Abstract
Understanding the impacts of herbicides on soil microbial communities is important, as these organisms mediate a wide range of ecosystem services. Here, we investigated whether the diversity and function of soil microbial communities were significantly influenced by one-off applications of atrazine, diuron, fluazifop-P-butyl, haloxyfop-P-methyl and pendimethalin as pure compounds at their recommended doses over multiple time points (1, 3, 7, 14, 30 and 60 days). Phylogenetic marker gene sequencing revealed that none of the herbicides influenced the numbers of bacterial and archaeal taxa or the evenness of their abundances. Similarly, none of the herbicides influenced the composition of bacterial and archaeal communities, except for diuron, fluazifop-P-methyl and pendimethalin, which were associated with larger relative abundances of a small number of OTUs on day 30 only. Functionally, none of the herbicides significantly influenced fluorescein diacetate hydrolysis (FDA) and beta-glucosidase activities or the induced respiratory responses of soil microbial communities to a range of substrates. These data indicate that the active herbicide ingredients tested may have minimal non-target effects when applied once at their recommended dose. Given their frequent use, it is important to next consider whether these herbicides have more pronounced effects at higher doses and application frequencies.
1. Introduction
By controlling weeds, herbicides play an increasingly crucial role in maintaining global food security. Nonetheless, they can harm non-target organisms, thereby reducing soil biodiversity and the provision of microbially mediated ecosystem services [1,2,3,4]. At present, information concerning the effects of herbicides on soil microbial communities is strongly biased towards the world’s most widely used herbicide, glyphosate. Hence, the effects of many other herbicides on soil microbial communities are poorly understood and require investigation. This is particularly the case when considering the effects of the active constituents of commercially available herbicides, which are the only components that are typically disclosed.
Atrazine, diuron, fluazifop-P-butyl, haloxyfop-P-methyl and pendimethalin are used extensively worldwide. At present, there are critical knowledge gaps for some herbicide actives that have only been investigated as commercial formulations containing a range of unknown compounds (e.g., fluazifop-P-butyl and pendimethalin). In addition, most studies concerning the effects of herbicide actives on soil microbial diversity are based on culturing, microbial fatty acid analyses or the fingerprinting of phylogenetic marker genes [3]. High-throughput sequencing facilitates better measurements of microbial diversity, particularly in terms of comparing the richness and evenness of communities and identifying any taxa that respond to herbicide treatments.
In this study, we investigated the effects of one-off applications of atrazine, diuron, fluazifop-P-butyl, haloxyfop-P-methyl and pendimethalin at recommended doses (Table 1) on the diversity and function of microbial communities associated with soil samples collected from a banana plantation in the wet tropics of northeast Queensland, Australia. Soils were incubated in containers for 60 days and communities were characterised at multiple time points. The degradation of herbicides was characterised over time using LC-MS/MS, and their potential impacts on the functioning of microbial communities were characterised using multiple substrate utilisation (MicroResp) and enzyme activity assays. Finally, the diversity of bacterial and archaeal communities was characterised using 16S rRNA gene amplicon sequencing.

Table 1.
Application rates for each herbicide active.
2. Results
2.1. Herbicide Degradation over Time
Linear regression revealed that the concentrations of atrazine (R2 = 51%, p < 0.001), diuron (R2 = 82%, p < 0.001), fluazifop-P-butyl (R2 = 84%, p < 0.001) and haloxyfop-P-methyl (R2 = 88%, p < 0.001) in soil declined over time (Figure 1). The concentration of pendimethalin, however, was similar throughout the experiment (Figure 1).
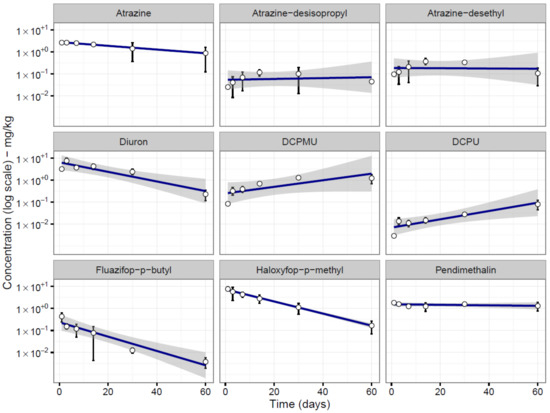
Figure 1.
LC–MS analysis of soil samples spiked with five herbicides (n = 4). DCPMU: 3–(3,4–dichlorophenyl)–1–methylurea and DCPU: 1–(3,4–dichlorophenyl)urea.
For atrazine and diuron, we were also able to trace the formation of degradation by-products over time. For atrazine, the degradation by-products atrazine-desisopropyl and atrazine-desethyl peaked at 14 days and then declined (Figure 1). For diuron, the concentration of the degradation by-product 3-(3,4-dichlorophenyl)-1-methylurea (DCPMU) peaked after 14 days incubation, then remained stable, while that of 1-(3,4-dichlorophenyl)urea (DCPU) increased throughout the experiment (Figure 1).
2.2. Soil Microbial Activity
Relative to the control, none of the herbicides were associated with significant changes in the respiratory responses of soil microbial communities to any of the added substrates when considered individually or combined (Figure 2A). Similarly, potential betaglucosidase and FDA activity did not differ significantly from the controls (Figure 2B).
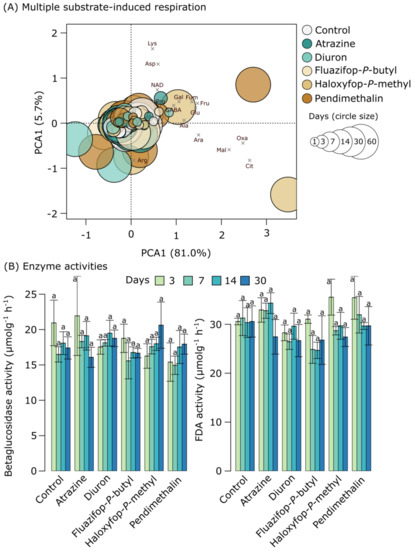
Figure 2.
(A) An ordination highlighting differences in the respiratory responses of soil microbial communities to the addition of 15 different substrates as measured using MicroResp. (B) Bar plots representing the mean betaglucosidase and FDA activity in each treatment over time. Error bars represent standard errors of the means, and the letters above the bars indicate significant differences (p < 0.05) between treatments according to ANOVA with post hoc Tukey tests.
2.3. Soil Microbial Diversity
Soil bacterial communities were dominated by representatives of Acidobacteriota, Actinobacteriota, Bacteroidota, Firmicutes, Nitrospirota, and Proteobacteria (Figure 3). The most dominant archaeal populations were members of Crenarchaeota (Figure 3).
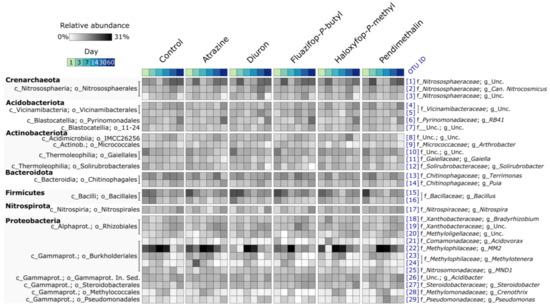
Figure 3.
Heatmap summarising the relative abundances of bacterial and archaeal OTUs present at ≥1% mean relative abundance within any treatment group. Relative abundances are Hellinger transformed. The blue numbers in brackets are OTU IDs.
The alpha diversity of bacterial and archaeal communities, as represented by Simpson’s Diversity Index, and the numbers of observed (Sobs) and predicted (Chao1) OTUs, was significantly influenced by time (p < 0.001, PERMANOVA), but not by herbicide application.
Similarly, the composition of bacterial and archaeal communities, as represented by Hellinger-transformed OTU relative abundances, was significantly influenced by time (p < 0.001, PERMANOVA), but not by herbicide application (Figure 4). This finding was supported by separate analyses within each time point, except on Day 30, when significant differences in community composition were apparent between herbicides (p = 0.007).
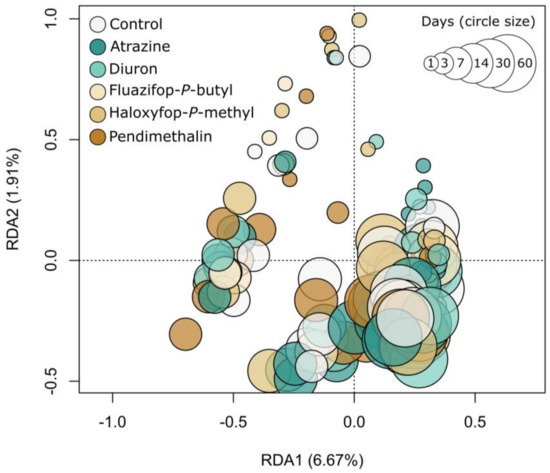
Figure 4.
A distance-based redundancy analysis ordination highlighting differences in the composition (Hellinger-transformed OTU relative abundances) of bacterial and archaeal communities between treatments.
Indicator analysis revealed that the differences between herbicide treatments on Day 30 were associated with a Viciamibacteraceae (OTU5), an Acidimicrobiia (OTU8), a Gaiellales (OTU10), and a Steroidobacter (OTU27) population (Figure 5) Relative to the control, diuron application was associated with a significantly larger relative abundance of the Viciamibacteraceae (OTU5) population, and fluazifop-P-butyl and pendimethalin were associated with larger relative abundances of the Steroidobacter (OTU27) population (Figure 5).
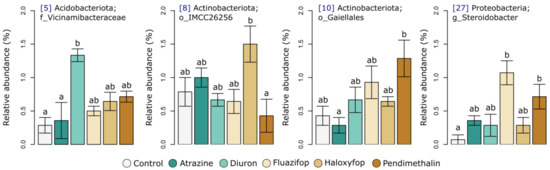
Figure 5.
Bar plots representing the mean relative abundances of OTUs that were identified as significant indicators of herbicides on day 30. The blue numbers in square brackets are OTU IDs and are consistent with the heatmap. Error bars represent standard errors of the means, and the letters above the bars indicate significant differences (p < 0.05) between treatments according to ANOVA with post hoc Tukey tests.
3. Discussion
Our results indicate that while all herbicides, except pendimethalin, were being degraded throughout the experiment, significant changes in microbial diversity and function were not observed. The only exceptions to this were on day 30, when the relative abundances of a few OTUs were larger in diuron-, fluazifop-P-methyl- or pendimethalin-treated soils.
For atrazine at recommended application rates, our findings are consistent with previous studies that found no changes in FDA [5] and beta-glucosidase [6] activity, as well as substrate utilisation patterns [7]. Similarly, using 16S rRNA gene amplicon sequencing, Yale et al. [8] and Fernandez et al. [9] found no overall significant effects of atrazine addition on the richness, evenness and composition of soil bacterial communities. In contrast, Mahía et al. [6] and Briceño et al. [10] observed significant changes in microbial community structure at 5 mg/kg (using phospholipid fatty acid analyses) or 1, 2 and 3 mg/kg (using denaturing gradient gel electrophoresis of 16S rRNA gene amplicons), respectively. Lastly, Percich and Lockwood [11] found that 10 mg atrazine per kilogram soil (approximately recommended rate) had no significant impacts on the numbers of bacteria and fungi, but larger doses (30 or 100 mg/kg) increased microbial counts for at least two months.
For diuron, our results are less consistent with previous studies. For example, while Tejada et al. [12] observed negligible effects of diuron at its recommended rate on some enzyme activities (urease and phosphatase), temporary reductions in betaglucosidase activity were detected. Furthermore, El Fantroussi et al. [13] observed significant decreases in the numbers of culturable bacteria, changes in bacterial community structure (DGGE), and shifts in substrate utilisation patterns (BIOLOG) in response to diuron, albeit at twice the recommended dose. Not all studies, however, demonstrate significant impacts of diuron on bacterial communities. For example, using 16S rRNA gene amplicon sequencing, Angly et al. [14] observed no significant changes in the diversity of bacterial communities upon exposure to environmentally realistic levels of diuron in Great Barrier Reef coastal waters.
Fluazifop-P-butyl is the active ingredient of the post-emergent herbicide Fusilade. We are not aware of studies that focus on fluazifop-P-butyl as a pure compound, but the effects of Fusilade on soil microbial diversity and function have been investigated in several studies [15,16,17]. At one and ten times the recommended Fusilade dose, Darine et al. [15] observed a reduction in acid phosphatase, urease and FDA activity and a change in bacterial community structure as indicated by a terminal-restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA gene amplicons. Likewise, Santos et al. [16] observed a reduction in soil microbial biomass carbon 12 and 51 days after Fusilade application, and Abdel-Mallek et al. [17] found significantly more fungi in soils with five and ten times the recommended Fusilade dose, albeit only temporally. Importantly though, at the recommend dose, microbial communities were not influenced by Fusilade® application [17]. Hence, the impacts on microbial communities observed in other studies may be attributable to high doses or the presence of one or more ingredients present in Fusilade other than fluazifop-P-butyl.
In response to the addition of haloxyfop-P-methyl at twice the recommended dose, Tu [18] observed negligible effects on phosphatase and urease activities, but the stimulation of dehydrogenase activity. Hence, in terms of enzyme activity, our results are roughly consistent. The effects of haloxyfop-P-methyl on the diversity of bacterial communities have not been extensively researched. The only other study that we are aware of is that of Liang et al. [19], who used 16S rRNA gene amplicon sequencing to characterise bacterial communities in the rhizosphere of Sparina alterniflora. This study demonstrated significant changes in rhizosphere bacterial diversity and community composition, albeit only within the first week and at higher than recommended doses [19]. Furthermore, it remains unclear whether the effects observed by Liang et al. [19] result from direct exposure to haloxyfop-P-methyl or from changes in rhizodeposition from the host plant.
Stomp contains the active ingredient pendimethalin, and its effects on soil microbial diversity and function have been investigated in serval studies [20,21]. As with fluazifop-P-butyl, however, we are not aware of similar studies that focus on the effects of pendimethalin as a pure compound. In one study, one or two times the recommended Stomp dose had no significant impact on the numbers or activities of soil microorganisms [20]. Similarly, Stomp applied at approximately five times the recommended dose was shown to have no effects on soil microbial biomass, phosphatase activity and community-level substrate utilisation patterns [21]. Nayak et al. [22], however, observed significant stimulatory and suppressive effects of Stomp on the numbers of soil bacteria and fungi. Likewise, Chikoye et al. [23] found that Stomp applied at approximately double or four times the recommended dose reduced the nodulation and arbuscular mycorrhizal colonisation of soybeans and cowpeas.
4. Conclusions
In response to recommended application rates of atrazine, diuron, fluazifop-P-butyl, haloxyfop-P-methyl and pendimethalin as pure compounds, we observed no significant effects on: (1) the induced respiratory responses of soil microbial communities to a range of substrates, (2) the potential enzyme activities of soil microbial communities, (3) the alpha diversity of bacterial and archaeal communities, or (4) the composition of bacterial and archaeal communities, except on day 30 when the relative abundances of a small number of OTUs were larger in diuron-, fluazifop-P-methyl- or pendimethalin-treated soils. In other words, no significant effects from the addition of atrazine or haloxyfop-P-methyl were detected, and only minor transient effects were observed for other herbicide actives. In our 60-day, laboratory-based experiment, these findings indicate that single applications of these herbicide actives at their recommended dose pose little threat to soil microbial diversity and function. In reality, however, herbicides are applied more frequently, and as commercial formulations containing additional compounds that may also affect microbial communities. Furthermore, these chemicals are applied on farms with different management histories, soils and environmental conditions. These factors may strongly influence the impacts of herbicides on soil microbial communities. Hence, while our results are broadly consistent with previous studies, further work is needed to quantify the impacts of herbicide actives relative to additives, dose and application frequency, soil type and environmental conditions, as well as management history.
5. Materials and Methods
5.1. Experimental Design and Soil Sampling
Soil was sampled with a corer (auger) of 5 cm diameter to 10 cm depth from a Musa (AAA Group, Cavendish Subgroup) ‘Williams’ plantation in East Palmerston, Queensland, Australia (S 17°35′32′′ E 145°49′58′′), then transported to the Centre for Wet Tropics Agriculture, South Johnstone, Australia for further processing. The soil had a pH in water of 6.7, a clay loam texture (38% sand, 30% silt, 33% clay), was sieved to 4 mm and was adjusted to 50% water-holding capacity. It was then loaded, in 1.6 kg samples, into 2 L plastic containers with lids that allowed gas exchange and rested at 27 °C for 14 days prior to the application of treatments.
Each of the treatments (five herbicide actives and a control) were replicated four times, arranged in a randomized block design, and included atrazine, diuron, fluazifop-P-butyl, haloxyfop-P-methyl, and pendimethalin, which were all purchased from Sigma-Alrich. The actives were each dissolved in 5 mL methanol, and the control was a 5 mL methanol-only treatment. Each compound was applied at a dose equal to the upper recommended rate of the corresponding commercial herbicide (Table 1). These rates were calculated by first multiplying the quantity of active compound that would be applied per cm2 of soil at the upper recommended rate by the exposed surface area of soil in each container (408 cm2), and then multiplying this number by four, as the depth of soil in each container was 4 cm and the bulk density of the soil was c. 1 g/cm3. All treatments were applied to the soil surface using a fine mist sprayer, and the containers were incubated at 27 °C for 60 days.
Soil cores were collected from each container after 1, 3, 7, 14, 30 and 60 days using sterile 50 mL plastic tubes and transferred to −20 °C storage for DNA extraction or maintained at 4 °C for 2–4 days for enzyme assays and MicroRespTM.
5.2. Liquid Chromatography–Mass Spectrometry–Mass Spectrometry (LC-MSMS)
The internal standard (IS) atrazine-d5 was purchased from Toronto Research Chemical Inc. (Ontario, Canada). Methanol (analytical grade) and acetonitrile (HPLC grade) were purchased from Labscan. Glacial acetic acid was purchased from Merck KGaA. Individual herbicide or internal standard stock solutions were prepared at 1 mg/mL in water (MilliQ) or methanol. Working solutions were prepared at 1 and 5 mg/mL for each herbicide and at 2 and 40 μg/mL for IS. The degradation of fluazifop-P-butyl, pendimethalin, haloxyfop-P-methyl, atrazine, and diuron was confirmed using the QuEChERS method due to its simplicity and robustness in combining chemical extraction, liquid chromatography and mass spectrometry. To 5 g dried, sieved (4 mm) soil sample (4 replicates per time-point) in a Falcon tube (50 mL) was added a 50 µL solution of atrazine-d5 (IS) at 40 µg/mL directly onto the soil matrix. After 5 min, water (3 mL) and MeCN (7 mL) were added, followed by acetic acid (100 µL). The turbid solution was then subjected to vortexing (15 s) and then mechanical shaking (30 min) to prevent lumps and aggregation. To the omogenized solution was then added the QuEChERS salt pouch content (Agilent pre-weighted: MgSO4, NaCl, NaCit, Na2Cit) portion-wise, followed by vortexing (15 s) and centrifugation (4000 rpm, 5 min). The supernatant (1 mL) was collected and dispensed in a 2 mL dispersive solid-phase extraction (d-SPE) tube (Agilent pre-weighted: C18 25 mg, PSA 25 mg and MgSO4 150 mg). The cartridge was vortexed (1 min) and centrifuged (4000 rpm). From the d-SPE cartridge was collected supernatant (0.5 mL), and water (MilliQ, 0.5 mL) was added to make up the sample for LC-MSMS analysis (final IS concentration 0.1 µg/mL).
Herbicides were determined by HPLC-MS/MS using an AB/Sciex API5500Q mass spectrometer (AB/Sciex, Concord, ON, Canada) equipped with an electrospray (TurboV) interface coupled to a Shimadzu Nexera HPLC system (Shimadzu Corp., Kyoto, Japan). Separation was achieved using a 4 μm 50 × 2.0 mm Phenomenex Synergi Fusion RP column (Phenomenex, Torrance, CA, USA) run at 45 °C and a flow rate of 0.4 mL min−1 with a linear gradient starting at 8% B for 0.5 min, ramped to 100% B in 8.0 min, and then held at 100% for 4.0 min, followed by equilibration at 8% B for 4.0 min. (A = 1% methanol in HPLC grade water, B = 95% methanol in HPLC grade water, both containing 0.1% acetic acid). The mass spectrometer was operated in the positive ion, multiple reaction-monitoring mode using nitrogen as the collision gas with two transitions monitored for each analyte. Positive samples were confirmed by retention time and by comparing transition intensity ratios between the sample and an appropriate concentration standard from the same run. Samples were reported as positive if the two transitions were present, retention time was within 0.15 min of the standard, and the relative intensity of the confirmation transition was within 20% of the expected value. Quantitation was achieved by comparing the response of a particular compound in a sample to standard solutions using a five-point calibration. The value reported was that for the quantitation transition. Using a 0.5 μL injection, the limit of detection for this method was typically less than 1.0 μg/L, with a reporting limit of 3 μg/L in the extract. The response was linear to at least 300 μg/L.
5.3. Enzyme Assays
Beta-glucosidase assays were performed as a measure of organic matter degradation potential using a modified method of Eivazi and Tabatabai [24], in which the modified universal buffer and toluene were replaced with McIlvaine buffer (pH 6, 0.2 M dibasic sodium phosphate solution and 0.1 M citric acid) and 0.1% Tween buffer, respectively. Fluorescein diacetate (FDA) hydrolysis assays were used as a measure of total microbial enzyme activity according to the method of Schnürer and Rosswall [25]. Briefly, 2 mL deionized water was added to 5 g soil in 50 mL centrifuge tubes and incubated at 27 °C for 7 days. Post-incubation, soils were shaken for 30 min in 20 mL 60 mM potassium phosphate buffer and 200 μL fluorescein diacetate solution (2000 μg/mL). Reactions were terminated by the addition of 20 mL of acetone.
5.4. MicroResp
The induced respiratory responses of soil organisms associated with 400 mg of each soil sample to 15 substrates were measured using MicroResp [26]. The substrates included carboxylic acids (citric acid, oxalic acid, L-malic acid), amino acids (L-alanine, DL-aspartic acid, γ-aminobutyric acid, L-lysine hydrochloride, L-arginine) and carbohydrates (L-arabinose, D-fructose, D-galactose, D-glucose, N-acetyl-D-glucosamine, and D-(+)-trehalose dehydrate). Sterile distilled water was added to controls.
5.5. Microbial Diversity
DNA extraction, PCR and sequencing: DNA was extracted from 250 mg of thawed soil using the Power Soil DNA Isolation kit (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. Universal bacterial and archaeal 16S rRNA genes were amplified by polymerase chain reaction (PCR) using the primers 926F (5′-AAA CTY AAA KGA ATT GRC GG-3′) and 1392wR (5′-ACG GGC GGT GWG TRC-3′) [27], each modified on the 5′ end to contain the Illumina overhang adapter for compatibility with the P5 and i7 Nextera XT indices, respectively. PCRs were performed on 1.5 µL DNA samples in 1X PCR Buffer minus Mg2+ (Invitrogen), 100 µM of each of the dNTPs (Invitrogen), 300 µM of MgCl2 (Invitrogen), (Invitrogen), 0.625 U Taq DNA Polymerase (Invitrogen), and 250 µM of each primer, made up to a total volume of 25 µL with molecular biology grade water. Thermocycling conditions were as follows: 94 °C for 3 min; then 35 cycles of 94 °C for 45 s, 55 °C for 30 s, 72 °C for 1 min 30 s; followed by 72 °C for 10 min. Amplifications were performed using a Veriti 96-well thermocycler (Applied Biosystems). Amplicons were purified using Agencourt AMPure magnetic beads and dual-indexed using the Nextera XT Index Kit (Illumina) as per the manufacturer’s instructions. Indexed amplicons were purified using Agencourt AMPure XP beads and then quantified using a PicoGreen dsDNA Quantification Kit (Invitrogen). Equal concentrations of each sample were pooled and sequenced on an Illumina MiSeq at The University of Queensland’s Institute for Molecular Biosciences (UQ, IMB) using 30% PhiX Control v3 (Illumina) and a MiSeq Reagent Kit v3 (600 cycle; Illumina) according to the manufacturer’s instructions.
5.6. Processing of Sequence Data
Sequencing data were quality filtered using the QIIME v. 1.9.1 script multiple_split_libraries.py with the homopolymer filter deactivated [28]. Forward reads were then concatenated and checked for chimeras against the SILVA SSU (v138) [29] database using UCHIME ver. 3.0.617 [30]. Sequences were clustered at 97% similarity using UCLUST v. 1.2.22 [31]. The SILVA SSU (v138) taxonomy was then assigned to the representative OTU sequences using BLASTN (v2.3.0+) [32]. Data were rarefied, and the mean numbers of observed OTUs, the estimated total OTUs (Chao 1) as well as Simpson’s Diversity Index values were calculated using QIIME.
5.7. Statistical Analyses
The influence of herbicides on univariate response variables, such as FDA activity, beta-glucosidase activity, and bacterial and archaeal community richness (observed OTUs and Chao1 predicted OTUs) and evenness (Simpson’s Diversity Index), were analysed using analysis of variance with Tukey’s Honest Significant Difference (HSD) tests for the post hoc comparison of means. The influence of herbicides on multivariate responses, such as Hellinger-transformed bacterial and archaeal OTU relative abundance profiles and multiple substrate utilisation profiles, were assessed using permutational multivariate analysis of variance (PERMANOVA). All analyses were performed using R.
Author Contributions
A.B.P. and P.G.D. designed the experiment and obtained funding. T.K. ran the experiment and performed the enzyme and MicroResp assays. C.F. extracted DNA and carried out the PCR and sequencing of 16S rRNA gene amplicons. F.P. and G.E. performed the LC-MSMS analyses. P.G.D. analysed the data and wrote the manuscript with input from other authors. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Horticulture Innovation Australia Limited using the research and development banana industry levy and funds from the Australian Government, with co-investment from the Queensland Government and the University of Queensland, through project: BA13001—Scoping herbicide impacts on banana production and soils health. The funders and authors declare no conflicts of interest.
Data Availability Statement
All data are available on request.
Acknowledgments
We gratefully acknowledge Hongwei Liu for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural soils, pesticides and microbial diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbo, P.; Rainio, M.; Saikkonen, K. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The effects of glyphosate, glufosinate, paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef]
- Tyler, H.L.; Khalid, S.; Jackson, C.R.; Moore, M.T. Determining potential for microbial atrazine degradation in agricultural drainage ditches. J. Environ. Qual. 2013, 42, 828. [Google Scholar] [CrossRef][Green Version]
- Mahía, J.; González-Prieto, S.J.; Martín, A.; Bååth, E.; Díaz-Raviña, M. Biochemical properties and microbial community structure of five different soils after atrazine addition. Biol. Fertil. Soils. 2011, 47, 577–589. [Google Scholar] [CrossRef]
- Marchand, A.L.; Piutti, S.; Lagacherie, B.; Soulas, G. Atrazine mineralization in bulk soil and maize rhizosphere. Biol. Fertil. Soils. 2002, 35, 288–292. [Google Scholar] [CrossRef]
- Yale, R.L.; Sapp, M.; Sinclair, C.J.; Moir, J.W.B. Microbial changes linked to the accelerated degradation of the herbicide atrazine in a range of temperate soils. Environ. Sci. Pollut. Res. 2017, 24, 7359–7374. [Google Scholar] [CrossRef]
- Fernandes, A.F.T.; Wang, P.; Staley, C.; Aparecida Silva Moretto, J.; Miguel Altarugio, L.; Chagas Campanharo, S.; Guedes Stehling, E.; Jay Sadowsky, M. Impact of atrazine exposure on the microbial community structure in a Brazilian tropical latosol soil. Microbes Environ. 2020, 35, ME19143. [Google Scholar] [CrossRef]
- Briceño, G.; Jorquera, M.A.; Demanet, R.; Mora, M.L.; Durán, N.; Palma, G. Effect of cow slurry amendment on atrazine dissipation and bacterial community structure in an agricultural Andisol. Sci. Total Environ. 2010, 408, 2833–2839. [Google Scholar] [CrossRef]
- Percich, J.A.; Lockwood, J.L. Interaction of atrazine with soil microorganisms: Population changes and accumulation. Can. J. Microbiol. 1978, 24, 1145–1152. [Google Scholar] [CrossRef]
- Tejada, M.; Morillo, E.; Gómez, I.; Madrid, F.; Undabeytia, T. Effect of controlled release formulations of diuron and alachlor herbicides on the biochemical activity of agricultural soils. J. Hazard Mater. 2017, 322, 334–347. [Google Scholar] [CrossRef]
- El Fantroussi, S.; Verschuere, L.; Verstraete, W.; Top, E.M. Effect of phenylurea herbicides on soil microbial communities estimated by analysis of 16S rRNA gene fingerprints and community-level physiological profiles. Appl. Environ. Microbiol. 1999, 65, 982–988. [Google Scholar] [CrossRef]
- Angly, F.E.; Pantos, O.; Morgan, T.C.; Rich, V.; Tonin, H.; Bourne, D.G.; Mercurio, P.; Negri, A.P.; Tyson, G.W. Diuron tolerance and potential degradation by pelagic microbiomes in the Great Barrier Reef lagoon. PeerJ 2016, 4, e1758. [Google Scholar] [CrossRef]
- Darine, T.; Alaeddine, C.; Fethi, B.; Ridha, M. Fluazifop-P-butyl (herbicide) affects richness and structure of soil bacterial communities. Soil Biol. Biochem. 2015, 81, 89–97. [Google Scholar] [CrossRef]
- Santos, J.B.; Jakelaitis, A.; Silva, A.A.; Costa, M.D.; Manabe, A.; Silva, M.C.S. Action of two herbicides on the microbial activity of soil cultivated with common bean (Phaseolus vulgaris) in conventional-till and no-till systems. Weed Res. 2006, 46, 284–289. [Google Scholar] [CrossRef]
- Abdel-Mallek, A.Y.; Abdel-Kader, M.I.A.; Omar, S.A. Effect of the herbicide fluazifop-butyl on fungal populations and activity in soil. Water Air Soil Pollut. 1996, 86, 151–157. [Google Scholar] [CrossRef]
- Tu, C.M. Effect of three newer pesticides on microbial and enzymatic activities in soil. Bull. Environ. Contam. Toxicol. 2004, 49, 120–128. [Google Scholar] [CrossRef]
- Liang, Q.; Yan, Z.; Li, X. Influence of the herbicide haloxyfop-R-methyl on bacterial diversity in rhizosphere soil of Spartina alterniflora. Ecotoxicol. Environ. Saf. 2020, 194, 110366. [Google Scholar] [CrossRef]
- Kocčárek, M.; Artikov, H.; Vorříšek, K.; Borůvka, L. Pendimethalin degradation in soil and its interaction with soil microorganisms. Soil Water Res. 2016, 11, 213–219. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A.; Wright, D.J. Persistence of chlorpyrifos, fenamiphos, chlorothalonil, and pendimethalin in soil and their effects on soil microbial characteristics. Bull. Environ. Contam. Toxicol. 2002, 69, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Prusty, J.C.; Monhanty, S.K. Effect of herbicides on bacteria, fungi and actinomycetes in sesame (Sesamum indicum) soil. Ind. J. Agric. Sci. 1994, 64, 888–890. [Google Scholar]
- Chikoye, D.; Abaidoo, R.; Fontem, L.A. Response of weeds and soil microorganisms to imazaquin and pendimethalin in cowpea and soybean. Crop Prot. 2014, 65, 168–172. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- Engelbrektson, A.; Kunin, V.; Wrighton, K.C.; Zvenigorodsky, N.; Chen, F.; Ochman, H.; Hugenholtz, P. Experimental factors affecting PCR-based estimates of microbial species richness and evenness. ISME J. 2010, 4, 642–647. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).