Abstract
Methane (CH4) has attracted attention as not only one of the hydrogen carriers in terms of energy density, but also synthetic natural gas. In nature, the decomposition of organic compounds is performed with bacterial ecosystems that can produce CH4. Clostridium cellulovorans as a decomposer was cultivated with pig manure (PM) as an unused biomass in this study. As a result of high-performance liquid chromatography (HPLC) analysis, while formate and lactate were decreased in the C. cellulovorans medium containing 0.5% PM, acetate and butyrate were increased in it. Accordingly, in order to compare with the effect of carbon sources for methane production, the cocultivation of C. cellulovorans and the methanogenesis of Methanosarcina mazei or microbial flora of methane production (MFMP) was carried out in the C. cellulovorans medium. As a result, only the cocultivation with C. cellulovorans and MFMP showed methane production in 0.5% acetate medium. Moreover, in comparison with a carbon source in either 1% acetate or 1% methanol medium, MFMP was only cultivated after being precultivated with 0.5% glucose medium for 12 h. The results revealed that MFMP with a 1% methanol medium produced methane approximately eight times higher than with 1% acetate medium. After cultivation with 1% acetate or 1% methanol, next-generation sequencing (NGS) analysis of MFMP was carried out. Interestingly, Methanofollis (0.211%), belonging to methanogens through the CO2 reduction pathway, was dominant in the 1% acetate medium for 72 h cultivation, while Methanosarcina siciliae (1.178%), M. barkeri (0.571%), and Methanofollis (0.490%) were major species in 1% methanol medium for 72 h cultivation. Since Methanosarcina spp. belong to acetoclasts (acetoclastic pathway), methanol could promote the growth of Methanosarcina spp., rather than acetate. Therefore, it seems that Methanosarcina spp. may play a key methanogenesis role in MFMP. Thus, these results will provide important information for low-cost biomethane production.
1. Introduction
Anaerobic digestion (AD) consists of a series of biochemical processes such as hydrolysis, fermentation (acidogenesis), acetogenesis and methanogenesis performed by various interacting microorganisms, including bacteria such as acidogens and acetogens, and archaea (methanogens). It is also clear that the cumulative CH4 production from the three different substrates varied significantly and was not in agreement with the expected, according to the theoretical value calculated (Table 1) (formate 82.35 N mLCH4/gVS, acetate 273.17 N mLCH4/gVS, H2/CO2 414.81 N mLCH4/gVS) [1]. Since methanogenesis is the final step in anaerobic carbon transformation and is of critical concern in thawing permafrost peatland systems where CH4 release is increasing rapidly, predicting the magnitude of carbon loss as CO2 or CH4 is hampered by our limited knowledge of the microbial metabolism of organic matter in these environments [2]. Genome-centric metagenomic analysis of microbial communities provides the necessary information to examine how specific lineages transform organic matter during permafrost thaw [3]. The biomethanation process in nature relies on the microbial interactions between three main metabolic groups of anaerobes: fermentative, acetogenic, and methanogenic microorganisms [4,5,6]. Whereas the first two groups decompose complex organic matters to acetate—H2 and CO2, which are the key precursors for methanogenesis, methanogens further convert these metabolites to CH4 by two major routes: the acetoclastic pathway and the CO2 reduction pathway [7]. On the other hand, although the growth behavior of a donor bacterium, Sulfurospirillum multivorans, in the modified Methanococcus voltae (acceptor) medium with pyruvate alone as substrate was similar to that in the medium originally used for the cultivation of S. multivorans, the morphology of S. multivorans cells was unaltered in the M. voltae medium and independent from the type of cultivation—fermentatively or respiratory [8]. In this case, the new medium with lactate as the sole growth substrate instead of formate and acetate could not promote growth for pure S. multivorans cultures. Furthermore, 15 mM lactate was consumed in approximately 2 weeks, while methane was produced in the corresponding coculture, indicating lactate fermentation by S. multivorans and H2 transfer to M. voltae as a syntrophic partner. Therefore, the coculture system seems to include system unique advantages, composition, products, and interaction mechanisms.

Table 1.
Methanogenic reactions from typical substrates.
The elaboration of the underlying mechanism in microbial communities, such as the exchange of intermediate metabolites, cell-to-cell electrical connections, communications, etc., would guide the design of artificial microbial consortia and further improve the robustness and stability of the cocultivation systems [9,10,11,12]. Therefore, these artificial microbial consortia interact mutually through the interaction of synergism, commensalism, competition, mutualism, and so on [12]. Diverse microbial communities within the same or different species have been set up to realize more complicated tasks [8,13,14]. In particular, the greatest advantage of coculture systems consists of the combination of the metabolic capacity of two or more microorganisms that allows for the utilization of more complex substrates and the production of specific products [14]. In addition, the treatment of wastewater, biodegradation of textile azo dye and disposal of contaminated soil have also recently been applied to produce biofuels, bulk chemicals, and natural products by cocultivation systems [15,16,17,18,19,20,21,22,23,24,25,26].
Cellulose is most abundant on the Earth and is not easily degraded and utilized. In addition to cellulosic sources, various other carbohydrates, carbon monoxide and syngas can also be processed using these systems [27]. The cellulolytic system of Clostridium cellulovorans mainly consists of a cellulosome that synergistically collaborates with non-complexed enzymes [28,29]. IBE (isopropanol-butanol-ethanol) fermentation by the cocultivation of C. cellulovorans and C. beijerinckii was performed using mandarin orange wastes [30], and methane was produced from sugar beet pulp [31] and mandarin orange peel under cocultivation with C. cellulovorans and methanogens [32]. Furthermore, two coculture models combining C. cellulovorans with Methanosarcina barkeri Fusaro or M. mazei Gö1 were established for the direct conversion of cellulose to CH4 [33]. Coculturing C. cellulovorans with M. barkeri or M. mazei not only enabled the direct conversion of cellulose to CH4, but also stabilized pH for C. cellulovorans, resulting in a metabolic shift and enhanced cellulose degradation. The other approach involved implementing nanotechnology in combination with C. cellulovorans through a consolidated bioprocessing (CBP) method to produce hydrogen from raw corn cob [34].
In this study, we observed the cocultivation of C. cellulovorans and M. mazei or microbial flora of methane production (MFMP) for the different carbon sources between sugars such as glucose and cellobiose, which are the products from cellulose degraded by C. cellulovorans, and acetate metabolized from glucose through the TCA cycle. Furthermore, pig manure (PM) was used for the C. cellulovorans cultivation and was analyzed with organic acids. In addition, we investigated the cultivation manner of MFMP in comparison with acetate and methanol as the sole carbon source. Finally, 16S rRNA analysis in MFMP was performed by next-generation sequencing (NGS) after cultivations with acetate or methanol as a carbon source.
2. Materials and Methods
2.1. Microorganism
C. cellulovorans 743B (ATCC35296) was anaerobically grown as described previously [28], with pig manure (PM) (Mie University, Tsu, Japan) as a carbon source. M. mazei (DSM# 3647) was purchased from the German Collections of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and was cultivated with the JCM230 medium [35].
2.2. Culture Conditions
Moreover, 0.5% (w/v) PM, 0.5% (w/v) glucose, 0.5% acetic acid (FUJIFILM Wako Chemicals, Osaka, Japan), and 0.5% (w/v) cellobiose (Sigma, St. Louis, MO, USA) were used as the sole carbon source in 10 mL or 50 mL of C. cellulovorans media and was anaerobically cultivated. The microbial flora of methane production (MFMP) was obtained from methane fermentation digested liquid in January 2017 in Gifu, Japan [32]. C. cellulovorans was precultured with 0.5% cellobiose for 12 h at 37 °C and M. mazei and MFMP were precultured with 0.5% glucose for 12 h at 37 °C, respectively. Cocultivation was performed as approximately 1000 RLU of C.c cells and approximately 20,000 RLU of MFMP cells (C. cellulovorans:MFMP = 1:20) and approximately 1000 RLU of C. cellulovorans cells and approximately 3000 RLU of M. mazei cells (C. cellulovorans:M. mazei = 1:3), respectively. In comparison with cocultivations with 1% acetate and 1% methanol media, C. cellulovorans was precultured with 0.5% cellobiose for 12 h at 37 °C and M. mazei and MFMP were precultured with 0.5% glucose for 12 h at 37 °C, respectively. The precultured cells were inoculated into each medium with 1% acetate or 1% methanol. After the cells were collected by centrifugation, the supernatants and the cells were used for each experiment.
2.3. 16S rRNA Sequencing
Samples for bacterial cells cultivated in the culture medium were crashed by Shake Master Neo (bms, Tokyo, Japan) and DNA was extracted by Fast DNA spin kit (MP Bio, Irvine, CA, USA). iSeq 100 (Illumina, San Diego, CA, USA) was used for sequencing under the condition of 2 × 150 bp. The 16S Metagenomics App performed the taxonomic classification of 16S rRNA targeted amplicon reads using a version of the GreenGenes taxonomic database curated by Illumina. The primer sequences used in the protocol were: PCR1_Forward (50 bp): 5′–TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG–3′ and PCR1_Reverse (55 bp): 5′–GTCTCGTGG GCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC–3′, respectively. The 16S rRNA sequences of MFMP previously reported [31] were deposited in the DDBJ database (accession no. DRR160954).
2.4. Gas and Organic Acid Concentrations
The total gas amount and the concentration of organic acids were measured as previously described [31]. The produced gas after the cultivation was recovered by downward displacement of water by a syringe (Terumo, Tokyo, Japan) and measured by gas chromatography (Shimadzu, Kyoto, Japan). The concentration of organic acids was measured by high-performance liquid chromatography with a UV detector (Shimadzu, Kyoto, Japan). The data represent at least three independent experiments.
3. Results
3.1. Cultivation of C. cellulovorans with Pig Manure
In order to promote the utilization of pig manure (PM) as an unused biomass, the cultivation of C. cellulovorans was carried out. PM was pretreated with 0.45 μm filter to remove the inhibitor for bacterial cell growth and 0.5% (w/v) pretreated PM was used as the sole carbon source in the C. cellulovorans medium. C. cellulovorans was inoculated into the PM medium and then organic acids were measured by HPLC. The results suggested that C. cellulovorans was able to grow in the 0.5% PM medium and that acetate and butyrate were increased, while formate and lactate decreased after increasing once at 1-day post-cultivation (Figure 1). Total concentrations of acetate and butyrate at 14 days were approximately 2300 mg/L and 820 mg/L, respectively, showing that PM would be an excellent biomass for methanogenesis.
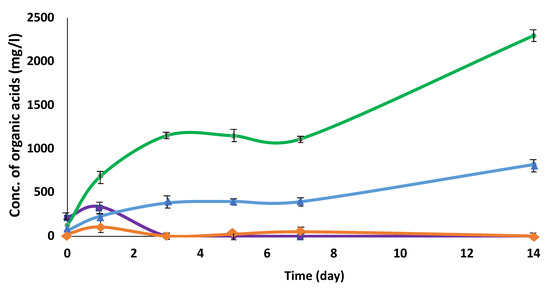
Figure 1.
Measurement of organic acids from 0.5% pig manure (PM) cultivated by C. cellulovorans. Symbols: rhombus, formate; square, lactate, circle, acetate; triangle, butyrate. The data represent at least three independent experiments.
3.2. Cocultivation of C. cellulovorans with Methanogens or M. mazei
CH4 production by coculturing C. cellulovorans–methanogens (MFMP) was examined with 0.5% (w/v) glucose, 0.5% (w/v) cellobiose, and 0.5% (v/v) acetate, respectively, while the cocultivation of C. cellulovrorans–M. mazei was conducted with 0.5% cellobiose as the sole substrate. As shown in Figure 2A, the cell growth in each coculture was observed and showed different patterns. On the other hand, the cocultivation of C. cellulovorans-MFMP showed CH4 production only with 0.5% acetate, whereas the cocultivation of C. cellulovorans-M. mazei with the 0.5% cellobiose medium led to no methanogenesis during the cultivation period, showing that M. mazei could never use cellobiose for its growth (Figure 2B). These results suggested that methanogenesis promotes not sugars such as glucose or cellobiose but acetate as the carbon source.
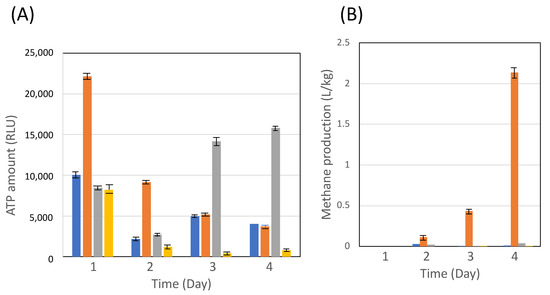
Figure 2.
Measurement of ATP amount (RLU) (A) and methane production (B) with cocultivation of C. cellulo vorans and MFMP or M. mazei. Bars: blue, 0.5% cellobiose cultivated with C. cellulovorans and MFMP; orange, 0.5% acetate cultivated with C. cellulovorans and MFMP; gray, 0.5% glucose cultivated with C. cellulovorans and MFMP; yellow, 0.5% cellobiose cultivated with C. cellulovorans and M. mazei. The data represent at least three independent experiments.
3.3. Effect of Carbon Sources with Methanogens
In order to produce CH4 efficiently, MFMP was examined with the culture media of 1.0% (v/v) acetate and 1.0% (v/v) methanol, respectively (Figure 3). The cell growth in the medium of 1.0% acetic acid was at peak at 1 day, while that in the medium of 1.0% methanol was at peak at 16 days (Figure 3A). On the other hand, CH4 production on the methanol medium was increased from 8 days, and then the maximum production of methane was at peak at 16 days (Figure 3B). In case of the acetic acid medium, CH4 production was lower than that of the methane medium, resulting in the difference in the metabolic pathway of methanogenesis in MFMP. These results indicated that methanogenesis easily occurs for not acetate but methanol, and the production of methane by 1.0% methanol was 8 times higher than that by 1.0% acetate.
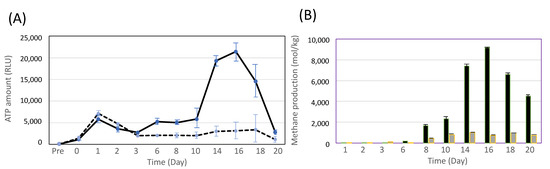
Figure 3.
Measurement of ATP amount (RLU) (A) and methane production (B) in MFMP cultivation. (A) black line, 1% methanol; wavey line, 1% acetic acid. (B) black bar, 1% methanol; gray bar, 1% acetic acid. The data represent at least three independent experiments.
3.4. Identification of Methanogens Cultivated with the Different Carbon Sources
MFMP was precultivated with 0.5% glucose medium for 12 h at 37 °C and then 1000 RLU of MFMP cells was inoculated into the C. cellulovorans medium containing 1% acetate or 1% methanol at 37 °C for 72 h. After DNA extraction from the growth cells of each medium, 16S rRNA analyses were carried out by a next-generation sequencer. As shown in Figure 4, Methanofollis was the majority of the archaea and was 0.211% in 1% acetate medium for 72 h cultivation. On the other hand, Methanofollis in 1% methanol medium was found, i.e., 0.007% for 24 h cultivation and 0.490% for 72 h cultivation, respectively. On the other hand, Methanosarcina barkeri was a typical methanogen and was 0.011% for 24 h cultivation and 0.015% for 72 h cultivation, respectively, in 1% acetate medium. Interestingly, for 72 h cultivation, 0.004% of M. mazei was found in 1% methanol medium, while 0.571% of M. barkeri was detected in the same medium. These results indicated that the growth of methanogens was dependent on the carbon sources, and the growth trends of individual methanogens seemed remarkably different under the sole carbon sources.
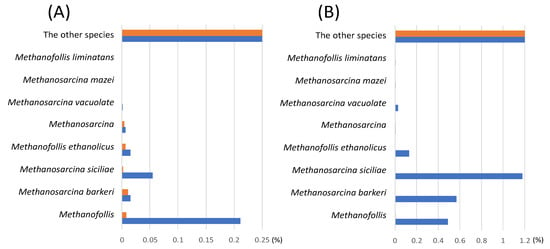
Figure 4.
Relative abundance ratio of archaea in MFMP after cultivated with the different carbon sources. After 16S rRNA analysis, the identified archaea were compared. (A) 1% acetate cultivation; (B) 1% methanol cultivation. Bars indicate orange (after 24 h cultivation) and blue (after 72 h cultivation), respectively. The others show the rest of total percentage.
4. Discussion
With simultaneous renewable energy production, anaerobic digestion (AD) has been widely applied for organic solid waste or wastewater treatment. AD consists of a series of biochemical processes (i.e., hydrolysis, fermentation (acidogenesis), acetogenesis and methanogenesis) performed by various interacting microorganisms, containing bacteria (i.e., acidogens, acetogens) and archaea (methanogens). Through these processes, polymers such as carbohydrates, lipids and proteins are ultimately converted into CH4 and CO2. In some cases, hydrolysis as the first step is considered as the rate-limiting step by affecting the overall process kinetics, especially when treating recalcitrant fibrous materials. In other cases, especially when the feedstock consists of easily biodegradable organic matter, methanogenesis is considered as the rate-limiting pathway. About 25.31 million tons of food wastes from consumer households, food manufacturing and retail in Japan was generated in 2018 [36]. In addition to maximizing social and economic benefits, such a management process for appropriate food waste should be implemented to minimize the environmental impacts. Although recycling food waste is preferred to composting and converting to animal feed in Japan, the composting of food waste is still in high demand from farmers, due to the relatively low price and a shortage of cropland for application [37,38,39]. Therefore, since the most successful application at the commercial scale has so far been AD, which has been widely adopted for waste treatment, a plentiful source of organic compounds such as pig manure (PM) can be used as feedstock in AD. Namely, since the fermented liquid feed (FLF) for pigs contains several nutrients required for bacterial growth, recycling food waste has been considered a possible alternative for many years. Additionally, due to the accumulation of volatile fatty acids (VFAs), PM with a high buffering capacity possibly protects AD against failures [40,41,42]. The food-waste mixing ratio for methane yield reported that the feedstock composition of 60:40 (volatile solid basis) significantly enhanced methane yield [43]. On the other hand, another group reported that the co-substrate using vegetable processing wastes could improve methane yield up to three-fold with a feedstock ratio of 50:50 (dry weight basis) [44]. Thus, since several potential co-substrates have been examined to assess the effect of varying feedstock compositions on improving AD process performance and increasing methane yield, the VFAs of the C. cellulovorans medium containing PM were measured in this study. As a result, acetic acid (approx. 2300 mg/mL) and butyric acid (approx. 820 mg/mL) were accumulated for 14 days, respectively (Figure 1). In this study, since the high concentration of ammonia might inhibit bacterial activity in AD [45,46,47,48,49], in order to enhance methane production, the filtration (0.45 μm filter) was used for the pretreatment of PM before the inoculation of C. cellulovorans. By adjusting the ratio of carbon-to-nitrogen (C/N), the co-digestion of PM with organic wastes including high carbon dilute seemed to enhance the macro- and micro-nutrient balance in the feedstocks and improve the inhibitory effect of ammonia [50,51]. On the other hand, cow manure (CM) is rich in nutrients and can provide strong buffer capacity, and thus CM seems more robust than other manures in AD [52]. Therefore, the alleviation of ammonia inhibition when CM is used in AD seems not that urgent and should not be the priority of co-digestion. Additionally, CM is categorized as lignocellulosic waste due to its high amount of lignocellulose (50% in dry matter), which is relatively low in other types of manure [53]. Hence, to make full use of CM to produce more methane via co-digestion, attention should be paid to how to improve the degradation of recalcitrant lignocellulose in CM. In addition, the current study determined biogas production in single-stage and two-stage AD using sheep manure (SP) as substrate and yak rumen fluid as the inoculum. Yak rumen fluid is rich in hydrolytic bacteria [54] and, consequently, its inclusion should improve the degradation of lignocellosic biomass, leading to high biogas production.
All archaeal metagenome-assembled genomes (MAGs) could reveal the reconstruction of pathways related to methanogenesis and relevant energy conservation systems [55]. Methane production from H2/CO2 or acetate as sole substrates has also been widely reported. On the contrary, only scarce knowledge on formate as a methanogenic substrate is available despite its reported important role in interspecies electron transfer. Furthermore, it could be evaluated by the average RPKM of genes in each KEGG module among the holistic microbial community activity [56]. However, all methanogenic environments in natural conditions are mixed cultures, such as anaerobic granules, municipal waste digesters, soil and anaerobic aquatic systems. Additionally, almost all of the kinetic studies have not been combined with an analysis of the microbial composition, which has a direct impact on CH4 production kinetics parameters. Thus, by maintaining the methanogenic activity of the microbial community, such a syntrophic behavior is required to synthesize numerous metabolites. An overall shift of the microbial activity was observed in the majority of the KEGG modules after H2 addition. Moreover, H2 also enhanced the activity of the glyoxylate cycle and the biosynthesis of lipids and specific amino acids. In addition to H2, formate, as a similarly formed product during fermentative metabolism, is an important electron carrier in the syntrophic fatty acid-degrading methanogenic consortia [13]. In fact, formate was in low concentration and immediately consumed in the PM medium (Figure 1). Therefore, other anaerobes might utilize both formate and H2 as an electron donor for methanogenesis or sulfate respiration.
Clostridium coculture systems are typically used to produce biofuels such as H2 and CH4, solvents, and organic acids [57]. Because cellulosic materials are commonly found in nature [18], the specific metabolic capacities of cellulolytic strains and producers in coculture systems have attracted significant attention and offered many long-term prospects for development. Furthermore, since the combination of genome-centric metagenomics and metatranscriptomics has successfully revealed individual functional roles of microbial members in methanogenic microcosms, these results assigned a multi-trophic role to Methanosarcina ssp., suggesting its ability to perform simultaneous methanogenesis from acetate, CO2 and methanol/methylamine [55]. The MFMP used in this study originally consisted of C. butyricum (0.005%), identified as the same genus of C. celulovorans and M. mazei (1.34%) found among methanogens [32]. Furthermore, other methanogens such as Methanosaetaceae, Methanosaeta, and Methanospirillaceae were also identified in MFMP. The genus Methanosaeta, which utilizes only acetate, was a large portion of the ratio, next to Methanosarcina. On the other hand, 1% acetate or 1% methanol was used as the sole carbon source for MFMP cultivation in this study. As a result, while Methanosarcina siciliae (1.178%), M. barkeri (0.571%), and Methanofollis (0.490%) were major species in the 1% methanol medium for 72 h cultivation, Methanofollis (0.211%) was dominant in the 1% acetate medium for 72 h cultivation (Figure 4). It is thought that all methanogens are physiologically specialized and able to scavenge the electrons from H2, formate, acetate, and methanol, having CH4 as the final product [49]. The Clostridium coculture system can also produce CH4 in addition to producing H2 and solvents, in particular the coculture of cellulolytic Clostridia and methanogens, including M. barkeri Fusaro, M. mazei, and Methanothermobacter thermautotrophicus. The methanogens utilized H2 and CO2, acetate, and even formate that was generated by the cellulolytic Clostridia from cellulose to produce CH4 [33,58]. In this study, CH4 production by cellobiose was not found in the cocultivation of C. cellulovorans-M. mazei (C.c:M.m = 1:3), while only acetate led to methanogenesis in the cocultivation of C. cellulovorans-MFMP (Figure 2). In addition, since M. barkeri was more dominant than M. mazei in MFMP cultivation according to the 16S rRNA analysis (Figure 4), it seemed that Methanosarcina spp. may play a key role on the methanogenesis of MFMP. So far, it has been reported that CH4 production was investigated with sugar beet pulp [16] and mandarin orange peel [17] in the cocultivation of C. cellulovorans-MFMP (C.c:MFMP = 1:20). Therefore, carbon sources such as acetic acid and methanol were compared by the production of CH4 in this study. As expected, CH4 production from methanol was approximately eight times higher than that from acetic acid, related to the cell growth of MFMP (Figure 3). Thus, methanogens seemed to be altered in their flora, dependent on the sole carbon source.
5. Conclusions
In this study, C. cellulovorans was cultivated with PM and the cocultivation of C. cellulovoroans-M. mazei or C. cellulovorans-MFMP was performed with different carbon sources. Since the cultivation of C. cellulovorans with PM had much acetic acid, it was thought to be one an excellent biomass for methane production. On the other hand, methanol was the best carbon source for CH4 production with MFMP. Regarding the next-generation sequence analysis of MFMP for 72 h cultivation, while Methanofollis (0.211%) was dominant in the 1% acetic acid medium, Methanosarcina siciliae (1.178%), M. barkeri (0.571%), and Methanofollis (0.490%) were major species in 1% methanol medium. Therefore, it seemed that Methanosarcina spp. may play a key role in the methanogenesis of MFMP.
Author Contributions
Conceptualization, Y.T.; methodology, H.T.; writing—original draft, H.S. and F.O.; writing—review and editing, Y.T.; supervision, Y.T.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was a part supported by Chubu Electric Power Co., Inc., Mitsui Chemicals, Inc., and Yanmar Holdings Co., Ltd. The Company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Hiroki Matsui and Tomomi Ban at Mie University for providing PM. The authors also thank Mayu Hayashi and Midori Kosaka at Tamaru’s laboratory for technical support of experimental studies.
Conflicts of Interest
The authors have no conflict of interest relevant to the content of this article.
References
- Pan, X.; Angelidaki, I.; Alvarado-Morales, M.; Liu, H.; Liu, Y.; Huang, X.; Zh, G. Methane production from formate, acetate and H2/CO2; focusing on kinetics and microbial characterization. Bioresour. Technol. 2016, 218, 796–806. [Google Scholar] [CrossRef]
- Christensen, T.R.; Johansson, T.; Åkerman, H.J.; Mastepanov, M.; Malmer, N.; Friborg, T.; Crill, P.; Svenssonet, B.H. Thawing sub-arctic permafrost: Effect sonvegetation and methane emissions. Geophys. Res. Lett. 2004, 31, L04501. [Google Scholar] [CrossRef]
- Woodcroft, B.J.; Singleton, C.M.; Boyd, J.A.; Evans, P.N.; Emerson, J.B.; Zayed, A.F.Z.; Hoelzle, R.D.; Lamberton, T.O.; Mccalley, C.K.; Hodgkins, S.B.; et al. Genome-centric view of carbon processing in thawing permafrost. Nature 2018, 560, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. 2008, 23, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Kaster, A.K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Garcia, J.L.; Patel, B.K.C.; Ollivier, B. Taxonomic, phylogenetic, and ecological diversity of methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef]
- Deppenmeier, U.; Müller, V.; Gottschalk, G. Pathways of energy conservation in methanogenic archaea. Arch. Microbiol. 1996, 165, 149–163. [Google Scholar] [CrossRef]
- Kruse, S.; Goris, T.; Westermann, M.; Adrian, L.; Diekert, G. Hydrogen production by Sulfurospirillum species enables syntrophic interactions of Epsilonproteobacteria. Nat. Commun. 2018, 9, 4872. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Segre, J.A. Signaling in host-associated microbial com- munities. Cell 2016, 164, 1288–1300. [Google Scholar] [CrossRef]
- Kenny, D.J.; Balskus, E.P. Engineering chemical interactions in microbial communities. Chem. Soc. Rev. 2018, 47, 1705–1729. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Ding, M.Z.; Song, H.; Wang, E.X.; Liu, Y.; Yuan, Y.J. Design and construction of synthetic microbial consortia in China. Synth. Syst. Biotechnol. 2016, 1, 230–235. [Google Scholar] [CrossRef]
- De Bok, F.A.; Plugge, C.M.; Stams, A.J. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and applications of Clostridium co-culture systems in biotechnology. Front. Microbiol. 2020, 11, 560223. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Hu, W.; Li, Y. Biodegradation mechanisms and kinetics of azo dye 4BS by a microbial consortium. Chemosphere 2004, 57, 293–301. [Google Scholar] [CrossRef]
- Khouni, I.; Marrot, B.; Amar, R.B. Treatment of reconstituted textile waste-water containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep. Purif. Technol. 2012, 87, 110–119. [Google Scholar] [CrossRef]
- Safonova, E.; Kvitko, K.V.; Iankevitch, M.I.; Surgko, L.F.; Afti, I.A.; Reisser, W. Biotreatment of industrial wastewater by selected algal-bacterial consortia. Eng. Life Sci. 2004, 4, 347–353. [Google Scholar] [CrossRef]
- Xu, X.H.; Liu, X.M.; Zhang, L.; Mu, Y.; Zhu, X.Y.; Fang, J.Y.; Li, S.P.; Jiang, J.D. Bioaugmentation of chlorothalonil-contaminated soil with hydrolytically or reductively dehalogenating strain and its effect on soil microbial community. J. Hazard. Mater. 2018, 351, 240–249. [Google Scholar] [CrossRef]
- Sabra, W.; Dietz, D.; Tjahjasari, D.; Zeng, A.P. Biosystems analysis and engineering of microbial consortia for industrial biotechnology. Eng. Life Sci. 2010, 10, 407–421. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Eiteman, M.A.; Lee, S.A.; Altman, R.; Altman, E. A substrate-selective co-fermentation strategy with Escherichia coli produces lactate by simultaneously consuming xylose and glucose. Biotechnol. Bioeng. 2009, 102, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.X.; Ding, M.Z.; Ma, Q.; Dong, X.T.; Yuan, Y.J. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb. Cell Fact. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidtheck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Xin, F.X.; He, J.Z. Characterization of a thermostable xylanase from a newly isolated Kluyvera species and its application for biobutanol production. Bioresour. Technol. 2013, 135, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Goyal, G.; Chen, W. Surface display of a functional minicelluloome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl. Environ. Microbiol. 2010, 76, 7514–7520. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. ISME J. 2016, 11, 327–336. [Google Scholar] [CrossRef]
- Charubin, K.; Bennett, R.K.; Fast, A.G.; Papoutsakis, E.T. Engineering Clostridium organisms as microbial cell-factories: Challenges & opportunities. Metab. Eng. 2018, 50, 173–191. [Google Scholar] [PubMed]
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Nakanishi, A.; Kawade, Y.; Yamamoto, K.; Uemura, M.; Fujita, Y.; Doi, R.H.; Ueda, M. Genome sequence of the cellulosome-producing mesophilic organism Clostridium cellulovorans 743B. J. Bacteriol. 2010, 192, 901–902. [Google Scholar] [CrossRef]
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Nakanishi, A.; Matsushima, C.; Doi, R.H.; Ueda, M. Comparison of the mesophilic cellulosome- producing Clostridium cellulovorans genome with other cellulosome-related clostridial genomes. Micro. Biotechnol. 2011, 4, 64–73. [Google Scholar] [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Direct IBE fermentation from mandarin orange wastes by combination of Clostridium cellulovorans and Clostridium beijerinckii. AMB Express 2019, 9, 1. [Google Scholar] [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Biomethane production from sugar beet pulp under cocultivation with Clostridium cellulovorans and methanogens. AMB Express 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tamaru, Y. The second-generation biomethane from mandarin orange peel under cocultivation with methanogens and the armed Clostridium cellulovorans. Fermentation 2019, 5, 95. [Google Scholar] [CrossRef]
- Lu, H.; Ng, S.-K.; Jia, Y.; Cai, M.; Lee, P.K.H. Physiological and molecular characterizations of the interactions in two cellulose-to-methane cocultures. Biotechnol. Biofuels 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Kim, B.S. Green hydrogen production through consolidated bioprocessing of lignocellulosic biomass using nanobiotechnology approach. Biores. Technol. 2022, 365, 128108. [Google Scholar] [CrossRef] [PubMed]
- Goevert, D.; Conrad, R. Effect of substrate concentration on carbon isotope fractionation during acetoclastic methanogenesis by Methanosarcina barkeri and M. acetivorans and in rice field soil. Appl. Environ. Microbiol. 2009, 75, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Ministry of the Environment. MOE Discloses the Estimated Amount of Japan’s Food Loss and Waste Generated in FY 2018. 2021. Available online: https://www.env.go.jp/en/headline/2515.html (accessed on 10 November 2022).
- Watanabe, E.; Seike, N.; Motoki, Y.; Inao, K.; Otani, T. Potential application of immunoassays for simple, rapid and quantitative detections of phytoavailable neonicotinoid insecticides in cropland soils. Ecotoxicol. Environ. Saf. 2016, 132, 288–294. [Google Scholar] [CrossRef]
- Cheung, H.N.B.; Huang, G.H.; Yu, H. Microbial-growth inhibition during composting of food waste: Effect of organic acids. Bioresour. Technol. 2010, 101, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Hirai, H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef]
- Wang, H.; Lim, T.T.; Duong, C.; Zhang, W.; Xu, C.; Yan, L.; Mei, Z.; Wang, W. Long-Term Mesophilic Anaerobic Co-Digestion of Swine Manure with Corn Stover and Microbial Community Analysis. Microorganisms 2020, 8, 188. [Google Scholar] [CrossRef]
- Córdoba, V.; Fernández, M.; Santalla, E. The effect of different inoculums on anaerobic digestion of swine wastewater. J. Environ. Chem. Eng. 2016, 4, 115–122. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Fernández, C.; Gómez, X.; Moran, A. Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol. Bioprocess Eng. 2011, 16, 1044–1052. [Google Scholar] [CrossRef]
- Dennehy, C.; Lawlor, P.G.; McCabe, M.S.; Cormican, P.; Sheahan, J.; Jiang, Y.; Zhan, X.; Gardiner, G.E. Anaerobic co-digestion of pig manure and food waste: Effects on digestate dewaterability, and microbial community dynamics. Waste Manag. 2018, 71, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; González-Fernández, C.; Gómez, X.; García-González, M.C.; Morán, A. Vegetable processing wastes addition to improve swine manure anaerobic digestion: Evaluation in terms of methane yield and SEM characterization. Appl. Energy 2012, 91, 36–42. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Macé, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 2006, 53, 7–22. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Biogas production from food waste via co-digestion and digestion- effects on performance and microbial ecology. Sci. Rep. 2017, 7, 17664. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the treatment of cattle manure. A review. C J. Carbon Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different live-stock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Rabee, A.E.; Sayed Alahl, A.A.; Lamara, M.; Ishaq, S.L. Fibrolytic rumen bacteria of camel and sheep and their applications in the bioconversion of barley straw to soluble sugars for biofuel production. PLoS ONE 2022, 17, e0262304. [Google Scholar] [CrossRef]
- Zhu, X.; Campanaro, S.; Treu, L.; Seshadri, R.; Ivanova, N.; Kougias, P.G.; Kyrpides, N.; Angelidaki, I. Metabolic dependencies govern microbial syntrophies during methanogenesis in an anaerobic digestion ecosystem. Microbiome 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, R.; Zhou, J.; He, A.; Xu, J.; Xin, F.; Zhang, W.; Ma, J.; Jiang, M.; Dong, W. Recent advances of biofuels and biochemicals production from sustainable resources using co-cultivation systems. Biotechnol. Biofuels 2019, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, D.; Morita, M.; Sasaki, K.; Watanabe, A.; Ohmura, N. Acceleration of cellulose degradation and shift of product via methanogenic co-culture of a cellulolytic bacterium with a hydrogenotrophic methanogen. J. Biosci. Bioeng. 2012, 114, 435–439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).