Agrobacterium tumefaciens-Mediated Gene Transfer in a Major Human Skin Commensal Fungus, Malassezia globosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
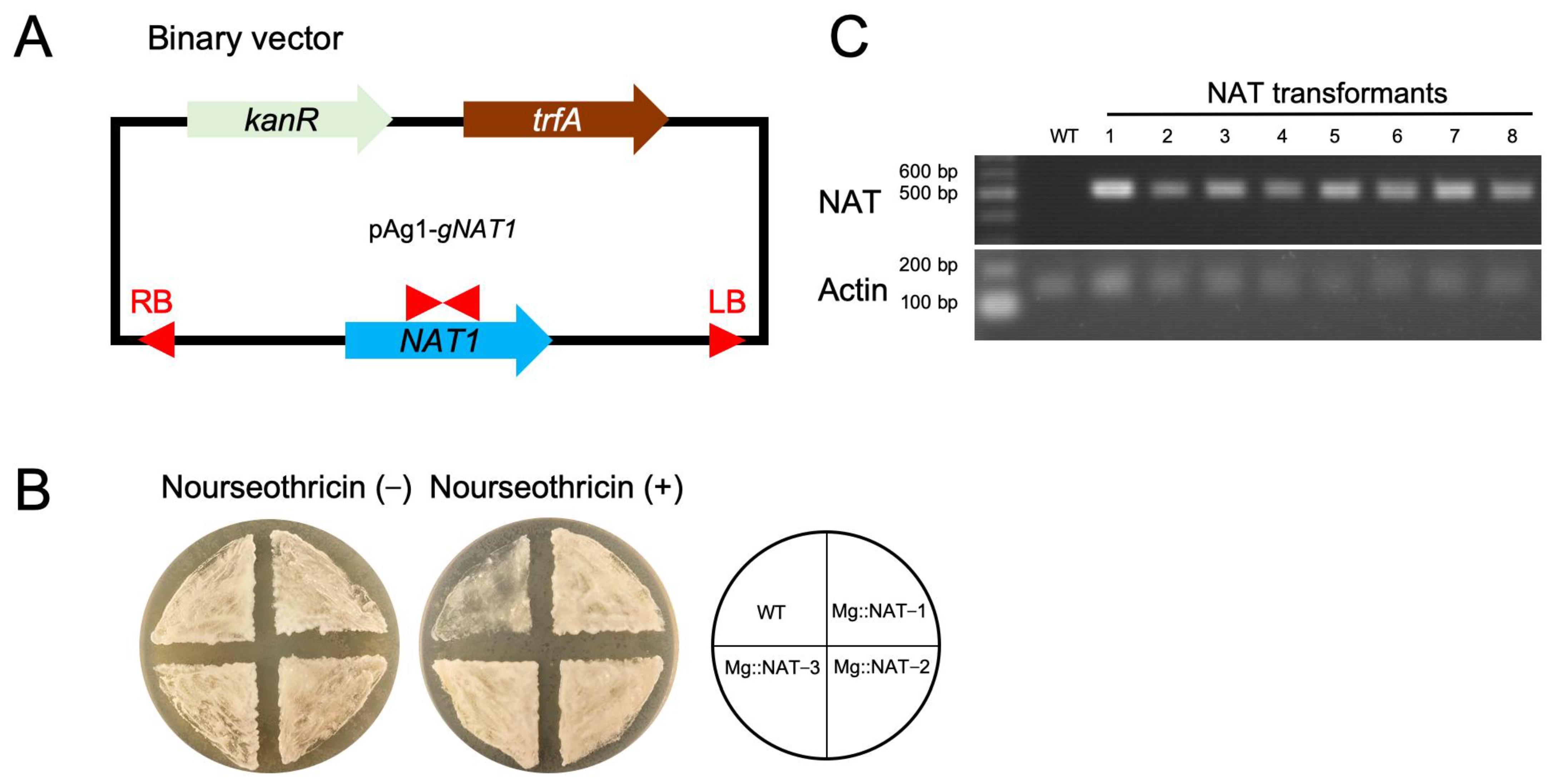
2.2. Construction of M. globosa Expressing the NAT1 Gene Using ATMT
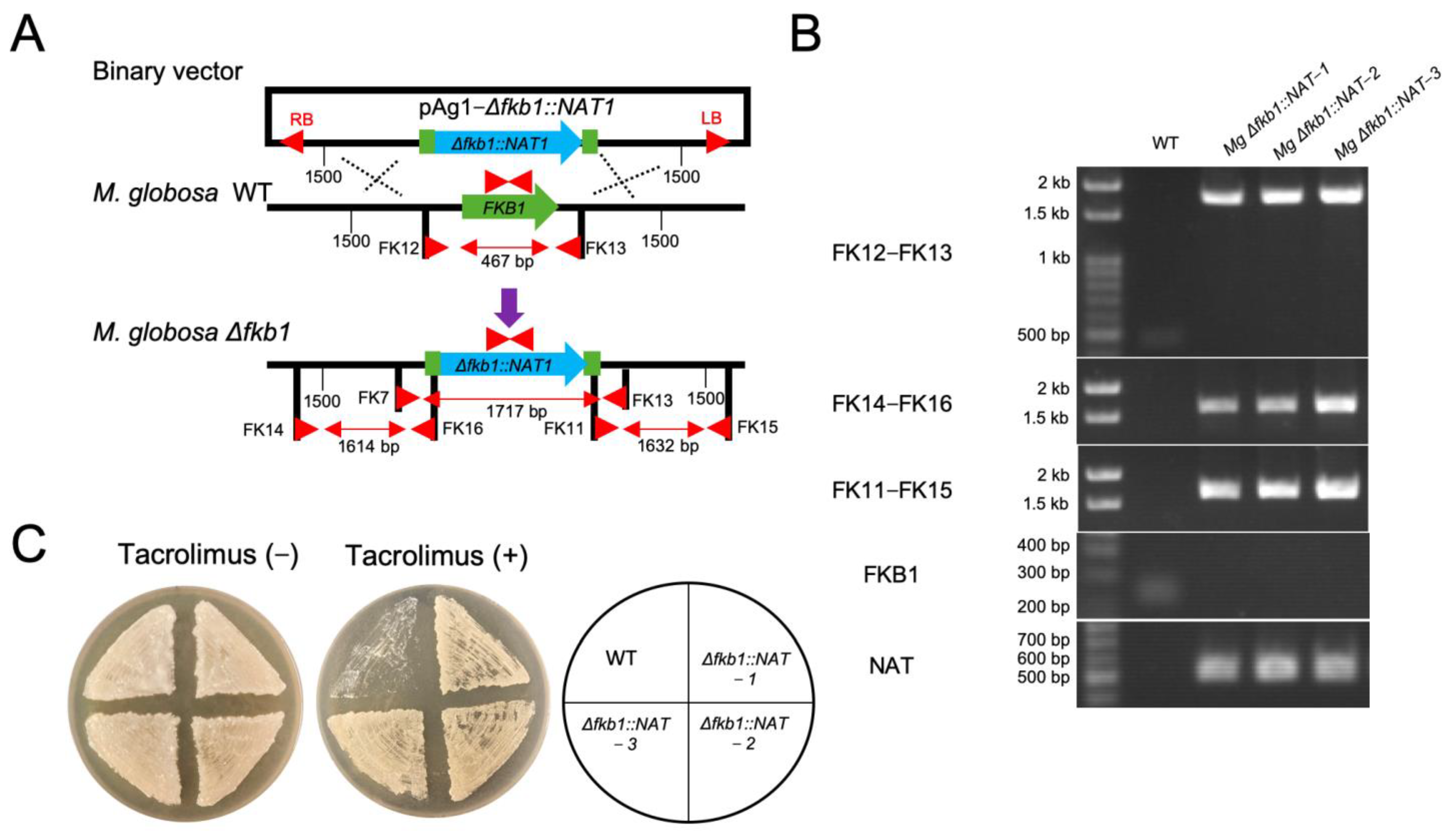
2.3. Targeted Gene Replacement in M. globosa via ATMT
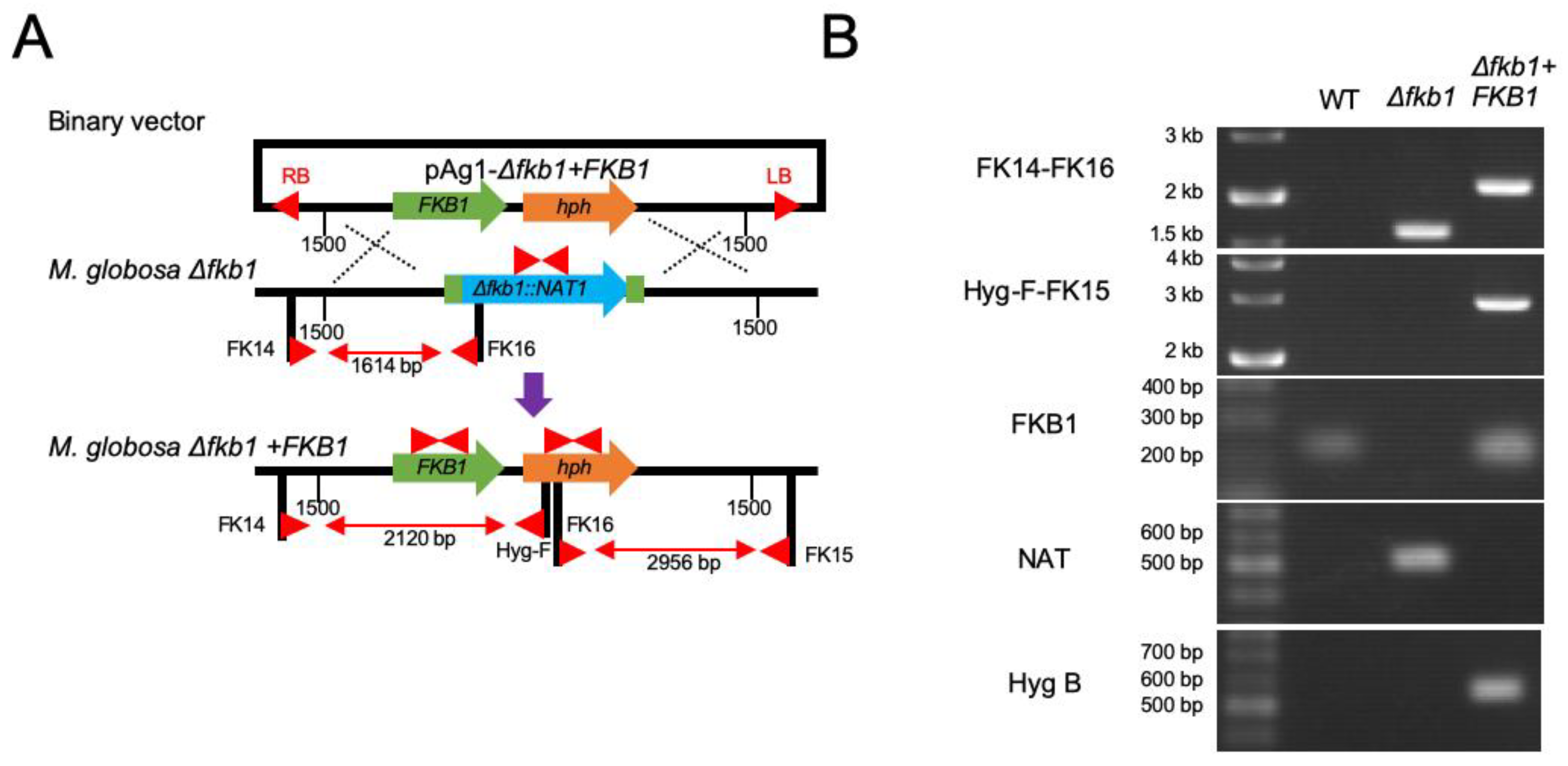
2.4. Reintroduction of the Target Gene into the M. globosa Δfkb1 Mutant
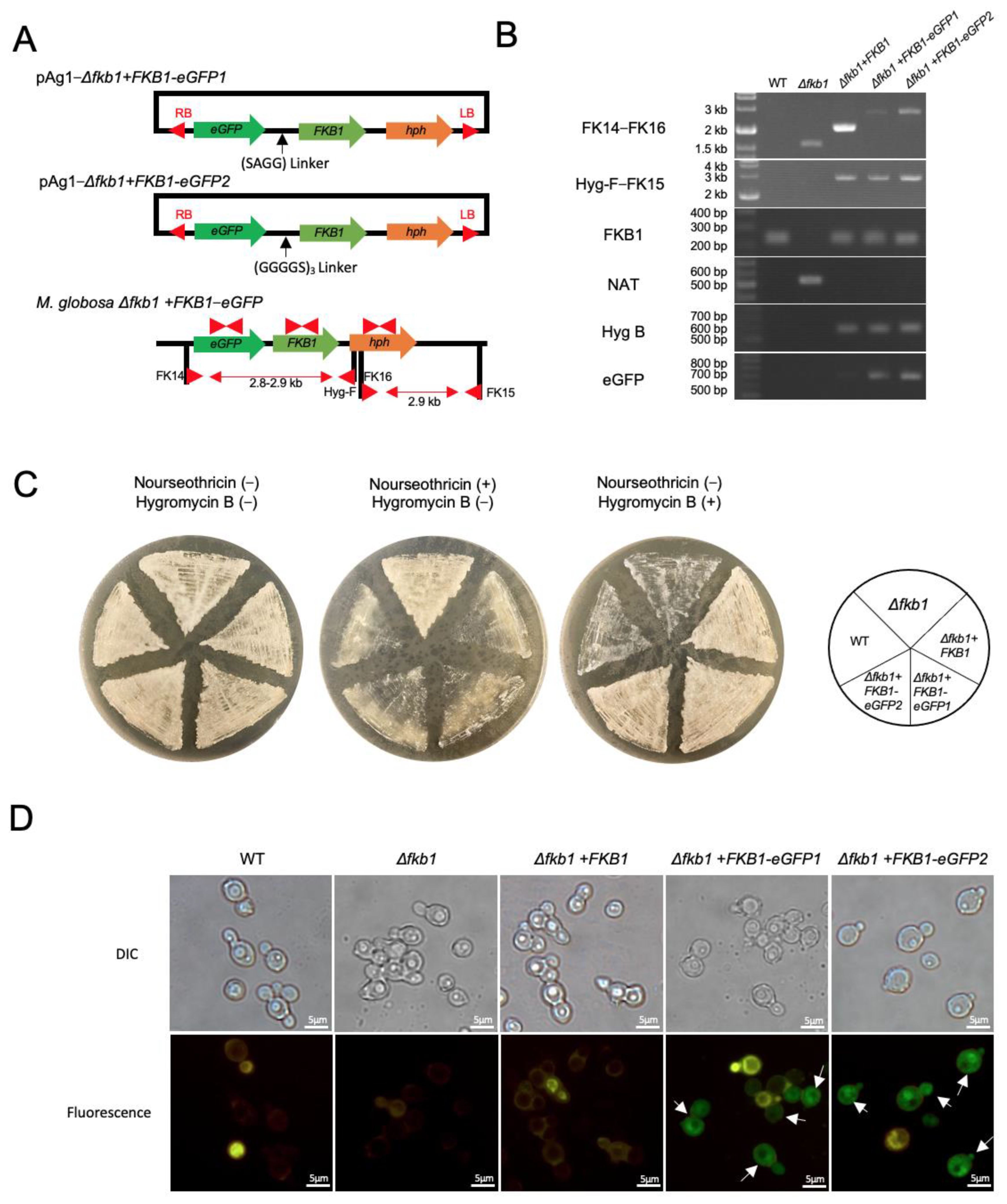
2.5. Construction of the FKB1-eGFP Fusion Gene in M. globosa
2.6. Calcineurin Inhibitor Susceptibility
3. Results
3.1. M. globosa Transformation Using ATMT
3.2. FKB1 Gene Replacement in M. globosa
3.3. Reintroduction of the FKB1 Gene into the M. globosa Δfkb1 Mutant
3.4. Construction of the FKB1-eGFP Fusion Gene and Fluorescence Microscopy
3.5. Drug Susceptibility to Calcineurin Inhibitors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; NIH Intramural Sequencing Center Comparative Sequencing Program; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Harada, K.; Saito, M.; Sugita, T.; Tsuboi, R. Malassezia species and their associated skin diseases. J. Dermatol. 2015, 42, 250–257. [Google Scholar] [CrossRef]
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L., Jr. Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 2018, 56, S10–S25. [Google Scholar] [CrossRef]
- Sugita, T.; Boekhout, T.; Velegraki, A.; Guillot, J.; Hađina, S.; Cabañes, F.J. Epidemiology of Malassezia-related skin diseases. In Malassezia and the Skin: Science and Clinical Practice; Velegraki, A., Boekhout, T., Mayser, P., Guého-Kellermann, E., Eds.; Springer: Heidelberg/Berlin, Germany, 2010; pp. 65–119. [Google Scholar]
- Dawson, T.L., Jr. Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J. Investig. Dermatol. Symp. Proc. 2007, 12, 15–19. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, Y.M.; Gemmer, C.M.; Kaczvinsky, J.R.; Kenneally, D.C.; Schwartz, J.R.; Dawson, T.L., Jr. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J. Investig. Dermatol. Symp. Proc. 2005, 10, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Grendelmeier, P.; Flückiger, S.; Disch, R.; Trautmann, A.; Wüthrich, B.; Blaser, K.; Scheynius, A.; Crameri, R. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J. Allergy Clin. Immunol. 2005, 115, 1068–1075. [Google Scholar] [CrossRef]
- Flückiger, S.; Fijten, H.; Whitley, P.; Blaser, K.; Crameri, R. Cyclophilins, a new family of cross-reactive allergens. Eur. J. Immunol. 2002, 32, 10–17. [Google Scholar] [CrossRef]
- Civila, E.S.; Vignale, R.; Sanjinés, A.; Conti-Diaz, I.A. Hyphal production by Pityrosporum ovale. Int. J. Dermatol. 1978, 17, 74–77. [Google Scholar] [CrossRef]
- Ianiri, G.; Averette, A.F.; Kingsbury, J.M.; Heitman, J.; Idnurm, A. Gene function analysis in the ubiquitous human commensal and pathogen Malassezia genus. mBio 2016, 7, e01853-16. [Google Scholar] [CrossRef]
- Ianiri, G.; Applen Clancey, S.; Lee, S.C.; Heitman, J. FKBP12-Dependent inhibition of calcineurin mediates immunosuppressive antifungal drug action in Malassezia. mBio 2017, 8, e01752-17. [Google Scholar] [CrossRef] [PubMed]
- Celis, A.M.; Vos, A.M.; Triana, S.; Medina, C.A.; Escobar, N.; Restrepo, S.; Wösten, H.A.; de Cock, H. Highly efficient transformation system for Malassezia furfur and Malassezia pachydermatis using Agrobacterium tumefaciens-mediated transformation. J. Microbiol. Methods. 2017, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cho, O.; Matsumoto, Y.; Yamada, T.; Sugita, T. Establishment of a gene recombination method for a major human skin commensal fungus, Malassezia restricta, using Agrobacterium tumefaciens-mediated gene transfer system. Med. Mycol. in press.
- Idnurm, A.; Bailey, A.M.; Cairns, T.C.; Elliott, C.E.; Foster, G.D.; Ianiri, G.; Jeon, J. A silver bullet in a golden age of functional genomics: The impact of Agrobacterium-mediated transformation of fungi. Fungal Biol. Biotechnol. 2017, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Yamazaki, H.; Yamasaki, Y.; Tateyama, Y.; Yamada, T.; Sugita, T. A novel silkworm infection model with fluorescence imaging using transgenic Trichosporon asahii expressing eGFP. Sci. Rep. 2020, 10, 10991. [Google Scholar] [CrossRef]
- Yamada, T.; Makimura, K.; Satoh, K.; Umeda, Y.; Ishihara, Y.; Abe, S. Agrobacterium tumefaciens-mediated transformation of the dermatophyte, Trichophyton mentagrophytes: An efficient tool for gene transfer. Med. Mycol. 2009, 47, 485–494. [Google Scholar] [CrossRef]
- Rojas, F.D.; Sosa, M.d.L.A.; Fernández, M.S.; Cattana, M.E.; Córdoba, S.B.; Giusiano, G.E. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med. Mycol. 2014, 52, 641–646. [Google Scholar] [CrossRef]
- Sugita, T.; Suto, H.; Unno, T.; Tsuboi, R.; Ogawa, H.; Shinoda, T.; Nishikawa, A. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J. Clin. Microbiol. 2001, 39, 3486–3490. [Google Scholar] [CrossRef]
- Morishita, N.; Sei, Y.; Sugita, T. Molecular analysis of Malassezia microflora from patients with pityriasis versicolor. Mycopathologia 2006, 161, 61–65. [Google Scholar] [CrossRef]
- Tajima, M.; Sugita, T.; Nishikawa, A.; Tsuboi, R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: Comparison with other diseases and healthy subjects. J. Invest. Dermatol. 2008, 128, 345–351. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, S.C.; Cardenas, M.E.; Heitman, J. Calcium-calmodulin-calcineurin signaling: A globally conserved virulence cascade in eukaryotic microbial pathogens. Cell Host Microbe. 2019, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, S.; Garcia, A.E.; Lee, S.C. Interactions of FK506 and rapamycin with FK506 binding protein 12 in opportunistic human fungal pathogens. Front. Mol. Biosci. 2020, 7, 588913. [Google Scholar] [CrossRef] [PubMed]
- Ianiri, G.; Coelho, M.A.; Ruchti, F.; Sparber, F.; McMahon, T.J.; Fu, C.; Bolejack, M.; Donovan, O.; Smutney, H.; Myler, P.; et al. HGT in the human and skin commensal Malassezia: A bacterially derived flavohemoglobin is required for NO resistance and host interaction. Proc. Natl Acad. Sci. USA 2020, 117, 15884–15894. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.R.; Ianiri, G.; Coelho, M.A.; Reza, M.H.; Thimmappa, B.C.; Ganguly, P.; Vadnala, R.N.; Sun, S.; Siddharthan, R.; Tellgren-Roth, C.; et al. Loss of centromere function drives karyotype evolution in closely related Malassezia species. eLife 2020, 9, e53944. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Aghaei Gharehbolagh, S.; Mafakher, L.; Salehi, Z.; Asgari, Y.; Hashemi, S.J.; Mahmoudi, S.; Nasimi, M.; Rezaie, S. Unveiling the structure of GPI-anchored protein of Malassezia globosa and its pathogenic role in pityriasis versicolor. J. Mol. Model. 2021, 27, 246. [Google Scholar] [CrossRef]
- Aghaei Gharehbolagh, S.; Kordbacheh, P.; Hashemi, S.J.; Daie Ghazvini, R.; Asgari, Y.; Agha Kuchak Afshari, S.; Seyedmousavi, S.; Rezaie, S. MGL_3741 gene contributes to pathogenicity of Malassezia globosa in pityriasis versicolor. Mycoses 2018, 61, 938–944. [Google Scholar] [CrossRef]
- Hiragun, T.; Ishii, K.; Hiragun, M.; Suzuki, H.; Kan, T.; Mihara, S.; Yanase, Y.; Bartels, J.; Schröder, J.M.; Hide, M. Fungal protein MGL_1304 in sweat is an allergen for atopic dermatitis patients. J. Allergy Clin. Immunol. 2013, 132, 608–615.e4. [Google Scholar] [CrossRef]
- Takahagi, S.; Tanaka, A.; Hide, M. Sweat allergy. Allergol. Int. 2018, 67, 435–441. [Google Scholar] [CrossRef]
| M. globosa Strains | Relevant Genotype | Background | Reference |
|---|---|---|---|
| CBS 7966 | Wild-type | ||
| Mg::NAT-1 | NAT1 | CBS 7966 | This study |
| Mg::NAT-2 | NAT1 | CBS 7966 | This study |
| Mg::NAT-3 | NAT1 | CBS 7966 | This study |
| Mg ∆fkb1::NAT-1 | ∆fkb1::NAT1 | CBS 7966 | This study |
| Mg ∆fkb1::NAT-2 | ∆fkb1::NAT1 | CBS 7966 | This study |
| Mg ∆fkb1::NAT-3 | ∆fkb1::NAT1 | CBS 7966 | This study |
| Δfkb1 + FKB1 | ∆fkb1::NAT1 FKB1::hph | CBS 7966 ∆fkb1 | This study |
| Δfkb1 + FKB1-eGFP1 | ∆fkb1::NAT1 FKB1::eGFP::hph | CBS 7966 ∆fkb1 | This study |
| Δfkb1 + FKB1-eGFP2 | ∆fkb1::NAT1 FKB1::eGFP::hph | CBS 7966 ∆fkb1 | This study |
| M. globosa Strain | MIC (μg/mL) | |
|---|---|---|
| Tacrolimus | Cyclosporin A | |
| Wild-type | 0.06–0.12 | 8–16 |
| Δfkb1 | >100 | 8–16 |
| Δfkb1 + FKB1 | 0.06–0.12 | 8–16 |
| Δfkb1 + FKB1-eGFP1 | 0.06–0.12 | 8–16 |
| Δfkb1 + FKB1-eGFP2 | 0.06–0.12 | 8–16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, O.; Matsumoto, Y.; Yamada, T.; Sugita, T. Agrobacterium tumefaciens-Mediated Gene Transfer in a Major Human Skin Commensal Fungus, Malassezia globosa. Appl. Microbiol. 2022, 2, 827-836. https://doi.org/10.3390/applmicrobiol2040063
Cho O, Matsumoto Y, Yamada T, Sugita T. Agrobacterium tumefaciens-Mediated Gene Transfer in a Major Human Skin Commensal Fungus, Malassezia globosa. Applied Microbiology. 2022; 2(4):827-836. https://doi.org/10.3390/applmicrobiol2040063
Chicago/Turabian StyleCho, Otomi, Yasuhiko Matsumoto, Tsuyoshi Yamada, and Takashi Sugita. 2022. "Agrobacterium tumefaciens-Mediated Gene Transfer in a Major Human Skin Commensal Fungus, Malassezia globosa" Applied Microbiology 2, no. 4: 827-836. https://doi.org/10.3390/applmicrobiol2040063
APA StyleCho, O., Matsumoto, Y., Yamada, T., & Sugita, T. (2022). Agrobacterium tumefaciens-Mediated Gene Transfer in a Major Human Skin Commensal Fungus, Malassezia globosa. Applied Microbiology, 2(4), 827-836. https://doi.org/10.3390/applmicrobiol2040063






