| Nostoc carneum | EPS | Xylose (+), Glucose, Sul, Uronic Acid | Excellent Anti-oxidant | [62] |
| Anabaena cylindrica 10C | EPS | Galactose, Glucose, GlucoseA, Mannose, Fucose, Xylose | PHB+PHV Production | [63] |
| Anabaena flos-aquae | EPS | Glucose, Galactose, Xylose, Ribose | Recycling of growth media | [64] |
| Chlorogloeopsis spp. | EPS | Rhamnose, Galactose, Arabinose, Glucose, Mannose, Fucose | Inhibition of Avarol toxicity | [65] |
| Chroococcus submarinus | EPS | Rhamnose, Galactose, Arabinose, Glucose, GlucoseA, GalactoseA, Mannose, Fucose, Xylose, Ribose | - | [66] |
| Leptolyngbya sp. | EPS | Rhamnose, Glucose, Galactose, GalactoseA, Fucose, Ribose, Xylose | Persistence on lithoid surfaces | [67] |
| Nostoc insulare | EPS | Arabinose, Glucose, GlucoseA | High viscosity | [68] |
| Oscillatoria spp. | EPS | Glucose, Xylose, Ribose | Intrinsic viscosity | [69] |
Sulfated sugars are also highly present in Cyanobacterial EPSs (around 85% of all elucidated structures contain sulphated monomers). In physiological conditions, sulphated biopolymers are anionic (pKa around 2.6, being more acidic than uronic acid moieties). The presence of sulphated sugars and uronic acid units increases the hydrophilicity of the biopolymers, thereby increasing their solubility in water [70].
Both the sulphate groups and the uronic acids contribute to the anionic nature of EPSs, conferring a negative charge on the overall macromolecule and also modulating its adherence properties. Moreover, these anionic moieties are also known for their ability to chelate metallic cations. This capacity is correlated with the steric hindrance around the anionic parts. This hinders their availability, their distribution within the macromolecule, and the subsequent geometry of their metallic complexes [71,72,73,74].
On the other hand, some monomers increase EPSs’ lipophilicity. Deoxy sugars, such as fucose (6-deoxy-L-galactose) or rhamnose, in which one hydroxyl group has been replaced by a hydrogen atom, are highly present in Cyanobacterial EPSs. Fisher et al. reported that the chemical structure of Chroococcus minutus B 41.79 EPS was composed of 14 different units, including uronic acids and deoxy sugars [75]. According to the review by De Philippis and Vincenzini (27), the EPS produced by Cyanothece IR20 was composed by more than 80% of deoxy sugars, which conferred a high hydrophobicity to the EPS [76].
Acetylated sugars, where the hydroxyl group has been acetylated, are more lipophilic than the corresponding HO-monomer. The presence of peptides also increases their lipophilicity.
Overall, the hydrophile/lipophile balance of EPSs depends on the proportion of hydrophilic monomers (uronic acids and sulphated ones) versus the amount of acetylated and desoxy monomers. It is notable that cyanothece CE4 EPS collected from Italian saltworks displayed a high proportion of uronic acids (80.1%) along with deoxy sugars [76]. This composition, with its high level of lipophilic EPSs in combination with a strong charged moiety, contributes to the emulsifying capacity of such EPSs.
Overall, the composition of Cyanobacterial EPSs is essential for controlling their hyddophilic/lipophilic balance (HLB)’s compactness or flexibility. This determines their rheological properties and capacity to interact with other compounds such as cations [42,77,78,79,80]. Figure 4 summarizes the components that influence EPS HLB.
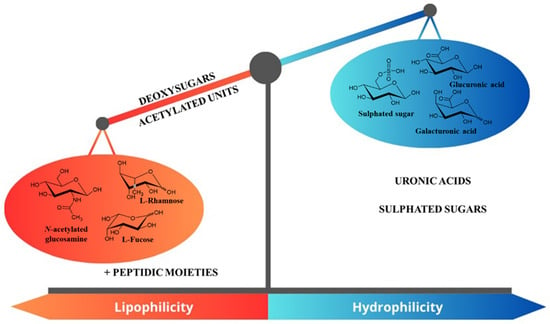
Figure 4.
Summary of monomers that influence the global HLB of Cyanobacterial EPS.
It was found that polymers will acquire a negative charge from uronic acids, and pullulan-like polysaccharides from Cyanobacteria (Anabaena and Nostoc), which resemble the yeast polysaccharide pullulan in structure, are capable of withstanding desiccation and are also used as reserve food sources [81,82]. The EPSs of glucose, galactose, glucuronic acid, and succinoglycan have been used in Synechocystis spp. to stimulate cell motility, which can lead to gliding motility, which is a kind of movement that is necessary for the chemotactic access of the cells. Cyanobacteria’s EPSs are rich in glucose, galactosamine, and glucosamine. The initial exploration of Cyanospira capsulata EPSs revealed novel N-acetylglucosamine (GIcNAc) and the rare acidic sugar 4-O-[1-carboxyethyl] mannose (4-1 actylman).
A major limitation of Cyanobacterial EPSs is that they are difficult to characterize because of their unique structural complexity. To determine the specific chemical structures and contents of EPSs, extensive optimization of the EPS characterization process and sophisticated analytical techniques are required. Since cyanobacterial EPSs are heterogeneous, a thorough investigation might be challenging. This heterogeneity is caused by variations in their chain length and complex branching patterns. In addition, it is necessary to establish effective extraction and purification techniques for each common Cyanobacterial EPS. The use of different strain-specific EPS extraction methods results in different approaches to using them as standard practice for other Cyanobacterial EPSs. It has been found that the genetic manipulation of cyanobacteria has a significant effect on EPS production. Therefore, a number of investigations have been carried out to modify Cyanobacterial EPSs’ characteristics and production [83,84,85]. However, our understanding of the genetic and enzymatic systems associated with EPS formation is still a very small portion of what has been derived from the complete mechanism.
4. The Factors That Induce the Production of Cyanobacterial EPS
This section will focus specifically on the role of temperature, light, and the availability of nutrients such as phosphorus, carbon, or nitrogen, as well as water accessibility, salinity, and pH. These parameters can influence Cyanobacteria development and, therefore, EPS production. In the case of a bioreactor, rheological factors are also important to consider. Indeed, EPS production affects the culture medium. Aeration and/or mixing need to be controlled to ensure the homogeneity of the solution and the distribution of the nutrients. Each parameter must be managed to enhance Cyanobacteria growth and develop an efficient bioprocess for Cyanobacterial EPS production.
It is important to note that Cyanobacterial EPSs exhibit a high degree of chemical diversity. From a process point of view, any change in environmental conditions and the culture process could impact the structure of the produced EPS. This section will present selected studies, discuss their effects on Cyanobacterial growth, and attempt to rationalize the data generated by these studies. Globally, these sometimes-divergent reports underline the challenge of establishing a performant bioprocess.
Cyanobacteria are light-powered. The light content influences Cyanobacteria growth, as the phototrophic metabolism provides the energy needed for Cyanobacteria development. Illumination is one of the major limiting factors controlling Cyanobacterial growth [18]. Light intensity (µE), light/dark cycles (L/Dh), and light quality are essential parameters to conduct the bioprocess. Very few reports deal with a systematic study of light influence. It was observed that, in the described processes, some processes used continuous illumination with low or high intensities. In contrast, others selected a balanced L/D cycle up to 12 h/12 h. [82,86,87]. Idris et al. reported that blue-light LED is a stimulus for the growth of the microalgae Chlorella vulgaris [88]. However, the conclusion is not so evident for Cyanobacteria. Yang et al. assessed the role of light in the growth of Cyanobacteria [89]. In this study, the authors established the relationship between two harmful Cyanobacterial spp. (Anabaena spp. and Microcystis spp.) and their associated bacterial assemblages during large-scale cultivation under varied illumination conditions [89]. They performed the experiments under natural light, blue-filtered light, and green-filtered light, as well as under dark conditions. Anabaena spp. and Microcystis spp. were cultivated in a transparent acrylic tank containing BG-11 medium and a nitrogen source composed of sodium nitrate and ferric ammonium citrate. A set of parameters was recorded over 15 days. The natural light condition (control group) displayed considerable cell growth over the first growth phase of Cyanobacteria (from 0 h to 144 h), which was attributed to the proliferation of Anabaena spp. Then, from 144 h to 360 h, the cell proliferation of Microcystis spp. occurred under natural light conditions but also under blue-light filtered conditions. Moreover, the bacterial communities’ compositions were significantly influenced by the irradiated wavelength range. This experiment proved that the dominant Cyanobacterial genus in the cultivation system can be tuned by varying the light regimen.
However, light’s effect cannot be decorrelated with its impact on environmental and water quality parameters. Indeed, a differential pathway to nitrogen assimilation has been observed between natural light illumination and a blue-filtered light. Nitrogen reductase was applied under natural light conditions, whereas nitrogenase (i.e., the alternative pathway) can be activated under blue-filtered light. In addition, Yang et al. rationalized the impact of environmental conditions on their obtained bacterial communities using a NMDS plot (Figure 5) [89]. The authors clustered the experimental data based on the similarity of this microorganism’s composition. The arrows represent the influences of parameters on cluster formation. This study strongly suggests the role of nutrients (orthophosphate PO43−, nitrite NO2−, and nitrate NO3− anions and ammonium NH4+), the quantity of dissolved oxygen (DO), the influence of pH, and the importance of rheological properties (turbidity). Depending on the values of these parameters, the bacterial community profile strongly differed, including its EPS content.
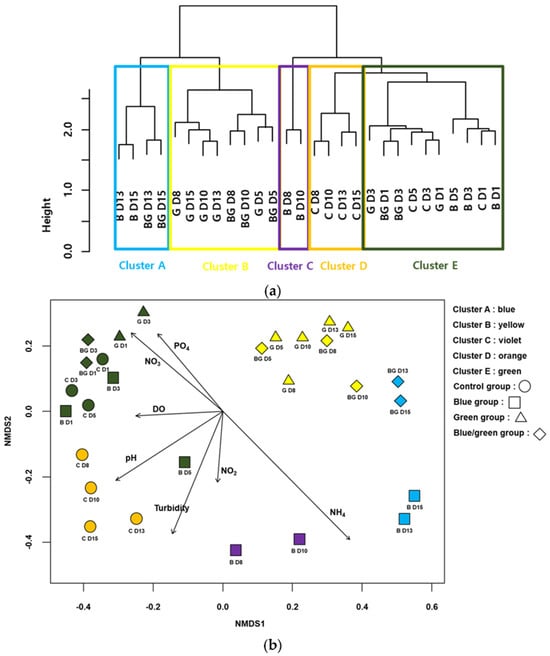
Figure 5.
Cluster analysis based on Cyanobacteria-associated bacteria assemblages. (a) Non-metric multidimensional scaling (NMDS) ordination of samplings with Cyanobacteria-associated bacteria assemblages. (b) Arrows indicate water quality variables significantly related to assemblage compositions (p < 0.05). The longer the arrow length, the higher the correlation coefficients with the NMDS axis. The first letter in the figures indicates the experimental condition (i.e., C: control, B: blue film, G: green film, BC: blue and green light) and the number represents the experimental date (D1: 24 h, D3: 72 h, D5: 14 h, D8: 196 h, D10: 240 h, D13: 312 h, and D15: 360 h). Data from [89] (CC BY 4.0).
The salinity of water is also a crucial parameter. Generally, under salt stress (about [NaCl]~0.5 M), Cyanobacteria produce larger amounts of EPS [65,90,91], but some reports exhibited no effect of a NaCl concentration increase or even a negative effect [76,92].
Temperature is a well-known propeller for many enzymatic processes. Cyanobacteria are quite special, as they can adapt to a wide range of temperatures, from below 15 °C to above 40 °C. Generally, in bioprocesses, a constant temperature within the mesophile range is applied. Very few reports deal with the effect of temperature on Cyanobacteria growth and composition. In 2018, an experimental approach was conducted by Savadova et al. [93]. Studying four strains (Planktothrix agardhii, Aphanizomenon gracile, Chrysosporum bergii, and Sphaerospermopsis aphanizomenoides), the authors proved that temperature had an impact on the growth rate of all tested Cyanobacteria. Temperature-modulated toxins and oligopeptide production in the strains were studied. Moreover, alien strains adapted well to temperature changes and were more productive than native strains [93]. These conclusions are similar to those of Moreno [94] but contrast with those of Otero and Vincenzini [95], who did not observe any impact of temperature variation. In addition, Nicolaus reported a slight decrease in performance for EPS production in Spirulina spp. at temperatures over 30 °C [65].
Carbon content is also an interesting parameter to consider. In 2020, Dineshbabu et al. [96] reported that Phormidium valderianum BDU 20041, a marine filamentous cyanobacterium, fixed the carbon and displayed a higher growth rate and biomass productivity from 3% to 15% CO2 compared to ambient air. However, predicting how communities will respond to changes in CO2 concentration is species-dependent [97]. A particular case concerns the development of subaerial phototrophic biofilms. Due to their ability to fix carbon dioxide (CO2), Cyanobacteria can colonize bare stone and form subaerial biofilms, mainly composed of a matrix of self-produced extracellular polymeric substances (EPS) [98]. Variations in atmospheric CO2 levels may affect the physiology of these microorganisms, as they use CO2 as a source of carbon. However, in a subaerial biofilm, experimental studies proved that EPSs produced by biofilms, measured as the carbohydrate fraction, did not differ clearly between low or high carbon content. This was in relation to either the total or relative number of CFUs [98]. This result highlighted the difference between Cyanobacteria and microalgae, as green microalgae of the genus Chlorella were significantly affected by elevated CO2 concentrations [99].
In 2019, Heimann et al. reported the effect of CO2 on the growth performance and biochemical profiles of the freshwater diazotrophic cyanobacterium Tolypothrix sp., for which 15% CO2-supplementation stimulated carbohydrate production by at least 35% compared to the control, whereas the protein levels did not change. In this study, it is notable that the quality of water, in particular its contamination with metallic residues, did not impact the production rate of Cyanobacteria and their metabolites [100].
Overall, many interconnected parameters influence Cyanobacterial EPS production. The temperature, pH, nutrients, illumination, and rheology need to be controlled to develop an efficient bioprocess for such Cyanobacterial EPSs (Figure 6).
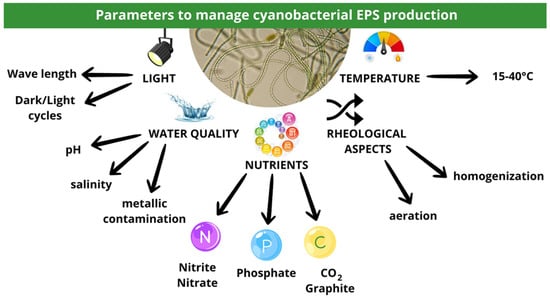
Figure 6.
An overview of the main parameters controlling Cyanobacterial EPS production.
5. Biological Properties of Cyanobacterial EPS on Pathogens
A variety of biological activities can be attributed to Cyanobacterial EPSs, depending on their monosaccharide composition as well as the anionic charged molecules [101]. Different studies have revealed that Cyanobacterial EPS promotes the growth of certain bacteria, inhibits the growth of others, and acts as a barrier against pathogens [102]. Microbial EPSs are considered non-digestible dietary fibers and are used as a prebiotic supplement to regulate gut microbial homeostasis [103]. Cyanobacterial EPSs are a fermentable polysaccharide that is active both as a fermentable polysaccharide and as an antimicrobial against several pathogenic bacteria and fungi [104,105]. The antimicrobial properties of EPS are attributed to the presence of sulphate groups and anionic charges within them. For example, EPSs from various Arthrospira spp. have broad spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria. Different monosaccharide compositions of Cyanobacterial EPSs are presumably responsible for the differences in antibacterial activity [106].
Bhatnagar et al. reported that EPSs from Anabaena spp., and Tolypothrix tenuis have inhibitory properties against wound pathogens such as E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa. This is possibly due to the presence of a sulphate group in the EPSs [107]. The EPSs of Gloeocapsa spp. Were shown to possess strong antibacterial activities against pathogenic bacteria including S. aureus, E. coli, and Salmonella enteritidis, as well as Candida albicans. The EPSs of Synechocystis spp. inhibited the growth of P. aeruginosa [108]. There is also evidence that Cyanobacterial EPSs can act as a quorum-sensing inhibitor, thereby preventing pathogenic microbes from forming biofilms [109]. For example, Phormidium marinum EPSs prevent the formation of C. albicans biofilm, rather than promoting the growth of this fungus [110].
Synechococcus spp. EPSs, being derived from a marine cyanobacterium, have been found to have anti-quorum sensing abilities against aquatic bacterial pathogens such as Vibrio harveyi and Vibrio vulnificus. This is due to the fact that they inhibit the complex Vibrio biofilm architecture [111]. The EPSs of Chlorococcus spp. R-10 have been shown to have antibacterial activity against S. aureus and B. subtilis, both Gram+ bacteria, and minimal inhibition of C. albicans, which is a Gram+ bacterium. It has been shown that three Gram+ bacteria (E. faecalis, B. cereus, and S. epidermidis) as well as two Gram-negative bacteria (P. hauseri and A. calcoaceticus) were resistant to EPS effects [112].
Recently, researchers have revealed the complex structure of polysaccharides found in Cyanobacteria’s cell wall. The sulfated polysaccharides found in marine Cyanobacteria are rare and rarely generated. They possess probiotic, prebiotic, immunological, and antiviral properties that make them unique [113,114]. Sulfated polysaccharides’ varied structural makeup shows exceptional resistance to the COVID-19 virus (SARS-CoV-2) [115]. These polysaccharides inhibit virus transcription and translation in the host cell. This enhances the host’s antiviral response as they interfere with the virus’s attachment, adsorption, and reproduction within the host cell [116,117]. Sulfated polysaccharide calcium spirulan from Arthrospira platensis chelates calcium ions and has antiviral action while maintaining its unique molecular structure [118,119]. EPSs from Synechocystis spp. and Gloecapsa spp. Gacheva 2007/R-06/1 have shown antibacterial activity against food-borne diseases. A fibrin network is formed by hydrophilic EPSs with anionic groups on their surfaces in bleeding wounds [107]. It was found that EPSs derived from Anabaena spp. and Tolypothrix spp. showed sustained inhibition of wound infections caused by E. coli, S. aureus, P. aeruginosa, and B. licheniformis [119].
6. Biological Properties of Cyanobacterial EPS on Immune/Inflammatory Responses
Cyanobacterial EPS modulates host immune responses, including cytokines and the immune response. Cyanobacterial EPSs can boost the immune response activity, including macrophages, natural killer cells, and T cells, which can lead to an increased immune response and a stronger defense against infections and illnesses [120,121]. EPSs can reduce inflammation and prevent overreactions of the immune system in allergic reactions, autoimmune diseases, and chronic inflammation [122]. Some Cyanobacterial EPSs neutralize inflammation-causing oxidative stress [123]. In addition to their antioxidant properties, Cyanobacterial polysaccharides have potential uses in food and medicine [124,125]. As a way to reduce ROS production, stronger DPPH and hydroxyl radical scavenging activities as well as increased levels of SOD, GSH, and MDA in N. flagelliforme cells have been observed in Cyanobacterial EPSs [125].
It is challenging to determine the relationship between the structure of Cyanobacterial polymers and their biological activity due to a lack of structural knowledge [126]. Recently, an analysis of monosaccharide profiles using chromatographic techniques indicated that the anionic charge and presence of sulfate groups explain their substantial biological activity [126,127]. Several studies have shown that Cyanobacterial EPSs have a favorable anti-oxidative effect such as those of Anabaena spp. [128], Arthrospira spp. [129], Isochrysis galbana [130], Nostoc carneum [62], Porphyridium spp., [131] P. cruentum [132], Rhodella reticulata [133], Schizochytrium spp. [134], and Spirulina platensis [135,136], and their sulphate groups exhibits high antioxidant properties with sulphate content [137].
Two EPS fractions (EPSa and EPSb) from B. animalis RH have been shown to possess strong antioxidative properties, in an in vivo model of aging mice, induced by D-galactose. Both EPS fractions prevented linoleic acid peroxidation and exhibited strong free radical scavenging activity in serums, livers, and brains, suggesting excellent anti-aging properties [138].
The cell wall polysaccharides of Cyanobacteria have recently been studied in an attempt to uncover their interesting complex structure. In Leptolyngbya spp. EPS, monosaccharides including glucose, rhamnose, mannose, and glucose are present along with proteins (24.20% ± 1.54), lipids (9.36% ± 1.21), and carbohydrates (66.44% ± 2.14) [59]. The monosaccharide backbone of Porphyridium cruentum’s cell wall polysaccharides (xylose, galactose, glucose, and glucuronic acid) has immune-stimulating properties in prawns. These monosaccharides have been shown to stimulate the non-specific immune response by enhancing hyaline, granular cells, and phagocytosis, as well as respiratory burst activity [139]. Additionally, sulphated polysaccharides and their derivatives minimize drag as well as being hypolipidaemic or hypoglycemic. Aside from that, sulphated polysaccharides and their derivatives also act as hypolipidaemics or hypoglycaemics and drag-reducers [140,141]. Probiotic Lactobacillus EPS stimulates both pro-inflammatory (Th1) and anti-inflammatory (Th2, Treg) functions and regulates inflammation by decreasing TNF-α-induced IL-8 production in epithelial Caco-2 cells [142,143].
The gut plays a major role in immune responses. Some Cyanobacterial EPSs can improve gut health by serving as prebiotics and fostering the development of healthy gut microbiota [144]. Cyanobacterial EPS may influence inflammatory reactions. It is possible for these characteristics to vary depending on the strain of Cyanobacteria and the composition of the EPS [145]. Some Cyanobacterial EPSs inhibit the synthesis of different pro-inflammatory mediators (cytokines and chemokines) [146]. The effect of Cyanobacterial EPS on the inflammatory process can be modulated by its ability to regulate the immune response. In addition, EPSs are capable of reducing tissue damage and excessive inflammation by balancing and regulating the immune system [147].
EPSs from Cyanobacteria have the ability to reduce the body’s oxidative stress by acting as antioxidants. Oxidative stress may contribute to inflammatory reactions, and research has shown that EPSs may reduce inflammation by reducing oxidative stress [148]. Cyanobacterial EPSs act as a protective agent against inflammatory disorders such as rheumatoid arthritis and IBD. They alleviate the intensity of the illness and its symptoms [146]. Inflammation can be controlled by maintaining a healthy gut microbiome [149]. The modulation of immune responses to allergens by Cyanobacterial EPS has shown potential for reducing allergy responses and allergic inflammation [150].
Cyanobacterial EPSs can inhibit pathogen development and invasion [101]. It has been observed that Nostoc EPS can stimulate macrophages to release pro-inflammatory cytokines like TNF-α and IL-6, as well as other critical immune components like prostaglandins (PG) and NO, which indicates that this biopolymer enhances early congenital immunological responses [151]. EPSs, which are significant adhesins, positively impact the ability of beneficial bacteria to colonize the intestinal epithelium. Intestinal epithelial cells attach to EPS, making it possible for microorganisms such as Lactobacillus and Bifidiobacterium to colonize the gastrointestinal tract and avoid dysbiosis-induced disruption in the gut microbiome. Additionally, beneficial microbiota can adhere to the intestinal mucosa and prevent pathogen adhesion, as well as prevent the growth of harmful intestinal bacteria [152]. A balanced immune response can be regulated by Cyanobacterial EPS, thus avoiding immune responses that are either hyperactive or repressed, which could increase inflammation over time [147].
7. Role of Cyanobacterial EPS in Biotechnological Applications
EPSs from Cyanobacteria have attracted interest due to their diverse biotechnological uses. These applications cover a variety of industries, such as food processing, agriculture, bioremediation, medicine, and more. Potential uses for Cyanobacterial EPSs include thickeners, stabilizers, and gelling agents in the food and pharmaceutical sectors. They are used in pharmaceutical formulations and food items to enhance their consistency and texture.
8. The Potential Applications of Cyanobacterial EPS in the Food Sector
Cyanobacterial EPSs are non-toxic carbohydrate polymers which exhibit a high structural complexity and biocompatibility, making them useful for food industrial applications such as emulsifiers, stabilizers, and gelling agents, as well as for increasing viscosity in products such as ice cream, cakes, jellies, beverages, and sauces [101].
Due to its stable physical properties, Cyanobacterial EPS can be used as a texture enhancer substance for fermented products, especially fermented milk, and to enhance the mouth-feel properties of sauces, soups, and desserts [153]. EPS derived from Cyanobacteria is similar to commercial-grade xanthan gum in terms of its pseudoplasticity, elasticity, and rheological properties. Thus, EPS can serve as a stabilizer agent in packed foods. For example, EPSs from Spirulina platensis and Anabaena spp. are potential alternatives to xanthan gums [119]. Cyanobacteria such as Anabaena, Nostoc, and Spirulina are known for their nutritional properties. Thus, EPSs can be used as food additives. Additionally, Cyanobacterial EPSs can be used as emulsifiers in foods, and stabilize water- and oil-based components like mayonnaise and salad dressings [154]. In some food items, EPS can be an alternative to fat and lower the total fat level while preserving desired sensory qualities. To improve the texture of some foods without gluten, Cyanobacterial polypeptides can be added. They can be added to dairy-free and vegan goods to enhance their creamy texture, such as plant-based milk substitutes [155].
Many of the EPSs produced by Cyanobacteria have prebiotic qualities that encourage the development of healthy gut microbiota. They can be added to functional meals and drinks and improve customer perception. Food items that use EPSs as a source of dietary fiber should have increased nutritional value. They can be added to functional meals and drinks to offer advantages to the body including stronger immunity and better digestive health [156]. By increasing the taste of sweetness in meals, Cyanobacterial EPSs can reduce the amount of excess sugar in food products. Many EPSs can improve flavor perception, which makes them useful for both savory and sweet food items. To promote freshness and moisture retention in baked goods, EPSs from Lactobacilli spp. enhance the texture and reduce the stalling rate in bread and crumb hardness [157]. Similarly, Cyanobacterial EPSs can improve product quality and shelf life.
Food packaging materials used with polysaccharides, proteins, and fats are considered edible packaging materials. These materials can be applied directly to food as a thin coating or direct wrapping without any alteration or loss of nutrients [158]. The biophysical and structural properties of Cyanobacterial EPSs make them resistant to environmental gases and yield high tensile properties and elongation, which can prevent mechanical damage to a small degree [159]. To increase the shelf life and decrease spoiling of some commercial foods, EPSs can be used to make edible films and coatings. This is especially important when it comes to fruits, vegetables, and sweets. For instance, Synechocystis spp. EPSs have about a 73.3% emulsification index along with inhibition of fungus, Fusarium verticillioides (70.2%), and Fusarium spp. (61.4%), which is an excellent alternative to plastic food covering [160]. This nature of edible films/coatings acts as a barrier to moisture and other environmental reactions that can reduce the volatile or sensitive nutritive qualities of foods [161]. This includes flavors, vitamins, and antioxidants [161]. Films made with such physically stable EPSs from Cyanobacteria prevent deterioration and extend the shelf-life of foods. In addition, Chlorella vulgaris biomass has been used in cookie production as a natural colorant and texture enhancer and as a substitute for food-grade synthetic chemicals [162].
9. The Potential Applications of Cyanobacterial EPS in the Pharmaceutical Sector
Many Cyanobacterial EPSs have been used as a source of film covers for drug encapsulation and the administration of certain medications due to their stability and biocompatibility [163]. Hydrogels can be created from Cyanobacterial EPSs which can be used for a variety of applications. The biocompatibility of EPSs and hydrogel-controlled release characteristics enable the creation of drug delivery systems with customized release profiles. The chemical and biological characteristics of Cyanobacterial EPSs are comparable to mammalian glycosaminoglycan. These properties make them attractive as pharmaceutical excipients and for drug delivery systems [164].
EPSs are widely used in healthcare and cosmetic products due to their ability to retain moisture and condition the skin. Wound healing can be aided by their capacity to produce bioactive substances, enable gas exchange, and maintain a moist wound environment [165]. EPS hydrogels using FeCl3 gelation have enhanced biocompatibility and significant wound healing abilities. EPS hydrogels derived from Nostoc aid in the migration of fibroblasts, which is crucial to the advancement of the regeneration process. They help restore and restructure injured skin [81,107].
EPSs conjugated with medication can effectively target tissue, are well tolerated by tissues, and are less likely to result in tissue damage or adverse reactions, particularly in cases of gastrointestinal disorders. Along with this, EPS-conjugated nanoparticles can be customized to recognize and bind specific cell types or tissues [166,167]. A hybrid hydrogel was produced by combining the Thamniochloris variabilis EPS with synthetic polyethene-glycol diacrylate (PEGDa), which embeds detoxification enzymes with catalytic cysteine residues such as the detoxification enzyme thiosulfate-cyanide sulfur transferase (TST). This shows the possibility of fabricating effective, functional, and non-cytotoxic hydrogels with enzymatic activity for therapeutic applications [163]. In the microencapsulation of vitamin B12 and EPS (extracellular carbohydrate polymer) from Cyanothece spp., CCY 0110 was combined with Arabic gum, which resulted in a highly intestinal release of vitamin B12 [168].
The ability to regulate the release of drugs is an important feature of many therapeutic applications, which can be enhanced with the use of Cyanobacterial EPS suspensions [42]. Drugs can be protected against environmental conditions that limit their efficiency, including pH variations, enzymatic breakdown, temperature, and exposure to light, by being encapsulated inside an EPS coat [169]. This prolongs medications’ shelf life and stability. Aside from that, carboxyl groups in EPSs contribute to the production of hydrogels with pH-sensitive swelling behavior [163]. These hydrogels may exhibit an increased pore size at basic pH and permit protein release from the matrix in the intestinal environment [163]. This may improve protein drug absorption [163]. Hydrogels are three-dimensional, hydrophilic polymers that absorb and retain water. The hydrogel derived from Cryptococcus laurentii 70766 EPSs exhibited comparable gelation kinetics, swelling ratios, rheological characteristics, and an extended degradation profile. This was compared to traditional hydrogels based on alginate. Furthermore, there was no discernible negative impact on the survival and proliferation of human macrophage and fibroblast cells [170]. Unlike traditional hydrogels, which are often made from synthetic molecules, recent research has demonstrated that Cyanobacterial EPSs have the potential to be employed in the creation of hydrogels that are both eco-friendly and biocompatible. Cyanobacterial EPS-mediated hydrogels have been demonstrated to be effective as delivery systems for different medications [163].
Some Cyanobacterial EPSs possess mucoadhesive properties, making them effective for mucosal medical administration [171]. These properties enhance the absorption and bioavailability of medication by enabling drug carriers to stick to mucosal surfaces. EPSs can contribute to stabilizing nanoparticles in a colloidal state and reducing metal content [172]. Additionally, EPS-tagged drugs are easily delivered to the appropriate target sites, simply adhering to the mucosal surfaces, resulting in the drug reaching and reacting with them [173].
10. Conclusions
The Western diet is highly associated with processed foods and saturated fats which trigger IBD, a chronic inflammatory disease. This type of diet is characterized by a high caloric content that is associated with a significant decrease in gut microbiota biodiversity. EPSs are one of the strategies to enhance the gut microbiota and immune-inflammatory response deregulation in IBD patients. EPSs, also known as prebiotics, are produced by commensal bacteria such as Lactobacillus and Bifidobacterium. They are critical in several aspects, including strengthening the gut microbiota, eliminating pathogenic microorganisms, modulating immune responses, and promoting gut health. EPS improves the function of the intestinal barrier and mucus secretion, as well as IgA dimer production in Peyer’s patches. These approaches enable improving the abundance of beneficial bacteria populations in the gut, and can help reduce the risk of developing IBD. Additionally, Cyanobacteria can be considered a promising source of novel EPSs and have potential pharmaceutical and therapeutic applications. The majority of Cyanobacterial EPSs belong to the heteropolysaccharide class of biopolymers, consisting of six or more monomers. The presence of uronic acids and sulphate groups in EPSs is an important factor that gives EPSs an anionic charge that is not seen in other prokaryotic species. This feature may impact their physico-chemical characteristics and biological properties. Cyanobacteria EPSs have antimicrobial properties due to the presence of sulphate groups and anionic charges. Further, EPSs from Cyanobacteria have a wide range of biotechnological applications. These applications include food processing, agriculture, bioremediation, medicine, and many more. Cyanobacterial EPSs can be used as thickeners, stabilizers, and gelling agents in the food and pharmaceutical industries. They are used in pharmaceutical formulations and food items to enhance consistency and texture. In terms of future research directions, the development of scalable procedures will be key to improving the efficiency of EPS production in the future. This includes characterizing and evaluating Cyanobacteria viability and their EPS production on a commercial scale. In addition, it involves refining and improving techniques [174]. There is still much to be explored in relation to the ecological significance of Cyanobacterial EPSs in freshwater and aquatic habitats, as well as biofilms. Understanding how these EPSs support Cyanobacteria resistance in various environmental conditions will facilitate the development of high-quality and significant Cyanobacteria-associated items. Considering how EPSs might affect Cyanobacteria’s adherence to surfaces and their ability to form biofilms, as well as their interaction with other microorganisms under various conditions, it would be important to look at the role of EPSs in biofilm formation and structure for a better understanding of EPS function [175]. The development of novel methods for the characterization and use of Cyanobacterial EPSs could be achieved due to innovations in the fields of microbiology, biotechnology, chemistry, and materials science. The present review focuses on the role of EPS in IBD, with a special focus on EPSs derived from Cyanobacteria. This review also covers the biological properties of Cyanobacterial EPSs in immuno-inflammatory responses and against pathogens as well as its role in biotechnological applications. Overall, Cyanobacterial EPSs have therapeutic potential against IBD due to their anti-inflammatory and immunoregulatory properties that can reduce inflammation, regulate the immune response, and restore gut microbiota. Thus, EPSs could be expected to be used in the diet and lifestyle of IBD patients.
Author Contributions
D.M., S.N., M.B. and S.J. writing—original draft preparation; S.J. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heyman, M.B.; Kirschner, B.S.; Gold, B.D.; Ferry, G.; Baldassano, R.; Cohen, S.A.; Winter, H.S.; Fain, P.; King, C.; Smith, T.; et al. Children with early-onset inflammatory bowel disease (IBD): Analysis of a pediatric IBD consortium registry. J. Pediatr. 2005, 146, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Mullen, A.; Whelan, K. Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr. Rev. 2015, 73, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Khandpur, N.; Rossato, S.L.; Lochhead, P.; Lopes, E.W.; Burke, K.E.; Richter, J.M.; Song, M.; Ardisson Korat, A.V.; Sun, Q.; et al. Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1323–e1337. [Google Scholar] [CrossRef]
- Ji, Y.; Sakata, Y.; Tso, P. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Vors, C.; Geloen, A.; Chauvin, M.A.; Soulage, C.; Lambert-Porcheron, S.; Peretti, N.; Alligier, M.; Burcelin, R.; Laville, M.; et al. Emulsified lipids increase endotoxemia: Possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011, 22, 53–59. [Google Scholar] [CrossRef]
- Jawhara, S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms 2023, 11, 1556. [Google Scholar] [CrossRef]
- Martin, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medynska-Przeczek, A.; Wedrychowicz, A.; Skoczen, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Knowles, E.J.; Castenholz, R.W. Effect of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms. FEMS Microbiol. Ecol. 2008, 66, 261–270. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Chung, K.S.; Shin, J.S.; Lee, J.H.; Park, S.E.; Han, H.S.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Protective effect of exopolysaccharide fraction from Bacillus subtilis against dextran sulfate sodium-induced colitis through maintenance of intestinal barrier and suppression of inflammatory responses. Int. J. Biol. Macromol. 2021, 178, 363–372. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-kappaB inflammatory pathways in human colon cancer. Microb. Cell Fact. 2018, 17, 29. [Google Scholar] [CrossRef]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-kappaB/MAPK pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Paik, W.; Alonzo, F., 3rd; Knight, K.L. Probiotic Exopolysaccharide Protects against Systemic Staphylococcus aureus Infection, Inducing Dual-Functioning Macrophages That Restrict Bacterial Growth and Limit Inflammation. Infect. Immun. 2019, 87, e00791-18. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, C.; Hayakawa, A.; Matsumoto, K.; Katoh, T.; Yamamoto, K.; Hisa, K. Exopolysaccharides Produced by Leuconostoc mesenteroides Strain NTM048 as an Immunostimulant to Enhance the Mucosal Barrier and Influence the Systemic Immune Response. J. Agric. Food Chem. 2015, 63, 7009–7015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, K.; Qi, W.; Zhou, Y.; Hong, T.; Xiong, T.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 Enhances Colonic Mucosal Homeostasis by Controlling Epithelial Cell Differentiation and c-Jun/Muc2 Signaling. J. Agric. Food Chem. 2019, 67, 9831–9839. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Shin, J.S.; Rhee, Y.K.; Cho, C.W.; Lee, M.K.; Hong, H.D.; Lee, K.T. In vitro and in vivo immunostimulatory activity of an exopolysaccharide-enriched fraction from Bacillus subtilis. J. Appl. Microbiol. 2015, 118, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Gorska, S.; Sandstrom, C.; Wojas-Turek, J.; Rossowska, J.; Pajtasz-Piasecka, E.; Brzozowska, E.; Gamian, A. Structural and immunomodulatory differences among lactobacilli exopolysaccharides isolated from intestines of mice with experimentally induced inflammatory bowel disease. Sci. Rep. 2016, 6, 37613. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef]
- Kiray, E.; Raheel, N.M. A New Approach to Exopolysaccharides of Post Probiotic Lactobacillus paracasei L1 Strain: Anti-quarum Sensing Activity. Balk. Med. J. 2023, 40, 351–357. [Google Scholar] [CrossRef]
- Ksonzekova, P.; Bystricky, P.; Vlckova, S.; Patoprsty, V.; Pulzova, L.; Mudronova, D.; Kubaskova, T.; Csank, T.; Tkacikova, L. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Xu, R.; Aruhan; Xiu, L.; Sheng, S.; Liang, Y.; Zhang, H.; Liu, Y.; Tong, H.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus buchneri TCP016 Attenuate LPS- and d-GalN-Induced Liver Injury by Modulating the Gut Microbiota. J. Agric. Food Chem. 2019, 67, 11627–11637. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; Francois, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Altus, S.; Tay, J.W.; Meehl, J.B.; Johnson, E.B.; Bortz, D.M.; Cameron, J.C. Mechanical regulation of photosynthesis in cyanobacteria. Nat. Microbiol. 2020, 5, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef]
- Jones, A.C.; Monroe, E.A.; Eisman, E.B.; Gerwick, L.; Sherman, D.H.; Gerwick, W.H. The unique mechanistic transformations involved in the biosynthesis of modular natural products from marine cyanobacteria. Nat. Prod. Rep. 2010, 27, 1048–1065. [Google Scholar] [CrossRef]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1440–1443. [Google Scholar] [CrossRef]
- Allonsius, C.N.; van den Broek, M.F.L.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine Cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaur, R.; Bansal, A.; Kapur, S.; Sundaram, S. Biotechnological exploitation of cyanobacteria and microalgae for bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–259. [Google Scholar]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from cyanobacteria: Strategies for bioprocess development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Grenn, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Singh, S.; Sran, S.K.; Pinnaka, A.K.; Choudhury, A.R. Purification, characterization and functional properties of exopolysaccharide from a novel halophilic Natronotalea sambharensis sp. nov. Int. J. Biol. Macromol. 2019, 136, 547–558. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; De Philippis, R. Exocellular polysaccharides in Microalgae and Cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In The Physiology of Microalgae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 565–590. [Google Scholar]
- Hu, C.; Liu, Y.; Paulsen, B.S.; Petersen, D.; Klaveness, D. Extracellular carbohydrate polymers from five desert soil algae with different cohesion in the stabilization of fine sand grain. Carbohyd. Polym. 2003, 54, 33–42. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Decho, A.W. Biochemical characterizationof cyanobacterial extracellular polymers (EPS) from modern marine stromatolites. Prep. Biochem. Biotechnol. 2000, 30, 321–330. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Decho, A.W. Isolation and biochemical characterization of extracellular polymeric secretions (EPS) from modern soft marine stromatolites (bahamas) and its inhibitory effect on CaCO3 precipitation. Prep. Biochem. Biotechnol. 2002, 32, 51–63. [Google Scholar] [CrossRef]
- Wang, H.M.; Loganathgan, D.; Linhardt, R.J. Determination of the pKa of glucuronic acid and the carboxy groups of heparin by 13C-nuclear-magnetic-resonance spectroscopy. Biochem. J. 1991, 278, 689–695. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef]
- Ruangsomboon, S.; Chidthaisong, A.; Bunnag, B.; Inthorn, D.; Harvey, N.W. Lead (Pb2+) adsorption characteristics and sugar composition of capsular polysaccharides of cyanobacterium Calothrix marchica. Songklanakarin J. Sci. Technol. 2007, 29, 529–541. [Google Scholar]
- Yoshimura, H.; Kotake, T.; Aohara, T.; Tsumuraya, Y.; Ikeuchi, M.; Ohmori, M. The role of extracellular polysaccharides produced by the terrestrial cyanobacterium Nostoc sp. strain HK-01 in NaCl tolerance. J. Appl. Phycol. 2012, 24, 237–243. [Google Scholar] [CrossRef]
- Yoshimura, H.; Ikeuchi, M.; Ohomori, M. Cell surface-associated proteins in the filamentous cyanobacterium Anabaena sp. strain PCC 7120. Microbes Environ. 2012, 27, 538–543. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Batsalova, T.G.; Dzhambazov, B.M.; Ognyanov, M.H.; Denev, P.N.; Antonova, D.V.; Wold, C.W.; Yanakieva, I.Z.; Teneva, I.I.; Paulsen, B.S. Immunomodulating polysaccharide complexes and antioxidant metabolites from Anabaena laxa, Oscillatoria limosa and Phormidesmis molle. Algal Res. 2021, 60, 102538. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Yeh, H.-Y.; Liao, Z.-H.; Hung, S.-W.; Chen, B.; Lee, P.-T.; Nan, F.-H.; Shih, W.-L.; Chang, C.-C.; Lee, M.-C. An in vitro study shows the potential of Nostoc commune (Cyanobacteria) polysaccharides extract for wound-healing and anti-allergic use in the cosmetics industry. J. Funct. Foods 2021, 87, 104754. [Google Scholar] [CrossRef]
- Gongi, W.; Gomez Pinchetti, J.L.; Cordeiro, N.; Ouada, H.B. Extracellular polymeric substances produced by the Thermophilic Cyanobacterium Gloeocapsa gelatinosa: Characterization and assessment of their antioxidant and metal-chelating activities. Mar. Drugs 2022, 20, 227. [Google Scholar] [CrossRef]
- Gongi, W.; Cordeiro, N.; Pinchetti, J.L.G.; Ouada, H.B. Functional, rheological, and antioxidant properties of extracellular polymeric substances produced by a thermophilic cyanobacterium Leptolyngbya sp. J. Appl. Phycol. 2022, 34, 1423–1434. [Google Scholar] [CrossRef]
- Senousy, H.H.; El-Sheekh, M.M.; Saber, A.A.; Khairy, H.M.; Said, H.A.; Alhoqail, W.A.; Abu-Elsaoud, A.M. Biochemical analyses of ten cyanobacterial and microalgal strains isolated from egyptian habitats, and screening for their potential against some selected phytopathogenic fungal strains. Agronomy 2022, 12, 1340. [Google Scholar] [CrossRef]
- Ahmed, M.; Moerdijk-Poortvliet, T.C.; Wijnholds, A.; Stal, L.J.; Hasnain, S. Isolation, characterization and localization of extracellular polymeric substances from the cyanobacterium Arthrospira platensis strain MMG-9. Eur. J. Phycol. 2014, 49, 143–150. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abou-ElWafa, G.S.; Shaaban-Dessuuki, S.A.; Hassan, N.I. Characterization and antioxidant activity of exopolysaccharide secreted by Nostoc carneum. Int. J. Pharmacol. 2015, 11, 432–439. [Google Scholar] [CrossRef]
- Lama, L.; Nicolaus, B.; Calandrelli, V.; Manca, M.C.; Romano, I.; Gambacorta, A. Effect of growth conditions on endo-and exopolymer biosynthesis in Anabaena cylindrica 10 C. Phytochemistry 1996, 42, 655–659. [Google Scholar] [CrossRef]
- Moore, B.; Tischer, R. Extracellular polysaccharides of algae: Effects on life-support systems. Science 1964, 145, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Panico, A.; Lama, L.; Romano, I.; Manca, M.C.; De Giulio, A.; Gambacorta, A. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochemistry 1999, 52, 639–647. [Google Scholar] [CrossRef]
- Richert, L.; Golubic, S.; Guédès, R.L.; Ratiskol, J.; Payri, C.; Guezennec, J. Characterization of exopolysaccharides produced by cyanobacteria isolated from Polynesian microbial mats. Curr. Microbiol. 2005, 51, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; De Philippis, R. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Volk, R.-B.; Venzke, K.; Blaschek, W. Structural investigation of a polysaccharide released by the cyanobacterium Nostoc insulare. J. Appl. Phycol. 2007, 19, 255–262. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef]
- Nunes, C.S.; Rufato, K.B.; Souza, P.R.; de Almeida, E.A.M.S.; da Silva, M.J.V.; Scariot, D.B.; Nakamura, C.V.; Rosa, F.A.; Martins, A.F.; Muniz, E.C. Chitosan/chondroitin sulfate hydrogels prepared in [Hmim][HSO4] ionic liquid. Carbohydr. Polym. 2017, 170, 99–106. [Google Scholar] [CrossRef]
- Brown, M.J.; Lester, J.N. Role of bacterial extra-cellular polymers in metal uptake in pure bacterial culture and activated sludge. Water Res. 1982, 16, 1539–1548. [Google Scholar] [CrossRef]
- Mancuso Nichols, C.A.; Bowman, J.P.; Fuezennec, J. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef]
- De Philippis, R.; Ena, A.; Paperi, R.; Sili, C.; Vincenzini, M. Assessment of the potential of Nostoc strains from Pasteur culture collection for the production of polysaccharides of applied interest. J. Appl. Phycol. 2000, 12, 401–407. [Google Scholar] [CrossRef]
- Micheletti, E.; Pereria, S.; Mannelli, F.; Moradas-Ferreira, P.; Tamagnini, P.; De Philippis, R. Sheathless mutant of the cyanobacterium Gloeothece sp. strain PCC 6909 with increased capacity to remove copper ions from aqueous solutions. Appl. Environ. Microbiol. 2008, 74, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Schlosser, U.G.; Pohl, P. Exopolysaccharide production by cyanobacteria grown in closed photobioreactors and immobilized using white cotton towelling. J. Appl. Phycol. 1997, 9, 205–213. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Muthuraj, M.; Bhunia, B.; Bandyopadhyay, T.K.; Annapurna, K.; Sahu, M.; Indrama, T. Biosynthesis, purification and structure-property relationships of new cyanobacterial exopolysaccharides. Polym. Test. 2020, 89, 106592. [Google Scholar] [CrossRef]
- Bhunia, B.; Uday, U.S.P.; Oinam, G.; Mondal, A.; Bandyopadhyay, T.K.; Tiwari, O.N. Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr. Polym. 2018, 179, 228–243. [Google Scholar] [CrossRef]
- Neu, T.R.; Dengler, T.; Jann, B.; Poralla, K. Structural studies of an emulsion-stabilizing exopolysaccharide produced by an adhesive hydrophobic Rhodococcus strain. J. Gen. Microbiol. 1992, 138, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Sheperd, R.; Rockey, J.; Sutherland, I.W.; Roller, S. Novel Bioemulsifiers from microorganisms for use in foods. J. Biotechnol. 1995, 40, 207–217. [Google Scholar] [CrossRef]
- Alvarez, X.; Alves, A.; Ribeiro, M.P.; Lazzari, M.; Coutinho, P.; Otero, A. Biochemical characterization of Nostoc sp. exopolysaccharides and evaluation of potential use in wound healing. Carbohydr. Polym. 2021, 254, 117303. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Khangembam, R.; Shamjetshabam, M.; Sharma, A.S.; Oinam, G.; Brand, J.J. Characterization and optimization of bioflocculant exopolysaccharide production by cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA990 in culture conditions. Appl. Biochem. Biotechnol. 2015, 176, 1950–1963. [Google Scholar] [CrossRef]
- Maeda, K.; Okuda, Y.; Enomoto, G.; Watanabe, S.; Ikeuchi, M. Biosynthesis of a sulfated exopolysaccharide, synechan, and bloom formation in the model cyanobacterium Synechocystis sp. strain PCC 6803. eLife 2021, 10, e66538. [Google Scholar] [CrossRef]
- Santos, M.; Pacheco, C.C.; Yao, L.; Hudson, E.P.; Tamagnini, P. CRISPRi as a Tool to Repress Multiple Copies of Extracellular Polymeric Substances (EPS)-Related Genes in the Cyanobacterium Synechocystis sp. PCC 6803. Life 2021, 11, 1198. [Google Scholar] [CrossRef]
- Pereira, S.B.; Santos, M.; Leite, J.P.; Flores, C.; Eisfeld, C.; Buttel, Z.; Mota, R.; Rossi, F.; De Philippis, R.; Gales, L.; et al. The role of the tyrosine kinase Wzc (Sll0923) and the phosphatase Wzb (Slr0328) in the production of extracellular polymeric substances (EPS) by Synechocystis PCC 6803. Microbiologyopen 2019, 8, e00753. [Google Scholar] [CrossRef]
- Singh, S.; Das, S. Screening, production, optimization and characterization of cyanobacterial Polysaccharide. World J. Microbiol. Biotechnol. 2011, 27, 1971–1980. [Google Scholar] [CrossRef]
- Khangembam, R.; Tiwari, O.N.; Kalita, M. Production of exopolysaccharides by the cyanobacterium Anabaena sp. BTA992 and application as bioflocculants. J. Appl. Biol. Biotechnol. 2016, 4, 8–11. [Google Scholar]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef]
- Yang, T.; Lee, C.S.; Cho, J.Y.; Bae, M.J.; Kim, E.J. Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions. Microorganisms 2022, 10, 2150. [Google Scholar] [CrossRef]
- Su, C.; Chi, Z.; Lu, W. Optimization of medium and cultivation conditions for enhanced exopolysaccharide yield by marine Cyanothece sp 113. Chin. J. Oceanol. Limnol. 2007, 25, 411–417. [Google Scholar] [CrossRef]
- Sudo, H.; Burgess, J.G.; Takemasa, H.; Nakamura, N.; Matsunaga, T. Sulfated exopolysaccharide production by the halophilic cyanobacterium Aphanocapsa halophytia. Curr. Microbiol. 1995, 30, 219–222. [Google Scholar] [CrossRef]
- De Philippis, R.; Sili, C.; Tassinato, G.; Vincenzini, M.; Materassi, R. Effects of growth conditions on exopolysaccharide production by Cynospira capsulata. Bioresour. Technol. 1991, 38, 101–104. [Google Scholar] [CrossRef]
- Savadova, K.; Mazur-Marzec, H.; Karosienė, J.; Kasperovičienė, J.; Vitonytė, I.; Toruńska-Sitarz, A.; Koreivienė, J. Effect of Increased Temperature on Native and Alien Nuisance Cyanobacteria from Temperate Lakes: An Experimental Approach. Toxins 2018, 10, 445. [Google Scholar] [CrossRef]
- Moreno, J.; Vargas, M.A.; Olivares, H.; Rivas, J.; Guerrero, M.G. Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J. Biotechnol. 1998, 60, 175–182. [Google Scholar] [CrossRef]
- Otero, A.; Vincenzini, M. Nostoc (Cyanophyceae) goes nude: Extracellular polysaccharides serve as a sink for reducing power under unbalanced C/N metabolism. J. Phycol. 2004, 40, 74–81. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Uma, V.S.; Mathimani, T.; Prabaharan, D.; Uma, L. Elevated CO2 impact on growth and lipid of marine cyanobacterium Phormidium valderianum BDU 20041—Towards microalgal carbon sequestration. Biocatal. Agric. Biotechnol. 2020, 25, 101606. [Google Scholar] [CrossRef]
- Raven, J.A.; Gobler, C.J.; Hansen, P.J. Dynamic CO2 and pH levels in coastal, estuarine, and inland waters: Theoretical and observed effects on harmful algal blooms. Harm. Algae 2020, 91, 101594. [Google Scholar] [CrossRef]
- Vázquez-Nion, D.; Fuentes, E.; Prieto, B. Effect of Inorganic Carbon Concentration on the Development of Subaerial Phototrophic Biofilms on Granite. Coatings 2020, 10, 1049. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Labavitch, J.M.; Vandergheynst, J.S. Elevated CO2 concentration impacts cell wall polysaccharide composition of green microalgae of the genus Chlorella. Lett. Appl. Microbiol. 2015, 60, 1–7. [Google Scholar] [CrossRef]
- Velu, C.; Cires, S.; Brinkman, D.L.; Heimann, K. Effect of CO2 and metal-rich waste water on bioproduct potential of the diazotrophic freshwater cyanobacterium. Heliyon 5 2019, 4, e01549. [Google Scholar] [CrossRef]
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Bhatnagar, A. Diversity of polysaccharides in cyanobacteria. Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 1. In Microbial Diversity in Normal & Extreme Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 447–496. [Google Scholar]
- Oerlemans, M.M.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Fanning, S.; Hall, L.J.; van Sinderen, D. Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes 2012, 3, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P. Biomolecules from microalgae and cyanobacteria: Applications and market survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Challouf, R.; Trabelsi, L.; Ben Dhieb, R.; El Abed, O.; Yahia, A.; Ghozzi, K.; Ben Ammar, J.; Omran, H.; Ben Ouada, H. Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospira platensis. Braz. Arch. Biol. Technol. 2011, 54, 831–838. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Parwani, L.; Sharma, V.; Ganguly, J.; Bhatnagar, A. Exopolymers from Tolypothrix tenuis and three Anabaena sp. (Cyanobacteriaceae) as novel blood clotting agents for wound management. Carbohydr. Polym. 2014, 99, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton, L.; Abdelkafi, S.; Fendri, I. Potential of exopolysaccharide from Porphyridium marinum to contend with bacterial proliferation, biofilm formation, and breast cancer. Mar. Drugs 2021, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Santhakumari, S.; Kannappan, A.; Pandian, S.K.; Thajuddin, N.; Rajendran, R.B.; Ravi, A.V. Inhibitory effect of marine cyanobacterial extract on biofilm formation and virulence factor production of bacterial pathogens causing vibriosis in aquaculture. J. Appl. Phycol. 2016, 28, 313–324. [Google Scholar] [CrossRef]
- Vasileva, I.; Toshkova-Yotova, T.; Georgieva, Z.; Karcheva, Z.; Petrova, D.; Chaneva, G.; Yocheva, L. Effect of temperature and light on the biochemical profile and antimicrobial activity of Chroococcus sp. R-10 (Cyanoprocaryota). Oxid. Commun. 2021, 44, 723–736. [Google Scholar]
- Falaise, C.; François, C.; Travers, M.-A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y. Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar. Drugs 2016, 14, 159. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural polysaccharide nanomaterials: An overview of their immunological properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Liu, X.; Elbahri, M. Antiviral polysaccharide and antiviral peptide delivering nanomaterials for prevention and treatment of SARS-CoV-2 caused COVID-19 and other viral diseases. J. Control. Release 2023, 358, 476–497. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.; Gallo, A.; Schommartz, T.; Handke, W.; Nagel, C.-H.; Günther, P.; Brune, W.; Reich, K. Calcium spirulan derived from Spirulina platensis inhibits herpes simplex virus 1 attachment to human keratinocytes and protects against herpes labialis. J. Allergy Clin. Immunol. 2016, 137, 197–203.e3. [Google Scholar] [CrossRef]
- Parwani, L.; Bhatt, M.; Singh, J. Potential biotechnological applications of cyanobacterial exopolysaccharides. Braz. Arch. Biol. Technol. 2021, 64, e21200401. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef]
- Kumari, M.; Dasriya, V.L.; Nataraj, B.H.; Nagpal, R.; Behare, P.V. Lacticaseibacillus rhamnosus-derived exopolysaccharide attenuates d-Galactose-Induced oxidative stress and inflammatory brain injury and modulates gut microbiota in a mouse model. Microorganisms 2022, 10, 2046. [Google Scholar] [CrossRef]
- Parwani, L.; Bhatnagar, M.; Bhatnagar, A.; Sharma, V. Antioxidant and iron-chelating activities of cyanobacterial exopolymers with potential for wound healing. J. Appl. Phycol. 2014, 26, 1473–1482. [Google Scholar] [CrossRef]
- Şengül, N.; Işık, S.; Aslım, B.; Uçar, G.; Demirbağ, A.E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-T.; Wang, S.; Han, N.; Li, M.-Y.; Li, J.; Li, Y.-R.; Jia, S.-R.; Han, P.-P. Effects of H2O2 acclimation on the growth, polysaccharide production and tolerance performance of Nostoc flagelliforme. Algal Res. 2023, 70, 102968. [Google Scholar] [CrossRef]
- Uhliariková, I.; Matulová, M.; Capek, P. Optimizing acid hydrolysis for monosaccharide compositional analysis of Nostoc cf. linckia acidic exopolysaccharide. Carbohydr. Res. 2021, 508, 108400. [Google Scholar] [CrossRef] [PubMed]
- Alberto Vieira Costa, J.; Franco Lucas, B.; Gabrielle Pires Alvarenga, A.; Botelho Moreira, J.; Greque de Morais, M. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; kanti Bandyopadhyay, T.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Majdoub, H.; Mansour, M.B.; Chaubet, F.; Roudesli, M.S.; Maaroufi, R.M. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim. Biophys. Acta 2009, 1790, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Bergman, M.; Van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Chen, B.; You, W.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010, 26, 833–840. [Google Scholar] [CrossRef]
- Wang, S.-y.; Jiang, Y.; Meng, C.; Ouyang, Y.-H.; Lin, X.-Z. Preparation of extracellular polysaccharide from fermentation liquor of marine microalgae Schizochytrium and study on the bioactivities. J. Fuzhou Univ. 2011, 39, 786–791. [Google Scholar]
- Herrero, M.; Martín-Álvarez, P.J.; Senorans, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Li, L.; Gao, Y.; Dai, Y.; Yang, Y.; Wang, X. Scavenging effects of Spirulina and polysaccharides Spirulina platensis on active oxygens and its antioxidation in vitro. Chem. Bioeng. 2007, 24, 55–57. [Google Scholar]
- Elkomy, R.G.; Ismail, M.M. Crude sulfated polysaccharides extracted from marine cyanobacterium Oscillatoria simplicissima with evaluation antioxidant and cytotoxic activities. Iran. J. Microbiol. 2021, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 2011, 17, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Risjani, Y.; Mutmainnah, N.; Manurung, P.; Wulan, S.N.; Yunianta. Exopolysaccharide from Porphyridium cruentum (purpureum) is not toxic and stimulates immune response against vibriosis: The assessment using zebrafish and white shrimp Litopenaeus vannamei. Mar. Drugs 2021, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; De Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Caetano, P.A.; do Nascimento, T.C.; Fernandes, A.S.; Nass, P.P.; Vieira, K.R.; Junior, M.R.M.; Jacob-Lopes, E.; Zepka, L.Q. Microalgae-based polysaccharides: Insights on production, applications, analysis, and future challenges. Biocatal. Agric. Biotechnol. 2022, 45, 102491. [Google Scholar] [CrossRef]
- Ciszek-Lenda, M.; Nowak, B.; Śróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37. Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef]
- Finamore, A.; Roselli, M.; Imbinto, A.; Seeboth, J.; Oswald, I.P.; Mengheri, E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PLoS ONE 2014, 9, e94891. [Google Scholar] [CrossRef]
- Gouda, M.; Tadda, M.A.; Zhao, Y.; Farmanullah, F.; Chu, B.; Li, X.; He, Y. Microalgae bioactive carbohydrates as a novel sustainable and eco-friendly source of prebiotics: Emerging health functionality and recent technologies for extraction and detection. Front. Nutr. 2022, 9, 806692. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 2010, 17, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory activity of exopolysaccharides from Phormidium sp. ETS05, the most abundant cyanobacterium of the therapeutic euganean thermal muds, using the zebrafish model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-inflammatory activity of bioactive compounds from microalgae and cyanobacteria by focusing on the mechanisms of action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, T.; Yuan, X.; Dong, Y.; Liu, C. Biocidal H2O2 treatment emphasizes the crucial role of cyanobacterial extracellular polysaccharides against external strong oxidative stress. Environ. Sci. Pollut. Res. 2023, 30, 60654–60662. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Matulová, M.; Šutovská, M.; Barboríková, J.; Molitorisová, M.; Kazimierová, I. Chlorella vulgaris α-L-arabino-α-L-rhamno-α, β-D-galactan structure and mechanisms of its anti-inflammatory and anti-remodelling effects. Int. J. Biol. Macromol. 2020, 162, 188–198. [Google Scholar] [CrossRef]
- Uhliariková, I.; Šutovská, M.; Barboríková, J.; Molitorisová, M.; Kim, H.J.; Park, Y.I.; Matulová, M.; Lukavský, J.; Hromadková, Z.; Capek, P. Structural characteristics and biological effects of exopolysaccharide produced by cyanobacterium Nostoc sp. Int. J. Biol. Macromol. 2020, 160, 364–371. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of Surface Exopolysaccharides from Bifidobacterium and Lactobacillus within the Intestinal Environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Shetty, P.H. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Ananya, A.K.; Ahmad, I.Z. Cyanobacteria “the blue green algae” and its novel applications: A brief review. Int. J. Innov. Appl. Stud. 2014, 7, 251. [Google Scholar]
- Li, J.; Ai, L.; Xu, F.; Hu, X.; Yao, Y.; Wang, L. Structural characterization of exopolysaccharides from Weissella cibaria NC516. 11 in distiller grains and its improvement in gluten-free dough. Int. J. Biol. Macromol. 2022, 199, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Muninathan, C.; Guruchandran, S.; Viswanath Kalyan, A.J.; Ganesan, N.D. Microbial exopolysaccharides: Role in functional food engineering and gut-health management. Int. J. Food Sci. Technol. 2022, 57, 27–34. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.; Wu, F.; Jin, Y.; Xu, X.; Gänzle, M.G. Comparison of the functionality of exopolysaccharides produced by sourdough lactic acid bacteria in bread and steamed bread. J. Agric. Food Chem. 2020, 68, 8907–8914. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Jakobsen, A.N.; Lerfall, J. Sustainable edible packaging systems based on active compounds from food processing byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 198–226. [Google Scholar] [CrossRef]
- Mishra, P. Cyanobacterial Exopolysaccharide as Natural Sources for Food Packaging Applications. Innov. Food Technol. Curr. Perspect. Future Goals 2020, 171–184. [Google Scholar] [CrossRef]
- Morales-Jiménez, M.; Gouveia, L.; Yáñez-Fernández, J.; Castro-Muñoz, R.; Barragán-Huerta, B.E. Production, preparation and characterization of microalgae-based biopolymer as a potential bioactive film. Coatings 2020, 10, 120. [Google Scholar] [CrossRef]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Bellini, E.; Ciocci, M.; Savio, S.; Antonaroli, S.; Seliktar, D.; Melino, S.; Congestri, R. Trichormus variabilis (Cyanobacteria) biomass: From the nutraceutical products to novel EPS-cell/protein carrier systems. Mar. Drugs 2018, 16, 298. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.K.; Sornkamnerd, S.; Kaneko, T. Development of functional bionanocomposites using cyanobacterial polysaccharides. Chem. Rec. 2018, 18, 1167–1177. [Google Scholar] [CrossRef]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef]
- Hadji, H.; Bouchemal, K. Advances in the treatment of inflammatory bowel disease: Focus on polysaccharide nanoparticulate drug delivery systems. Adv. Drug Deliv. Rev. 2022, 181, 114101. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Iqbal, H.M. Microbial exopolysaccharide-based nano-carriers with unique multi-functionalities for biomedical sectors. Biologia 2021, 76, 673–685. [Google Scholar] [CrossRef]
- Hamidi, M.; Jafari, H.; Siminska-Stanny, J.; Okoro, O.V.; Fatimi, A.; Shavandi, A. Anionic exopolysaccharide from Cryptococcus laurentii 70766 as an alternative for alginate for biomedical hydrogels. Int. J. Biol. Macromol. 2022, 212, 370–380. [Google Scholar] [CrossRef]
- Razzaghi, M.; Navvabi, A.; Homaee, M.B.; Sani, R.; Michaud, P.; Homaei, A. Exopolysaccharides in Drug Delivery Systems. Microb. Exopolysaccharides Nov. Signif. Biomater. 2021, 143–199. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Dineshkumar, K.; Yang, Y.-H. Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit. Rev. Microbiol. 2017, 43, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Datta, S. Bacterial exopolysaccharides in drug delivery applications. J. Drug Deliv. Sci. Technol. 2022, 74, 103557. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245 Pt B, 1674–1683. [Google Scholar] [CrossRef]
- Cheah, Y.T.; Chan, D.J.C. Physiology of microalgal biofilm: A review on prediction of adhesion on substrates. Bioengineered 2021, 12, 7577–7599. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).