The Impact of Lytic Viruses on Bacterial Carbon Metabolism in a Temperate Freshwater Reservoir (Naussac, France)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling Procedure
2.2. Limnological Variables
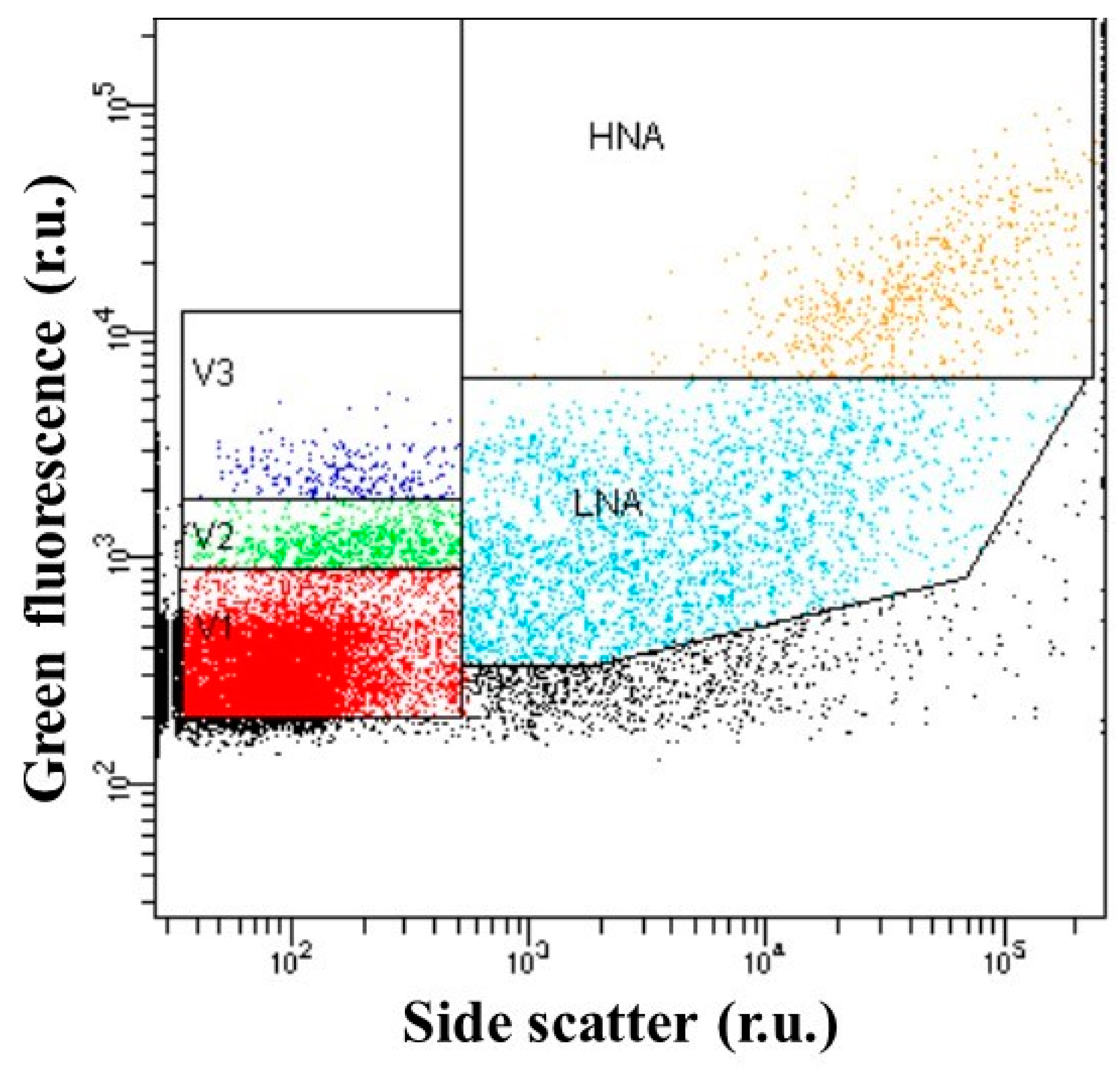
2.3. Flow Cytometry Enumeration of Viral and Bacterial Population
2.4. Bacterial Carbon Metabolism
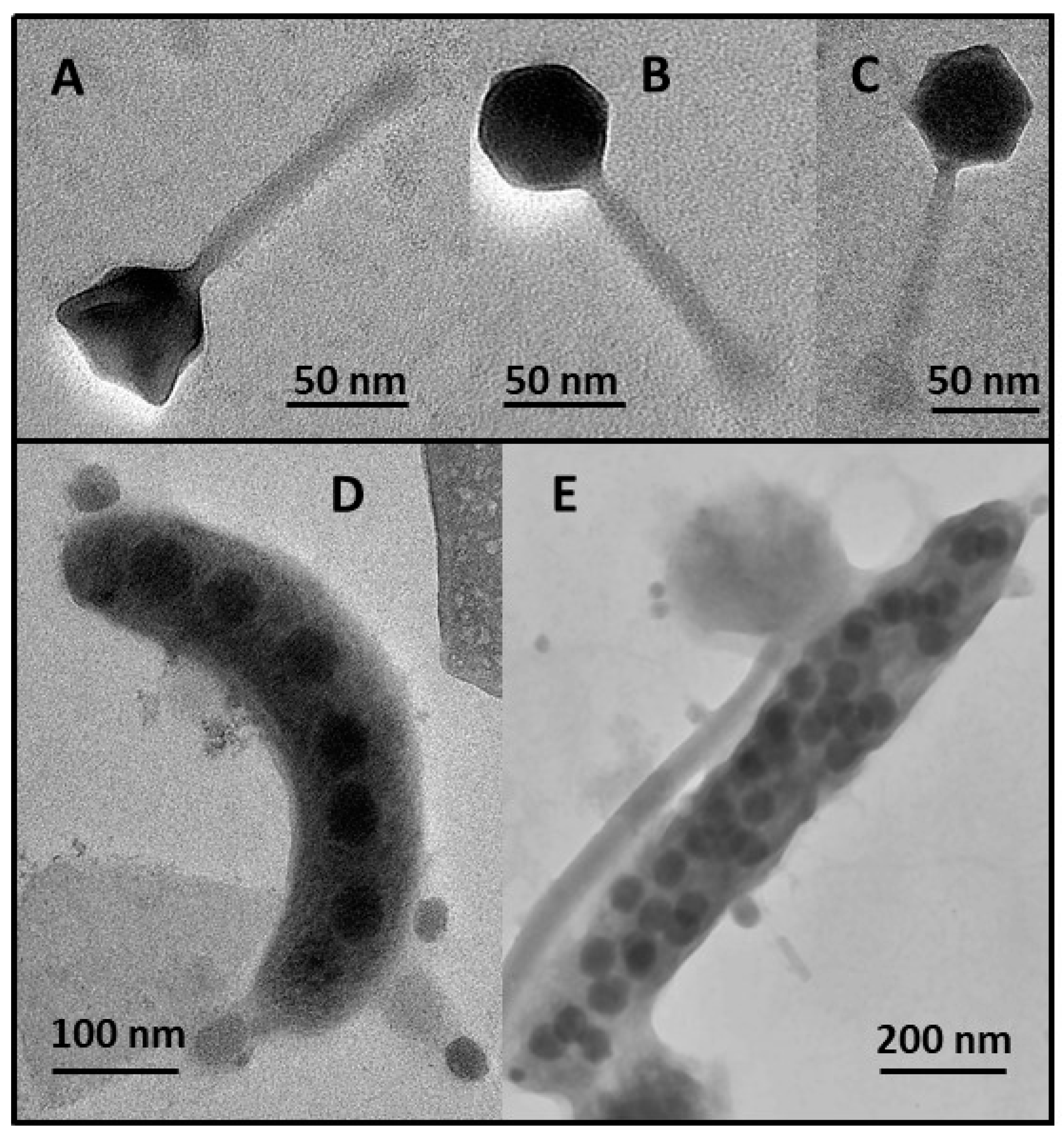
2.5. Phage Bacteriolysis, Burst Size Estimates and Morphometric Characteristics
2.6. Data Analyses
3. Results
3.1. Water Quality Characteristics
3.2. Flow Cytometry Counts of Viruses and Bacteria
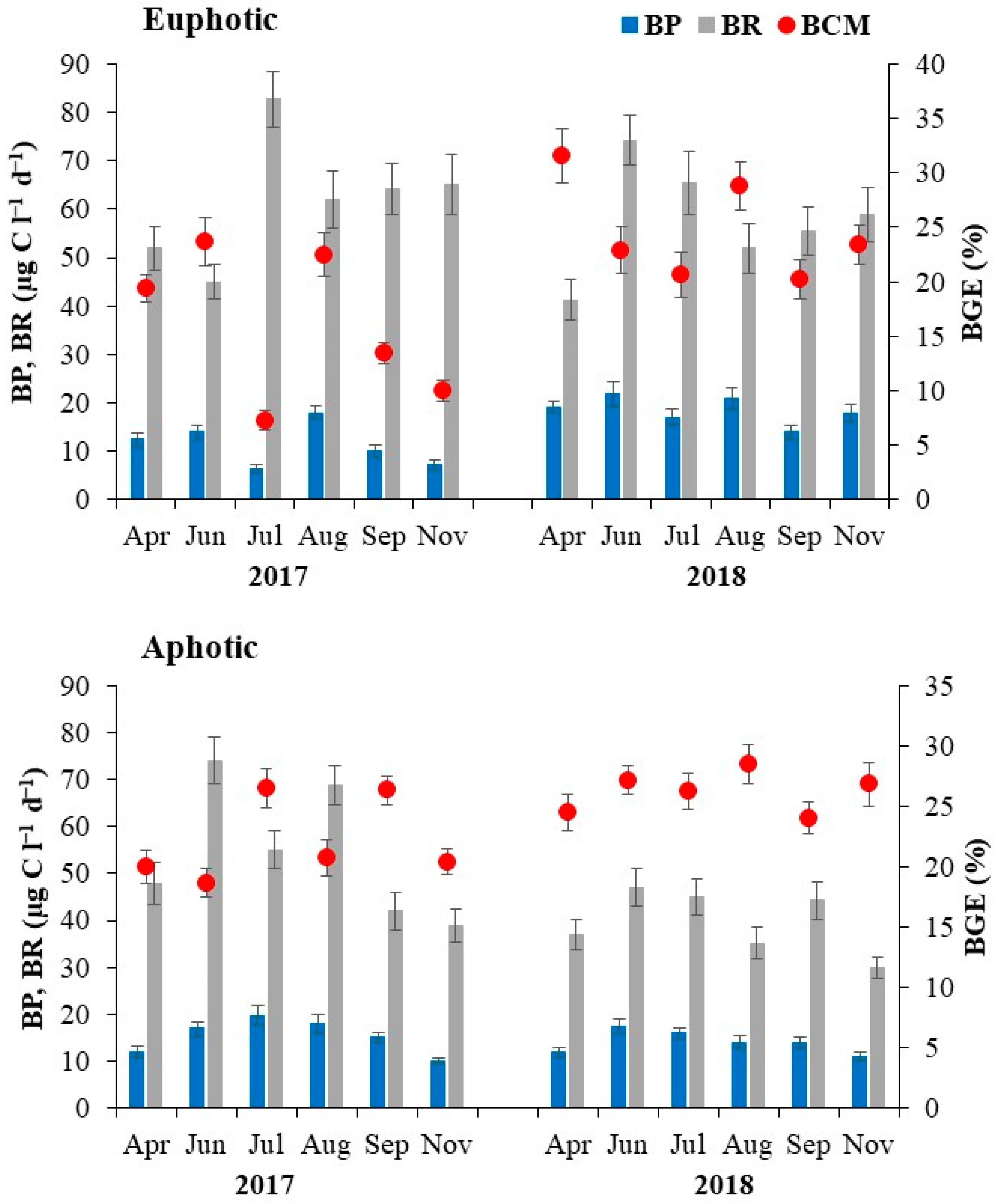
3.3. Bacterial Production (BP), Respiration (BR) and Carbon Metabolism (BCM)
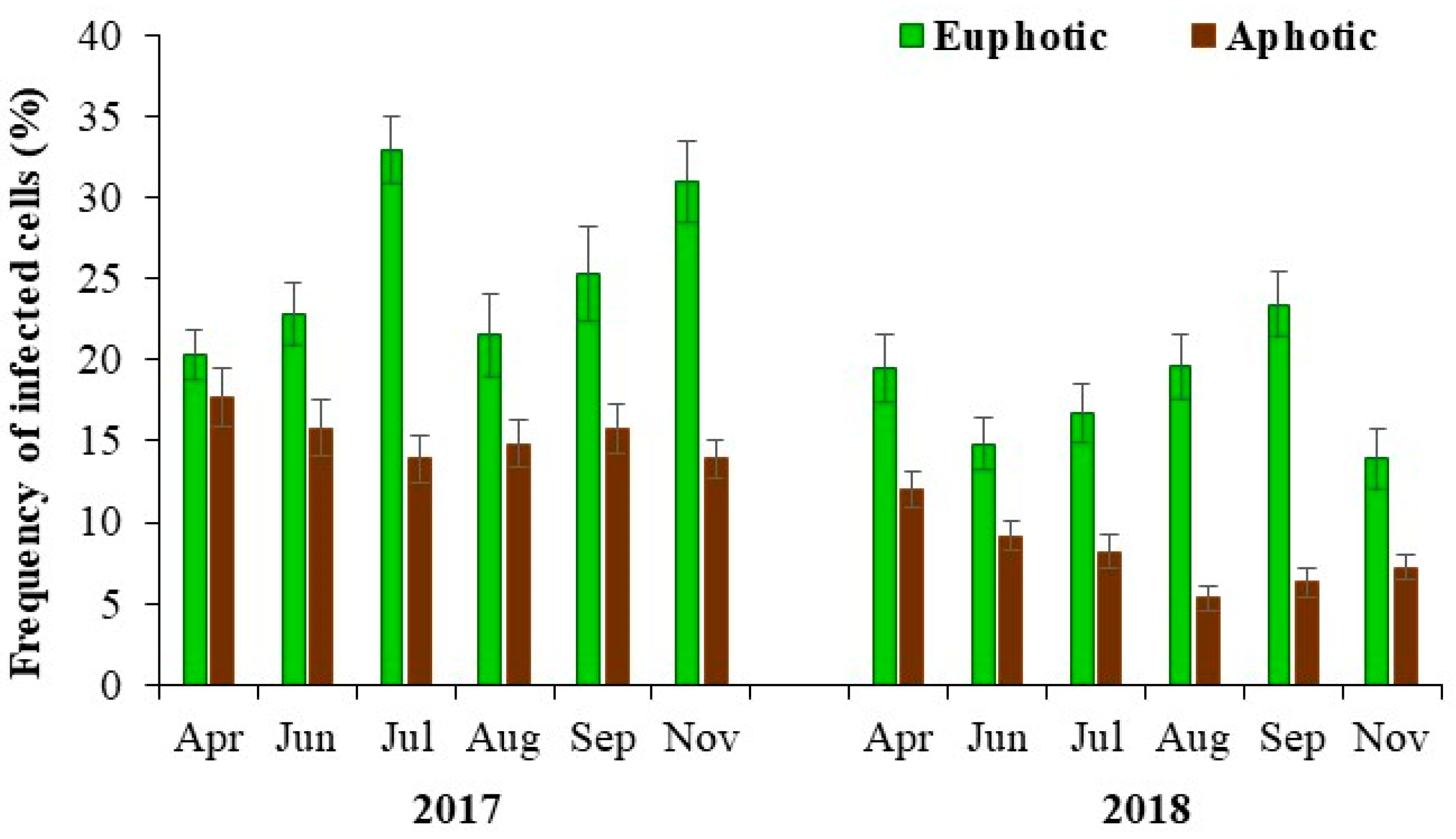
3.4. Lytic Viral Infection and Mortality
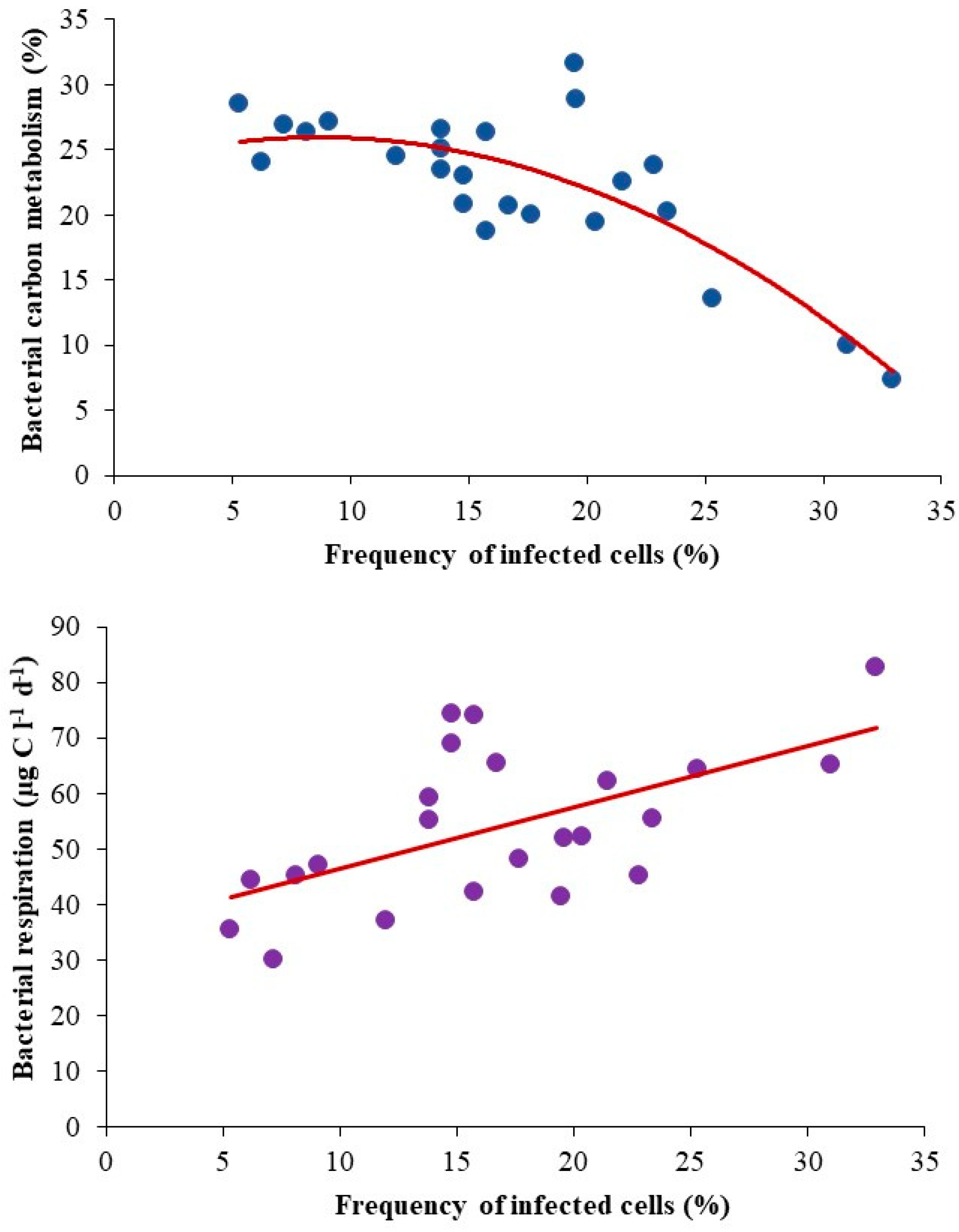
3.5. Viral Impact on Bacterial Population and Carbon Metabolism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweichhart, J.S. Prokaryotic viruses: Intriguing players in the aquatic stream. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- Jacquet, S.; Zhong, X.; Peduzzi, P.; Thingstad, T.F.; Parikka, K.J.; Weinbauer, M.G. Virus interactions in the aquatic world. In Viruses of Microorganisms; Hylan, P., Abedon, S.T., Eds.; Caister Academic Press: Poole, UK, 2018; Chapter 6; pp. 115–142. [Google Scholar]
- Zhang, Q.Y.; Gui, J.F. Diversity, evolutionary contribution and ecological roles of aquatic viruses. Sci. China Life Sci. 2018, 61, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G.; Höfle, M.G. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 1998, 64, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Ram, A.S.; Palesse, S.; Colombet, J.; Thouvenot, A.; Sime-Ngando, T. The relative importance of viral lysis and nanoflagellate grazing for prokaryote mortality in temperate lakes. Freshw. Biol. 2014, 59, 300–311. [Google Scholar]
- Berdjeb, L.; Pollet, T.; Domaizon, I.; Jacquet, S. Effect of grazers and viruses on bacterial community structure and production in two contrasting trophic lakes. BMC Microbiol. 2011, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.-Y.; Gong, G.-C.; Sanders, R.W.; Huang, J.-K. Contribution of viral lysis and nanoflagellate grazing to bacterial mortality in the inner and outer regions of the Changjiang river plume during summer. J. Plankt. Res. 2013, 35, 1283–1293. [Google Scholar] [CrossRef]
- Fischer, U.; Velimirov, B. High control of bacterial production by viruses in a eutrophic oxbow lake. Aquat. Microb. Ecol. 2002, 27, 1–12. [Google Scholar] [CrossRef]
- Pradeep Ram, A.S.; Colombet, J.; Perriere, F.; Thouvenot, A.; Sime-Ngando, T. Viral regulation of prokaryotic carbon metabolism in a hypereutrophic freshwater reservoir ecosystem (Villerest, France). Front. Microbiol. 2016, 7, 81. [Google Scholar] [CrossRef]
- Shen, S.; Shimizu, Y. Seasonal variation in viral infection rates and cell sizes of infected prokaryotes in a large and deep freshwater lake (Lake Biwa, Japan). Front. Microbiol. 2021, 12, 624980. [Google Scholar] [CrossRef]
- del Giorgio, P.A.; Cole, J.J. Bacterial growth efficiency in natural aquatic ecosystems. Annu. Rev. Ecol. Syst. 1998, 29, 503–541. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Langenheder, S.; Lindstrom, E.S. Influence of dissolved organic matter source on lake bacterioplankton structure and function-implications for seasonal dynamics of community composition. FEMS Microb. Ecol. 2006, 56, 406–417. [Google Scholar] [CrossRef][Green Version]
- Berman, T.; Yacobi, Y.Z.; Parparov, A.; Gal, G. Estimation of long-term respiration and growth efficiency in Lake Kinneret. FEMS Microbiol. Ecol. 2010, 71, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Comte, J.; del Giorgio, P.A. Links between resources, C metabolism and the major components of bacterioplankton community structure across a range of freshwater systems. Environ. Microbiol. 2009, 11, 1704–1706. [Google Scholar] [CrossRef]
- Amado, A.M.; Meirelles-Pereira, F.; Vidal, L.O.; Sarmento, H.; Suhett, A.L.; Farjalla, V.F.; Cotner, J.B.; Roland, F. Tropical freshwater ecosystems have lower growth efficiency than temperate ones. Front. Microbiol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Motegi, C.; Nagata, T.; Miki, T.; Weinbauer, M.G.; Legendre, L.; Rassoulzadegan, F. Viral control of bacterial growth efficiency in marine pelagic environments. Limnol. Oceanogr. 2009, 54, 1901–1910. [Google Scholar] [CrossRef]
- Xu, J.; Jing, H.; Sun, M.; Harrison, P.J.; Liu, H. Regulation of bacterial metabolic activity by dissolved organic carbon and viruses. J. Geophys. Res. Biogeosci. 2013, 118, 1573–1583. [Google Scholar] [CrossRef]
- Pradeep Ram, A.S.; Sime-Ngando, T. Differential effects of viruses on growth efficiency of freshwater bacterioplankton in eutrophic relative to non-eutrophic lakes. Microorganisms 2023, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, W.; Cai, L. The fate and biogeochemical cycling of viral elements. Nat. Rev. Microbiol. 2014, 12, 850–851. [Google Scholar] [CrossRef]
- Maurice, C.F.; Bouvier, T.; Comte, J.; Guillemette, F.; del Giorgio, P.A. Seasonal variation of phage life strategies and bacterial physiological states in the three northern temperate lakes. Environ. Microbiol. 2010, 12, 628–641. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Likens, G.E. Limnological Analysis, 2nd ed.; Springer: New York, NY, USA, 1995. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington DC, USA, 1998. [Google Scholar]
- Kosten, S.; Huszar, V.L.M.; Mazzeo, N.; Scheffer, M.; Sternberg, L.S.L.; Jeppesen, E. Lake and water shed characteristics rather than climate influence nutrient limitation in shallow lakes. Ecol. Appl. 2009, 19, 1791–1804. [Google Scholar] [CrossRef]
- Davis, C.E.; Blackbird, S.; Wolff, G.; Woodward, M.; Mahaffey, C. Seasonal organic matter dynamics in a temperate shelf sea. Prog. Oceanogr. 2019, 177, 101925. [Google Scholar] [CrossRef]
- Brussaard, C.; Payet, J.P.; Winter, C.; Weinbauer, M. Quantification of aquatic viruses by flow cytometry. In Manual of Aquatic Viral Ecology; Wilhelm, S.W., Weinbauer, M.G., Suttle, C.A., Eds.; ASLO: San Antonio, TX, USA, 2010; Chapter 11; pp. 102–109. [Google Scholar]
- Kirchman, D.L. Calculating microbial growth rates from data on production and standing stocks. Mar. Ecol. Prog. Ser. 2002, 233, 303–306. [Google Scholar] [CrossRef]
- Jugnia, L.B.; Tadonléké, R.D.; Sime-Ngando, T.; Devaux, J. The microbial food web in the recently flooded reservoir: Diel fluctuations in bacterial biomass and metabolic activity in relation to phytoplankton and flagellate grazers. Microb. Ecol. 2000, 40, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Carignan, R.; Blais, A.M.; Vis, C. Measurement of primary production and community respiration in oligotrophic lakes using the Winkler method. Can. J. Fish. Aquat. Sci. 1998, 55, 1078–1084. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Winter, C.; Höfle, M.G. Reconsidering transmission electron microscopy-based estimates of viral infection of bacterioplankton using conversion factors derived from natural communities. Aquat. Microb. Ecol. 2002, 27, 103–110. [Google Scholar] [CrossRef]
- Binder, B. Reconsidering the relationship between virally induced bacterial mortality and frequency of infected cells. Aquat. Microb. Ecol. 1999, 18, 207–215. [Google Scholar] [CrossRef]
- Carlson, R. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Kalinowska, K.; Guspiel, A.; Kiersztyn, B.; Chrost, R.J. Factors controlling bacteria and protists in selected Mazurian eutrophic lakes (North-eastern Poland) during spring. Aquat. Biosys. 2013, 9, 9. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, TX, USA, 2001; 1006p. [Google Scholar]
- Guildford, S.J.; Hecky, R.E. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef]
- Allesson, L.; Andersen, T.; Dorsch, P.; Eiler, A.; Wei, J.; Hessen, D.O. Phosphorous availability promotes bacterial DOC-mineralisation, but not cumulative CO2-production. Front. Microbiol. 2020, 11, 569879. [Google Scholar]
- Makino, W.; Cotner, J.B. Elemental stoichiometry of a heterotrophic bacterial community in a freshwater lake: Implications for growth- and resource-dependent variations. Aquat. Microb. Ecol. 2004, 34, 33–41. [Google Scholar] [CrossRef][Green Version]
- Scott, J.T.; Cotner, J.B.; LaPara, T.M. Variable stoichiometry and homeostatic regulation of bacterial biomass elemental composition. Front. Microbiol. 2012, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Šimek, K.; Pernthaler, J.; Weinbauer, M.G.; Hornak, K.; Dolan, J.R.; Nedoma, J.; Mašín, M.; Amann, R. Changes in bacterial community composition, dynamics and viral mortality rates associated with enhanced flagellate grazing in a meso-eutrophic reservoir. Appl. Environ. Microbiol. 2001, 67, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gu, X.; Te, S.H.; Goh, S.G.; Mani, K.; He, Y.; Gin, K.Y.H. Occurrence and distribution of viruses and picoplankton in tropical freshwater bodies determined by flow cytometry. Water Res. 2019, 149, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, A.I.; Zabotkina, Z.A. Virioplankton as an important component of plankton in the Volga reservoirs. Biosyst. Divers. 2021, 29, 151–159. [Google Scholar] [CrossRef]
- Martínez Martínez, J.; Swan, B.K.; Wilson, W.H. Marine viruses, a genetic reservoir revealed by targeted viromics. ISME J. 2014, 8, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Schattenhofer, M.; Wulf, J.; Kostadinov, I.; Glöckner, F.O.; Zubkov, M.V.; Fuchs, B.M. Phylogenetic characterization of picoplanktonic populations with high and low nucleic acid content in the North Atlantic Ocean. Syst. Appl. Microbiol. 2011, 34, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.R.; Besmer, M.D.; Langenegger, T.; Beck, K.; Walser, J.-C.; Ackermann, M.; Burgmann, H.; Hammes, F. Phylogenetic clustering of small low nucleic acid content bacteria across diverse freshwater ecosystems. ISME J. 2018, 12, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, X.; Yu, L.; Xu, D.; Jiao, N. Elevated contribution of low nucleic acid prokaryotes and viral lysis to the prokaryotic community along the nutrient gradient from an estuary to open ocean transect. Front. Microbiol. 2020, 11, 612053. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Vage, S.; Storesund, J.E.; Sandaa, R.A.; Giske, J. A theoretical analysis of how strain-specific viruses can control microbial species diversity. Proc. Natl. Acad. Sci. USA 2014, 111, 7813–7818. [Google Scholar] [CrossRef]
- Parikka, K.J.; Le Romancer, M.; Wauters, N.; Jacquet, S. Deciphering the virus-to-prokaryote ratio (VPR): Insights into virus-host relationships in a variety of ecosystems. Biol. Rev. 2017, 92, 1081–1100. [Google Scholar] [CrossRef]
- Peduzzi, P.; Shiemer, F. Bacteria and viruses in the water column of a tropical freshwater reservoirs. Environ. Microbiol. 2004, 6, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Ram, A.S.; Palesse, S.; Colombet, J.; Sabart, M.; Perriere, F.; Sime Ngando, T. Variable viral and grazer control of prokaryotic growth efficiency in temperate freshwater lakes (French Massif Central). Microbiol. Ecol. 2013, 66, 906–916. [Google Scholar]
- Kopylov, A.I.; Kosolapov, D.B.; Zabotkina, Z.A. Virus impact on heterotrophic bacterioplankton of water reservoirs. Microbiology 2011, 80, 228–236. [Google Scholar] [CrossRef]
- Parvathi, A.; Zhong, X.; Pradeep Ram, A.S.; Jacquet, S. Dynamics of auto- and heterotrophic picoplankton and associated viruses in Lake Geneva. Hydrol. Earth Syst. Sci. 2014, 18, 1073–1087. [Google Scholar] [CrossRef]
- Parsons, R.J.; Breitbart, M.; Lomas, M.W.; Carlson, C.A. Ocean time-series reveals recurring seasonal patterns of virioplankton dynamics in the northwestern Sargasso Sea. ISME J. 2012, 6, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Prairie, Y.T. Bacterial metabolism and growth efficiency in lakes: The importance of phosphorus availability. Limnol. Oceanogr. 2004, 49, 137–147. [Google Scholar] [CrossRef]
- Garcia-Martin, E.E.; Daniels, C.J.; Davidson, K.; Lozano, J.; Mayers, K.M.J.; McNeill, S.; Mitchell, E.; Poulton, A.J.; Purdie, D.A.; Tarran, G.A.; et al. Plankton community respiration and bacterial metabolism in a north Atlantic shelf sea during spring bloom development (April 2015). Prog. Oceanogr. 2019, 177, 101873. [Google Scholar] [CrossRef]
- Apple, J.K.; del Giorgio, P.A. Organic substrate quality as the link between bacterioplankton carbon demand and growth efficiency in a temperate salt-marsh estuary. ISME J. 2007, 1, 729–742. [Google Scholar] [CrossRef]
- Vidal, L.O.; Graneli, W.; Daniel, C.B.; Heiberg, L.; Roland, F. Carbon and phosphorus regulating bacterial metabolism in oligotrophic boreal lakes. J. Plank. Res. 2011, 33, 1747–1756. [Google Scholar] [CrossRef]
- Biddanda, B.; Ogdahl, M.; Cotner, J. Dominance of bacterial metabolism in oligotrophic relative to eutrophic waters. Limnol. Oceanogr. 2001, 46, 730–739. [Google Scholar] [CrossRef]
- Eiler, A.; Langenheder, S.; Bertilsson, S.; Tranvik, L.J. Heterotrophic bacterial growth efficiency and community structure at different natural organic carbon concentrations. Appl. Environ. Microbiol. 2003, 69, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Findji, O.; Maltis, A.; Lefèvre, D.; Rochelle-Newall, E.; Lemée, R.; Weinbauer, M.; Gattuso, J.-P. Viral effects on bacterial respiration, production and growth efficiency: Consistent trends in the Southern Ocean and the Mediterranean Sea. Deep Sea Res. Part II 2008, 55, 790–800. [Google Scholar] [CrossRef]
- Jasna, V.; Pradeep Ram, A.S.; Pravathi, A.; Sime-Ngando, T. Differential impact of lytic viruses on prokaryotic morphopopulations in a tropical estuarine system (Cochin estuary, India). PLoS ONE 2018, 13, e0194020. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Ram, A.S.; Colombet, J.; Perriere, F.; Thouvenot, A.; Sime-Ngando, T. Viral and grazer regulation of prokaryotic growth efficiency in temperate freshwater pelagic environments. FEMS Microb. Ecol. 2015, 91, 1–12. [Google Scholar] [CrossRef][Green Version]
- Wilhelm, S.W.; Suttle, C.A. Viruses and nutrient cycles in the sea. Bioscience 1999, 49, 781–788. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Øvreas, L.; Egge, J.K.; Lovdal, T.; Heldal, M. Use of non-limiting substrates to increase size; a generic strategy to simultaneously optimize uptake and minimize predation in pelagic osmotrophs? Ecol. Lett. 2005, 8, 675–682. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Hornak, K.; Jezbera, J.; Nedoma, J.; Dolan, J.R.; Simek, K. Synergistic and antagonistic effects of viral lysis and protistan grazing on bacterial biomass, production and diversity. Environ. Microbiol. 2007, 9, 777–788. [Google Scholar] [CrossRef]
- Keshri, J.; Pradeep Ram, A.S.; Colombet, J.; Perriere, F.; Thouvenot, A.; Sime-Ngando, T. Differential impact of lytic viruses on the taxonomic resolution of freshwater bacterioplankton community structure. Wat. Res. 2017, 124, 129–138. [Google Scholar] [CrossRef]

| Parameters | Mean (Range) a | p Value b | |
|---|---|---|---|
| Euphotic (EUP) | Aphotic (APH) | ||
| Water temperature (°C) | 15.7 (7.2–24.9) | 8.1 (4.8–10.2) | 0.001 |
| pH | 7.9 (7.5–9.0) | 7.4 (6.2–7.8) | 0.003 |
| Turbidity (FNU) | 1.1 (0.1–3.3) | 3.7 (0.5–9.0) | 0.05 |
| Conductivity (µs cm−1) | 55.7 (43.3–65.9) | 46.2 (40.7–52.0) | 0.001 |
| Dissolved oxygen (mg L−1) | 9.5 (8.1–12.0) | 6.5 (0.3–11.4) | 0.02 |
| Ammonium (µmol L−1) | 4.6 (2.8–11.7) | 3.4 (2.8–5.0) | NS |
| Nitrate + Nitrite (µmol L−1) | 27.1 (14.8–42.4) | 31.4 (9.9–47.5) | NS |
| Total phosphate (µmol L−1) | 0.4 (0.3–0.8) | 1.6 (0.3–5.2) | 0.03 |
| Orthophosphate (µmol L−1) | 0.1 (0.1–0.2) | 0.8 (0.1–4.8) | 0.05 |
| Total organic carbon (µmol L−1) | 447.7 (360.0–525.5) | 436.2 (370.3–493.9) | NS |
| Dissolved organic carbon (µmol L−1) | 411.5 (333.6–496.1) | 400.0 (343.0–472.6) | NS |
| Total dissolved nitrogen (µmol L−1) | 74.7 (37.5–186.9) | 59.5 (52.8–77.1) | NS |
| C:N ratio | 6.8 (2.1–12.9) | 7.2 (5.4–8.7) | NS |
| N:P ratio | 82.1 (37.9–139.2) | 60.6 (4.7–157.3) | NS |
| C:P ratio | 1127 (524–1440) | 701 (70–1393) | 0.05 |
| Chlorophyll-a (µg L−1) | 3.2 (1.0–9.1) | Not determined | NS |
| Parameters | Mean (Range) a | p Value b | |
|---|---|---|---|
| Euphotic (EUP) | Aphotic (APH) | ||
| Viral abundance (106 VLPs mL−1) | 9.2 (2.7–28.7) | 3.3 (1.5–6.5) | 0.03 |
| V1 viral group (106 VLPs mL−1) | 7.8 (2.3–22.7) | 2.6 (1.3–5.2) | 0.05 |
| V2 viral group (106 VLPs mL−1) | 1.4 (0.1–5.8) | 0.2 (0.04–0.7) | NS |
| V3 viral group (106 VLPs mL−1) | 0.2 (0.04–1.0) | 0.04 (0.01–0.06) | NS |
| Bacterial abundance (106 cells mL−1) | 2.1 (0.6–6.4) | 1.0 (0.4–2.4) | 0.05 |
| Low-nucleic-acid bacteria (106 cells mL−1) | 1.6 (0.6–4.7) | 0.6 (0.4–1.1) | NS |
| High-nucleic-acid bacteria (106 cells mL−1) | 0.5 (0.003–1.7) | 0.2 (0.003–0.8) | NS |
| Virus-to-bacteria ratio | 4.3 (2.4–5.9) | 3.5 (2.8–4.3) | 0.02 |
| Bacterial production (µg C L−1 d−1) | 14.9 (6.5–22.0) | 15.1 (11.0–19.8) | NS |
| Bacterial respiration (µg C L−1 d−1) | 59.8 (41.3–82.7) | 47.8 (30.0–74.1) | 0.03 |
| Bacterial carbon metabolism (%) | 20.2 (7.0–31.5) | 24.5 (18.7–28.5) | NS |
| Frequency of infected cells (%) | 21.8 (13.9–32.9) | 11.7 (5.3–17.7) | 0.001 |
| Burst size (virus cell−1) | 34.2 (12.0–79.0) | 18.4 (6.0–43.0) | 0.001 |
| VA | BA | FIC | BP | BR | |
|---|---|---|---|---|---|
| BA | 0.95 *** | ||||
| FIC | 0.80 *** | 0.75 *** | |||
| BP | −0.64 *** | −0.58 ** | −0.75 *** | ||
| BR | 0.51 ** | 0.43 * | 0.59 ** | NS | |
| BCM | −0.79 *** | −0.72 *** | −0.81 *** | 0.67 ** | −0.71 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeep Ram, A.S.; Mauduit, M.-E.; Colombet, J.; Perriere, F.; Thouvenot, A.; Sime-Ngando, T. The Impact of Lytic Viruses on Bacterial Carbon Metabolism in a Temperate Freshwater Reservoir (Naussac, France). Appl. Microbiol. 2023, 3, 1407-1423. https://doi.org/10.3390/applmicrobiol3040095
Pradeep Ram AS, Mauduit M-E, Colombet J, Perriere F, Thouvenot A, Sime-Ngando T. The Impact of Lytic Viruses on Bacterial Carbon Metabolism in a Temperate Freshwater Reservoir (Naussac, France). Applied Microbiology. 2023; 3(4):1407-1423. https://doi.org/10.3390/applmicrobiol3040095
Chicago/Turabian StylePradeep Ram, Angia Sriram, Marie-Eve Mauduit, Jonathan Colombet, Fanny Perriere, Antoine Thouvenot, and Télesphore Sime-Ngando. 2023. "The Impact of Lytic Viruses on Bacterial Carbon Metabolism in a Temperate Freshwater Reservoir (Naussac, France)" Applied Microbiology 3, no. 4: 1407-1423. https://doi.org/10.3390/applmicrobiol3040095
APA StylePradeep Ram, A. S., Mauduit, M.-E., Colombet, J., Perriere, F., Thouvenot, A., & Sime-Ngando, T. (2023). The Impact of Lytic Viruses on Bacterial Carbon Metabolism in a Temperate Freshwater Reservoir (Naussac, France). Applied Microbiology, 3(4), 1407-1423. https://doi.org/10.3390/applmicrobiol3040095








