Abstract
Mucosal vaccination offer an advantage over systemic inoculation from the immunological viewpoint. The development of an efficient vaccine is now a priority for emerging diseases such as COVID-19, that was declared a pandemic in 2020 and caused millions of deaths globally. Lactic acid bacteria (LAB) especially Lactobacillus are the vital microbiota of the gut, which is observed as having valuable effects on animals’ and human health. LAB produce lactic acid as the major by-product of carbohydrate degradation and play a significant role in innate immunity enhancement. LAB have significant characteristics to mimic pathogen infections and intrinsically possess adjuvant properties to enhance mucosal immunity. Increasing demand and deliberations are being substantially focused on probiotic organisms that can enhance mucosal immunity against viral diseases. LAB can also strengthen their host’s antiviral defense system by producing antiviral peptides, and releasing metabolites that prevent viral infections and adhesion to mucosal surfaces. From the perspectives of “one health” and the use of probiotics, conventional belief has opened up a new horizon on the use of LAB as antivirals. The major interest of this review is to depict the beneficial use of LAB as antivirals and mucosal immunity enhancers against viral diseases.
1. Introduction
The animal and human body carry a variety of microorganisms, which are collectively referred as microbiota. The term ‘gut microbiota’ describes the collection of microorganisms colonizing the gastrointestinal tract (GIT). All of the microbes of the enteric microbiota often have a symbiotic correlation with their host, providing nutrients and protection from invading pathogenic organisms. However, opportunistic enteric pathogens can also be present in the enteric microbiota. These microorganisms cause infections when the host is immunocompromised [1]. Probiotics, which are living microorganisms, have a positive impact on the host by re-establishing the gut microbiota when taken orally in appropriate amounts; meanwhile, synergistic combinations of probiotics and prebiotics are called synbiotics [2]. The animal and human mucosal surfaces are exposed to various pathogens that cause diseases. So, prevention of the pathogen’s entry onto the mucosal surfaces is critical for disease prevention. Probiotics such as lactic acid bacteria (LAB) prohibit the entry of viruses and other pathogens and significantly benefit animal and human health [3].
Depending on the characteristics of bacteria, LAB are Gram-positive, non-motile, non-spore-forming, generally rods or cocci, and have a strong tolerance to low pH, are facultative anaerobic except Bifidobacteria, which are obligatory anaerobes, and catalase-negative organisms that produce lactic acid by the degradative metabolism of carbohydrates [4]. LAB have been classified into more than 60 genera, most of which are used as probiotics, including Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, Enterococcus, and Weissella, and phylogenetic classes such as Bacillus, Clostridia, Enterococcus, Streptococcus, and Mollicutes [2,5,6]. As probiotics are essential for a good and healthy life, LAB may have many beneficial effects by improving the intestinal microbiota balance [7,8,9,10].
Many LAB strains have been identified as possessing multifunctional characteristics such as high fermentation capability and can modulate the immune system against invading pathogens [11]. Most of the LAB species are considered probiotics; however, some of the LAB species, such as Streptococcus mutans, are serious pathogens of periodontal-associated diseases such as dental caries. It is also responsible for infective endocarditis (IE), which primarily occurs in cases with underlying heart disease [12,13]. The immunomodulatory effects and escalation of mucosal immunity by LAB may be accomplished by generating more mucin in the mucosa, developing a biofilm to mask the receptors for the attachment of viruses, and the activation of dendritic cells (DCs) [14]. Along with these events, the production of cytokines such as interleukin (IL)-6, IL-12, and gamma interferon (IFN-γ) and the activation of natural killer (NK) cells are responsible for the clearance of pathogens [14]. In the present review, we outlined and addressed the significance of LAB as mucosal immunity enhancer and antivirals, and presented new research and development objectives for probiotic-based oral vaccinations for emerging viral diseases.
2. LAB as Immunomodulators and Mucosal Immunity Enhancers
The immune system, which consists of the acquired and innate immune systems, works to neutralize invading viruses and other pathogens. Researchers reported that DCs play an important role in bridging innate and adaptive antiviral immunity. Numerous viruses are continually attacking the body. Epithelial surfaces, such as the skin and the mucosal linings of the digestive, respiratory, and urogenital tracts, which are home to DCs, are the first line of defense against pathogens, especially viruses [15]. When these barriers are breached, pathogens are captured by DCs, which are activated and attach to lymphoid organs where the proper specialized immune responses are initiated [15]. Mucosal immunity is the capacity to induce the protective immune response within mucosae where pathogens enter and initiate infections [16,17]. Animals and humans could initiate both systemic and mucosal immunity by recognizing pathogens as foreign objects for their neutralization. The difference between mucosal immunity and systemic immunity is the production of secretory immunoglobulin IgA (sIgA) which is more resistant to protease enzymes [18,19]. For protective mucosal immunity, participation of all kinds of mucosal immune cells are necessary for producing protective IgA antibodies. This process can be divided into entrance sites, where the pathogens adhere to the mucosal surface, and effector sites, where the plasma cells make antibodies that trigger a local immune response, as shown in Figure 1 [16,20].
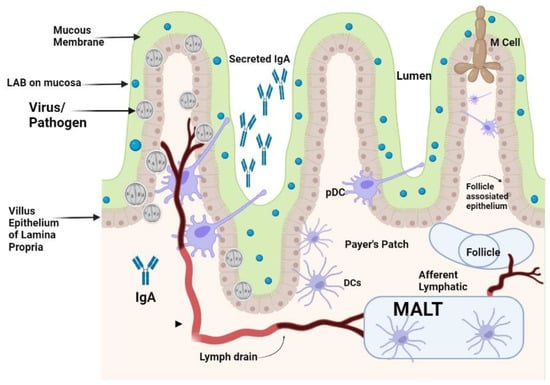
Figure 1.
Effect of orally administered LAB on activation of gut-induced mucosal immunity.
The LAB strains significantly impact on the process of DCs’ activation and the subsequent immunological responses. It has been demonstrated that murine DCs can respond differently depending on the strain of LAB, and this is exacerbated further by the fact that these responses can vary even amongst DC subtypes [21,22,23]. It has been reported that Lactobacillus modulates the maturation and function of DCs, macrophages, and CD4+FoxP3+ regulatory T cells (Tregs) as well as the differentiation of CD4+CD8+ and CD4+FoxP3+ T cells in Peyer’s patches (PPs) [24,25]. The counterattack of pathogens is carried out by specialized DCs of mucosa in the mesenteric lymph nodes also called membrane-associated lymphoid tissues (MALTs). These lymphoid tissues are located beneath the mucosal epithelium of the intestine. These MALTs are similar to peripheral lymph nodes with an abundant supply of B cells and M cells for capturing the invading pathogens [26]. LAB could also initiate the cellular response by differentiation of DCs, and production of cytokines, that could favor the differentiation of näive T cells into Tregs, which are a specialized T cell subpopulation with specific regulatory mechanisms that inhibit the core components of adaptive and innate immune responses [27]. Tregs can drive the depression of an excessive response of effector T cells either by Th1, Th2, or Th17 and maintain mucosal immune homeostasis [10]. Differentiated DCs perform a significant role in the triggering of the immune system against challenging viruses by attaching to them. These DCs are mainly located in the MALTs of the mucosal membrane of the intestine along with some draining lymphoid nodes in the mucosal membrane of the gastrointestinal tract. Plasmacytoid DCs (pDCs) and conventional DCs (cDCs) are the types of DCs presenting at the mucosal membrane. The pDCs are less commonly found in the blood circulation, the mucosal membrane of GIT, and the lymphoid tissue that produces IFN-α [28]. The DCs in the mucosal membrane are classified into CX3CR1+CD103+ DCs with fractalkine (FKN) receptors and CX3CR1+ DCs. Among these DCs, CX3CR1+ DCs have long stellate extensions which elongate from epithelial cells to the antigen found in the lumen of the gut and they usually do not migrate to another place. The mucosal immunity is thought to be organized within the MALTs, thus the antigen must be transported from the lumen to the MALTs by DCs for Tregs to initiate the immune response [29]. As a result of priming, a cascade of cytokines such as TGF-β, IL-6, IL-10, IL-12, IL-23, and other molecules are produced. These cytokines started a cascade of other interleukins’ production and priming of T-helper cells to produce Th1, Th2, Th17, and other T regulatory cells for the neutralization of invading pathogens [30]. Gut microbiota dysbiosis increases the susceptibility of an individual to various diseases. Emerging evidence suggests that LAB are beneficial for the control of RV and SARS-CoV-2 infections. Probiotics are known for restoring stable gut microbiota through the interactions and coordination of the intestinal innate and adaptive immunity [31]. The researchers have reported on the effective protection of LAB against gastrointestinal viruses that originated from clinical cases in humans [32]. The activation of antiviral peptides and the production of mucin by intestinal epithelial cells, and the activation of the local innate immune system lead to an increase in sIgA antibodies for neutralization of the challenge [32]. RV infection deteriorates the mucosal barrier of the GIT [33]. In the clinical cases of RV infection, when Lacticaseibacillus rhamnosus GG (LGG) is administered orally, it could prohibit diarrhea caused by RV infection by mucosal immunity enhancement [33]. The treated cases of LGG reduced the adverse effects of RV on the barrier function in the GIT of piglets, improved relatively the intestinal microbiota, lowered autophagy, increased apoptosis of epithelial cells in the ileum, and retarded the viral multiplication in the intestinal epithelial cells (IECs) [33,34]. It has also been shown that combinations of probiotics and immunization work together to effectively change the gut microbiota. An oral RV vaccine’s immunogenicity is increased by Lactobacillus acidophilus, which also improves the production of IgG and IgA antibodies. Probiotics such as Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 also modulate dendritic cell responses via distinct Toll-like receptor (TLR) signaling, and function as immunostimulants for the RV vaccine [31].
LAB given orally travel down into the intestinal tract surviving through the stomach and are entrapped in the mucus layer secreted in the villi of the small intestine. Lactobacilli from enteric cells could come into contact with the mucosal epithelium. IgA that is secreted by sensed plasma cells in the epithelial membrane is secreted via the IgA receptor into the gut lumen and could be a superintended factor in bacterial presence. Pathogens that come in contact with the apical surface of the mucosal membrane might be sensed by DCs that can capture the viruses by entailment through their protrusions between enterocytes without breaching the integrity of the epithelial layer. The PPs found in the enteric wall are major contact sites where pathogens and antigens are prone to attach to enteric cells. M-cells in the epithelium transport pathogens present inside the lumen to the membrane-associated lymphoid tissue (MALTs) where pathogens are neutralized. DCs that are present in the area of the PPs can uptake and phagocytose viruses and transport them to the MALTs, where they can directly modulate immune responses that are activated by the potential pathogens.
2.1. Immunomodulatory Responses of LAB on Cytokines and Immune Cells
Many kinds of cytokines are produced by mucosal epithelial cells of the intestine such as TGF-β, IL-10, IL-12, IL-25, and IL-33, immune cells including NK cells, antigen-presenting cells (APCs), and T cells which are regulated by LAB resulting in enhanced mucosal immunity [32]. These cytokines and cells retard the viral invasion and increase innate immune response [32]. L. plantarum 06CC2 increased the immunomodulatory effect by escalating the mRNA expression of IFN-γ and IL-12 in the PPs. In PPs, macrophages release IL-12 to activate NK cells after recognizing the viruses in the intestinal lumen. Activated NK cells modulate macrophages by releasing IFN-γ, which is an activator for macrophages and DCs. Both IFN-γ and IL-12 have significant effects on antiviral mucosal immunity. Macrophages and NK cells mutually promote virus clearance in addition to direct phagocytosis of viruses and the virus-infected cells [35]. The molecular mechanisms of LAB as probiotics in animals for the production of mucosal immunity are still undefined. Researchers have conducted studies to explain the mucosal transcriptomic responses of healthy animals to the orally administered Lactobacillus strains. As expected, differential genomic expression was observed in the oral administration of L. acidophilus L10, LGG, and L. casei CRL-431 [36]. It is further reported that L. acidophilus L10 modulates IL-23 signaling and has a harmonious role in immune protection against RV infection. L. acidophilus L10 upregulates the proclamation of Th1-specific IFN-induced chemokines, such as CXCL11 and CXCL10, and IFN-stimulated genes (ISGs) [32].
2.2. PRRs-Associated Immunomodulation
Pattern-recognition receptors (PRRs) on outer mucosal surfaces have substantial interactions between pathogens and hosts. These are specialized attachment surfaces by which host cells recognize pathogens. The PRRs that are modulated by LAB are comprised of Toll-like receptors (TLRs). The members of TLRs can recognize invading pathogens such as viruses, and bacteria [37]. LAB can promote an innate immune response through the Gram-positive cell wall peptidoglycan and lipotechoic acid, which activate the TLR-2, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and C-type lectin receptors [38,39]. It has been reported that LGG is beneficial for diarrhea caused by RV infection through the TLR3 signaling pathway [37]. LAB-activated NOD2 and TLR2 receptors mediate the significant innate immune response to prevent RV invasions [37]. In addition, several studies have shown that the immunomodulatory activities of the probiotic mixture. For instance, LAB such as Bifidobacterium infantis R0033, L. helveticus R0052, and Bifidobacterium bifidum R0071 have significant effect on downregulating the proclamation of inflammatory cytokines, such as IL-6, IL-8, and IL-1β. The major impact on these cytokines includes upregulating the expression of TLR3 and mitogen-activated protein kinase and downregulating nuclear factor kappa B (NF-κB) expression [40]. The EPSs produced by LAB also stimulate APCs, and activation of TLRs specifically through TLR2 and TLR4 signaling pathways [41,42,43]. The PRRs presenting on the outer cell surface could also serve as LAB receptors that can change cell signaling and transcription factors and are also responsible for inducing and enhancing cytokine production to counter invading pathogens.
Researchers reported that IECs sense the dsRNA of the virus via PRRs including RIG-I, TLR3, and MDA-5 [44]. After the identification of dsRNA by those receptors, innate cellular responses are initiated to neutralize infections. The initiating of PRRs in response to invading viruses gives rise to the production of chemokines, cytokines, IFNs, and ISGs that play significant roles in manifesting an antiviral environment and viral spread [44]. Many researchers have demonstrated that EPSs produced by LAB can favorably modulate PRRs-associated modulatory response in GIT by controlling the functions of IECs [45,46].
In GIT, IgA and sIgA play significant roles in the neutralization of invading viruses as the first line of defense. This IgA exhibits antiviral properties by collaborating with non-specific defense mechanisms [47,48]. In pig intestinal epithelium (PIE) cells, TLR3 activation by L. rhamnosus CRL1505 and L. plantarum CRL1506 significantly influence the production of IFN-γ, IFN-β, IL-8, MCP-1, and IL-6 [46,49]. It has been demonstrated that L. rhamnosus CRL1505 and L. plantarum CRL1506 also differentially regulate the expression of chemokines, cytokines, and adhesion molecules on the surface of PIE cells.
4. LAB as Mucosal Vaccine Vectors
To maintain the good health of animals and humans, LAB have been introduced as a vector in animal feed and human food as beneficial organisms to prevent many lethal viral and bacterial diseases by modulating the immune system, as shown in Figure 3 [5]. Diseases caused by various gastrointestinal viruses remain a big challenge for farmed animals and for humans [107]. Vaccination is the most important option to prevent viral infections in farm animals, but differences between pandemics and vaccine strains make vaccination less effective. Moreover, vaccine development for novel viral strains is a difficult task. Lactobacilli have been used as vehicles for the delivery of vaccines to counter many viral diseases [108]. The choice of LAB as a vaccine vector is based on a variety of characteristics that render them very appealing as a possible means of vaccine delivery. Dietary LAB organisms have a very long history of safe administration through the oral route [109]. Additionally, LAB are able to colonize cavities such as the mouth, the urogenital, and GIT, where they play a critical role in maintaining a balanced normal microflora. In addition, LAB have an absence of lipopolysaccharides (LPS) in their cell wall that virtually eliminates the risk of endotoxic shock and survival inside the stomach due to acid resistance [109]. The commensal and dietary types of Lactobacillus strains are used as inherent vaccine vectors that give beneficial effects to animals and humans [110].
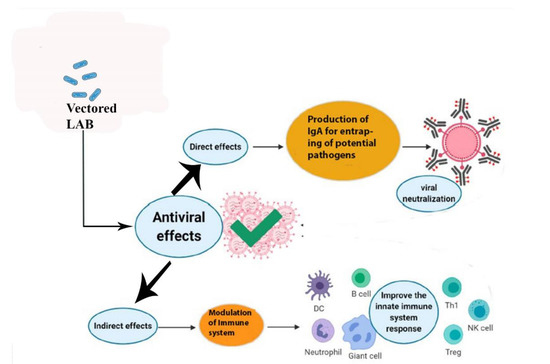
Figure 3.
Direct and indirect effects of vectored lactic acid bacteria delivered orally in the enhancement of mucosal immunity. Lactic acid bacteria (LAB) when used as probiotics orally display direct and indirect effects on the health of animals and humans by preventing the attachment of potential pathogens to the mucosal surface of the intestine. LAB also modulate the innate immune system to produce antibodies and immune cells for the neutralization of viruses.
Many researchers have reported that Lactobacillus gasseri is the most advantageous species, and is considered the model organism to be used as a vaccine vector, because of the unchallenging manipulation of its genome. This has made L. gasseri more beneficial for biotechnological use, covering a range from the production of recombinant proteins to the expression and delivery of modified chimera and bioactive molecules to the mucosal surfaces [108,111]. Characteristics of LAB such as high resistance to the acidic environment of the stomach, the ability to remain in the GIT without colonizing, less immunogenicity, and the lack of lipopolysaccharides in its cell wall, which reduces the chances of endotoxin shock, making such organisms highly versatile to be used as vectors, including in immunization programs [109,112].
In pigs, the composition of the gut microbiota might change the host’s immune response against invading viruses and other pathogens. Similar patterns have been seen in various viral infections, including the African swine fever virus and enteric viruses [113,114]. The production of various kinds of anti-viral peptides by LAB has also been reported by many researchers against viral infections [113,115]. The mechanism of antigen delivery to targeted DCs has tremendous potential for new-age vaccine development. LAB such as L. lactis, L. acidophilus, L. gasseri, and L. casei have great potential as a vector for the delivery of molecules orally to induce a mucosal immune response and production of IgA for many viral and pathogenic diseases. This advancement has a great leap to the conventional process of attenuating pathogens for vaccine development.
LAB have conserved pathogen-associated molecular patterns (PAMPs) such as peptidoglycans, cell wall polysaccharides, lipoteichoic acid (LTA), surface-associated adhesion molecules of Gram-positive bacteria, and lipoproteins which are anchored in the cell cytoplasm membrane [19,116]. It should be taken into account that various strains of LAB differ in their immune regulatory properties, which can have significant roles in intrinsic use as vectors. In particular, their ability to attach to the mucosal surfaces is a principal characteristic.
4.1. Suitability of LAB as Vectors
LAB have become increasingly significant in therapeutic uses such as anti-viral, immunomodulatory, anti-inflammatory, anti-oxidant, anti-tumor, anti-diabetic, enhanced colonization of pathogens, anti-hypertensive, and cholesterol-lowering actions, as shown in Figure 4.
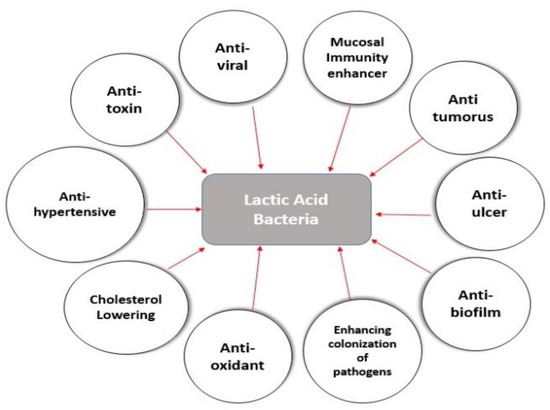
Figure 4.
Health and nutritional benefits of lactic acid bacteria in the schematic diagram. LAB have a number of beneficial health effects on animals and humans. LAB typically colonize the GIT and then reinforces the host defense systems with its anti-microbial, immunomodulatory, anti-inflammatory, anti-oxidant, anti-tumor, anti-viral, anti-diabetic, anti-ulcer, and cholesterol-lowering properties; probiotic microorganisms have now become incredibly valuable in therapeutic applications against emerging viruses.
Commensal LAB strains can give healthful effects with the capability of sticking to the wall of the GIT, urethra, and vagina. Dietary strains lead to struggling with the microbiota in GIT, which can result in antigen disclosure for a longer duration. Both in vitro and in vivo techniques have been applied to screen sticky Lactobacillus strains to be used as vaccine vectors. Data have indicated that the colonization of LAB in GIT is site-specific [110]. The optimal expression of gene sequences, targeting sequences, transcription, and translation in LAB are strain-dependent. Recent advancements in genomic sequence included the development of recombinant plasmid expression vectors with enhanced stoutness and integrated genomic systems with targeted specific loci. These developments are mostly contingent on various non-replicative or chimeric conjugative transposons [110]. This approach may lead to components that pose an antibiotic resistance marker, with inactivated chromosomal genes necessary for immune regulation [110]. Autophagy is one of the significant areas of research by oncologists nowadays. This phenomenon is based on the type of cancer cells involved. Some cells of the body have been subjected to autophagy as a result of treatments and some other tumorous cells may be treated with some other different forms of cell death. It has been demonstrated that the LAB-produced EPSs can regulate the autophagy gene Beclin-1 and also interrelate with apoptosis-related genes [117].
4.2. Limitations and Risks of LAB as Oral Vaccines
LAB are generally recognized as safe (GRAS) and also contain various essential properties that make the way more difficult for their effective use as advantageous vaccine carriers, but a lot of risks persist that need to be disclosed and resolved before their use is in the best interests of humans.
The vaccine which consists of a live attenuated organism contains antigen encoding genes that are either present on the plasmid or amalgamated on an organism’s DNA. In both circumstances, the concerns with the protection, such as the eventual result of the genetically modified plasmid in the vaccine, must be appraised. However, the vaccine use of a specified host range plasmid replication system retards the horizontal pass-on of the plasmid to other microbes and prevents unwanted perseverance of the plasmid. Research should evaluate which cells absorb and express the DNA, the destiny of the DNA in that cell, and the time required in which the DNA persists in the cells. The dose of the modified DNA should also be probed to clarify the defensible amount of plasmid gathered peripherally to the target cells. Another significant difficulty for the use of the LAB as a vaccine is the propensity of these bacteria to survive if released in a natural environment which is a highly arguable issue and safeguards must be rigorously maintained to avoid their spread. To reduce this risk, the use of an auxotrophic chimera should be a priority for LAB used as vectors. Without appropriate growing conditions, such an LAB chimaera cannot spontaneously proliferate in the environment.
5. Conclusions and Future Perspectives
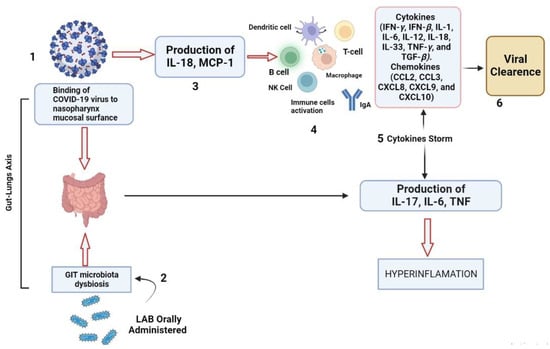
Viral diseases are linked with dysbiosis of the intestinal microbiota leading to severe GIT infections. So, oral probiotic-based treatments are becoming significant in the prevention of viral infections. Probiotics can regulate host immunity and counteract the “cytokine storm” production during viral infections such as COVID-19. However, probiotic-based treatment against novel viruses such as SARS-CoV-2 infections in the field is still an open research question. Viral infections in the respiratory tract are one of the rapidly surging global diseases with high morbidity rates. The intensity can range from a mild upper respiratory tract infection to a severe chronic infection of the mucosal layer of the lower respiratory tract and multi-organ failure in some cases. Activation of the immune system is one of the best prophylactic techniques to lower the severity of such viral diseases. Oral administration of probiotics has various advantages such as strengthening the gut barrier function, balancing the composition of the gut microbiota, and initiation of protective immune responses against invading viruses and pathogens. Bacterial vector vaccines have been studied experimentally for more than a decade; however, interestingly, no live, recombinant bacterial vectors are available for commercial human or veterinary use. Various constraints exist, such as the safety of vaccine strains remains a major issue at the level of vaccinated individuals and environmental spread. To date, the results obtained with LAB are very encouraging as they show that these non-pathogenic, non-invasive bacterial vectors are capable of taking antigens to the mucosal and systemic immune systems initiating specific antibody responses in serum and secretions. While both GIT colonizers and non-colonizers seem to work equally well by the systemic and respiratory routes, the importance of colonization or adhesion in oral administration against viruses is still under appraisal. In the process of recombinant LAB vaccine development, various key points need to be addressed. It is important to pursue a detailed analysis of the immune response generated in relation to the mode of antigen presentation and the delivery route and to further improve the effectiveness of LAB as antigen carriers in order to compare them to the other bacterial vectors under development. It is also necessary to attain knowledge about the antigen production level in vivo, the adhesion mechanism of LAB with GIT walls, and the fate of LAB when administered orally. Last but not least, as in the case of probiotics, well-controlled studies have to be performed in humans or animals in order to clarify the colonization capacity of properly selected Lactobacillus strains and their interaction with the immune system and the endogenous microbiota of the host.
Author Contributions
Conceptualization and approval: H.-J.Q.; original draft preparation: A.M.; review and editing: Y.S., Y.W., J.H. and M.U.Z.K. All the authors equally contributed to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (Grant no. 2021YFD1801403).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
LAB: Lactic acid bacteria; EPSs: Exo-polysaccharides; HoPSs: Homo-polysaccharides; HePSs: Hetro-polysaccharides; DCs: Dendritic cells; APCs: Antigen-presenting cells; GIT: Gastrointestinal tract; IECs: Intestinal epithelial cells; HIV: Human immunodeficiency virus; HSV: Herpes simplex virus.
References
- Lamberte, L.E.; Van Schaik, W. Antibiotic Resistance in the Commensal Human Gut Microbiota. Cur. Opin. Microbiol. 2022, 68, 102150. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Schrezenmeir. Probiotics, Prebiotics, and Synbiotics. In Food Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 1–66. [Google Scholar] [CrossRef]
- Singh, K.; Rao, A. Probiotics: A Potential Immunomodulator in COVID-19 Infection Management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.; Malcata, F.X. Bifidobacterium spp. and Lactobacillus acidophilus: Biological, Biochemical, Technological and Therapeutical Properties Relevant for Use as Probiotics. Trends Food Sci. Technol. 1999, 10, 139–157. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and Probiosis: Antimicrobial Activity of Lactic Acid Bacteria with a Focus on Their Antiviral and Antifungal Properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef] [PubMed]
- Garrity, G.M.; Holt, J.G. The Road Map to the Manual. In Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2001; pp. 119–166. [Google Scholar] [CrossRef]
- Tsuda, H.; Miyamoto, T. Production of Exo-polysaccharide by Lactobacillus plantarum and the Prebiotic Activity of Exo-polysaccharide. Food Sci. Technol. Res. 2010, 16, 87–92. [Google Scholar] [CrossRef]
- Chong, E.S.L. A Potential Role of Probiotics in Colorectal Cancer Prevention: Review of Possible Mechanisms of Action. World J. Microbiol. Biotechnol. 2014, 30, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A Comprehensive Review of Anticancer, Immunomodulatory and Health Beneficial Effects of the Lactic Acid Bacteria Exo-polysaccharides. Carbhydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Toushik, S.H.; Mizan, M.F.; Hossain, M.I.; Ha, S.D. Fighting with Old Foes: The Pledge of Microbe-Derived Biological Agents to Defeat Mono and Mixed Bacterial Biofilms Concerning Food Industries. Trends Food Sci. Technol. 2020, 99, 413–425. [Google Scholar] [CrossRef]
- Nomura, R.; Matayoshi, S.; Otsugu, M.; Kitamura, T.; Teramoto, N.; Nakano, K. Contribution of Severe Dental Caries Induced by Streptococcus mutans to the Pathogenicity of Infective Endocarditis. Infect. Immun. 2020, 88, e00897-19. [Google Scholar] [CrossRef]
- Patel, S.; Majumder, A.; Goyal, A. Potentials of Exopolysaccharides from Lactic Acid Bacteria. Indian J. Microbiol. 2012, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I. New Insights on Antiviral Probiotics; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Larsson, M.; Beignon, A.S.; Bhardwaj, N. DC-Virus Interplay: A Double-Edged Sword. Semin. Immunol. 2004, 16, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Perdigon, G. The Probiotic Bacterium Lactobacillus casei Induces Activation of the Gut Mucosal Immune System through Innate Immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Kim, K.; Ashrafudoulla, M.; Mizan, M.F.; Roy, P.K.; Nahar, S.; Kim, Y.; Ha, S.D. Korean Kimchi Derived Lactic Acid Bacteria Inhibit Foodborne Pathogenic Biofilm Growth on Seafood and Food Processing Surface Materials. Food Contr. 2021, 129, 108276. [Google Scholar] [CrossRef]
- Sutherland, D.B.; Fagarasan, S. IgA Synthesis: A Form of Functional Immune Adaptation Extending beyond Gut. Curr. Opin. Immunol. 2012, 24, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.L.; Sahay, B.; Mohamadzadeh, M. New Generation of Oral Mucosal Vaccines Targeting Dendritic Cells. Curr. Opin. Chem. Biol. 2013, 17, 918–924. [Google Scholar] [CrossRef]
- Mestecky, J.; McGhee, J. Prospects for Human Mucosal Vaccines. Adv. Exp. Med. Biol. 1992, 327, 13–23. [Google Scholar] [CrossRef]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum Strain YU from Fermented Foods Activates Th1 and Protective Immune Responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef]
- Christensen, H.R.; Frokier, H.; Pestka, J.J. Lactobacilli Differentially Modulate Expression of Cytokines and Maturation Surface Markers in Murine Dendritic Cells. J. Immunol. 2002, 168, 171–178. [Google Scholar] [CrossRef]
- Liu, H.Y.; Giraud, A.; Seignez, C.; Ahl, D.; Guo, F.; Sedin, J.; Walden, T.; Oh, J.H.; Van Pijkeren, J.P.; Holm, L.; et al. Distinct B Cell Subsets in Peyer’s Patches Convey Probiotic Effects by Limosilactobacillus reuteri. Microbiome 2021, 9, 198. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.H.; Yang, G.; Duan, J.L.; Yang, L.Y.; Chen, L.X.; Hou, S.L.; Huang, X.G. Oral Administration of Lactobacillus delbrueckii Enhances Intestinal Immunity through Inducing Dendritic Cell Activation in Suckling Piglets. Food Funct. 2022, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Pellon, A.; Barriales, D.; Pena-Cearra, A.; Castelo-Careaga, J.; Palacios, A.; Lopez, N.; Atondo, E.; Pascual-Itoiz, M.A.; Martin-Ruiz, I.; Sampedro, L. The Commensal Bacterium Lactiplantibacillus plantarum Imprints Innate Memory-Like Responses in Mononuclear Phagocytes. Gut Microbes 2021, 13, 1939598. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, K.A.; Bennett, K.M.; Lo, D.D. Mucosal Vaccine Design and Delivery. Annu. Rev. Biomed. Eng. 2012, 14, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T Cells (Tregs) and Their Therapeutic Potential against Autoimmune Disorders Advances and Challenges. Hum. Vaccin. Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Vallon Eberhard, A.; Elinav, E.; Aychek, T.; Shapira, Y.; Luche, H.; Fehling, H.J.; Hardt, W.D.; Shakhar, G.; Jung, S. Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origins and Functions. Immunity 2009, 31, 502–512. [Google Scholar] [CrossRef]
- Chinen, T.; Rudensky, A.Y. The Effects of Commensal Microbiota on Immune Cell Subsets and Inflammatory Responses. Immunol. Rev. 2012, 245, 45–55. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Chong, L.C.; Hor, Y.Y.; Lew, L.C.; Rather, I.A.; Choi, S.B. Role of Probiotics in the Management of COVID-19: A Computational Perspective. Nutrients 2022, 14, 274. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of Probiotic Lactobacilli and Bifidobacteria Effects, Immune Responses, and Rotavirus Vaccines and Infection in Different Host Species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef]
- Mao, X.; Gu, C.; Hu, H.; Tang, J.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Tian, G. Dietary Lactobacillus rhamnosus GG Supplementation Improves the Mucosal Barrier Function in the Intestine of Weaned Piglets Challenged by Porcine Rotavirus. PLoS ONE 2016, 11, e0146312. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, L.; Zhang, Y.; Liu, F.; Li, G.; Wen, K.; Kocher, J.; Yang, X.; Sun, J. Probiotic Lactobacillus rhamnosus GG Mono Association Suppresses Human Rotavirus-Induced Autophagy in the Gnotobiotic Piglet Intestine. Gut Pathog. 2013, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Takeshita, M.; Kikuchi, Y.; Dashnyam, B.; Kawahara, S.; Yoshida, H.; Watanabe, W.; Muguruma, M.; Kurokawa, M. Efficacy of Oral Administration of Heat-Killed Probiotics from Mongolian Dairy Products against Influenza Infection in Mice: Alleviation of Influenza Infection by Its Immunomodulatory Activity through Intestinal Immunity. Int. Immunopharmacol. 2011, 11, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Troost, F.; Van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.; Kleerebezem, M. Human Mucosal in vivo Transcriptome Responses to Three Lactobacilli Indicate How Probiotics May Modulate Human Cellular Pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Aoki Yoshida, A.; Saito, S.; Fukiya, S.; Aoki, R.; Takayama, Y.; Suzuki, C.; Sonoyama, K. Lactobacillus rhamnosus GG Increases Toll-Like Receptor 3 Gene Expression in Murine Small Intestine ex vivo and in vivo. Benef. Microbes 2016, 7, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Bruijns, S.C.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S Layer Protein A of Lactobacillus acidophilus NCFM Regulates Immature Dendritic Cell and T Cell Functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef]
- Rice, T.A.; Brenner, T.A.; Percopo, C.M.; Ma, M.; Keicher, J.D.; Domachowske, J.B.; Rosenberg, H.F. Signaling via Pattern Recognition Receptors NOD2 And TLR2 Contributes to Immunomodulatory Control of Lethal Pneumovirus Infection. Antiviral Res. 2016, 132, 131–140. [Google Scholar] [CrossRef][Green Version]
- Jeong, D.; Kim, D.H.; Kang, I.B.; Kim, H.; Song, K.Y.; Kim, H.S.; Seo, K.H. Characterization and Antibacterial Activity of a Novel Exo-polysaccharide Produced by Lactobacillus kefiranofaciens DN1 Isolated from Kefir. Food Control 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Kotzamanidis, C.; Kourelis, A.; Litopoulou-Tzanetaki, E.; Tzanetakis, N.; Yiangou, M. Evaluation of Adhesion Capacity, Cell Surface Traits and Immunomodulatory Activity of Presumptive Probiotic Lactobacillus Strains. Int. J. Food Microbiol. 2010, 140, 154–163. [Google Scholar] [CrossRef]
- Weiss, G.; Rasmussen, S.; Zeuthen, L.H.; Nielsen, B.N.; Jarmer, H.; Jespersen, L.; Frokiær, H. Lactobacillus acidophilus Induces Virus Immune Defence Genes in Murine Dendritic Cells by a Toll-Like Receptor-2-Dependent Mechanism. Immunology 2010, 131, 268–281. [Google Scholar] [CrossRef]
- Kitazawa, H.; Villena, J. Modulation of Respiratory TLR3 Antiviral Response by Probiotic Microorganisms: Lessons Learned from Lactobacillus rhamnosus CRL1505. Front. Immunol. 2014, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.B.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Albarracin, L.; Kobayashi, H.; Iida, H.; Sato, N.; Nochi, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Transcriptomic Analysis of the Innate Antiviral Immune Response in Porcine Intestinal Epithelial Cells: Influence of Immunobiotic Lactobacilli. Front. Immunol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Perdigon, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of Oral Administration of the Exo-polysaccharide Produced by Lactobacillus kefiranofaciens on the Gut Mucosal Immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Bjerke, K.; Kett, K.; Kvale, D.; Rognum, T.; Scott, H.; Sollid, L.; Valnes, K. Production and Secretion of Immunoglobulins in the Gastrointestinal Tract. Ann. Allergy 1987, 59, 21–39. [Google Scholar]
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.G.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus Strains Differentially Modulate Antiviral Immune Response in Porcine Intestinal Epithelial and Antigen-Presenting Cells. BMC Microbiol. 2014, 14, 126. [Google Scholar] [CrossRef]
- Bernal, P.; Llamas, M.A. Promising Biotechnological Applications of Antibiofilm Exopolysaccharides. Microb. Biotechnol. 2012, 5, 670–673. [Google Scholar] [CrossRef]
- Abedfar, A.; Hossininezhad, M. Overview of The Most Important Characterization of Exopolysaccharides Produced by Probiotics Bacteria and Their Biological Function. J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 47–55. [Google Scholar] [CrossRef]
- Deepak, V.; Ram Kumar Pandian, S.; Sivasubramaniam, S.D.; Nellaiah, H.; Sundar, K. Optimization of Anticancer Exopolysaccharide Production from Probiotic Lactobacillus acidophilus By Response Surface Methodology. Prep. Biochem. Biotechnol. 2016, 46, 288–297. [Google Scholar] [CrossRef]
- Liu, C.T.; Chu, F.J.; Chou, C.C.; Yu, R.C. Antiproliferative and Anticytotoxic Effects of Cell Fractions and Exo-polysaccharides from Lactobacillus casei 01. Mutat. Res. 2011, 721, 157–162. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Vahed, S.Z.; Barzegari, A.; Saadat, Y.R.; Goreyshi, A.; Omidi, Y. Leuconostoc Mesenteroides Derived Anticancer Pharmaceuticals Hinder Inflammation and Cell Survival in Colon Cancer Cells by Modulating NF-κB/AKT/PTEN/MAPK Pathways. Biomed. Pharmacother. 2017, 94, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. P Production, Properties, and Industrial Food Application of Lactic Acid Bacteria-Derived Exo-polysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- West, T. Effect of Temperature on Bacterial Gellan Production. World J. Microbiol. Biotechnol. 2003, 19, 649–652. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of Exo-polysaccharides from Lactic Acid Bacteria as Biotechnological Tools in Food, Pharmaceutical, and Medical Applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Ogel, Z.B.; Ozturk, H.I. Antiviral Mechanisms Related to Lactic Acid Bacteria and Fermented Food Products. Biotechnol. Studies 2020, 29, 18–28. [Google Scholar] [CrossRef]
- Alsaadi, L.G.; Baker, B.A.; Kadhem, B.M.; Mahdi, L.H.; Mater, H.N. Exopolysaccharide as Antiviral, Antimicrobial and as Immunostimulants: A Review. Plant Arch. 2020, 20, 5859–5875. [Google Scholar]
- Mousavi, E.; Makvandi, M.; Teimoori, A.; Ataei, A.; Ghafari, S.; Samarbaf-Zadeh, A. Antiviral Effects of Lactobacillus crispatus against HSV-2 in Mammalian Cell Lines. J. Chin. Med. Assoc. 2018, 81, 262–267. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Xiao, H.; Killip, M.J.; Staeheli, P.; Randall, R.E.; Jackson, D. The Human Interferon-Induced MxA Protein Inhibits Early Stages of Influenza A Virus Infection by Retaining the Incoming Viral Genome in the Cytoplasm. J. Virol. 2013, 87, 13053–13058. [Google Scholar] [CrossRef]
- Botic, T.; Dano, T.; Weingartl, H.; Cencic, A. A Novel Eukaryotic Cell Culture Model to Study Antiviral Activity of Potential Probiotic Bacteria. Int. J. Food Microbiol. 2007, 115, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral Potential of Lactic Acid Bacteria and their Bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Sheikh, F.N.; Jamal, S.; Ezeh, J.K.; Akhtar, A. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cureus 2020, 12, e7355. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Salminen, S.; Isolauri, E. Specific Probiotics in Reducing the Risk of Acute Infections in Infancy: A Randomised, Double-blind, Placebo-Controlled Study. Br. J. Nutr. 2008, 101, 1722–1726. [Google Scholar] [CrossRef]
- King, S.; Tancredi, D.; Lenoir Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; Linder, J.A.; Shane, A.L.; Merenstein, D. Does Probiotic Consumption Reduce Antibiotic Utilization for Common Acute Infections: A Systematic Review and Meta-Analysis. Eur. J. Public Health 2019, 29, 494–499. [Google Scholar] [CrossRef]
- Antunes, A.E.; Vinderola, G.; Xavier-Santos, D.; Sivieri, K. Potential Contribution of Beneficial Microbes to Face the COVID-19 Pandemic. Food Res. Intl. 2020, 136, 109577. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between Probiotics and Pathogenic Microorganisms in Hosts and Foods: A Review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Montazeri-Najafabady, N.; Kazemi, K.; Gholami, A. Recent Advances in Antiviral Effects of Probiotics: Potential Mechanism Study in Prevention and Treatment of SARS-CoV-2. Biologia 2022, 1–18. [Google Scholar] [CrossRef]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2021, 72, 293–299. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Baud, D.; Agri, V.D.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Starosila, D.; Rybalko, S.; Varbanetz, L.; Ivanskaya, N.; Sorokulova, I. Anti-Influenza Activity of Bacillus subtilis Probiotic Strain. Antimicrob. Agents Chemother. 2017, 61, e00539-17. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Quartieri, A.; Prandi, B.; Raimondi, S.; Leonardi, A.; Rossi, M.; Ulrici, A.; Gatti, M.; Sforza, S.; Nocetti, M.; et al. Characterization of Peptide Fraction from Digested Parmigiano Reggiano Cheese and Its Effect on Growth of Lactobacilli and Bifidobacteria. Int. J. Food Microbiol. 2017, 255, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on Antivirally Active Sulfated Polysaccharides: From Structure Activity Analysis to Clinical Evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exo-polysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Ermolenko, E.; Desheva, Y.; Kolobov, A.; Kotyleva, M.; Sychev, I.; Suvorov, A. Anti- Influenza Activity of Enterocin B in vitro and Protective Effect of Bacteriocinogenic Enterococcal Probiotic Strain on Influenza Infection in Mouse Model. Probiotics Antimicrob. Proteins 2019, 11, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Muhialdin, B.J.; Zawawi, N.; Razis, A.F.; Bakar, J.; Zarei, M. Antiviral Activity of Fermented Foods and Their Probiotic Bacteria towards Respiratory and Alimentary Tracts Viruses. Food Control 2021, 127, 108140. [Google Scholar] [CrossRef]
- Ang, L.; Too, H.; Tan, E.; Chow, T.; Shek, P.; Tham, E.; Alonso, S. Antiviral Activity of Lactobacillus Reuteri Protects against Coxsackievirus A and Enterovirus 71 Infection in Human Skeletal Muscle and Colon Cell Lines. Virol. J. 2016, 13, 111. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, Y.-T.; Le Ngo, V.; Cho, Y.-H.; Ko, E.-J.; Hong, S.-M.; Kim, K.-H.; Jang, J.-H.; Oh, J.-S.; Park, M.-K.; et al. Heat-Killed Lactobacillus casei Confers Broad Protection against Influenza A Virus Primary Infection and Develops Heterosubtypic Immunity against Future Secondary Infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Ahn, Y.J.; Baek, S.H.; Kwon, D.H. Antiviral Activities of Cell-Free Supernatants of Yogurts Metabolites against Some RNA Viruses. Eur. Food Res. Technol. 2009, 228, 945–950. [Google Scholar] [CrossRef]
- Xin, K.Q.; Hoshino, Y.; Toda, Y.; Igimi, S.; Kojima, Y.; Jounai, N.; Ohba, K.; Kushiro, A.; Kiwaki, M.; Hamajima, K.; et al. Immunogenicity and Protective Efficacy of Orally Administered Recombinant Lactococcus lactis Expressing Surface-Bound HIV Env. Blood 2003, 102, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Mercenier, A. Mucosal Delivery of Therapeutic and Prophylactic Molecules Using Lactic Acid Bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Eichwald, C.; Burrone, O.; De Mendoza, D. Rotavirus VP7 Antigen Produced by Lactococcus lactis Induces Neutralizing Antibodies in Mice. J. Appl. Microbiol. 2005, 99, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Poo, H.; Han, D.P.; Hong, S.P.; Kim, K.; Cho, M.W.; Kim, E.; Sung, M.H.; Kim, C.J. Mucosal Immunization with Surface-Displayed Severe Acute Respiratory Syndrome Coronavirus Spike Protein on Lactobacillus casei Induces Neutralizing Antibodies in Mice. J. Virol. 2006, 80, 4079–4087. [Google Scholar] [CrossRef]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K. Effects of Oral Intake of pDC Stimulative Lactic Acid Bacterial Strain on Pathogenesis of Influenza-Like Illness and Immunological Response to Influenza Virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of Respiratory Syncytial Virus Infection with Probiotic Lactic Acid Bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef]
- Kajikawa, A.; Zhang, L.; LaVoy, A.; Bumgardner, S.; Klaenhammer, T.R.; Dean, G.A. Mucosal immunogenicity of genetically modified Lactobacillus acidophilus expressing an HIV-1 epitope within the surface layer protein. PLoS ONE 2015, 10, e0141713. [Google Scholar] [CrossRef]
- Vilander, A.C.; Dean, G.A. Adjuvant strategies for lactic acid bacterial mucosal vaccines. Vaccines 2019, 7, 150. [Google Scholar] [CrossRef]
- Ge, J.W.; Liu, D.Q.; Li, Y.J. Construction of Recombinant Lactobacilli Expressing the Core Neutralizing Epitope (COE) of Porcine Epidemic Diarrhea Virus and a Fusion Protein Consisting of COE and Escherichia coli Heat-Labile Enterotoxin B, and Comparison of the Immune Responses by Orogastric Immunization. Can. J. Microbiol. 2012, 58, 1258–1267. [Google Scholar]
- Indo, Y.; Kitahara, S.; Tomokiyo, M.; Araki, S.; Islam, M.A.; Zhou, B.; Albarracin, L.; Miyazaki, A.; Ikeda Ohtsubo, W.; Nochi, T.; et al. Ligilactobacillus salivarius Strains Isolated from the Porcine Gut Modulate Innate Immune Responses in Epithelial Cells and Improve Protection against Intestinal Viral Bacterial Superinfection. Front. Immunol. 2021, 12, 652923. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, G.; Wang, Q.; Wang, Z.; Yang, W.; Gu, W.; Shi, C.; Wang, J.; Huang, H.; Wang, C. Molecular Mechanisms Underlying Protection Against H9N2 Influenza Virus Challenge in Mice by Recombinant Lactobacillus plantarum with Surface Displayed HA2-LTB. J. Biotechnol. 2017, 259, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, J.; Guo, Y.; Yang, W.; Ye, L.; Shi, C.; Liu, Y.; Yang, G.; Wang, C. Construction and Immunological Evaluation of Recombinant Lactobacillus plantarum Expressing HN of Newcastle Disease Virus and DC-Targeting Peptide Fusion Protein. J. Biotechnol. 2015, 216, 82–89. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Patch, J.A.; Barron, A.E. Mimicry of Bioactive Peptides via Non-natural, Sequence-specific Peptidomimetic Oligomers. Cur. Opin. Chem. Biol. 2002, 6, 872–877. [Google Scholar] [CrossRef]
- Bosch, T.C.; Zasloff, M. Antimicrobial Peptides or How Our Ancestors Learned to Control the Microbiome. mBio 2021, 12, e01847-21. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; De Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 Inhibits Late Stages of HSV-1 and HSV-2 Replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Danova, S.; Ivanova, I. Antiinfluenza Virus Activity of a Bacteriocin Produced by Lactobacillus delbrueckii. App. Biochem. Biotechnol. 2000, 88, 285–298. [Google Scholar] [CrossRef]
- Hill, J.A.; Anderson, D.J. Human Vaginal Leukocytes and the Effects of Vaginal Fluid on Lymphocyte and Macrophage Defense Functions. Am. J. Obstet. Gynecol. 1992, 166, 720–726. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal Fluid, Bacteria Associated with Bacterial Vaginosis Can Be Suppressed with Lactic Acid but Not Hydrogen Peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef]
- Nahui Palomino, R.A.; Zicari, S.; Vanpouille, C.; Vitali, B.; Margolis, L. Vaginal Lactobacillus Inhibits HIV-1 Replication in Human Tissues ex vivo. Front. Microbiol. 2017, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Tyssen, D.; Wang, Y.; Hayward, J.; Agius, P.; DeLong, K.; Aldunate, M. Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid. mSphere 2018, 3, e00055-18. [Google Scholar] [CrossRef]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Deen, J. Global Trends in Infectious Diseases of Swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef] [PubMed]
- Tagliavia, M.; Nicosia, A. Advanced Strategies for Food Grade Protein Production: A New E. coli Lactic Acid Bacteria Shuttle Vector for Improved Cloning and Food Grade Expression. Microorganisms 2019, 7, 116. [Google Scholar] [CrossRef]
- Mercenier, A.; Müller-Alouf, H.; Grangette, C. Lactic Acid Bacteria as Live Vaccines. Curr. Issues Mol. Biol. 2000, 2, 17–25. [Google Scholar] [CrossRef]
- Szatraj, K.; Szczepankowska, A.K.; Chmielewska-Jenza, M. Lactic Acid Bacteria Promising Vaccine Vectors: Possibilities, Limitations, and Doubts. J. Appl. Microbiol. 2017, 123, 325–339. [Google Scholar] [CrossRef]
- Wells, J. Mucosal Vaccination and Therapy with Genetically Modified Lactic Acid Bacteria. Annu. Rev. Food Science Technol. 2011, 2, 423–445. [Google Scholar] [CrossRef]
- Pontes, D.S.; De Azevedo, M.S.; Chatel, J.M.; Langella, P.; Azevedo, V.; Miyoshi, A. Lactococcus Lactis a Live Vector: Heterologous Protein Production and DNA Delivery Systems. Protein Expr. Purif. 2011, 79, 165–175. [Google Scholar] [CrossRef]
- Zhang, J.; Rodríguez, F.; Navas, M.J.; Costa Hurtado, M.; Almagro, V.; Bosch Camos, L.; Lopoz, E.; Cuadrado, R.; Accensi, F.; Pina Pedrero, S.; et al. Fecal Microbiota Transplantation from Warthog to Pig Confirms the Influence of the Gut Microbiota on African Swine Fever Susceptibility. Sci. Rep. 2020, 10, 17605. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, W.; Sun, Z.; Zhu, C.; Werid, G.M.; Ibrahim, Y.M.; Zhang, W.; Pan, Y.; Shi, D.; Chen, H.; et al. Abundance of Lactobacillus in Porcine Gut Microbiota Is Closely Related to Immune Response Following PRRSV Immunization. Vet. Microbiol. 2021, 259, 109134. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H.; et al. GPR31-Dependent Dendrite Protrusion of Intestinal CX3CR1+ Cells by Bacterial Metabolites. Nature 2019, 566, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of Intestinal Homeostasis and Immunity with Probiotic Lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, S.H.; Jeon, J.H.; Kim, E.B.; Lee, N.K.; Beck, S.; Choi, Y.J.; Kang, S.K. Cytoplasmic Expression of Model Antigen with M Cell Targeting Moiety in Lactic Acid Bacteria and Implication of the Mechanism as a Mucosal Vaccine via Oral Route. Vaccine 2021, 39, 4072–4081. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).