A Brief Snapshot of Aspergillus Section Nigri Isolated from Brazilian Peanuts and Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Peanut Samples
2.2. Water Activity of Peanut Samples
2.3. Fungal Isolation and Counting
2.4. Morphological Characterization
2.5. Molecular Identification
2.6. Potential for OTA Production by Aspergillus Section Nigri Isolates
2.7. OTA Analysis in Peanut Samples
3. Results and Discussion
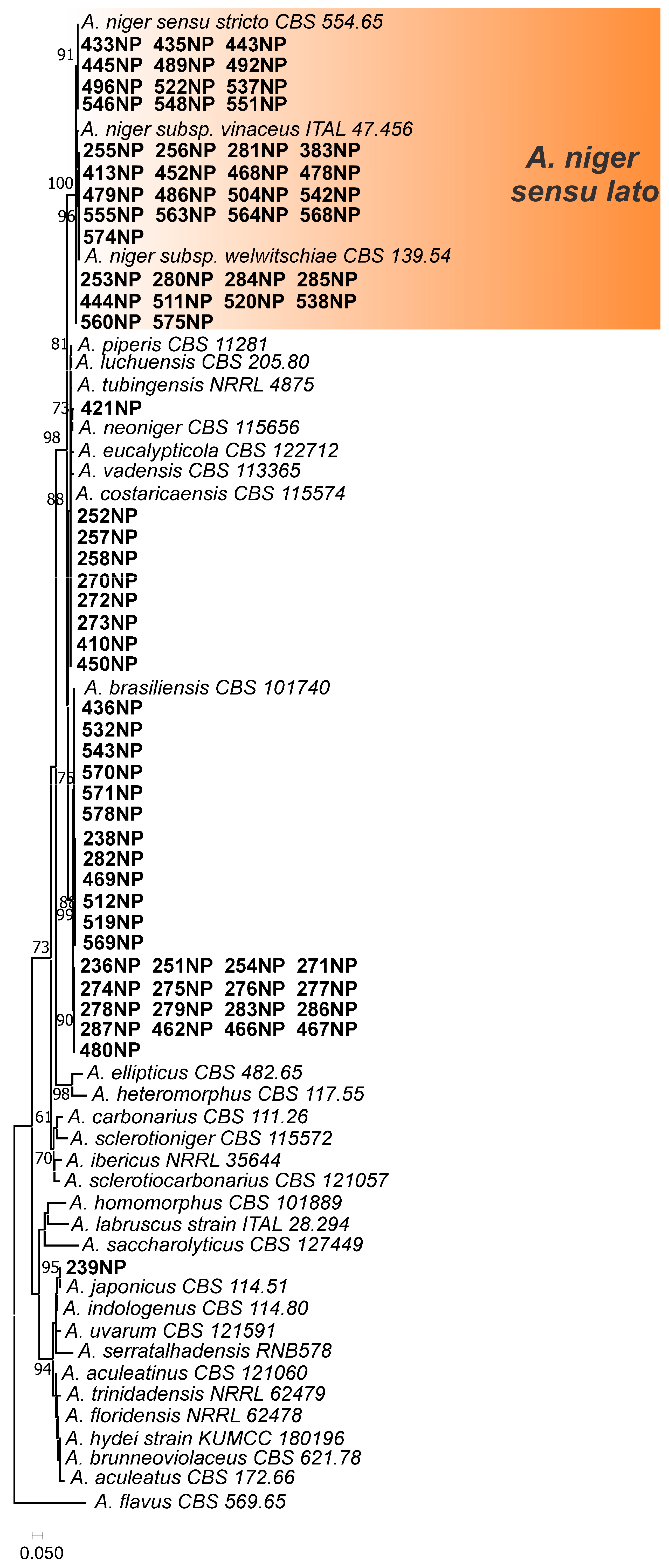
3.1. Aspergillus Section Nigri in Peanut Samples
3.2. Ocratoxigenic Potential of Aspergillus Section Nigri Isolates
3.3. Occurrence of Ochratoxin A in Peanuts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. United States Department of Agriculture—Foreign Agricultural Service. Production, Supply and Distribution. Available online: http://apps.fas.usda.gov/psdonline/psdQuery.aspx (accessed on 10 March 2023).
- Syed, F.; Arif, S.; Ahmed, I.; Khalid, N. Groundnut (peanut) (Arachis Hypogaea). In Oilseeds: Health Attributes and Food Applications, 1st ed.; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 93–122. [Google Scholar] [CrossRef]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Zorzete, P.; Baquião, A.C.; Atayde, D.D.; Reis, T.A.; Gonçalez, E.; Corrêa, B. Mycobiota, aflatoxins and cyclopiazonic acid in stored peanut cultivars. Food Res. Int. 2013, 52, 380–386. [Google Scholar] [CrossRef]
- Martins, L.M.; Sant’Ana, A.S.; Fungaro, M.H.P.; Silva, J.J.; Nascimento, M.d.S.d.; Frisvad, J.C.; Taniwaki, M.H. The biodiversity of Aspergillus section Flavi and aflatoxins in the Brazilian peanut production chain. Food Res. Int. 2017, 94, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 4th ed.; Springer: New York, NY, USA, 2022; pp. 1–645. [Google Scholar] [CrossRef]
- Magnoli, C.; Hallak, C.; Astoreca, A.; Ponsone, L.; Chiacchiera, S.; Dalcero, A.M. Occurrence of ochratoxin A-producing fungi in commercial corn kernels in Argentina. Mycopathologia 2006, 161, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.J.; Bertoldo, R.; Fungaro, M.H.P.; Massi, F.P.; Taniwaki, M.H.; Sant’Ana, A.S.; Iamanaka, B.T. Black aspergilli in Brazilian onions: From field to market. Int. J. Food. Microbiol. 2021, 337, 108958. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Hocking, A.D.; Pitt, J.I.; Kazi, B.A.; Emmett, R.W.; Scott, E.S. Australian research on ochratoxigenic fungi and ochratoxin A. Int. J. Food Microbiol. 2006, 111, S10–S17. [Google Scholar] [CrossRef]
- Ferranti, L.S.; Fungaro, M.H.P.; Massi, F.P.; Silva, J.J.; Penha, R.E.S.; Frisvad, J.C.; Taniwaki, M.H.; Iamanaka, B.T. Diversity of Aspergillus section Nigri on the surface of Vitis labrusca and its hybrid grapes. Int. J. Food Microbiol. 2018, 268, 53–60. [Google Scholar] [CrossRef]
- Sultan, Y.; Magan, N. Mycotoxigenic fungi in peanuts from different geographic regions of Egypt. Mycotox. Res. 2010, 26, 133–140. [Google Scholar] [CrossRef]
- Magnoli, C.; Astoreca, A.; Ponsone, M.L.; Fernández-Juri, M.G.; Barberis, C.; Dalcero, A.M. Ochratoxin A and Aspergillus section Nigri in peanut seeds at different months of storage in Córdoba, Argentina. Int. J. Food Microbiol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Larsen, T.O.; Thrane, U.; Meijer, M.; Varga, J.; Samson, R.A.; Nielsen, K.F. Fumonisin and ochratoxin production in industrial Aspergillus Niger strains. PLoS ONE 2011, 6, e23496. [Google Scholar] [CrossRef]
- FDA. Food and Drug Administration. Food Ingredient and Packaging Inventories. 2001. Available online: https://www.fda.gov/food/food-ingredients-packaging/food-ingredient-and-packaging-inventories (accessed on 10 March 2023).
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Stea, G.; Epifani, F.; Varga, J.; Frisvad, J.C.; Samson, R.A. Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol. 2011, 115, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the evaluation of carcinogenic risks to humans: Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. Int. Agency Res. Cancer 1993, 56, 489–521. [Google Scholar]
- Gutleb, A.C.; Morrison, E.; Murk, A.J. Cytotoxicity assays for mycotoxins produced by Fusarium strains: A review. Environ. Toxicol. Pharmacol. 2002, 11, 309–320. [Google Scholar] [CrossRef]
- Silva, J.J.; Iamanaka, B.T.; Ferranti, L.S.; Massi, F.P.; Taniwaki, M.H.; Puel, O.; Lorber, S.; Frisvad, J.C.; Fungaro, M.H.P. Diversity within Aspergillus niger clade and description of a new species: Aspergillus vinaceus sp. nov. J. Fungi 2020, 6, 371. [Google Scholar] [CrossRef]
- Tavaré, S.; Miura, R.M. Some probabilistic and statistical problems in the analysis of DNA sequences, lectures on mathematics in the life sciences. Am. Math. Soc. 1986, 17, 57–86. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Filtenborg, O.; Frisvad, J.C.; Svendsen, J.A. Simple screening method for molds producing intracellular mycotoxins in pure cultures. Appl. Environ. Microbiol. 1983, 45, 581. [Google Scholar] [CrossRef]
- EURACHEM. The Fitness for Purpose of Analytical Methods a Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Magnusson, B., Örnemark, U., Eds.; Eurachem Guides: Teddington, UK, 2014; ISBN 9789187461590. [Google Scholar]
- Varga, J.; Frisvad, J.C.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Szigeti, G.; Samson, R.A. New and revisited species in Aspergillus section Nigri. Stud. Mycol. 2011, 69, 1–17. [Google Scholar] [CrossRef]
- Hong, S.B.; Lee, M.; Kim, D.-H.; Varga, J.; Frisvad, J.C.; Perrone, G.; Gomi, K.; Yamada, O.; Machida, M.; Houbraken, J.; et al. Aspergillus luchuensis, an industrially important black Aspergillus in East Asia. PLoS ONE 2013, 8, e63769. [Google Scholar] [CrossRef] [PubMed]
- Chein, S.H.; Sadiq, M.B.; Datta, A.; Anal, A.K. Prevalence and identification of Aspergillus and Penicillium species isolated from peanut kernels in Central Myanmar. J. Food Saf. 2019, 39, e12686. [Google Scholar] [CrossRef]
- Palencia, E.R.; Hinton, D.M.; Bacon, C.W. The black Aspergillus species of maize and peanuts and their potential for mycotoxin production. Toxins 2010, 2, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Kocsubé, S.; Tóth, B.; Frisvad, J.C.; Perrone, G.; Susca, A.; Meijer, M.; Samson, R.A. Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution. Int. J. Syst. Evol. Microbiol. 2007, 57, 1925–1932. [Google Scholar] [CrossRef]
- Manikandan, P.; Varga, J.; Kocsubé, S.; Revathi, R.; Anita, R.; Dóczi, I.; Németh, T.M.; Narendran, V.; Vágvölgyi, C.; Bhaskar, M.; et al. Keratitis caused by the recently described new species Aspergillus brasiliensis: Two case reports. J. Med. Case Rep. 2010, 4, 68. [Google Scholar] [CrossRef]
- Garmendia, G.; Vero, S. Occurrence and biodiversity of Aspergillus section Nigri on ‘Tannat’ grapes in Uruguay. Int. J. Food Microbiol. 2016, 216, 31–39. [Google Scholar] [CrossRef]
- Gil-Serna, J.; García-Díaz, M.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Significance of Aspergillus niger aggregate species as contaminants of food products in Spain regarding their occurrence and their ability to produce mycotoxins. Food Microbiol. 2019, 82, 240–248. [Google Scholar] [CrossRef]
- Hassanin, N.I. Detection of mycotoxigenic fungi and bacteria in processed cheese in Egypt. Int. Biodeterior. Biodegrad. 1993, 31, 15–23. [Google Scholar] [CrossRef]
- Tawakkol, W.; Khafaga, N.I. Fungal contamination of meat and its environment with special reference to the strains producing aflatoxins, ochratoxins, proteinase and lipase enzymes. New Egypt. J. Microbiol. 2007, 17, 1–14. [Google Scholar] [CrossRef]
- Logrieco, A.F.; Haidukowski, M.; Susca, A.; Mulè, G.; Munkvold, G.P.; Moretti, A. Aspergillus section Nigri as contributor of fumonisin b(2) contamination in maize. Food Addit. Contam. Part A 2014, 31, 149–155. [Google Scholar] [CrossRef]
- Massi, F.P.; Sartori, D.; Ferranti, L.S.; Iamanaka, B.T.; Taniwaki, M.H.; Vieira, M.L.C.; Fungaro, M.H.P. Prospecting for the incidence of genes involved in ochratoxin and fumonisin biosynthesis in Brazilian strains of Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2016, 221, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Shulhani, R.; Rot, Y.; Zemach, H.; Belausov, E.; Grinberg-Baran, M.; Borenstein, M.; Pivonia, S.; Ezra, D.; Shtienberg, D. Aspergillus niger, the causal agent of black mould disease in date fruits, infects and colonizes flowers and young fruitlets. Plant Pathol. 2021, 70, 1195–1208. [Google Scholar] [CrossRef]
- Pantelides, I.S.; Aristeidou, E.; Lazari, M.; Tsolakidou, M.D.; Tsaltas, D.; Christofidou, M.; Kafouris, D.; Christou, E.; Ioannou, N. Biodiversity and ochratoxin A profile of Aspergillus Section Nigri populations isolated from wine grapes in Cyprus vineyards. Food Microbiol. 2017, 67, 106–115. [Google Scholar] [CrossRef]
- Silva, J.J.; Puel, O.; Lorber, S.; Ferranti, L.S.; Ortiz, L.F.; Taniwaki, M.H.; Iamanaka, B.T.; Fungaro, M.H.P. Occurrence and diversity of Aspergillus in commercial yerba mate elaborated for the Brazilian beverage ‘Chimarrão’. Food Res. Inter. 2019, 121, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Gits-Muselli, M.; Hamane, S.; Verillaud, B.; Cherpin, E.; Denis, B.; Bondeelle, L.; Touratier, S.; Alanio, A.; Garcia-Hermoso, D.; Bretagne, S. Different repartition of the cryptic species of Black aspergilli according to the anatomical sites in human infections, in a French University Hospital. Med. Mycol. 2021, 59, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Frisvad, J.C. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 2004, 50, 45–61. [Google Scholar]
- Perrone, G.; Gallo, A. Aspergillus species and their associated mycotoxins. Methods Mol. Biol. 2017, 1542, 33–49. [Google Scholar] [CrossRef]
- European Community Commission Regulation (EC). No 401/2006 Laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Uni. 2006, 70, 12–34. [Google Scholar]
- Battilani, P.; Pietri, A. Ochratoxin A in Grapes and Wine. Eur. J. Plant Pathol. 2002, 108, 639–643. [Google Scholar] [CrossRef]
- Abarca, M.L.; Accensi, F.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. J. Food Prot. 2003, 66, 504–506. [Google Scholar] [CrossRef]
- Joosten, H.M.L.J.; Goetz, J.; Pittet, A.; Schellenberg, M.; Bucheli, P. Production of ochratoxin a by Aspergillus carbonarius on coffee cherries. Int. J. Food Microbiol. 2001, 65, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Copetti, M.V.; Pereira, J.L.; Iamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Ochratoxigenic fungi and ochratoxin A in cocoa during farm processing. Int. J. Food Microbiol. 2010, 143, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Essawet, N.; Abushahma, H.; Inbaia, S.; Najii, A.; Amra, H.A. Natural incidence of aflatoxins and ochratoxin A nuts collected from local market in Tripoli. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1479–1486. [Google Scholar] [CrossRef]
| Determinations | Field (42 Samples) | Storage (10 Samples) |
|---|---|---|
| Range of water activity | 0.521–0.724 | 0.541–0.609 |
| Number of positive samples (%) | 27 (64%) | 10 (100%) |
| Range of Aspergillus section Nigri infection (%) | 0–42 | 6–34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, F.; Silva, J.J.; Iamanaka, B.T.; Martins, L.M.; Taniwaki, M.H. A Brief Snapshot of Aspergillus Section Nigri Isolated from Brazilian Peanuts and Soil. Appl. Microbiol. 2023, 3, 476-484. https://doi.org/10.3390/applmicrobiol3020033
Rodrigues F, Silva JJ, Iamanaka BT, Martins LM, Taniwaki MH. A Brief Snapshot of Aspergillus Section Nigri Isolated from Brazilian Peanuts and Soil. Applied Microbiology. 2023; 3(2):476-484. https://doi.org/10.3390/applmicrobiol3020033
Chicago/Turabian StyleRodrigues, Fernanda, Josué J. Silva, Beatriz T. Iamanaka, Ligia M. Martins, and Marta H. Taniwaki. 2023. "A Brief Snapshot of Aspergillus Section Nigri Isolated from Brazilian Peanuts and Soil" Applied Microbiology 3, no. 2: 476-484. https://doi.org/10.3390/applmicrobiol3020033
APA StyleRodrigues, F., Silva, J. J., Iamanaka, B. T., Martins, L. M., & Taniwaki, M. H. (2023). A Brief Snapshot of Aspergillus Section Nigri Isolated from Brazilian Peanuts and Soil. Applied Microbiology, 3(2), 476-484. https://doi.org/10.3390/applmicrobiol3020033






