Therapeutic Effects of a Dry Powder Prepared from the Green Microalga Coccomyxa sp. KJ in Mice Infected with Influenza A Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Material
2.2. Cell Line and Virus
2.3. Mice
2.4. Murine Influenza Infection
2.5. Neutralizing Antibody Assay
2.6. Statistics
3. Results
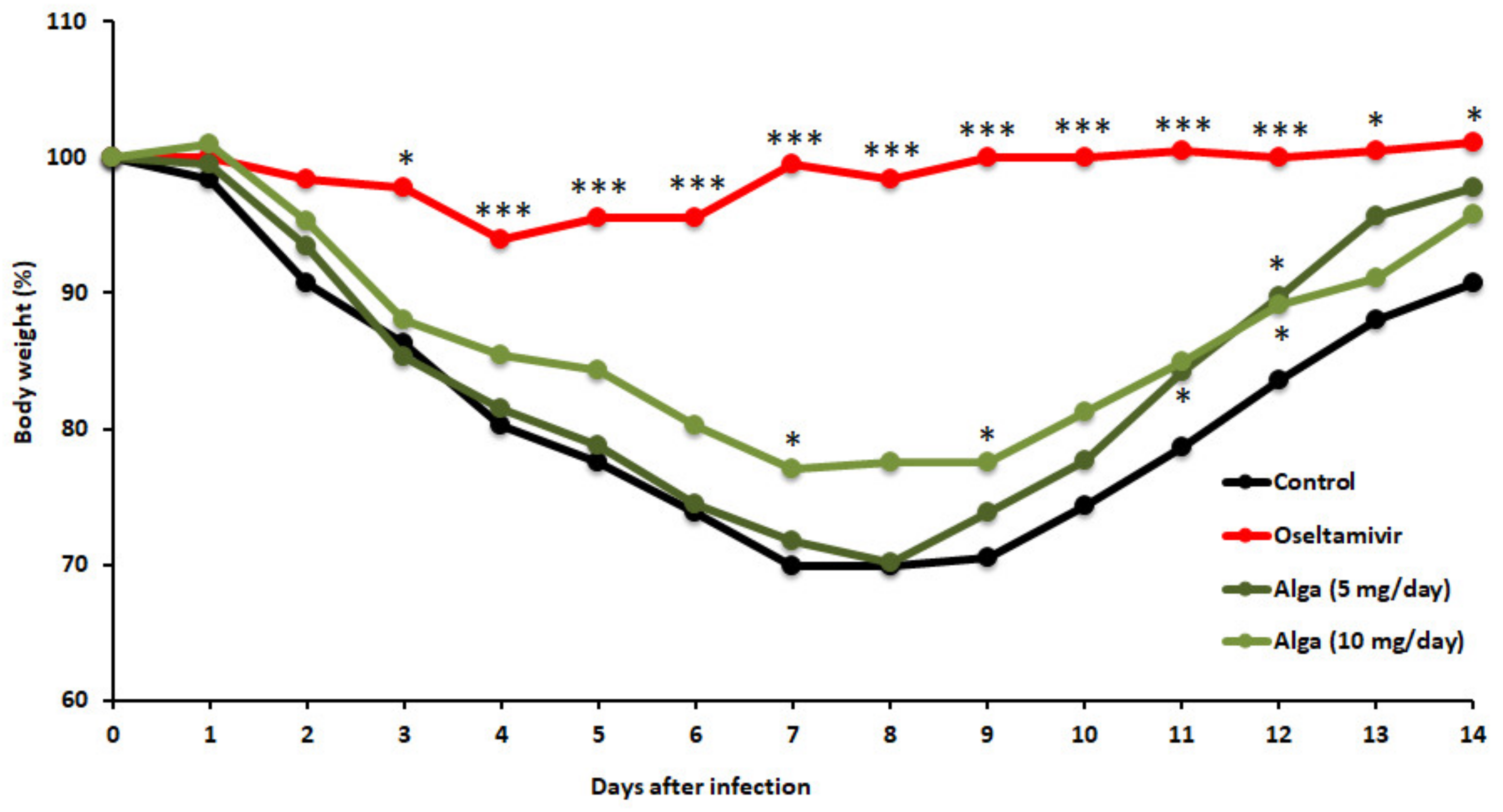
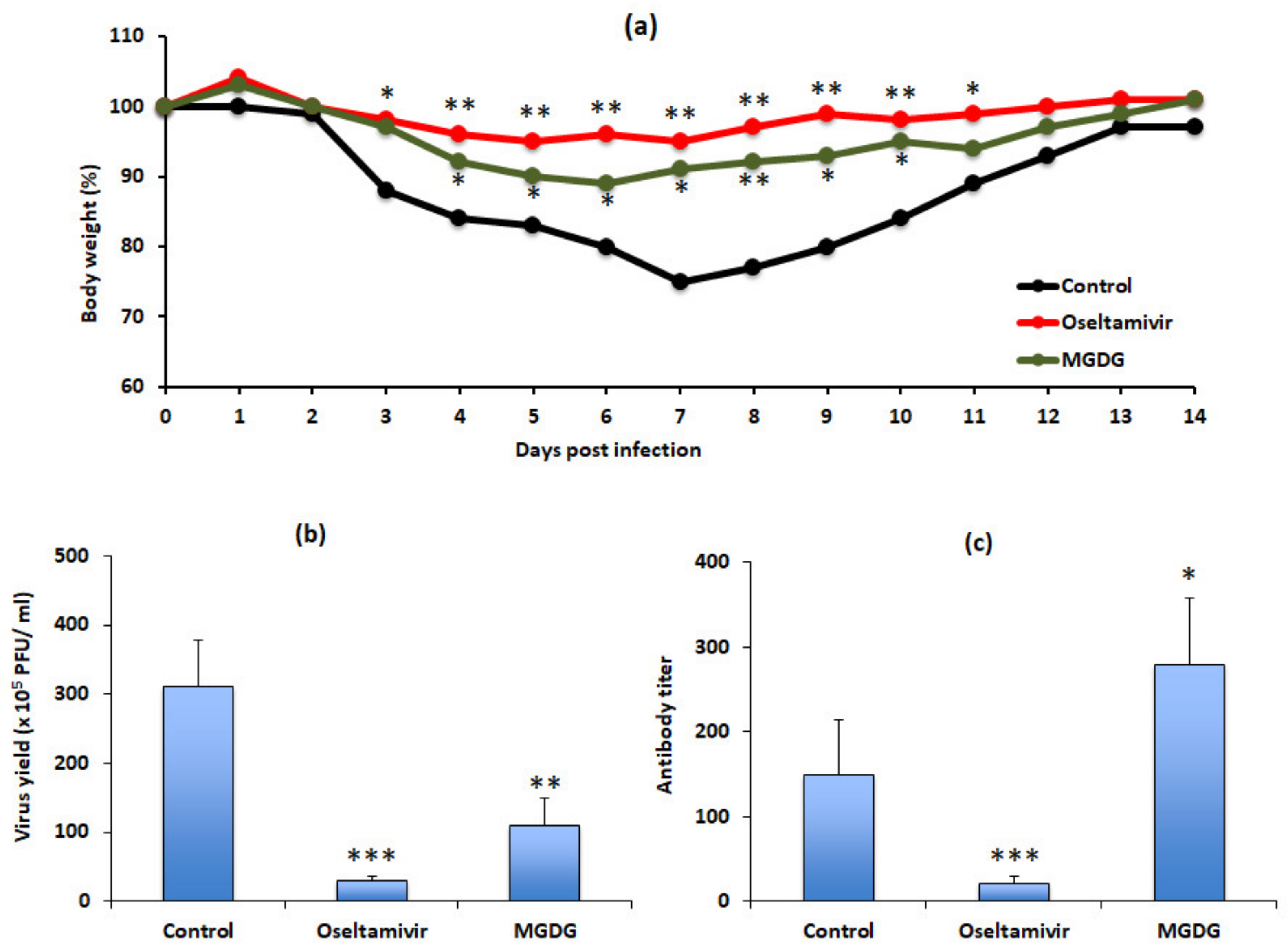
3.1. Algal Powder Attenuates Body Weight Decrease after IFV Infection
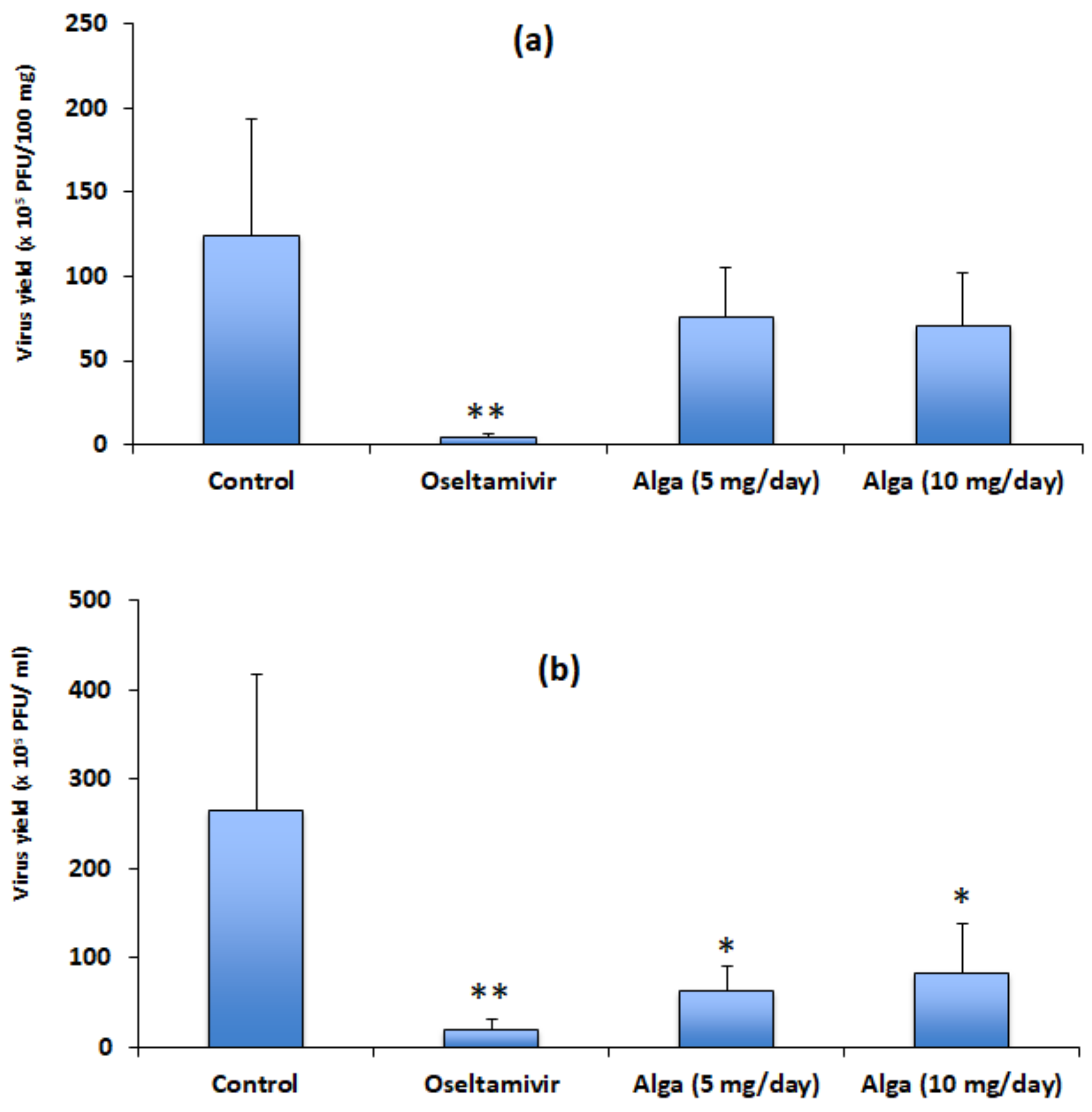
3.2. Algal Powder Inhibits Viral Replication
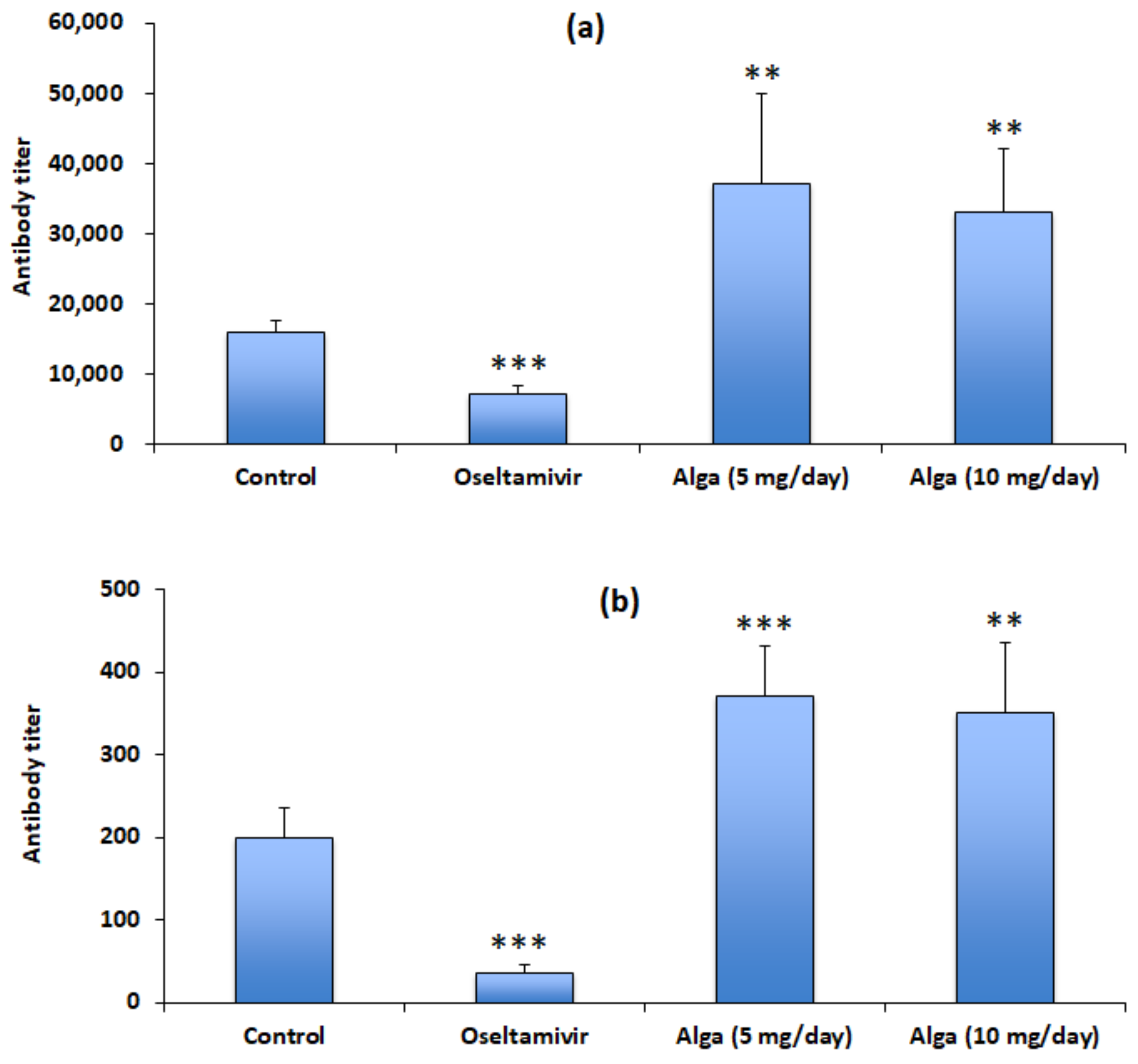
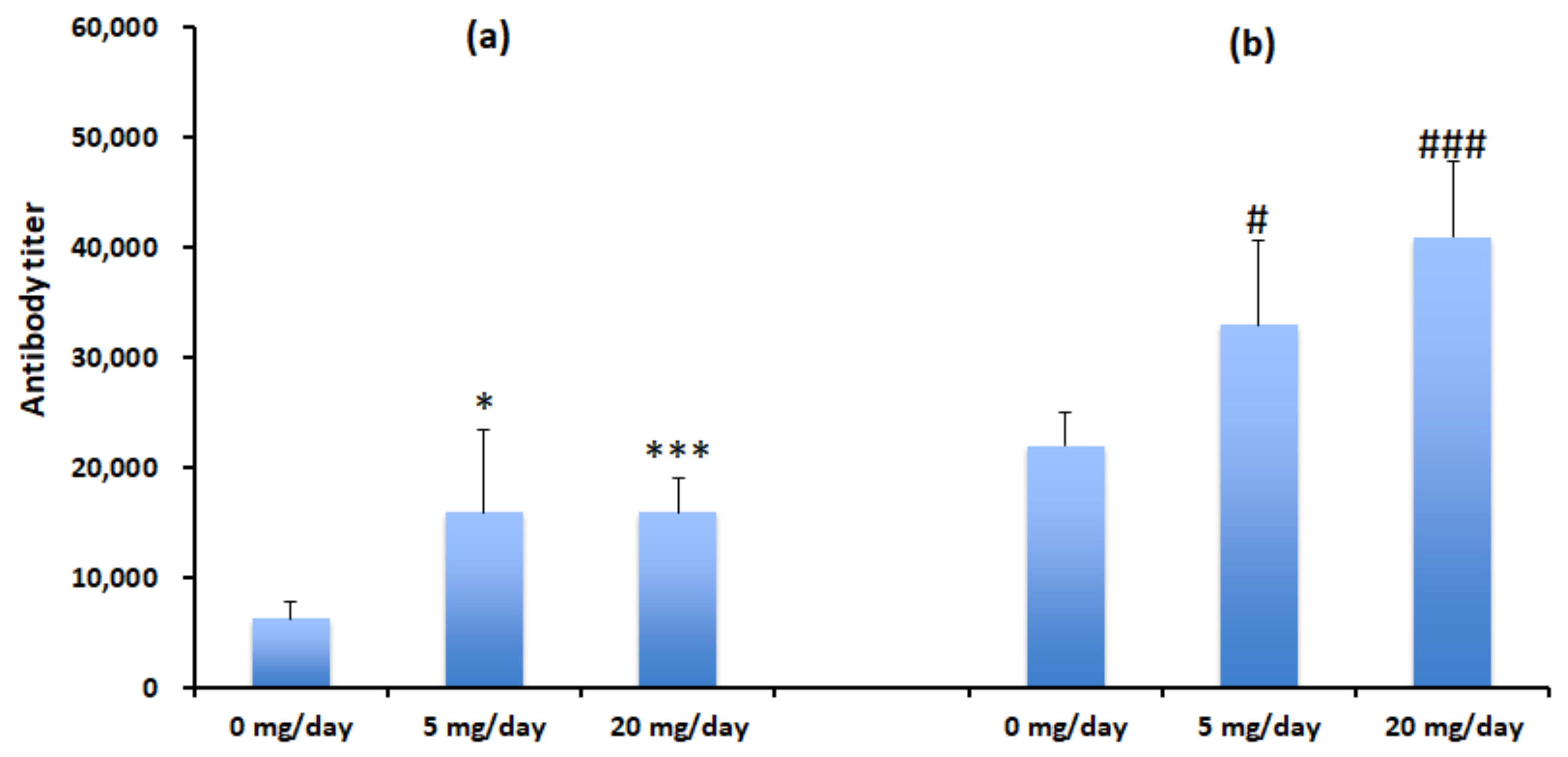
3.3. Algal Powder Increases Neutralizing Antibody Responses
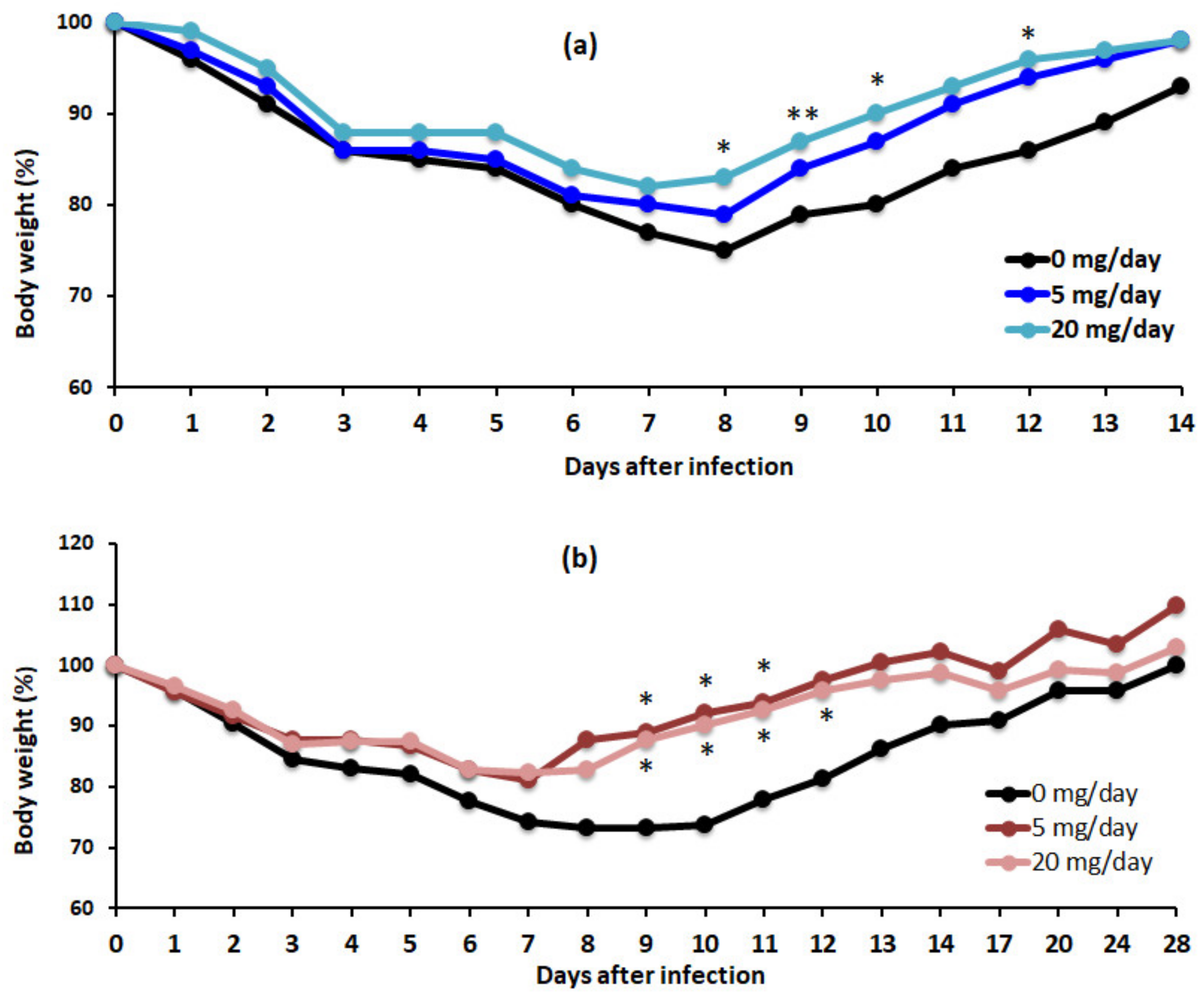
3.4. Algal Powder Increases Weight and Antibody Production at 20 mg/day
3.5. MGDG Accounts for Algal Powder’s Therapeutic Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taubenberger, J.K.; Morens, D.M. The pathology of influenza virus infections. Ann. Rev. Pathol. 2008, 3, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Grounder, A.P.; Boon, A.C.M. Influenza pathogenesis: The effect of host factors on severity of disease. J. Immunol. 2019, 202, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Hurt, A.C.; Kelly, H. Debate regarding oseltamivir use for seasonal and pandemic influenza. Emerg. Infect. Dis. 2016, 22, 949–955. [Google Scholar] [CrossRef]
- Heneghan, C.J.; Onakpoya, I.; Thompson, M.; Spencer, E.A.; Jones, M.; Jefferson, T. Zanamivir for influenza in adults and children: Systematic review of clinical study reports and summary of regulatory comments. BMJ 2014, 384, g2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Takashita, E.; Kawakami, C.; Morita, H.; Ogawa, R.; Fujisaki, S.; Shirakura, M.; Miura, H.; Nakamura, K.; Kishida, N.; Kuwahara, T.; et al. Detection of influenza A (H3N2) viruses exhibiting reduced susceptibility to the novel cap-dependent endonuclease inhibitor baloxavir in Japan, December 2018. Eurosurveillance 2019, 24, 1800698. [Google Scholar] [CrossRef] [Green Version]
- Satoh, A.; Kato, M.; Yamamoto, K.; Ishibashi, M.; Sekiguchi, H.; Kurano, N.; Miyachi, S. Characterization of the Lipid Accumulation in a New Microalgal Species, Pseudochoricystis ellipsoidea (Trebouxiophyceae). J. Jpn. Inst. Energy 2010, 89, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Kasai, Y.; Oshima, K.; Ikeda, F.; Abe, J.; Yoshimitsu, Y.; Harayama, S. Construction of a self-cloning system in the unicellular green alga Pseudochoricystis ellipsoidea. Biotechnol. Biofuels 2015, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Komatsu, S.; Kuno, H.; Asai, S.; Matsuura, I.; Kudkyal, V.R.; Kawahara, T. Virucidal and immunostimulating activities of monogalactosyl diacyglyceride from Coccomyxa sp. KJ, a green microalga, against murine norovirus and feline calicivirus. Mar. Drugs 2022, 20, 131. [Google Scholar] [CrossRef]
- Hayashi, K.; Asai, S.; Umezawa, K.; Kakizoe, H.; Miyachi, H.; Morita, M.; Akaike, T.; Kuno, H.; Komatsu, S.; Watanabe, T.; et al. Virucidal effect of monogalactosyl diacyglyceride from a green microalga, Coccomyxa sp. KJ, against clinical isolates of SARS-CoV-2 as assessed by a plaque assay. J. Clin. Lab. Anal. 2022, 36, e24146. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.-B.; Atsumi, K.; Kanazashi, M.; Shibayama, T.; Okamoto, K.; Kawahara, T.; Hayashi, T. In vitro and in vivo anti-herpes simplex virus activity of monogalactosyl diacylglyceride from Coccomyxa sp. KJ (IPOD FERM BP-22254), a green microalga. PLoS ONE 2019, 14, e0219305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Hayashi, K.; Nishiguchi, M.; Hayashi, T.; Iinuma, M. Resveratrol oligomer C-glucosides and antiviral resveratrol tetramers isolated from the stem bark of Shorea uliginosa. Phytochem. Lett. 2018, 28, 1–7. [Google Scholar] [CrossRef]
- Clements, M.L.; Betts, R.F.; Tierney, E.L.; Murphy, B.R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild virus. J. Clin. Microbiol. 1986, 24, 157–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tophan, D.J.; Tripp, R.A.; Doherty, P.C. CD8+ T cells clear influenza virus by perforin or Fas-dependent process. J. Immunol. 1997, 159, 5197–5200. [Google Scholar]
- Burleson, G.R.; Burleson, F.G. Influenza virus host resistance model. Methods 2007, 41, 31–37. [Google Scholar] [CrossRef]
- Lee, C.-C.; Yang, C.-Y.; Lin, L.-L.; Ko, T.-P.; Chang, A.H.-L.; Chang, S.S.-C.; Wang, A.H.-J. An effective neutralizing antibody against influenza virus H1N1 from human B cells. Sci. Rep. 2019, 9, 4546. [Google Scholar] [CrossRef]
- Padilla-Quirarte, H.O.; Lopez-Guerrero, D.V.; Gutierrez-Xicotencatl, L.; Esquivel-Guandarrama, F. Protective antibodies against influenza proteins. Front. Immunol. 2019, 10, 1677. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.M.; Ferres, M.; Labarca, J.A. Pandemic (H1N1) 2009 reinfection, Chile. Emerg. Infect. Dis. 2010, 16, 156–157. [Google Scholar] [CrossRef]
- Ohshima, S.; Komatsu, S.; Kashiwagi, H.; Goto, Y.; Ohno, Y.; Yamada, S.; Kanno, A.; Shimizu, T.; Seki, T.; Yasuda, A.; et al. Coccomyxa sp. KJ extract affects the fate of T cells stimulated by toxic shock syndrome toxin-1, a superantigen secreted by Staphylococcus aureus. Microbiol. Immunol. 2022, 12982. [Google Scholar] [CrossRef]
- Terasawa, M.; Hayashi, K.; Lee, J.-B.; Nishiura, K.; Matsuda, K.; Hayashi, T.; Kawahara, T. Anti-influenza A virus activity of rhamnan sulfate from green algae Monostroma nitidum in mice with normal and compromised immunity. Mar. Drugs 2020, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A Viruses. Carbohydr. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.-B.; Nakano, T.; Hayashi, T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013, 15, 302–309. [Google Scholar] [CrossRef]
- Lee, J.-B.; Koizumi, S.; Hayashi, K.; Hayashi, T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010, 81, 572–577. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hao, C.; Yunjia, Y.; Qin, L.; He, M.; Mao, W. Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr. Polym. 2018, 200, 43–53. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.; Ross, G.D. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Murakami, K.; Nishimura, I.; Nakano, T.; Obata, A. A sulfated polysaccharide, fucoidan, enhances the immunomodulatory effects of lactic acid bacteria. Int. J. Mol. Med. 2012, 29, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, H. Intestinal M cells. J. Biochem. 2016, 159, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Kuno, H.; Komatsu, S.; Lee, J.-B.; Kawahara, T. Therapeutic Effects of a Dry Powder Prepared from the Green Microalga Coccomyxa sp. KJ in Mice Infected with Influenza A Virus. Appl. Microbiol. 2022, 2, 481-491. https://doi.org/10.3390/applmicrobiol2030037
Hayashi K, Kuno H, Komatsu S, Lee J-B, Kawahara T. Therapeutic Effects of a Dry Powder Prepared from the Green Microalga Coccomyxa sp. KJ in Mice Infected with Influenza A Virus. Applied Microbiology. 2022; 2(3):481-491. https://doi.org/10.3390/applmicrobiol2030037
Chicago/Turabian StyleHayashi, Kyoko, Hitoshi Kuno, Satoko Komatsu, Jung-Bum Lee, and Toshio Kawahara. 2022. "Therapeutic Effects of a Dry Powder Prepared from the Green Microalga Coccomyxa sp. KJ in Mice Infected with Influenza A Virus" Applied Microbiology 2, no. 3: 481-491. https://doi.org/10.3390/applmicrobiol2030037
APA StyleHayashi, K., Kuno, H., Komatsu, S., Lee, J.-B., & Kawahara, T. (2022). Therapeutic Effects of a Dry Powder Prepared from the Green Microalga Coccomyxa sp. KJ in Mice Infected with Influenza A Virus. Applied Microbiology, 2(3), 481-491. https://doi.org/10.3390/applmicrobiol2030037




