Abstract
Zika virus (ZIKV) is an emerging flavivirus that is associated with neurological complications, such as neuroinflammatory Guillain Barré Syndrome in adults and microcephaly in newborns, and remains a potentially significant and international public health concern. The World Health Organization is urging the development of novel antiviral therapeutic strategies against ZIKV, as there are no clinically approved vaccines or drugs against this virus. Given the public health crisis that is related to ZIKV cases in the last decade, efficient strategies should be identified rapidly to combat or treat ZIKV infection. Several promising strategies have been reported through drug repurposing studies, de novo design, and the high-throughput screening of compound libraries in only a few years. This review summarizes the genome and structure of ZIKV, viral life cycle, transmission cycle, clinical manifestations, cellular and animal models, and antiviral drug developments, with the goal of increasing our understanding of ZIKV and ultimately defeating it.
1. Introduction
Zika virus (ZIKV) is a vector-borne flavivirus belonging to the Flaviviridae family and is closely related to several other flaviviruses that cause global disease, including dengue virus (DENV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), West Nile virus (WNV), and tick-borne encephalitis virus (TBEV) [1,2]. In 1947, ZIKV was first isolated from a febrile sentinel rhesus monkey in the Zika forest in Uganda and was detected in mosquitoes in the following year [3,4]. It was not until 1952 that the first human cases of ZIKV infection were reported in Uganda and the United Republic of Tanzania [5]. There was not much scientific awareness of ZIKV early after it was identified, and accordingly, it was understudied until the first reemergence of ZIKV occurred on the Yap Islands in Micronesia in 2007 [6]. Subsequently, an outbreak of ZIKV was reported in French Polynesia in 2013–2014 [7] and an epidemic in Brazil with ~130,000 suspected cases in late 2015 (Figure 1) [8,9]. In 2016, the World Health Organization declared the ZIKV epidemic as an international health emergency [10,11].
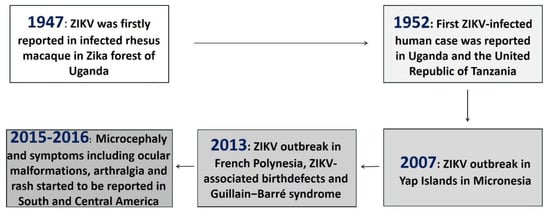
Figure 1.
Discovery of ZIKV and its emergence as a global health threat.
ZIKV infection is mostly asymptomatic and often causes a self-limiting febrile illness in 20% of adults [12]; however, it has been causally associated with congenital malformations and neurological disorders and has rapidly spread to more than 70 countries and territories, which has focused public attention on this emerging pathogen [13]. Of significant concern, no specific and effective vaccine or drug is currently available for ZIKV infection, which has motivated global and scientific efforts to develop countermeasures [14].
2. Genome and Structure of ZIKV
ZIKV is a single-stranded positive-sense RNA virus comprising of enveloped virions with a diameter of approximately 40–60 nm. The genome is approximately 10.7 kb in length and consists of open reading frames (ORFs) with flanking untranslated regions (UTRs) at both ends [15]. The ORF codes for a large polypeptide precursor, which is cleaved by host and virus proteases to generate structural and non-structural proteins from the N-terminus to the C-terminus (Figure 2A) [11].
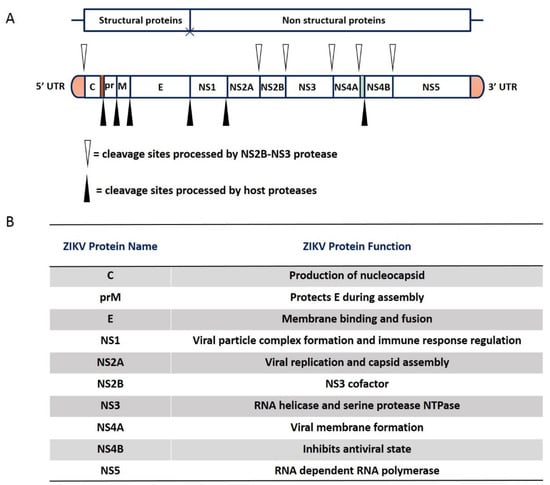
Figure 2.
Genome structure and proteins of ZIKV. (A). ZIKV RNA codes for a polyprotein that is co-translationally cleaved into yield 10 protein: three structural proteins (C, prM/M, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The white and black triangle represents cleavage sites that are processed by NS2B-NS3 protease and cleavage sites that are processed by host proteases, respectively. (B). The table summarizes the names and biological functions of structural and non-structural proteins from the 5′ to the 3′ untranslated regions. The three structural proteins are responsible for the virus particle formation and are involved in virus entry, assembly, and the release of virions into host cells. The other seven are nonstructural proteins play roles in viral genome replication and host immunity evasion.
The three N-terminal structural proteins, capsid protein (C), precursor of membrane (prM), and envelope protein (E) are the skeletal elements for the formation of virus particles. Among them, C combines with viral genomic RNA to form a nucleocapsid core, whereas prM and E are viral surface glycoproteins that are adsorbed to the host cytoplasmic membrane [16]. The M protein is expressed as a glycosylated prM and attached to the host-derived lipid envelope, whereas the E protein may or may not be glycosylated and this is a determinant of neuroinvasion, acting to increase both transepithelial and axonal transportation [17].
There are seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) at the C-terminus of the genome that participate in multiple stages and functions of the viral life cycle, such as RNA replication, virus particle assembly, and immune escape. Among the nonstructural proteins, NS1 has been identified to possess multiple functions in the viral life cycle including the viral particle complex formation, immune evasion, and pathogenesis, it is the most enigmatic protein of the flaviviruses [18,19]. Additionally, NS1 is a glycoprotein and its glycosylation is crucial for effective secretion, virulence, and viral replication [20,21]. Recent publications reported that the overall structure of the ZIKV NS1 proteins is highly similar to the WNV and DENV2-NS1 structures, with the same protein fold and domain arrangement [22,23,24]. Different flaviviruses may exert their NS2A functions in a virus-specific manner [25,26,27]; ZIKV NS2A has a single segment that inserts the ER membrane and six segments that are peripherally connected with the ER membrane that is essential for viral RNA synthesis and virion assembly [28]. NS2A also regulates the evasion of innate immunity, exhibiting an inhibitory effect on Type-I IFN induction at the step of TANK binding kinase 1 (TBK1) complex formation [29]. The flaviviral NS3 protein is divided into two functional domains: a C terminal domain, which contains RNA triphosphatase and helicase activities, and an N terminal protease domain, requiring a cofactor for appropriate function [30]. The protease cofactor, NS2B, is a small crucial transmembrane protein and is integral for proper folding and functions of the NS3 protease [31]. The viral protease active holoenzyme is the trypsin-like NS3 protease in complex with NS2B [32]. The two cleavage sites, NS2A/NS2B and NS2B/NS3, are mediated via NS3 in cis [33], while processing both NS3/NS4A and NS4A/NS5 are mediated by NS3 and NS3 in trans [34,35,36], as well as at internal sites with C, NS2A, NS3, and NS4A [37,38]. Additionally, the mature NS4A and NS4B proteins are produced by cleavage in two sites by NS2B/NS3 protease [34].
NS4A has a small molecular weight compared to other non-structural proteins and antagonizes interferons [39]. NS4B protein is a glycosylated membrane-associated viral protein that is critical for viral replication [40], it has been reported to interact with NS3, suggesting potential interactions with the replication complex [41]. In particular, NS5 is the most conserved and largest of the flavivirus proteins, it encodes an RNA-dependent RNA polymerase (RdRp) for genome replication, methyltransferase for 5′ RNA cap formation and methylation, and a Type I interferon antagonist (Figure 2B) [42].
3. Life Cycle of ZIKV
Similar to other known flaviviruses, the viral life cycle of ZIKV could be roughly divided into stages of binding, entry, translation, replication, assembly, and release (Figure 3) [43]. Briefly, the E protein is involved in the attachment of virus to receptors on the host cell membrane, and the virus is internalized through endocytosis that is mediated by clathrin proteins in the acid pH environment [20,44]. Several cell surface receptors facilitate ZIKV viral entry, such as Tyro3, DC-SIGN, AXL, and TIM-1 [45]. Subsequently, fusion of viral membrane with endosome membrane occurs, the positive-strand viral genomic RNA releases from nucleocapsid into cytoplasm, and the synthesis of negative-strand genomic RNA using viral positive-strand genome as template by RdRp [9]. On surface of endoplasmic reticulum, the negative-strand RNA is used to synthesize new viral positive-strand genomes and viral mRNAs, which are further translated into viral polyproteins via host machinery. The resulting polyprotein is cleaved into the three nonstructural and seven structural proteins by the viral protease as mentioned above [42]. The newly-synthetized positive RNAs could be recruited for further activities of translation/replication or incorporated into the virions, which initiates their assembly [46]. Immature virions are assembled in the endoplasmic reticulum and transfer to the Golgi apparatus through budding for maturation, until their final release into the extracellular space [15,46].
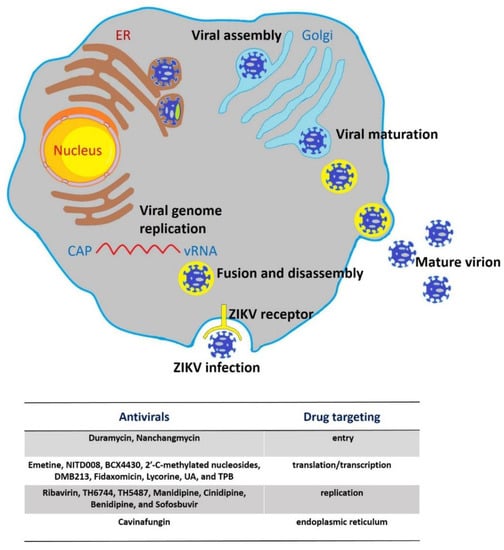
Figure 3.
Life cycle of ZIKV. Virions attach to the cell surface receptors and subsequently enter cells by receptor-mediated endocytosis and are internalized into clathrin-coated vesicles. Endosome acidification triggers conformational changes, fusion of viral membrane with endosome membrane, and particle disassembly and the viral genomic RNA is released from nucleocapsid into the cytoplasm. The ssRNA then is translated into a single polyprotein that is further processed co-translationally and post-translationally by viral and cellular proteases. This cleavage makes three structural proteins and seven nonstructural proteins. Viral genome replication initiates with negative-strand RNA synthesis, and further serves as a template for the synthesis of the positive-strand genomic RNA. Virus assembly take places in the endoplasmic reticulum (ER) surface by budding, and immature virus particles travel along the host secretory pathway through the trans-Golgi network, where virion maturation occurs followed by release from the cell via exocytosis. Additionally, the below table shows where the inhibitor/antivirals of ZIKV act during the ZIKV life cycle.
4. Transmission Cycle of ZIKV
Similar to DENV and YFV, ZIKV directly circulates between Aedes mosquitos and humans, and thus is capable of epidemic transmission. ZIKV circulation has been documented in two evolutionarily and ecologically distinct transmission cycles: an urban or human cycle, between peridomestic/domestic Aedes spp. mosquitoes and humans; and an enzootic, sylvatic cycle, where ZIKV circulates between non-human primates and Aedes spp. mosquitoes [1,47,48]. Epidemiological evidence suggests that Aedes aegypti is the main vector, and it spreads the virus after sucking the blood of ZIKV-infected individuals. It has high kinship behavior characteristics and is one of the most difficult vectors to control in the urban transmission cycle of ZIKV [49].
In addition to insect-borne transmission, ZIKV can also be transmitted by non-vector means, such as sexual transmission, mother–child transmission, and blood transfusion. ZIKV is generally transmitted between people through sexual contact, mostly from men to women, and viral RNA can be detected in the semen of men [50]. When ZIKV infects pregnant women, it infects the fetus through vertical transmission, and the virus is distributed in the placenta, amniotic fluid, and fetal brain, resulting in fetal intrauterine developmental restriction, congenital malformation, microcephaly, and miscarriage [51,52]. Further, Brazil published a case report of ZIKV transmission through blood transfusion [53]; most patients infected with ZIKV are asymptomatic, whereas approximately 3% of blood donors have positive ZIKV test results, and the virus can survive in whole blood for 2 months, making it easy to spread to blood donors. Because of the diversity and complexity of ZIKV transmission patterns, it is difficult to formulate strategies against ZIKV infection [54]. In addition to human-to-human transmission, it is unclear whether domestic animals could transmit ZIKV via sexual behavior among non-human primates or through mosquitoes, and whether it can be characterized by human sexual transmission needs to be further explored [55,56].
5. Clinical Manifestations of ZIKV Disease
ZIKV is related to other human flaviviruses that cause significant pathology including DENV, YFV, JEV, WNV, TBEV, and Saint Louis encephalitis virus (SLEV) and is most closely related to Spondweni virus (SPONV) [57]. In comparison to the encephalitic flaviviruses such as WNV and TBEV, ZIKV generally is less neuroinvasive in adult patients, and rarely leads encephalitis and meningitis [58]. Regarding the early prevalence of ZIKV infection in humans, approximately 20% of patients show influenza-like symptoms that are similar to self-limited fever, which can subside within a few days. The ZIKV pandemic in 2016 was associated with a variety of serious diseases including thrombocytopenic purpura and dyspnea, which might be related to pathogenic changes in the evolution of ZIKV [59].
In May 2015, the largest ZIKV outbreak in history occurred in Northeastern Brazil, and caused great public panic [60,61]. ZIKV can be transmitted from mother to child; the clinical phenotypes of the latter include cerebral calcification, cortical malformation, ventricular enlargement, delayed myelination [62], and cerebellar brainstem dysplasia [63]. A series of studies has shown that ZIKV has a tendency to infect neural progenitor cells, which might affect the development of the fetal brain, and obstruction is the main mechanism of microcephaly and fetal death [64,65]. Apart from its neurophilic features, ZIKV also results in visceral infection that is similar to DENV and YFV, which attacks the immune system in the body. During the ZIKV epidemic in French Polynesia, Guillain Barré Syndrome was reported many times. This suggests that ZIKV infection targets neurons and glial cells to mediate demyelination of the peripheral nervous system and the pathogenesis of axon malformation; thus, Guillain Barré Syndrome can occur simultaneously with or after ZIKV infection [62].
6. Cellular Models of ZIKV
A great deal of human primary cells and cell lines have been used to deeply explore the tropism and pathogenic mechanisms of ZIKV [4]. Primary cell infection has been demonstrated for neonatal keratinocytes, adult dendritic cells, amniotic epithelial cells, sertoli cells, trophoblast progenitor cells, astrocytes and microglial cells, cytotrophoblasts, and human endothelial stromal cells [66]. Recently, studies demonstrated that ZIKV directly infects human cortical neural progenitor cells with high-level efficiency, causing stunted cell growth and transcriptional dysregulation [67]. In term of cell lines, nonhuman primate cell lines, as well as various human cell lines and small animals cell lines have been used to study ZIKV infection and potential drugs. With regard to human cell lines, such as SF268, Caco-2, and JEG-3 cells, allowed ZIKV replication and formed cytopathic effects (CPE). Moreover, some cell lines, including Hela, Hek, and LNCaP cells, effectively infected ZIKV but did not show CPE [68,69]. Aedes aegypti and albopictus-derived cell lines have also been tested to date with scientific topics on the life cycle of ZIKV [70]. Another recently developed alternative is to culture human neurospheres and brain organoids, which permit ZIKV infection and exhibit reduced viability and growth resembling microcephaly [71,72].
7. Animal Models of ZIKV
An intensive effort by the global scientific community has resulted in the deployment of animal models for the study of multiple aspects of ZIKV biology. Similar to animal models of JEV infection, rodents and non-human primates are common animal models to study ZIKV. After ZIKV infects the human body, it partially acts on the STAT2 molecule of the downstream signaling pathway of the Type I interferon receptor through the NS5 protein, resulting in its degradation, which antagonizes the human immune response. However, ZIKV infection has no such effect on mice, which could explain why immunized mice cannot serve as the host of ZIKV [73,74]. Therefore, immunocompetent adult mice have a certain tolerance to lethal ZIKV strains. Newborn mice can be infected by Senegal and Puerto Rico ZIKV strains, which cause 30% mortality, whereas the latter causes a sharp decline in body weight, serious damage to the nervous system, and the death of newborn mice. In addition, T-cells of ZIKV-infected mice infiltrate the central nervous system, which is consistent with other neuroinvasive flavivirus infection phenotypes [73,75].
In adult mice with Type I interferon receptor deficiency (Ifnar1−/− mice), after infection with ZIKV, viruses are enriched in the brain, blood, spleen, kidney, liver, and eyes, which can recapitulate the characteristics of the vertical transmission of ZIKV through the placenta, limb paralysis, and invasive diseases of the nervous system. After ZIKV infection in male Ifnar1−/− mice, the structures of the testicular stroma and vas deferens are seriously damaged [73]. This mouse model is also applicable to the study of the WNV infection mechanism; specifically, Ifnar1−/− mice show severe pathological signs after infection with WNV and generally die within 3–5 days [76]. Adult mice with Type I and Type II interferon receptor knockout (AG129 mice) were infected with French Polynesia, Cambodia, or Porto strains via subcutaneous, intradermal, or peritoneal routes and showed signs of ataxia, tremor, and paralysis, and all three strains had lethal effects. Among them, the French Polynesia strain at a dose of only 1 PFU could cause the death of AG129 mice [77,78,79].
Immunodeficient mice with IRF3/IRF5/IRF7 interferon genes are more susceptible to the ZIKV strain Cambodia, which causes neurological damage, the apoptosis of neural progenitor cells, hind limb weakness, paralysis, and other signs, until the death of ZIKV-infected mice [80]. In addition, after injecting the interferon antibody MAR1-5A3 into wild-type pregnant mice, pregnant mice in this state could be infected with ZIKV. Viral load can be detected in both maternal and fetal bodies, and this animal model is suitable for exploring the mechanism of ZIKV infection [73]. It is worth noting that although this kind of immunodeficient mouse model is helpful for understanding the disease characteristics that are caused by ZIKV infection, it still has some limitations for comprehensively exploring the pathogenic mechanism and immune response to flaviviruses. For example, IFNα/β-dependent viral tropism and the initiation of lymphoid T- and B-cell pathways are not obvious in such animal models. To further explore the immune mechanism that is associated with ZIKV, more animal models need to be studied.
One day after rhesus monkeys are infected with the Polynesia strain of ZIKV, viral RNA could be isolated from their plasma, cerebrospinal fluid, saliva, and urine [81]. In the cynomolgus monkey model, viral RNA is present in the semen and saliva for more than 3 weeks [82]. In addition, ZIKV replication is active, but it does not cause embryo death when inoculating chicken embryos at embryonic age E13 with ZIKV. When the embryonic age was increased to E15 and E20, pathological characteristics such as brain malformation, a decline in brain growth and development, and increased ventricular volume were observed. The chicken embryo model might provide an important basis to study the pathogenic mechanism of ZIKV in developing embryos [83].
9. Conclusions and Future Perspective
Viral pandemics have seriously affected human production and life. Over the past 50 years, more than 300 new or recurrent infectious diseases have been reported as epidemics [123]. In recent years, outbreaks that are caused by ZIKV, DENV, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) have caused concern; however, there are no approved specific therapeutic drugs for these viruses.
YFV, DENV, WNV, and JEV cause an arthropod-borne disease in humans, known as Zika fever. Since the ZIKV outbreak in Brazil in 2015, several scientific communities have engaged in efforts to understand and control ZIKV infection. ZIKV can be transmitted through infected mosquitos, blood transfusion, urine, sexual behavior, and from mother to fetus. ZIKV causes congenital neurological disorders and replicates effectively in reproductive tissues, with the following symptoms: conjunctivitis, skin rash, joint pain, and fever, with an incubation period of approximately 7 days [124]. Some patients also have edema, diarrhea, and body weakness. Moreover, severe phenotypes, including congenital abnormalities in fetuses, microcephaly, and Guillain Barré syndrome are relevant to ZIKV infection, which greatly affect human health [125,126].
While ZIKV cases have dropped from their peak during the 2015–2017 outbreak, the threat of a resurgence of ZIKV due to vector migration, virus mutation, and human travel remains. Given this situation, there is an urgent need to explore novel antiviral targets and develop effective compounds for ZIKV infection [11]. The life cycle of ZIKV depends on host cytokines, cellular signals, and metabolic pathways targeting virus or cell components that are necessary for virus replication; thus, the design of specific ZIKV inhibitors is the primary core goal of modern drug research and development.
As discussed in this perspective, in recent years, extensive novel chemical entities and preclinical investigations of existing compounds have been performed to investigate their antiviral effect on ZIKV in vitro and in vivo system [127], for instance, anti-inflammatory and anticancer molecules, anti-parasitics, and antibiotics. The Table 1 is the summary of the antiviral strategies develop for ZIKV. However, few candidates with inhibitory activity of ZIKV in animal models have advanced into clinical trials, the result would be difficult to extrapolate to humans, it is hard for mostly the test antivirals to accomplish the entire drug development pipeline [128]. Further development of novel compounds as well as effective combination therapies may provide innovative avenues for the treatment of ZIKV infection. Additionally, as the emphatic target population for anti-ZIKV therapeutic strategies would be pregnant women and patients with other medical complications [129]. Therefore, there remains an urgent need for the development of additional effective candidates.

Table 1.
Summary of the antiviral strategies develop for ZIKV.
Furthermore, the DENV and YFV outbreaks also affect broad swaths of several endemic areas for ZIKV. Based on this situation, the development of antivirals that could broadly inhibit multiple flaviviruses infections, may represent an efficient and cost-effective strategy for the treatment of co-infections in endemic regions. Since flaviviruses entry and efficient propagation requires require a series of host factors and cellular metabolic pathways, this offers a valuable way to explore host targets as therapeutic tools that, in some instances, could be broad spectrum inhibitors, whose effect would be less prone to emergence of viral mutants [12,130]. Additionally, for prospective antiviral drug researchers, it is essential to contrast ZIKV with other flaviviruses relatives, with respect to viral pathogenesis and replication, which could lead to the development of antiviral drugs and vaccines for the treatment of ZIKV infection. In the increasingly competitive new drug market environment, it is particularly necessary to use short-term, efficient, and low-cost technology to obtain ideal drugs.
Author Contributions
Conceptualization, J.G. and Y.G.; validation, X.M. and X.X.; writing—original draft preparation, J.G.; writing—review and editing, J.G.; visualization, B.L. and M.W.; supervision, Y.W.; funding acquisition, J.G., X.X. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Health research project of Shaanxi Province (2022D040 to J.G.), the Science and Technology Planning Project of Shaanxi Provincial Education Department (22JK0545 to J.G.), the Foundation for starting scientific research of Doctor of Xi’an Medical University (2021DOC04 to J.G.), the National Science Foundation of China (32070069 to Y.W.), the Natural Science Foundation of Shaanxi Province (2019JZ-38 to X.X.), J.G. is a recipient of start-up postdoctoral fellowship from Xi’an Medical University. The APC was funded by the Foundation for starting scientific research of Doctor of Xi’an Medical University (2021DOC04 to J.G.).
Conflicts of Interest
The authors declare that they have no competing interest in the publication of this review.
References
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Gasco, S.; Muñoz-Fernández, M. A Review on the Current Knowledge on ZIKV Infection and the Interest of Organoids and Nanotechnology on Development of Effective Therapies against Zika Infection. Int. J. Mol. Sci. 2020, 22, 35. [Google Scholar] [CrossRef]
- Smithburn, K.C. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J. Immunol. 1952, 69, 223–234. [Google Scholar] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic char-acterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Hancock, W.T.; Marfel, M.; Bel, M. Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1960. [Google Scholar] [CrossRef] [PubMed]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Kulkarni, M.A.; Brownstein, J.S.; Mekaru, S.R.; Hay, S.I.; Groot, E.; et al. Anticipating the international spread of Zika virus from Brazil. Lancet 2016, 387, 335–336. [Google Scholar] [CrossRef]
- Bernatchez, J.A.; Tran, L.T.; Li, J.; Luan, Y.; Siqueira-Neto, J.L.; Li, R. Drugs for the Treatment of Zika Virus Infection. J. Med. Chem. 2020, 63, 470–489. [Google Scholar] [CrossRef]
- Imperato, P.J. The Convergence of a Virus, Mosquitoes, and Human Travel in Globalizing the Zika Epidemic. J. Community Health 2016, 41, 674–679. [Google Scholar] [CrossRef]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Deng, Y.-Q.; Zou, P.; Wang, Q.; Dai, Y.; Yu, F.; Du, L.; Zhang, N.-N.; Tian, M.; Hao, J.-N.; et al. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat. Commun. 2017, 8, 15672. [Google Scholar] [CrossRef] [PubMed]
- Bullard-Feibelman, K.M.; Govero, J.; Zhu, Z.; Salazar, V.; Veselinovic, M.; Diamond, M.S.; Geiss, B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017, 137, 134–140. [Google Scholar] [CrossRef]
- Cox, B.D.; Stanton, R.A.; Schinazi, R.F. Predicting Zika virus structural biology: Challenges and opportunities for interven-tion. Antivir. Chem. Chemother. 2015, 24, 118–126. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis. Microbiol. Mol. Biol. Rev. 2017, 81, e00055-16. [Google Scholar] [CrossRef]
- Neal, J.W. Flaviviruses are neurotropic, but how do they invade the CNS? J. Infect. 2014, 69, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Hilgenfeld, R. Zika virus NS 1, a pathogenicity factor with many faces. EMBO J. 2016, 35, 2631–2633. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.H.; dos Santos Alves, R.P.; Boscardin, S.B.; de Souza Ferreira, L.C. The dengue virus non-structural 1 protein: Risks and benefits. Virus Res. 2014, 181, 53–60. [Google Scholar] [CrossRef]
- Shi, Y.; Dai, L.; Song, H.; Gao, G.F. Structures of Zika Virus E & NS1: Relations with Virus Infection and Host Immune Responses. Front. Immunol. 2018, 1062, 77–87. [Google Scholar] [CrossRef]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Akey, D.L.; Konwerski, J.R.; Tarrasch, J.T.; Skiniotis, G.; Kuhn, R.J.; Smith, J.L. Extended surface for membrane association in Zika virus NS1 structure. Nat. Struct. Mol. Biol. 2016, 23, 865–867. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, H.; Qi, J.; Liu, Y.; Wang, H.; Su, C.; Shi, Y.; Gao, G.F. Contribution of intertwined loop to membrane association revealed by Zika virus full-length NS 1 structure. EMBO J. 2016, 35, 2170–2178. [Google Scholar] [CrossRef]
- Voßmann, S.; Wieseler, J.; Kerber, R.; Kümmerer, B.M. A basic cluster in the N terminus of yellow fever virus NS2A con-tributes to infectious particle production. J. Virol. 2015, 89, 4951–4965. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Khromykh, A.; Jones, M.; Westaway, E.G. Subcellular Localization and Some Biochemical Properties of the Flavivirus Kunjin Nonstructural Proteins NS2A and NS4A. Virology 1998, 245, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-H.; Tsai, M.-H.; Chao, D.-Y.; Yueh, A.; Peck, K.M.; Cockrell, A.S.; Yount, B.L.; Scobey, T.; Baric, R.S.; Heise, M.T. Scanning Mutagenesis Studies Reveal a Potential Intramolecular Interaction within the C-Terminal Half of Dengue Virus NS2A Involved in Viral RNA Replication and Virus Assembly and Secretion. J. Virol. 2015, 89, 4281–4295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, X.; Zou, J.; Xia, H.; Shan, C.; Chen, X.; Shi, P.-Y. Genetic and biochemical characterizations of Zika virus NS2A protein. Emerg. Microbes Infect. 2019, 8, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.G.; Vasconcelos, P.F.C.; et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Phoo, W.W.; Luo, D. Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol. Sin. 2014, 29, 74–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xing, H.; Xu, S.; Jia, F.; Yang, Y.; Xu, C.; Qin, C.; Shi, L. Zika NS2B is a crucial factor recruiting NS3 to the ER and activating its protease activity. Virus Res. 2019, 275, 197793. [Google Scholar] [CrossRef]
- Chambers, T.J.; Weir, R.C.; Grakoui, A.; McCourt, D.W.; Bazan, J.F.; Fletterick, R.J.; Rice, C.M. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc. Natl. Acad. Sci. USA 1990, 87, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Preugschat, F.; Yao, C.W.; Strauss, J.H. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J. Virol. 1990, 64, 4364–4374. [Google Scholar] [CrossRef]
- Cahour, A.; Falgout, B.; Lai, C.J. Cleavage of the dengue virus polyprotein at the NS3/NS4A and NS4B/NS5 junctions is mediated by viral protease NS2B-NS3, whereas NS4A/NS4B may be processed by a cellular protease. J. Virol. 1992, 66, 1535–1542. [Google Scholar] [CrossRef]
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991, 65, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mohan, P.M.; Padmanabhan, R. Processing and localization of Dengue virus type 2 polyprotein precursor NS3-NS4A-NS4B-NS5. J. Virol. 1992, 66, 7549–7554. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, S.; Joseph, J.S.; Daudenarde, S.; Gatchalian, J.; Cornillez-Ty, C.; Kuhn, P. Serotype-specific structural dif-ferences in the protease-cofactor complexes of the dengue virus family. J. Virol. 2010, 84, 3059–3067. [Google Scholar] [CrossRef]
- Chernov, A.V.; Shiryaev, S.A.; Aleshin, A.E.; Ratnikov, B.I.; Smith, J.W.; Liddington, R.C.; Strongin, A.Y. The Two-component NS2B-NS3 Proteinase Represses DNA Unwinding Activity of the West Nile Virus NS3 Helicase. J. Biol. Chem. 2008, 283, 17270–17278. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Cheng, C.-W.; Yang, T.-C.; Li, S.-W.; Cheng, M.-H.; Wan, L.; Lin, Y.-J.; Lai, C.-H.; Lin, W.-Y.; Kao, M.-C. Interferon antagonist function of Japanese encephalitis virus NS4A and its interaction with DEAD-box RNA helicase DDX42. Virus Res. 2008, 137, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Naik, N.G.; Wu, H.-N. Mutation of Putative N-Glycosylation Sites on Dengue Virus NS4B Decreases RNA Replication. J. Virol. 2015, 89, 6746–6760. [Google Scholar] [CrossRef]
- Umareddy, I.; Chao, A.; Sampath, A.; Gu, F.; Vasudevan, S.G. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 2006, 87, 2605–2614. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Baz, M.; Boivin, G. Antiviral Agents in Development for Zika Virus Infections. Pharmaceuticals 2019, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef]
- Van Leur, S.W.; Heunis, T.; Munnur, D.; Sanyal, S. Pathogenesis and virulence of flavivirus infections. Virulence 2021, 12, 2814–2838. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika Virus from Aedes Aegypti Mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Weaver, S.C. Flavivirus transmission focusing on Zika. Curr. Opin. Virol. 2017, 22, 30–35. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- Bonaldo, M.C.; Ribeiro, I.P.; Lima, N.S.; dos Santos, A.A.C.; Menezes, L.S.R.; da Cruz, S.O.D.; de Mello, I.S.; Furtado, N.D.; de Moura, E.E.; Damasceno, L.; et al. Isolation of Infective Zika Virus from Urine and Saliva of Patients in Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004816. [Google Scholar] [CrossRef]
- Song, B.H.; Yun, S.I.; Woolley, M.; Lee, Y.M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef]
- Victora, C.G.; Schuler-Faccini, L.; Matijasevich, A.; Ribeiro, E.; Pessoa, A.; Barros, F.C. Microcephaly in Brazil: How to in-terpret reported numbers? Lancet 2016, 387, 621–624. [Google Scholar] [CrossRef]
- Magnus, M.M.; Espósito, D.L.A.; Costa, V.A.D.; Melo, P.S.; Costa-Lima, C.; Fonseca, B.; Addas-Carvalho, M. Risk of Zika virus transmission by blood donations in Brazil. Hematol. Transfus. Cell Ther. 2018, 40, 250–254. [Google Scholar] [CrossRef]
- Lustig, Y.; Mendelson, E.; Paran, N.; Melamed, S.; Schwartz, E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Eurosurveillance 2016, 21, pii=30269. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugières, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Karimi, O.; Goorhuis, A.; Schinkel, J.; Codrington, J.; Vreden, S.G.S.; Vermaat, J.S.; Stijnis, C.; Grobusch, M.P. Thrombocyto-penia and subcutaneous bleedings in a patient with Zika virus infection. Lancet 2016, 387, 939–940. [Google Scholar] [CrossRef]
- Gyawali, N.; Bradbury, R.S.; Taylor-Robinson, A.W. The global spread of Zika virus: Is public and media concern justified in regions currently unaffected? Infect. Dis. Poverty 2016, 5, 37. [Google Scholar] [CrossRef]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martínez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N. WHO Zika Causality Working Group Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.; Bukhari, W.; Todd, A.; Gunn, W.; Huang, Q.S.; Timmings, P. Acute Zika infection with concurrent onset of Guil-lain-Barré Syndrome. Neurology 2016, 87, 1623–1624. [Google Scholar] [CrossRef]
- De Fatima Vasco Aragao, M.; van der Linden, V.; Brainer-Lima, A.M.; Coeli, R.R.; Rocha, M.A.; Sobral da Silva, P.; Durce Costa Gomes de Carvalho, M.; van der Linden, A.; Cesario de Holanda, A.; Valenca, M.M. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: Retrospective case series study. BMJ 2016, 353, i1901. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Diamond, M.S.; Mysorekar, I.U. Maternal-Fetal Transmission of Zika Virus: Routes and Signals for Infection. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2017, 37, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Troumani, Y.; Touhami, S.; Jackson, T.L.; Ventura, C.V.; Stanescu-Segall, D.M.; Errera, M.-H.; Rousset, D.; Bodaghi, B.; Cartry, G.; David, T.; et al. Association of Anterior Uveitis with Acute Zika Virus Infection in Adults. JAMA Ophthalmol. 2021, 139, 95. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.J.; Miranda Guarines, K.; Duarte Silva, A.J.; Sales Leal, L.R.; Mendes Félix, D.; Silva, A.; de Oliveira, S.A.; Junqueira Ayres, C.F.; Júnior, A.S.; de Freitas, A.C. In vitro and in vivo models for studying Zika virus biology. J. Gen. Virol. 2018, 99, 1529–1550. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef]
- Chan, J.F.; Yip, C.C.; Tsang, J.O.; Tee, K.M.; Cai, J.P.; Chik, K.K.; Zhu, Z.; Chan, C.C.; Choi, G.K.; Sridhar, S.; et al. Differential cell line susceptibility to the emerging Zika virus: Implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg. Microbes Infect. 2016, 5, e93. [Google Scholar] [CrossRef] [PubMed]
- Bierle, C.J.; Fernández-Alarcón, C.; Hernandez-Alvarado, N.; Zabeli, J.C.; Janus, B.C.; Putri, D.; Schleiss, M.R. Assessing Zika virus replication and the development of Zika-specific antibodies after a mid-gestation viral challenge in guinea pigs. PLoS ONE 2017, 12, e0187720. [Google Scholar] [CrossRef]
- Schultz, M.J.; Isern, S.; Michael, S.F.; Corley, R.B.; Connor, J.H.; Frydman, H.M. Variable Inhibition of Zika Virus Replication by Different Wolbachia Strains in Mosquito Cell Cultures. J. Virol. 2017, 91, e00339-17. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Patho-genesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Grant, A.; Ponia Sanket, S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz Megan, C.; Sánchez-Seco Mari, P.; Evans Matthew, J.; Best Sonja, M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Ireland, D.D.C.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 2016, 12, e1006004. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Daffis, S.; Brien, J.D.; Gainey, M.D.; Yokoyama, W.M.; Sheehan, K.C.F.; Murphy, K.M.; Schreiber, R.D.; Diamond, M.S. A Temporal Role of Type I Interferon Signaling in CD8+ T Cell Maturation during Acute West Nile Virus Infection. PLoS Pathog. 2011, 7, e1002407. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Duggal, N.K.; Bullard-Feibelman, K.; Veselinovic, M.; Romo, H.; Nguyen, C.; Rückert, C.; Brault, A.C.; Bowen, R.A.; Stenglein, M.; et al. Development and Characterization of Recombinant Virus Generated from a New World Zika Virus Infectious Clone. J. Virol. 2017, 91, e01765-16. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Regla-Nava, J.A.; Chai, G.; Sheets, N.; Tang, W.; Terskikh, A.V.; Shresta, S.; Gleeson, J.G. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell 2016, 19, 593–598. [Google Scholar] [CrossRef]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef] [PubMed]
- Osuna, C.E.; Lim, S.-Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.W.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, F.T.; Tesla, B.; Simchick, G.; Zhao, Q.; Hodge, T.; Brindley, M.A.; Stice, S.L. Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev. 2016, 25, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, E.; Einav, S. Combating emerging viral threats. Science 2015, 348, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, N.; Soma, R.; Hidano, S.; Watanabe, K.; Umekita, H.; Fukuda, C.; Noguchi, K.; Gendo, Y.; Ozaki, T.; Sonoda, A.; et al. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antivir. Res. 2017, 146, 1–11. [Google Scholar] [CrossRef]
- Kim, J.-A.; Seong, R.-K.; Kumar, M.; Shin, O.S. Favipiravir and Ribavirin Inhibit Replication of Asian and African Strains of Zika Virus in Different Cell Models. Viruses 2018, 10, 72. [Google Scholar] [CrossRef]
- Pettke, A.; Tampere, M.; Pronk, R.; Wallner, O.; Falk, A.; Berglund, U.W.; Helleday, T.; Mirazimi, A.; Puumalainen, M.-R. Broadly Active Antiviral Compounds Disturb Zika Virus Progeny Release Rescuing Virus-Induced Toxicity in Brain Organoids. Viruses 2020, 13, 37. [Google Scholar] [CrossRef]
- Richard, A.S.; Zhang, A.; Park, S.J.; Farzan, M.; Zong, M.; Choe, H. Virion-Associated Phosphatidylethanolamine Promotes TIM1-Mediated Infection by Ebola, Dengue, and West Nile Viruses. Proc. Natl. Acad. Sci. USA 2015, 112, 14682–14687. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef]
- Rausch, K.; Hackett, B.A.; Weinbren, N.L.; Reeder, S.; Sadovsky, Y.; Hunter, C.A.; Schultz, D.C.; Coyne, C.B.; Cherry, S. Screening Bioactives Reveals Nanchangmycin as a Broad Spectrum Antiviral Active against Zika Virus. Cell Rep. 2017, 18, 804–815. [Google Scholar] [CrossRef]
- Zhang, R.; Miner, J.J.; Gorman, M.J.; Rausch, K.; Ramage, H.; White, J.P.; Zuiani, A.; Zhang, P.; Fernandez, E.; Zhang, Q.; et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 2016, 535, 164–168. [Google Scholar] [CrossRef]
- Estoppey, D.; Lee, C.M.; Janoschke, M.; Lee, B.H.; Wan, K.F.; Dong, H.; Mathys, P.; Filipuzzi, I.; Schuhmann, T.; Riedl, R.; et al. The Natural Product Cavinafungin Selec-tively Interferes with Zika and Dengue Virus Replication by Inhibition of the Host Signal Peptidase. Cell Rep. 2017, 19, 451–460. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Guo, J.; Wang, P.; Zhang, L.; Xiao, G.; Wang, W. Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Gardinali, N.R.; Marchevsky, R.S.; Oliveira, J.M.; Pelajo-Machado, M.; Kugelmeier, T.; Castro, M.P.; Silva, A.C.; Pinto, D.P.; Fonseca, L.B.; Vilhena, L.S.; et al. Sofosbuvir shows a protective effect against vertical transmission of Zika virus and the associated congenital syndrome in rhesus monkeys. Antivir. Res. 2020, 182, 104859. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, M.; Lee, E.M.; Gorshkov, K. Emetine inhibits Zika and Ebola virus infections through two molecular mecha-nisms: Inhibiting viral replication and decreasing viral entry. Cell Discov. 2018, 4, 31. [Google Scholar] [PubMed]
- Yin, Z.; Chen, Y.L.; Schul, W.; Wang, Q.Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A.; et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef]
- Deng, Y.-Q.; Zhang, N.-N.; Li, C.-F.; Tian, M.; Hao, J.-N.; Xie, X.-P.; Shi, P.-Y.; Qin, C.-F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef]
- Julander, J.G.; Bantia, S.; Taubenheim, B.R.; Minning, D.M.; Kotian, P.; Morrey, J.D.; Smee, D.F.; Sheridan, W.P.; Babu, Y.S. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a Hamster model. Antimicrob. Agents Chemo-Ther. 2014, 58, 6607–6614. [Google Scholar] [CrossRef]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Siddharthan, V.; Evans, J.; Taylor, R.; Tolbert, K.; Apuli, C.; Stewart, J.; Collins, P.; Gebre, M.; Neilson, S.; et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017, 137, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hercík, K.; Kozak, J.; Šála, M.; Dejmek, M.; Hřebabecký, H.; Zborníková, E.; Smola, M.; Ruzek, D.; Nencka, R.; Boura, E. Adenosine triphosphate analogs can efficiently inhibit the Zika virus RNA-dependent RNA polymerase. Antivir. Res. 2017, 137, 131–133. [Google Scholar] [CrossRef]
- Xu, H.-T.; Hassounah, S.A.; Colby-Germinario, S.P.; Oliveira, M.; Fogarty, C.; Quan, Y.; Han, Y.; Golubkov, O.; Ibanescu, I.; Brenner, B.; et al. Purification of Zika virus RNA-dependent RNA polymerase and its use to identify small-molecule Zika inhibitors. J. Antimicrob. Chemother. 2017, 72, 727–734. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, J.; Huang, Y.; He, Z.; Luo, J.; Wu, Y.; Zheng, Y.; Wu, J.; Zhu, X.; Wang, H.; et al. Antibiotic fidaxomicin is an RdRp inhibitor as a potential new therapeutic agent against Zika virus. BMC Med. 2020, 18, 204. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, Biology, and Medicinal Potential of Narciclasine and its Congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef]
- Chen, H.; Lao, Z.; Xu, J.; Li, Z.; Long, H.; Li, D.; Lin, L.; Liu, X.; Yu, L.; Liu, W.; et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 2020, 546, 88–97. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Song, W.; Si, S.; Han, Y.; Jiang, J. Identification and characterization of Zika virus NS5 RNA-dependent RNA polymerase inhibitors. Int. J. Antimicrob. Agents 2019, 54, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, A.; Palermo, N.; Sahoo, B.R.; Yuan, Z.; Hu, D.; Annamalai, A.S.; Vu, H.L.; Correas, I.; Prathipati, P.K.; Destache, C.J.; et al. Discovery of a non-nucleoside RNA polymerase inhibitor for blocking Zika virus replication through in silico screening. Antivir. Res. 2018, 151, 78–86. [Google Scholar] [CrossRef]
- Rehman, A.; Ashfaq, U.A.; Javed, M.R.; Shahid, F.; Noor, F.; Aslam, S. The Screening of Phytochemicals Against NS5 Poly-merase to Treat Zika Virus Infection: Integrated Computational Based Approach. Comb. Chem. High Throughput Screen. 2022, 25, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-J.; Nguyen, T.T.H.; Kim, N.M.; Park, J.-S.; Jang, T.-S.; Kim, D. Inhibitory effect of flavonoids against NS2B-NS3 protease of ZIKA virus and their structure activity relationship. Biotechnol. Lett. 2017, 39, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Ishida, Y.; Oishi, S.; Fujii, N.; Watanabe, S.; Vasudevan, S.G.; Tajima, S.; Takasaki, T.; Suzuki, Y.; Ichiyama, K.; et al. Novel antiviral activity of bromocriptine against dengue virus replication. Antivir. Res. 2016, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Chik, K.K.-H.; Yuan, S.; Yip, C.C.-Y.; Zhu, Z.; Tee, K.-M.; Tsang, J.O.-L.; Chan, C.C.-S.; Poon, V.K.-M.; Lu, G.; et al. Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antivir. Res. 2017, 141, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhou, R.; Huang, C.; Zhang, R.; Wang, J.; Zhang, Y.; Ding, J.; Li, X.; Zhou, J.; Cen, S. Identification of Theafla-vin-3,3′-Digallate as a Novel Zika Virus Protease Inhibitor. Front. Pharmacol. 2020, 11, 514313. [Google Scholar] [CrossRef]
- Brecher, M.; Li, Z.; Liu, B.; Zhang, J.; Koetzner, C.A.; Alifarag, A.; Jones, S.A.; Lin, Q.; Kramer, L.D.; Li, H. A conformational switch high-throughput screening assay and allosteric inhibition of the flavivirus NS2B-NS3 protease. PLoS Pathog. 2017, 13, e1006411. [Google Scholar] [CrossRef]
- Zhang, F.; Hammack, C.; Ogden, S.C.; Cheng, Y.; Lee, E.M.; Wen, Z.; Qian, X.; Nguyen, H.N.; Li, Y.; Yao, B.; et al. Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016, 44, 8610–8620. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Abrams, R.P.M.; Yasgar, A.; Teramoto, T.; Lee, M.-H.; Dorjsuren, D.; Eastman, R.T.; Malik, N.; Zakharov, A.V.; Li, W.; Bachani, M.; et al. Therapeutic candidates for the Zika virus identified by a high-throughput screen for Zika protease inhibitors. Proc. Natl. Acad. Sci. USA 2020, 117, 31365–31375. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, J.F.-W.; Den-Haan, H.; Chik, K.K.-H.; Zhang, A.J.; Chan, C.C.-S.; Poon, V.K.-M.; Yip, C.C.-Y.; Mak, W.W.-N.; Zhu, Z.; et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antivir. Res. 2017, 145, 33–43. [Google Scholar] [CrossRef]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; A Koetzner, C.; Allen, C.; A Jones, S.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Sam, I.-C.; Chong, W.L.; Lee, V.S.; Chan, Y.F. Polysulfonate suramin inhibits Zika virus infection. Antivir. Res. 2017, 143, 186–194. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Lum, F.M.; Low, D.K.; Fan, Y.; Tan, J.J.; Lee, B.; Chan, J.K.; Renia, L.; Ginhoux, F.; Ng, L.F. Zika Virus Infects Human Fetal Brain Microglia and Induces Inflammation. Clin. Infect. Dis. 2017, 64, 914–920. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popovic, M.; Poljsak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodusek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.-K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Tan, L.; Cederquist, G.Y.; Fan, Y.; Hartley, B.J.; Mukherjee, S.; Tomishima, M.; Brennand, K.J.; Zhang, Q.; Schwartz, R.E.; et al. High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain. Cell Stem Cell 2017, 21, 274–283.e275. [Google Scholar] [CrossRef] [PubMed]
- White, M.K.; Wollebo, H.S.; David Beckham, J.; Tyler, K.L.; Khalili, K. Zika virus: An emergent neuropathological agent. Ann. Neurol. 2016, 80, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: Dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).