Recent Advances in the Application of Essential Oils as Potential Therapeutic Candidates for Candida-Related Infections
Abstract
1. Introduction
2. Mode of Action of EOs
2.1. Phenolic Terpenes
2.2. Cyclic Terpenes
2.3. Aldehyde Terpenes
3. Activity of EOs against Drug-Resistant Candida spp.
4. Combinative Therapies against Candidiasis Infections
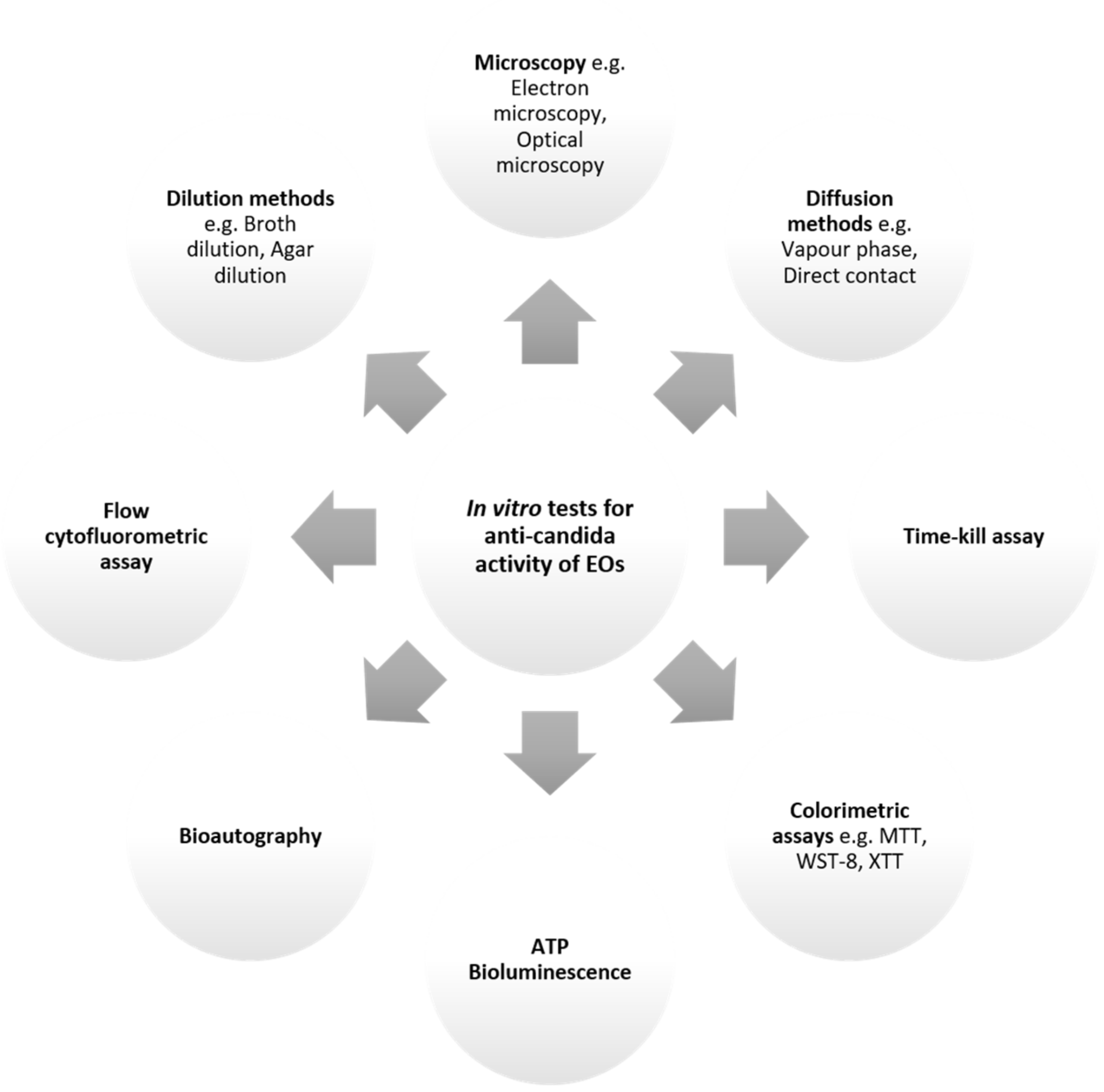
5. Approaches to Investigate Anti-Candida Activity of EOs
5.1. In Vitro Methods
5.1.1. Diffusion Assays
5.1.2. Dilution Assays
5.1.3. Time-Kill Assays
5.1.4. Colorimetric Methods
5.1.5. ATP Bioluminescence Assay
5.1.6. Direct Bioautography
5.1.7. Flow Cytofluorometric Method
5.1.8. Microscopy Assays
5.2. In Vivo Methods
5.2.1. Mammalian Models
Findings from In Vivo Studies Investigating Anti-Candida Activity of EOs
6. Clinical Trials of Therapeutic Formulations with EOs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.C.; Joosten, L.A.B.; Kullberg, B.J.; Netea, M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012, 80, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.P.; van de Veerdonk, F.L.; Kullberg, B.J.; Netea, M.G. Genetic susceptibility to Candida infections. EMBO Mol. Med. 2013, 5, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Zomorodian, K.; Nouraei, H.; Zareshahrabadi, Z.; Barzegar, S.; Zare, M.R.; Pakshir, K. Prevalence of superficial-cutaneous fungal infections in Shiraz, Iran: A five-year retrospective study (2015–2019). J. Clin. Lab. Anal. 2021, 35, e23850. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Invasive Candidiasis Statistics. Available online: https://www.cdc.gov/fungal/diseases/candidiasis/invasive/statistics.html (accessed on 25 May 2022).
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Fraser, V.J.; Jones, M.; Dunkel, J.; Storfer, S.; Medoff, G.; Claiborne Dunagan, W. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 1992, 15, 414–421. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Yusuff, H.; Porter, R. Fungal infections in adult patients on extracorporeal life support. Crit. Care 2018, 22, 98. [Google Scholar] [CrossRef]
- Deorukhkar, S.C.; Saini, S. Why Candida species have emerged as important nosocomial pathogens? Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 533–545. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Singh-Babak, S.D.; Babak, T.; Diezmann, S.; Hill, J.A.; Xie, J.L.; Chen, Y.L.; Poutanen, S.M.; Rennie, R.P.; Heitman, J.; Cowen, L.E. Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog. 2012, 8, e1002718. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-resistant Candida auris: ‘New kid on the block’ in hospital-associated infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Berman, J.; Novikov, A.; Bash, E.; Shachor-Meyouhas, Y.; Zakin, S.; Maor, Y.; Tarabia, J.; Schechner, V.; Adler, A.; et al. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg. Infect. Dis. 2017, 23, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential oils in medicine: Principles of therapy. Parassitologia 2008, 50, 89–91. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phyther. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Kole, C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios 1996, 86, 237–246. [Google Scholar] [PubMed]
- Uma, K.; Huang, X.; Kumar, B.A. Antifungal effect of plant extract and essential oil. Chin. J. Integr. Med. 2017, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Rapp, R.P. Changing strategies for the management of invasive fungal infections. Pharmacotherapy 2004, 24, 4S–28S. [Google Scholar] [CrossRef]
- Flores, F.C.; Beck, R.C.R.; da Silva, C.B. Essential oils for treatment for onychomycosis: A mini-review. Mycopathologia 2016, 181, 9–15. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]
- Pootong, A.; Norrapong, B.; Cowawintaweewat, S. Antifungal activity of cinnamaldehyde against Candida albicans. Southeast Asian J. Trop. Med. Public Health 2017, 48, 150–158. [Google Scholar]
- Khan, S.N.; Khan, S.; Iqbal, J.; Khan, R.; Khan, A.U. Enhanced killing and antibiofilm activity of encapsulated cinnamaldehyde against Candida albicans. Front. Microbiol. 2017, 8, 1641. [Google Scholar] [CrossRef]
- Leite, M.C.A.; De Brito Bezerra, A.P.; De Sousa, J.P.; Guerra, F.Q.S.; De Oliveira Lima, E. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.O.; De Medeiros Nóbrega, F.; De Oliveira, W.A.; De Oliveira Lima, E.; Albuquerque Menezes, E.; Afrânio Cunha, F.; De Fátima Formiga Melo Diniz, M. Anti-Candida albicans effectiveness of citral and investigation of mode of action. Pharm. Biol. 2012, 50, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- da SilvaRivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305. [Google Scholar]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. 2018, 56, 565–578. [Google Scholar]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the antifungal effect of red thyme oil (Thymus vulgaris L.) compounds in vapor phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol Induces Candida albicans apoptosis associated with Ca2+/Calcineurin pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Manzoor, N.; Khan, L.A. Antifungal activities of Ocimum sanctum essential oil and its lead molecules. Nat. Prod. Commun. 2010, 5, 345–349. [Google Scholar] [CrossRef]
- Lone, S.A.; Khan, S.; Ahmad, A. Inhibition of ergosterol synthesis in Candida albicans by novel eugenol tosylate congeners targeting sterol 14α-demethylase (CYP51) enzyme. Arch. Microbiol. 2020, 202, 711–726. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Latifah-Munirah, B.; Himratul-Aznita, W.H.; Mohd Zain, N. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front. Life Sci. 2015, 8, 231–240. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Khosravi, A.R.; Shokri, H.; Shirzadi, H. Potential effect of 2-isopropyl-5-methylphenol (thymol) alone and in combination with fluconazole against clinical isolates of Candida albicans, C. glabrata and C. krusei. J. Mycol. Med. 2018, 28, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Rhee, M.S. Short-term antifungal treatments of caprylic acid with carvacrol or thymol induce synergistic 6-log reduction of pathogenic Candida albicans by cell membrane disruption and efflux pump inhibition. Cell. Physiol. Biochem. 2019, 53, 285–300. [Google Scholar] [PubMed]
- Rajkowska, K.; Nowak, A.; Kunicka-Styczyńska, A.; Siadura, A. Biological effects of various chemically characterized essential oils: Investigation of the mode of action against: Candida albicans and HeLa cells. RSC Adv. 2016, 6, 97199–97207. [Google Scholar] [CrossRef]
- Mukherji, R.; Prabhune, A. Novel glycolipids synthesized using plant essential oils and their application in quorum sensing inhibition and as Antibiofilm agents. Sci. World J. 2014, 2014, 890709. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-resistant candida: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef]
- Ksiezopolska, E.; Gabaldón, T. Evolutionary emergence of drug resistance in candida opportunistic pathogens. Genes 2018, 9, 461. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Malik, A.; Ahmad, I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Fayed, B.; Hamoda, A.M.; Rawas-Qalaji, M.; Haider, M.; Soliman, S.S.M. Essential oil-based design and development of novel anti-Candida azoles formulation. Molecules 2020, 25, 1463. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Silva, S.; Henriques, M. Candida glabrata biofilms: How far have we come? J. Fungi. 2017, 3, 11. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I. Biofilm inhibition by Cymbopogon citratus and Syzygium aromaticum essential oils in the strains of Candida albicans. J. Ethnopharmacol. 2012, 140, 416–423. [Google Scholar] [CrossRef]
- Santomauro, F.; Donato, R.; Sacco, C.; Pini, G.; Flamini, G.; Bilia, A.R. Vapour and Liquid-Phase Artemisia annua Essential Oil Activities against Several Clinical Strains of Candida. Planta Med. 2016, 82, 1016–1020. [Google Scholar] [CrossRef]
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of Mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 125904. [Google Scholar] [CrossRef]
- Essid, R.; Hammami, M.; Gharbi, D.; Karkouch, I.; Hamouda, T.B.; Elkahoui, S.; Limam, F.; Tabbene, O. Antifungal mechanism of the combination of Cinnamomum verum and Pelargonium graveolens essential oils with fluconazole against pathogenic Candida strains. Appl. Microbiol. Biotechnol. 2017, 101, 6993–7006. [Google Scholar] [CrossRef]
- Orchard, A.; Van Vuuren, S.F.; Viljoen, A.M. Commercial essential oil combinations against topical fungal pathogens. Nat. Prod. Commun. 2019, 14, 151–158. [Google Scholar] [CrossRef]
- Angiolella, L. Synergistic activity of Pelargonium capitatum and Cymbopogon martini essential oils against C. albicans. Nat. Prod. Res. 2021, 53, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Giordani, R.; Regli, P.; Kaloustian, J.; Mikaïl, C.; Abou, L.; Portugal, H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phyther. Res. 2004, 18, 990–995. [Google Scholar]
- Dupont, B.; Drouhet, E. In vitro synergy and antagonism of antifungal agents against yeast-like fungi. Postgrad. Med. J. 1979, 55, 683–686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siau, H.; Kerridge, D. The effect of antifungal drugs in combination on the growth of Candida glabrata in solid and liquid media. J. Antimicrob. Chemother. 1998, 41, 357–366. [Google Scholar] [CrossRef][Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Can Başer, K.H.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef]
- Amat, S.; Baines, D.; Alexander, T.W. A vapour phase assay for evaluating the antimicrobial activities of essential oils against bovine respiratory bacterial pathogens. Lett. Appl. Microbiol. 2017, 65, 489–495. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009, 106, 1343–1349. [Google Scholar] [CrossRef]
- Peano, A.; Pasquetti, M.; Tizzani, P.; Chiavassa, E.; Guillot, J.; Johnson, E. Methodological issues in antifungal susceptibility testing of Malassezia pachydermatis. J. Fungi 2017, 3, 37. [Google Scholar] [CrossRef]
- Gehrt, A.; Peter, J.; Pizzo, P.A.; Walsh, T.J. Effect of increasing inoculum sizes of pathogenic filamentous fungi on MICs of antifungal agents by broth microdilution method. J. Clin. Microbiol. 1995, 33, 1302–1307. [Google Scholar] [CrossRef]
- Meletiadis, J.; Meis, J.F.G.M.; Mouton, J.W.; Verweij, P.E. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 2001, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, A.; Aberkane, A.; Petrikkou, E.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Analysis of the influence of tween concentration, inoculum size, assay medium, and reading time on susceptibility testing of Aspergillus spp. J. Clin. Microbiol. 2005, 43, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.N.; Stocker, S.A.; Culver, D.H.; Thornsberry, C. Comparison of the E test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J. Clin. Microbiol. 1991, 29, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.F.; Rubini, D.; Shanmugavelan, P.; Murugan, R.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Effects of patchouli and cinnamon essential oils on biofilm and hyphae formation by Candida species. J. Mycol. Med. 2018, 28, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal activity and potential mechanism of n-butylphthalide alone and in combination with fluconazole against Candida albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Pankey, G.; Ashcraft, D.; Kahn, H.; Ismail, A. Time-Kill Assay and Etest Evaluation for Synergy with Polymyxin B and Fluconazole against Candida glabrata. Antimicrob. Agents Chemother. 2014, 58, 5795. [Google Scholar] [CrossRef]
- Sylvester, P.W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol. Biol. 2011, 716, 157–168. [Google Scholar]
- Tavares, A.C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Lopes, M.C.; Canhoto, J.; Salgueiro, L.R. Essential oil of Daucus carota subsp. halophilus: Composition, antifungal activity and cytotoxicity. J. Ethnopharmacol. 2008, 119, 129–134. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Sepahvand, A.; Jahanbakhsh, S.; Ezatpour, B.; Ayatollahi Mousavi, S.A. Evaluation of antifungal activities of the essential oil and various extracts of Nigella sativa and its main component, thymoquinone against pathogenic dermatophyte strains. J. Mycol. Med. 2014, 24, e155–e161. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Enhanced killing of Candida krusei by polymorphonuclear leucocytes in the presence of subinhibitory concentrations of Melaleuca alternifolia and “mentha of Pancalieri” essential oils. Molecules 2019, 24, 3824. [Google Scholar] [CrossRef]
- Meshulam, T.; Levitz, S.M.; Christin, L.; Diamond, R.D. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2, 3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2h)-tetrazolium-5-carboxanilide (xtt). J. Infect. Dis. 1995, 172, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Nakajima, M.O.; O’Hara, K.; Sawai, T. Novel antibiotic susceptibility tests by the ATP-bioluminescence method using filamentous cell treatment. Antimicrob. Agents Chemother. 1998, 42, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D.; Angiolella, L.; Vavala, E.; Rachini, A.; Mondello, F.; Ragno, R.; Bistoni, F.; Vecchiarelli, A. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement. Altern. Med. 2011, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Llama-Palacios, A.; Marín, M.J.; Figuero, E.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. Validation of atp bioluminescence as a tool to assess antimicrobial effects of mouthrinses in an in vitro subgingival-biofilm model. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e86. [Google Scholar] [CrossRef]
- Petty, R.D.; Sutherland, L.A.; Hunter, E.M.; Cree, I.A. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J. Biolumin. Chemilumin. 1995, 10, 29–34. [Google Scholar] [CrossRef]
- Choma, I.M.; Grzelak, E.M. Bioautography detection in thin-layer chromatography. J. Chromatogr. A 2011, 1218, 2684–2691. [Google Scholar] [CrossRef]
- Ramani, R.; Ramani, A.; Wong, S.J. Rapid flow cytometric susceptibility testing of Candida albicans. J. Clin. Microbiol. 1997, 35, 2320–2324. [Google Scholar] [CrossRef]
- Ramani, R.; Chaturvedi, V. Flow cytometry antifungal susceptibility testing of pathogenic yeasts other than Candida albicans and comparison with the NCCLS broth microdilution test. Antimicrob. Agents Chemother. 2000, 44, 2752–2758. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.H.; Lee, J. Antibiofilm and antihyphal activities of cedar leaf essential oil, camphor, and fenchone derivatives against Candida albicans. Front. Microbiol. 2017, 8, 1476. [Google Scholar] [CrossRef]
- Desai, J.V. Candida albicans hyphae: From growth initiation to invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef]
- Thompson, D.S.; Carlisle, P.L.; Kadosh, D. Coevolution of morphology and virulence in Candida species. Eukaryot. Cell 2011, 10, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.H.; Montanari, L.B.; Martins, C.H.G.; Zaia, J.E.; Almeida, A.M.F.; Matsumoto, M.T.; Mendes-Giannini, M.J.S. Anticandidal Efficacy of Cinnamon Oil against Planktonic and Biofilm Cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia 2011, 172, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Khan, L.A.; Manzoor, N. Ocimum sanctum (L.) essential oil and its lead molecules induce apoptosis in Candida albicans. Res. Microbiol. 2014, 165, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Frenkel, M. Experimental in vivo models of Candidiasis. J. Fungi 2018, 4, 21. [Google Scholar] [CrossRef]
- Chamilos, G.; Lionakis, M.S.; Lewis, R.E.; Lopez-Ribot, J.L.; Saville, S.P.; Albert, N.D.; Halder, G.; Kontoyiannis, D.P. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J. Infect. Dis. 2006, 193, 1014–1022. [Google Scholar] [CrossRef]
- Amorim-Vaz, S.; Delarze, E.; Ischer, F.; Sanglard, D.; Coste, A.T. Examining the virulence of Candida albicans transcription factor mutants using Galleria mellonella and mouse infection models. Front. Microbiol. 2015, 6, 367. [Google Scholar] [CrossRef]
- Hirakawa, M.P.; Martinez, D.A.; Sakthikumar, S.; Anderson, M.Z.; Berlin, A.; Gujja, S.; Zeng, Q.; Zisson, E.; Wang, J.M.; Greenberg, J.M.; et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015, 25, 413–425. [Google Scholar] [CrossRef]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef]
- Pedroso, R.D.S.; Balbino, B.L.; Andrade, G.; Dias, M.C.P.S.; Alvarenga, T.A.; Pedroso, R.C.N.; Pimenta, L.P.; Lucarini, R.; Pauletti, P.M.; Januário, A.H.; et al. In vitro and in vivo Anti-Candida spp. Activity of plant-derived products. Plants 2019, 8, 494. [Google Scholar] [CrossRef]
- De Campos Rasteiro, M.M.; da Costa, P.C.B.P.; Araújo, F.F.; de Barros, P.P.; Rossoni, D.D.; Anbinder, L.L.; Jorge, C.O.C.; Junqueira, C.C. Essential oil of Melaleuca alternifolia for the treatment of oral candidiasis induced in an immunosuppressed mouse model. BMC Complement. Altern. Med. 2014, 14, 489. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, H.; Tian, J.; Zheng, Y.; Ban, X.; Zeng, J.; Mao, Y. In vitro and in vivo activities of essential oil from the seed of Anethum graveolens L. against Candida spp. Evid.-Based Complement. Altern. Med. 2011, 2011, 659704. [Google Scholar]
- De Toledo, L.G.; Ramos, M.A.D.S.; da Silva, P.B.; Rodero, C.F.; de Sá Gomes, V.; da Silva, A.N.; Pavan, F.R.; da Silva, I.C.; Oda, F.B.; Flumignan, D.L.; et al. Improved in vitro and in vivo anti-candida albicans activity of Cymbopogon nardus essential oil by its incorporation into a microemulsion system. Int. J. Nanomed. 2020, 15, 10481–10497. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Arellano, A.; López-Villegas, E.O.; Rodríguez-Tovar, A.V.; Zamilpa, A.; Jiménez-Ferrer, E.; Tortoriello, J.; Martínez-Rivera, M.A. Use of antifungal saponin SC-2 of Solanum chrysotrichum for the treatment of vulvovaginal candidiasis: In vitro studies and clinical experiences. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Shaltout, A.S.; Abd El Aziz, S.M.M.; Abbas, A.M.; Abd El Moneem, H.G.; Youness, E.M.; Arief, A.F.; Ali, M.F.; Abd El-hamid, B.N. Vaginal suppositories of cumin seeds essential oil for treatment of vaginal candidiasis: Formulation, in vitro, in vivo, and clinical evaluation. Eur. J. Pharm. Sci. 2021, 157, 105602. [Google Scholar] [CrossRef]
- Catalán, A.; Pacheco, J.G.; Martínez, A.; Mondaca, M.A. In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, J.A.; Zawawi, A.A. Efficacy of alcohol-based and alcohol-free melaleuca oral solution for the treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS. HIV Clin. Trials 2002, 3, 379–385. [Google Scholar]
- De Araújo MR, C.; Maciel, P.P.; Castellano LR, C.; Bonan PR, F.; Alves DD, N.; de Medeiros AC, D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021, 41, 349–357. [Google Scholar]
- Carmo, E.S.; Pereira, F.D.O.; Cavalcante, N.M.; Gayoso, C.W.; Lima, E.D.O. Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf-therapeutic pilot study. An. Bras. Dermatol. 2013, 88, 381–385. [Google Scholar] [CrossRef]
- Chalhoub, E.; Emami, E.; Freijé, M.; Kandelman, D.; Campese, M.; St-Georges, A.; Voyer, R.; Rompré, P.; Barbeau, J.; Leduc, A.; et al. Effectiveness of an alcohol-free essential oil-containing mouthwash in institutionalised elders receiving long-term care: A feasibility study. Gerodontology 2016, 33, 69–78. [Google Scholar] [CrossRef]
| Antifungal Compound | EOs | Mode of Action on Candida Species | References |
|---|---|---|---|
| Aldehydes | C=O | ||
| Cinnamaldehyde | Camphor, Cassia, Cinnamon. | ATPase inhibition Induces apoptosis Induction of oxidative stress Reduction of ergosterol biosynthesis | [29,30,31] |
| Citral | Lemon, Lime, Orange. | Induction of oxidative stress Inhibition of pseudohyphae formation | [32,33] |
| Cyclic Terpenes | C6 ring | ||
| α-pinene | Frankincense, Juniper, Pine, Rosemary. | Disruption of cellular membranes Reduced biofilm formation | [34] |
| β-pinene | Cannabis, Lavender, Mint, Pine. | Disruption of cellular membranes Reduced biofilm formation | [34] |
| Limonene | Lemon, Lemongrass, Lime, Orange. | Disruption of cellular membranes Induces apoptosis | [35] |
| p-cymene | Anise, Basil, Camphor, Cumin, Eucalyptus, Oregano, Thyme. | Disruption of cellular membranes Inhibition of germ tube formation | [36] |
| Phenols | -OH | ||
| Carvacrol | Oregano, Thyme, Wild Bergamot | Binds to sterol components of membranes | [37,38] |
| Eugenol | Basil, Cinnamon, Clove, Nutmeg. | Altered protein functionality Increases membrane fluidity and permeability Inhibits ergosterol biosynthesis Inhibits proton efflux | [39] |
| Linalool | Basil, Lavender, Rose, Sage. | Altered protein functionality Increases membrane fluidity and permeability Inhibits proton efflux | [39] |
| Menthol | Geranium, Mint, Sunflower, Tarragon. | Inhibition of ergosterol biosynthesis | [40] |
| Thymol | Citrus, Coriander, Oregano, Thyme, Wild Bergamot. | Altered protein functionality Inhibition of ergosterol biosynthesis | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, H.N.H.; Udoh, S.; Russell, G.; Okeyoyin, O.R.; Aftab, S.; Rodriguez, I.; Tabe, E.S.; Adukwu, E.C. Recent Advances in the Application of Essential Oils as Potential Therapeutic Candidates for Candida-Related Infections. Appl. Microbiol. 2022, 2, 397-413. https://doi.org/10.3390/applmicrobiol2020030
Tran HNH, Udoh S, Russell G, Okeyoyin OR, Aftab S, Rodriguez I, Tabe ES, Adukwu EC. Recent Advances in the Application of Essential Oils as Potential Therapeutic Candidates for Candida-Related Infections. Applied Microbiology. 2022; 2(2):397-413. https://doi.org/10.3390/applmicrobiol2020030
Chicago/Turabian StyleTran, Hoang N. H., Stephanie Udoh, Grace Russell, Oluwadamilola R. Okeyoyin, Sofia Aftab, Isabela Rodriguez, Ebot S. Tabe, and Emmanuel C. Adukwu. 2022. "Recent Advances in the Application of Essential Oils as Potential Therapeutic Candidates for Candida-Related Infections" Applied Microbiology 2, no. 2: 397-413. https://doi.org/10.3390/applmicrobiol2020030
APA StyleTran, H. N. H., Udoh, S., Russell, G., Okeyoyin, O. R., Aftab, S., Rodriguez, I., Tabe, E. S., & Adukwu, E. C. (2022). Recent Advances in the Application of Essential Oils as Potential Therapeutic Candidates for Candida-Related Infections. Applied Microbiology, 2(2), 397-413. https://doi.org/10.3390/applmicrobiol2020030






