DHA-Rich Aurantiochytrium Biomass, a Novel Dietary Supplement, Resists Degradation by Rumen Microbiota without Disrupting Microbial Activity
Abstract
:1. Introduction
2. Materials and Methods
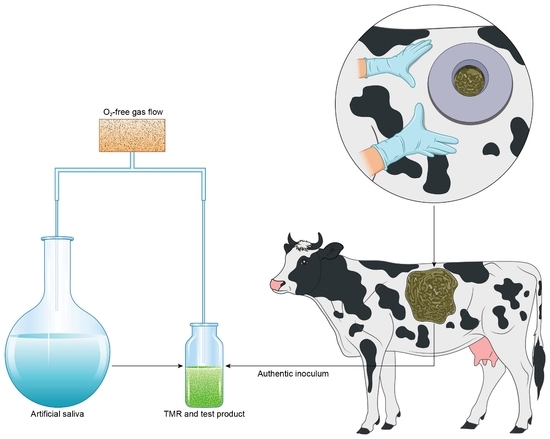
2.1. Basal Diet for Ex Vivo Fermentation
2.2. Rumen Ex Vivo Protocol
2.3. Ex Vivo Rumen Experiment I
2.4. Ex Vivo Rumen Experiment II
2.5. In Vivo Experiment with Rumen-Cannulated Cows—Animals, Trial Schedule, and Dietary Treatments
2.6. Sampling of Rumen Fluid and Milk
2.7. Analysis of Short-Chain Fatty Acids in Rumen Fluid
2.8. Extraction of Microbial DNA from Rumen Fluid Samples
2.9. qPCR Analysis of Rumen Microorganisms
2.10. LCFA Analysis of Rumen Fluid and Milk Samples
2.11. Statistical Analysis
2.11.1. Ex Vivo Results
2.11.2. In Vivo Results
3. Results
3.1. Comparison of Fatty Acid Profiles in Different Lipid Sources
3.2. Effect of Various Fatty Acid Sources on Rumen Fermentation Activity Ex Vivo—Experiment I
3.3. Resistance of the Major Fatty Acids to Attack by Rumen Bacteria Ex Vivo—Experiment I
3.4. Tolerance of Rumen Bacteria to Extreme Doses of AURA Supplement Ex Vivo—Experiment II
3.5. Resistance of Docosahexaenoic Acid to Rumen Bacterial Attack Ex Vivo—Experiment II
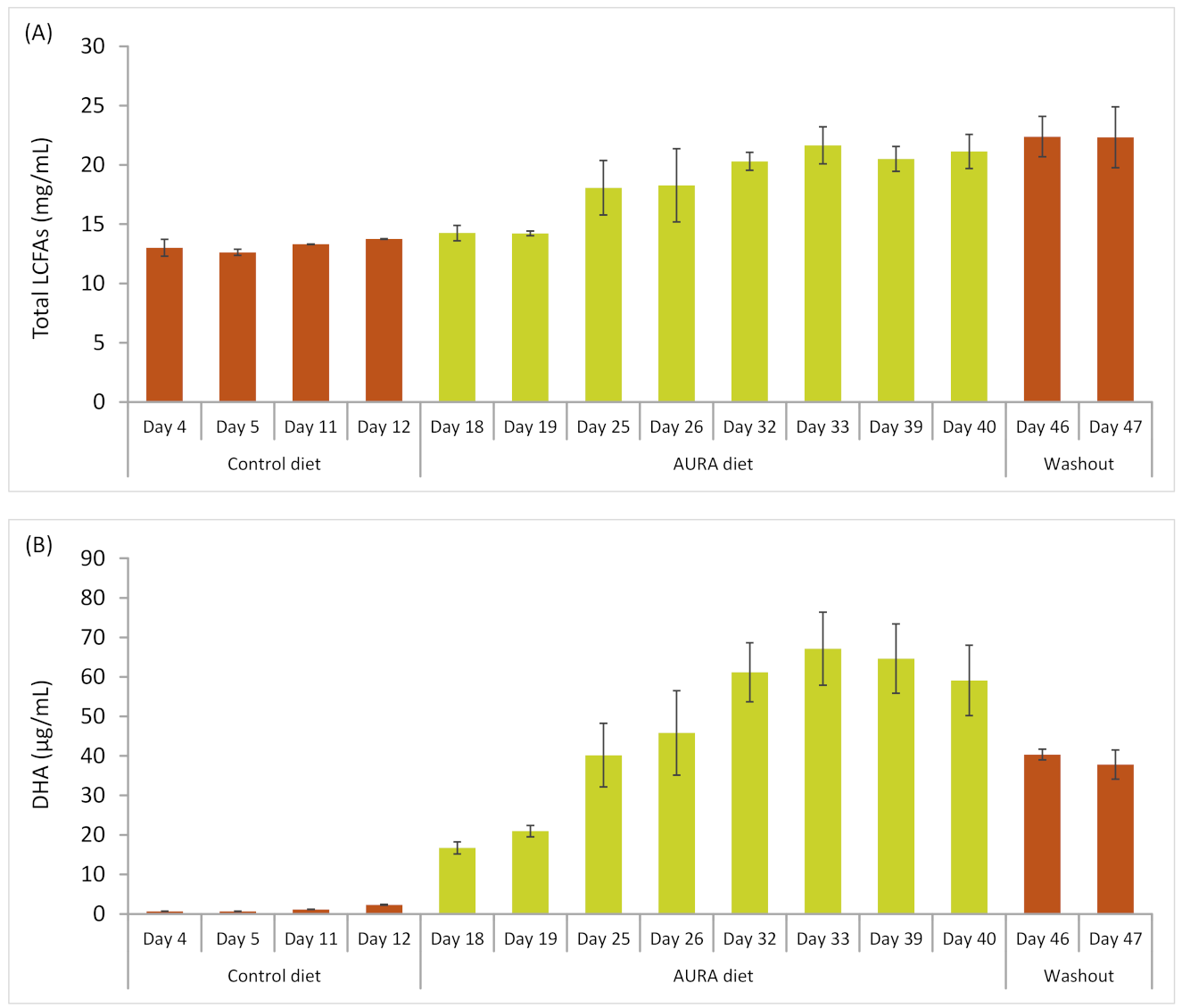
3.6. Effect of the AURA Product on LCFA Content in Dairy Cow Milk
3.7. Effect of the AURA Product on Rumen Microbiota and Metabolism in Cannulated Dairy Cows
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AURA | Aurantiochytrium limacinum |
| CDV | cardiovascular disease |
| DHA | docosahexaenoic acid |
| DM | dry matter |
| EDTA | ethylenediaminetetraacetic acid |
| EPA | eicosapentaenoic acid |
| EtOAc | ethyl acetate |
| FM | fish meal |
| FO | fish oil |
| HSD | honestly significant difference |
| LCFA | long-chain fatty acid |
| LC-PUFA | long-chain omega-3 polyunsaturated fatty acid |
| SCFA | short-chain fatty acid |
| VFA | volatile fatty acid |
References
- Feskens, E.J.M.; Sluik, D.; Van Woudenbergh, G.J. Meat Consumption, Diabetes, and Its Complications. Curr. Diabetes Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary Fat Intake and Risk of Alzheimer’s Disease and Dementia: A Meta-Analysis of Cohort Studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D. Food in the Anthropo-cene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Ascherio, A.; Colditz, G.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am. J. Clin. Nutr. 1999, 70, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahmani, P.; Ponnampalam, E.N.; Kraft, J.; Mapiye, C.; Bermingham, E.N.; Watkins, P.J.; Proctor, S.D.; Dugan, M.E. Bioactivity and health effects of ruminant meat lipids: Invited Review. Meat Sci. 2020, 165, 108114. [Google Scholar] [CrossRef] [PubMed]
- Hurtaud, C.; Faucon, F.; Couvreur, S.; Peyraud, J.-L. Linear relationship between increasing amounts of extruded linseed in dairy cow diet and milk fatty acid composition and butter properties. J. Dairy Sci. 2010, 93, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.; Bonnet, M.; Scollan, N. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Moran, C.; Morlacchini, M.; Keegan, J.D.; Warren, H.; Fusconi, G. Dietary supplementation of dairy cows with a docosahexaenoic acid-rich thraustochytrid, Aurantiochytrium limacinum: Effects on milk quality, fatty acid composition and cheese making properties. J. Anim. Feed. Sci. 2019, 28, 3–14. [Google Scholar] [CrossRef]
- Gebreyowhans, S.; Lu, J.; Zhang, S.; Pang, X.; Lv, J. Dietary enrichment of milk and dairy products with n-3 fatty acids: A review. Int. Dairy J. 2019, 97, 158–166. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef]
- Hanson, S.; Thorpe, G.; Winstanley, L.; Abdelhamid, A.S.; Hooper, L.; on behalf of the PUFAH group. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: Systematic review and meta-analysis of randomised trials. Br. J. Cancer 2020, 122, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, W.; Laufenberg, G. Enrichment of food products with polyunsaturated fatty acids by fish oil addition. Eur. Food Res. Technol. 2006, 222, 472–477. [Google Scholar] [CrossRef]
- Kim, E.J.; Huws, S.; Lee, M.; Wood, J.D.; Muetzel, S.; Wallace, R.J.; Scollan, N.D. Fish Oil Increases the Duodenal Flow of Long Chain Polyunsaturated Fatty Acids and trans-11 18:1 and Decreases 18:0 in Steers via Changes in the Rumen Bacterial Community. J. Nutr. 2008, 138, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingfield, K.J.; Kairenius, P.; Ärölä, A.; Paillard, D.; Muetzel, S.; Ahvenjärvi, S.; Vanhatalo, A.; Huhtanen, P.; Toivonen, V.; Griinari, J.M.; et al. Dietary Fish Oil Supplements Modify Ruminal Biohydrogenation, Alter the Flow of Fatty Acids at the Omasum, and Induce Changes in the Ruminal Butyrivibrio Population in Lactating Cows. J. Nutr. 2012, 142, 1437–1448. [Google Scholar] [CrossRef] [Green Version]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- AbuGhazaleh, A.; Potu, R.; Ibrahim, S. Short communication: The effect of substituting fish oil in dairy cow diets with docosahexaenoic acid-micro algae on milk composition and fatty acids profile. J. Dairy Sci. 2009, 92, 6156–6159. [Google Scholar] [CrossRef] [Green Version]
- Leyland, B.; Leu, S.; Boussiba, S. Are Thraustochytrids algae? Fungal Biol. 2017, 121, 835–840. [Google Scholar] [CrossRef]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Keegan, J.; Currie, D.; Knox, A.; Moran, C. Docosahexaenoic Acid Included in Layer Feed as an Unextracted Aurantiochytrium limacinum Biomass Is Efficiently Transferred to Eggs When Provided in Mash or Pellet Form. J. Appl. Poult. Res. 2019, 28, 1069–1077. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Fusconi, G. The effect of dietary supplementation with Aurantiochytrium lim-acinum on lactating dairy cows in terms of animal health, productivity, and milk composition. J. Anim. Physiol. Anim. Nutr. 2018, 102, 576–590. [Google Scholar] [CrossRef] [Green Version]
- Franklin, S.T.; Martin, K.R.; Baer, R.J.; Schingoethe, D.J.; Hippen, A.R. Dietary marine algae (Schizochytrium sp.) increases concentrations of conjugated linoleic, docosahexaenoic and transvaccenic acids in milk of dairy cows. J. Nutr. 1999, 129, 2048–2054. [Google Scholar] [CrossRef]
- Bichi, E.; Hervás, G.; Toral, P.G.; Loor, J.; Frutos, P. Milk fat depression induced by dietary marine algae in dairy ewes: Persistency of milk fatty acid composition and animal performance responses. J. Dairy Sci. 2013, 96, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.; Mosley, E.E. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Goering, H.K.; Van Soest, P.J. Forage fiber analyses: Apparatus, Ragents, Procedures, and Some Applications (No. 379). In Agricultural Research Service; US Department of Agriculture: Honolulu, HI, USA, 1970. [Google Scholar]
- Apajalahti, J.; Vienola, K.; Raatikainen, K.; Holder, V.; Moran, C.A. Conversion of branched-chain amino acids to corre-sponding isoacids—An in vitro tool for estimating ruminal protein degradability. Front. Vet. Sci. 2019, 6, 311. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Cadillo-Quiroz, H.; Brauer, S.; Yashiro, E.; Sun, C.; Yavitt, J.; Zinder, S. Vertical profiles of methanogenesis and methanogens in two contrasting acidic peatlands in central New York State, USA. Environ. Microbiol. 2006, 8, 1428–1440. [Google Scholar] [CrossRef]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Matsui, H.; Nakamura, M.; Benno, Y. Diet-Dependent Shifts in the Bacterial Population of the Rumen Revealed with Real-Time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, J.T.; Karnati, S.K.R.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an Assay to Quantify Rumen Ciliate Protozoal Biomass in Cows Using Real-Time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef] [Green Version]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Givens, D.I.; Cottrill, B.R.; Davies, M.; Lee, P.A.; Mansbridge, R.J.; Moss, A.R. Sources of n-3 polyunsaturated fatty acids ad-ditional to fish oil for livestock diets—A review. Nutr. Abstr. Rev. 2000, 70, 1–19. [Google Scholar]
- Moallem, U. Invited review: Roles of dietary n-3 fatty acids in performance, milk fat composition, and reproductive and immune systems in dairy cattle. J. Dairy Sci. 2018, 101, 8641–8661. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, G.; Goulas, C.; Apostolaki, E.; Abril, R. Effects of dietary supplements of algae, containing polyunsaturated fatty acids, on milk yield and the composition of milk products in dairy ewes. J. Dairy Res. 2002, 69, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Boeckaert, C.; Vlaeminck, B.; Fievez, V.; Maignien, L.; Dijkstra, J.; Boon, N. Accumulation of trans C 18:1 Fatty Acids in the Rumen after Dietary Algal Supplementation Is Associated with Changes in the Butyrivibrio Community. Appl. Environ. Microbiol. 2008, 74, 6923–6930. [Google Scholar] [CrossRef] [Green Version]
- Khas-Erdene, Q.; Wang, J.; Bu, D.; Wang, L.; Drackley, J.; Liu, Q.; Yang, G.; Wei, H.; Zhou, L. Short communication: Responses to increasing amounts of free α-linolenic acid infused into the duodenum of lactating dairy cows. J. Dairy Sci. 2010, 93, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.; Frutos, P.; Hervás, G.; Gómez-Cortés, P.; Juárez, M.; de la Fuente, M. Changes in milk fatty acid profile and animal performance in response to fish oil supplementation, alone or in combination with sunflower oil, in dairy ewes. J. Dairy Sci. 2010, 93, 1604–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toral, P.; Bernard, L.; Belenguer, A.; Rouel, J.; Hervás, G.; Chilliard, Y.; Frutos, P. Comparison of ruminal lipid metabolism in dairy cows and goats fed diets supplemented with starch, plant oil, or fish oil. J. Dairy Sci. 2016, 99, 301–316. [Google Scholar] [CrossRef]
- Reynolds, C.; Dürst, B.; Lupoli, B.; Humphries, D.; Beever, D. Visceral Tissue Mass and Rumen Volume in Dairy Cows During the Transition from Late Gestation to Early Lactation. J. Dairy Sci. 2004, 87, 961–971. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.; Keegan, J.; Jacques, K.; Hart, H. PSXI-4 Minimum inhibitory concentration of a heterotrophically grown, dried microalgae powder (All-G-Rich®️) and extracted microalgal oil against selected intestinal microorganisms. J. Anim. Sci. 2018, 96, 360–361. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyanathan, J.; Escobar, M.; Wallace, R.J.; Fievez, V.; Vlaeminck, B. Biohydrogenation of 22:6n-3 by Butyrivibrio proteoclasticus P18. BMC Microbiol. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toral, P.G.; Hervás, G.; Carreño, D.; Leskinen, H.; Belenguer, A.; Shingfield, K.; Frutos, P. In vitro response to EPA, DPA, and DHA: Comparison of effects on ruminal fermentation and biohydrogenation of 18-carbon fatty acids in cows and ewes. J. Dairy Sci. 2017, 100, 6187–6198. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Andrés, S.; Snelling, T.J.; López-Ferreras, L.; Yáñez-Ruíz, D.R.; Garcia-Estrada, C.; Lopez, S. Effect of Sunflower and Marine Oils on Ruminal Microbiota, In vitro Fermentation and Digesta Fatty Acid Profile. Front. Microbiol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J.H.A.; Salkinoja-Salonen, M.S. Absorption of pentachlorophenol (PCP) by bark chips and its role in microbial PCP degradation. Microb. Ecol. 1984, 10, 359–367. [Google Scholar] [CrossRef]
- Huws, S.A.; Lee, M.R.; Muetzel, S.M.; Scott, M.B.; Wallace, R.J.; Scollan, N.D. Forage type and fish oil cause shifts in rumen bacterial diversity. FEMS Microbiol. Ecol. 2010, 73, 396–702. [Google Scholar] [CrossRef] [Green Version]
- Sinedino, L.D.P.; Honda, P.M.; Souza, L.R.L.; Lock, A.L.; Boland, M.P.; Staples, C.R.; Thatcher, W.W.; Santos, J.E.P. Effects of supplementation with docosahexaenoic acid on reproduction of dairy cows. Reproduction 2017, 153, 707–723. [Google Scholar] [CrossRef] [Green Version]
- Carreño, D.; Toral, P.G.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.R.; Hervás, G.; McEwan, N.R.; Newbold, C.J.; Frutos, P. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Sci. Rep. 2019, 9, 11857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, H. Advances in the metabolism and regulation of lactic acids in the rumen. Pratacult. Sci. 2016, 33, 972–980. [Google Scholar]
- Durmic, Z.; Moate, P.J.; Eckard, R.; Revell, D.K.; Williams, R.; Vercoe, P.E. In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation. J. Sci. Food Agric. 2014, 94, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Dohme, F.; Danneels, M.; Raes, K.; Demeyer, D. Fish oils as potent rumen methane inhibitors and associated effects on rumen fermentation in vitro and in vivo. Anim. Feed. Sci. Technol. 2003, 104, 41–58. [Google Scholar] [CrossRef]
- Fievez, V.; Boeckaert, C.; Vlaeminck, B.; Mestdagh, J.; Demeyer, D. In vitro examination of DHA-edible micro-algae: 2. Effect on rumen methane production and apparent degradability of hay. Anim. Feed. Sci. Technol. 2007, 136, 80–95. [Google Scholar] [CrossRef]
- Henning, P.; Horn, C.; Steyn, D.; Meissner, H.; Hagg, F. The potential of Megasphaera elsdenii isolates to control ruminal acidosis. Anim. Feed. Sci. Technol. 2010, 157, 13–19. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
 AURA;
AURA;  AURA extract;
AURA extract;  FO.
FO.
 AURA;
AURA;  AURA extract;
AURA extract;  FO.
FO.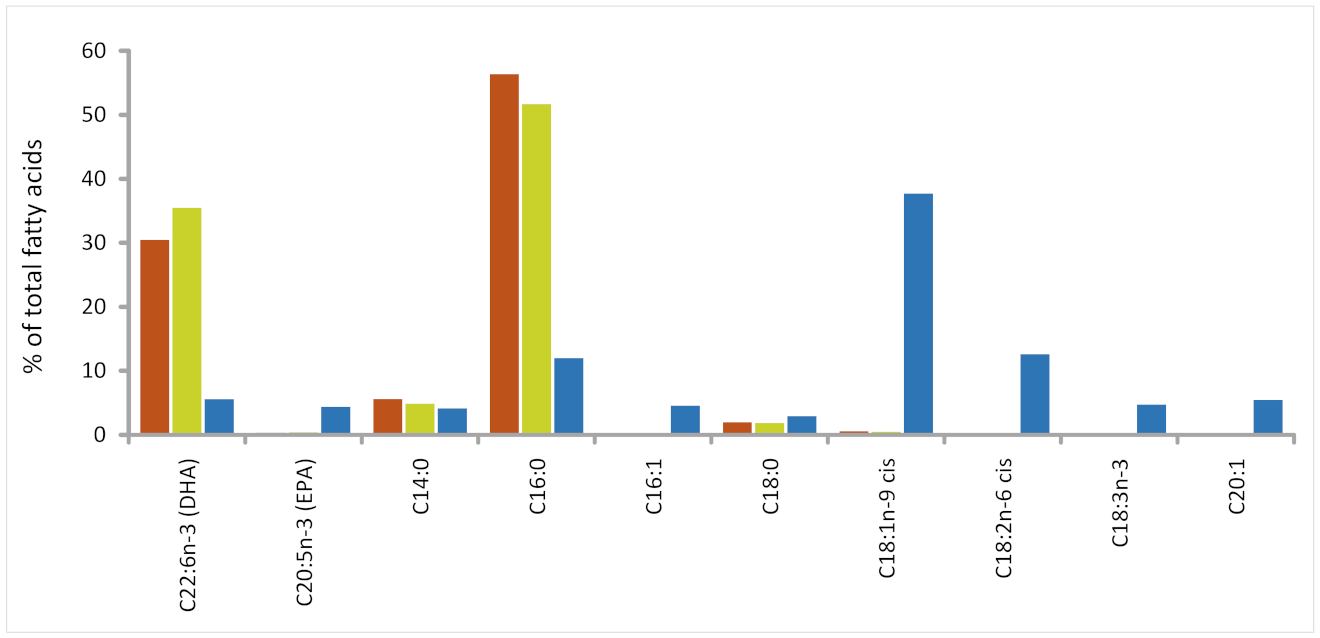
 acid concentration at 0 h;
acid concentration at 0 h;  acid concentration at 8 h. AURA concentration in fermentation medium: Dose 1 (0.08 mg/mL), Dose 2 (0.15 mg/mL), Dose 3 (0.30 mg/mL) and Dose 4 (0.60 mg/mL). FO doses in fermentation medium: Dose 1 (0.05 mg/mL), Dose 2 (0.11 mg/mL), Dose 3 (0.22 mg/mL) and Dose 4 (0.44 mg/mL).
acid concentration at 8 h. AURA concentration in fermentation medium: Dose 1 (0.08 mg/mL), Dose 2 (0.15 mg/mL), Dose 3 (0.30 mg/mL) and Dose 4 (0.60 mg/mL). FO doses in fermentation medium: Dose 1 (0.05 mg/mL), Dose 2 (0.11 mg/mL), Dose 3 (0.22 mg/mL) and Dose 4 (0.44 mg/mL).
 acid concentration at 0 h;
acid concentration at 0 h;  acid concentration at 8 h. AURA concentration in fermentation medium: Dose 1 (0.08 mg/mL), Dose 2 (0.15 mg/mL), Dose 3 (0.30 mg/mL) and Dose 4 (0.60 mg/mL). FO doses in fermentation medium: Dose 1 (0.05 mg/mL), Dose 2 (0.11 mg/mL), Dose 3 (0.22 mg/mL) and Dose 4 (0.44 mg/mL).
acid concentration at 8 h. AURA concentration in fermentation medium: Dose 1 (0.08 mg/mL), Dose 2 (0.15 mg/mL), Dose 3 (0.30 mg/mL) and Dose 4 (0.60 mg/mL). FO doses in fermentation medium: Dose 1 (0.05 mg/mL), Dose 2 (0.11 mg/mL), Dose 3 (0.22 mg/mL) and Dose 4 (0.44 mg/mL).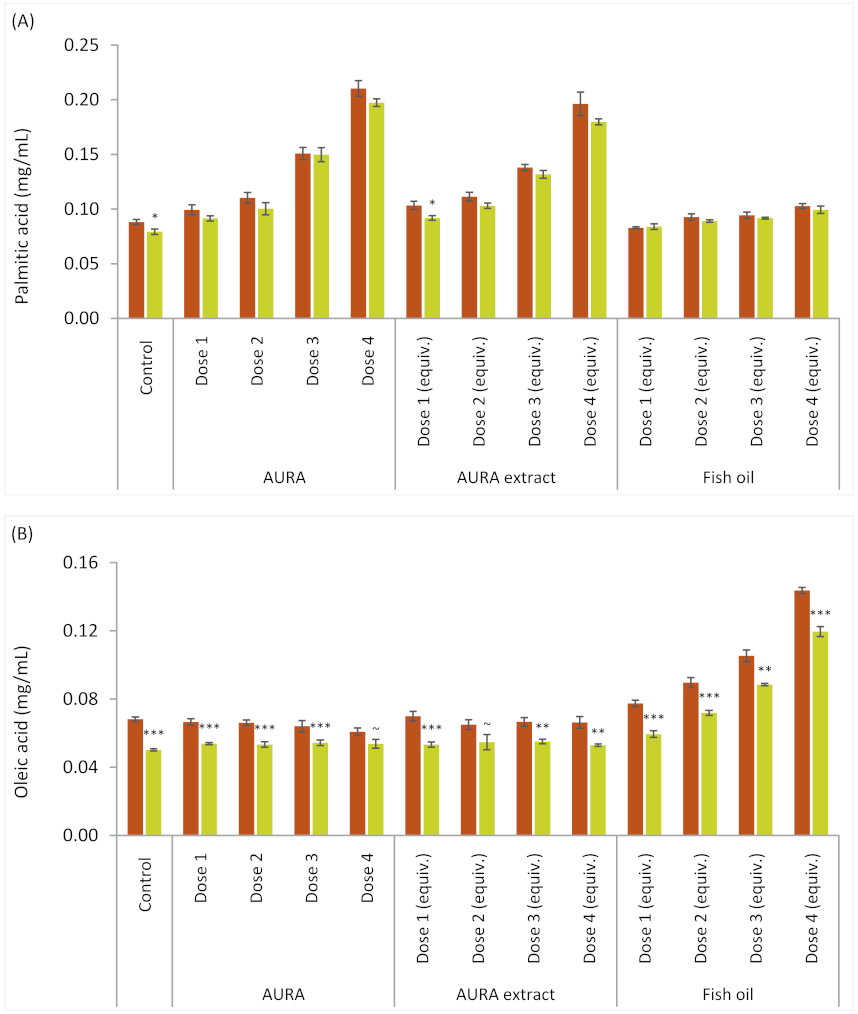

 AURA 0.25 mg/mL;
AURA 0.25 mg/mL;  AURA 0.63 mg/mL;
AURA 0.63 mg/mL;  AURA 1.50 mg/mL;
AURA 1.50 mg/mL;  AURA 2.50 mg/mL.
AURA 2.50 mg/mL.
 AURA 0.25 mg/mL;
AURA 0.25 mg/mL;  AURA 0.63 mg/mL;
AURA 0.63 mg/mL;  AURA 1.50 mg/mL;
AURA 1.50 mg/mL;  AURA 2.50 mg/mL.
AURA 2.50 mg/mL.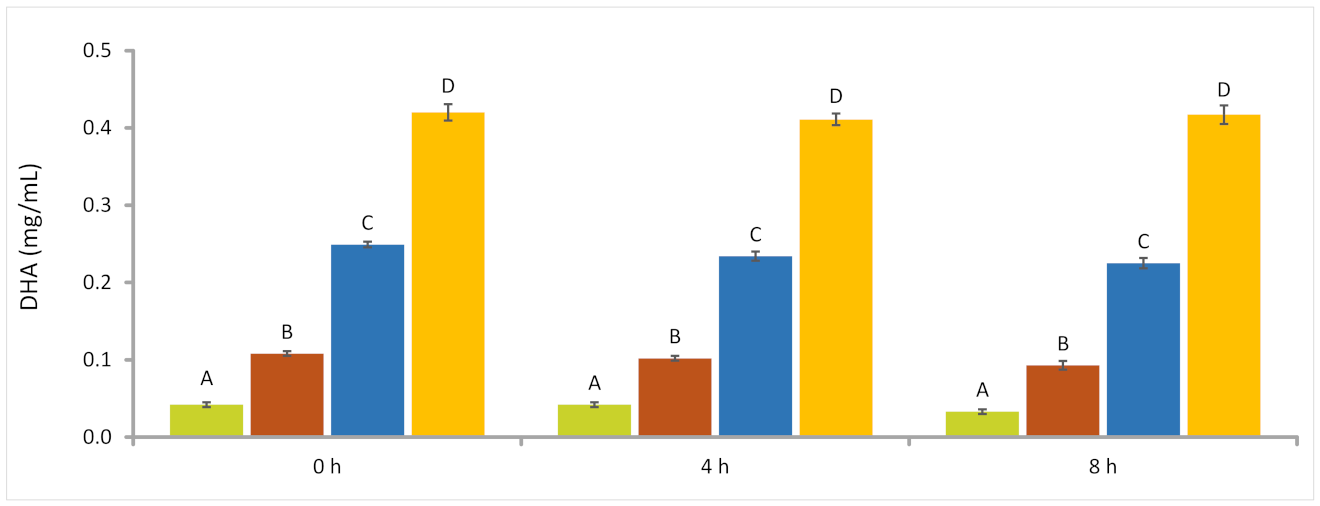
| Diet | Basal Substrate | Supplement Dosing | ||
|---|---|---|---|---|
| Grass Silage (mg DM) | Compound Feed(mg DM) | mg/mL * | LCFA % of Diet DM † | |
| Ex vivo experiment I | ||||
| Control | 150 | 100 | - | 0 |
| AURA dose 1 | 150 | 100 | 0.08 | 0.16 |
| AURA dose 2 | 150 | 100 | 0.15 | 0.32 |
| AURA dose 3 | 150 | 100 | 0.30 | 0.64 |
| AURA dose 4 | 150 | 100 | 0.60 | 1.27 |
| AURA extract dose 1 | 150 | 100 | ‡ | 0.16 |
| AURA extract dose 2 | 150 | 100 | ‡ | 0.32 |
| AURA extract dose 3 | 150 | 100 | ‡ | 0.64 |
| AURA extract dose 4 | 150 | 100 | ‡ | 1.27 |
| Fish oil dose 1 | 150 | 100 | 0.05 | 0.16 |
| Fish oil dose 2 | 150 | 100 | 0.11 | 0.32 |
| Fish oil dose 3 | 150 | 100 | 0.22 | 0.64 |
| Fish oil dose 4 | 150 | 100 | 0.44 | 1.27 |
| Ex vivo experiment II | ||||
| Control | 150 | 100 | - | 0 |
| AURA dose 1 | 150 | 100 | 0.25 | 1.0 |
| AURA dose 2 | 150 | 100 | 0.63 | 2.5 |
| AURA dose 3 | 150 | 100 | 1.50 | 5.7 |
| AURA dose 4 | 150 | 100 | 2.50 | 9.1 |
| Target Microorganism or Group | Primer Sequence (5′-3′) | Product Size (bp) | Reference |
|---|---|---|---|
| Total eubacteria | F: TCCTACGGGAGGCAGCAGT | 466 | [30] |
| R: GGACTACCAGGGTATCTAATCCTGTT | |||
| Methanogens | F: AATTGGCGGGGGAGCAC | 136 | [31] |
| R: GGCCATGCACCWCCTCTC | |||
| Lachnospiraceae | F: GACRGTACCTGACTAAGAAGC | 435 | Unpublished |
| R: TTTGAGTTTCATTCTTGCGAA | |||
| Ruminococcaceae | F: CGCACAAGCRGTGGAGT | 249 | Unpublished |
| R: ACCTTCCTCCGTTTTGTCAA | |||
| Bacteroides–Prevotella | F: GGTGTCGGCTTAAGTGCCAT | 140 | [32] |
| R: CGGAYGTAAGGGCCGTGC | |||
| Lactobacillus spp. | F: AGCAGTAGGGAATCTTCCA | 341 | [32] |
| R: CACCGCTACACATGGAG | |||
| Streptococcus spp. | F: GGGGATAACTATTGGAAACGATA | 118 | Unpublished |
| R: CCWACTAGCTAATACAACGCA | |||
| Veillonella spp. | F: AYCAACCTGCCCTTCAGA | 343 | [32] |
| R: CGTCCCGATTAACAGAGCTT | |||
| Selenomonas ruminantium | F: GATTAAAGATGGCCTCTACTTG | 253 | Unpublished |
| R: CGTCAACAGAGCTTTACGAG | |||
| Fibrobacter succinogenes | F: GGTATGGGATGAGCTTGC | 446 | [33] |
| R: GCCTGCCCCTGAACTATC | |||
| Megasphaera elsdenii | F: GGGTGAGTAACGCGTAAGCAA | 93 | Unpublished |
| R: CTGCCATGCGACAAAAAGAA | |||
| Total protozoa | F: GCTTTCGWTGGTAGTGTATT | 223 | [34] |
| R: CTTGCCCTCYAATCGTWCT |
| Diet | Gas Production (mL) | SCFA Concentration (mM) | |
|---|---|---|---|
| 0–8 h | 0 h | 8 h | |
| Control | 21.8 | 9.54 | 66.3 |
| AURA dose 1 | 21.2 | 9.40 | 64.8 |
| AURA dose 2 | 22.1 | 9.41 | 68.2 |
| AURA dose 3 | 21.5 | 9.34 | 64.6 |
| AURA dose 4 | 21.1 | 9.28 | 64.8 |
| AURA extract dose 1 (equiv.) | 21.6 | 9.09 | 65.9 |
| AURA extract dose 2 (equiv.) | 21.7 | 9.35 | 64.3 |
| AURA extract dose 3 (equiv.) | 21.7 | 9.29 | 67.2 |
| AURA extract dose 4 (equiv.) | 21.4 | 9.32 | 65.0 |
| Fish oil dose 1 (equiv.) | 22.6 | 9.31 | 67.3 |
| Fish oil dose 2 (equiv.) | 22.2 | 9.35 | 68.1 |
| Fish oil dose 3 (equiv.) | 22.0 | 9.28 | 68.3 |
| Fish oil dose 4 (equiv.) | 21.7 | 9.29 | 63.9 |
| SEM | 0.40 | 0.09 | 1.90 |
| p value ANOVA | 0.554 | 0.345 | 0.758 |
| Diet | Gas Production (mL) | SCFA Concentration(mM) | Total Bacteria(log10 16S rDNA Copies/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| 0–4 h | 0–8 h | 0 h | 4 h | 8 h | 0 h | 4 h | 8 h | |
| Control | 10.2 | 23.4 | 14.9 | 48.7 | 79.8 | 10.0 | 10.8 | 11.2 |
| AURA dose 1 | 10.3 | 23.5 | 14.9 | 49.8 | 78.7 | 10.0 | 11.1 | 11.1 |
| AURA dose 2 | 9.9 | 23.7 | 15.0 | 47.7 | 78.8 | 9.8 | 11.0 | 11.4 |
| AURA dose 3 | 10.5 | 22.7 | 14.6 | 51.1 | 78.3 | 10.0 | 11.0 | 11.1 |
| AURA dose 4 | 10.5 | 23.7 | 15.3 | 49.8 | 82.5 | 10.1 | 10.9 | 11.3 |
| SEM | 0.27 | 0.44 | 0.20 | 1.41 | 2.04 | 0.012 | 0.012 | 0.017 |
| p value ANOVA | 0.659 | 0.480 | 0.242 | 0.547 | 0.636 | 0.590 | 0.166 | 0.270 |
| Parameter | Control Diet a | AURA Diet a | Washout a | SEM | p Value ANOVA |
|---|---|---|---|---|---|
| Total eubacteria (log10 gene copies/mL) | 10.85 | 10.66 | 10.86 | 0.08 | 0.172 |
| Methanogens (log10 gene copies/mL) | 9.84 A | 9.46 B | 9.62 AB | 0.10 | 0.055 |
| Bacteroides–Prevotella (log10 gene copies/mL) | 10.31 | 10.28 | 10.49 | 0.09 | 0.221 |
| Ruminococcaceae (log10 gene copies/mL) | 9.38 | 9.25 | 9.47 | 0.10 | 0.299 |
| Lachnospiraceae (log10 gene copies/mL) | 10.07 | 9.88 | 10.11 | 0.12 | 0.437 |
| S. ruminantium (log10 gene copies/mL) | 8.41 | 8.40 | 8.67 | 0.09 | 0.150 |
| Lactobacillus spp. (log10 gene copies/mL) | 8.30 A | 7.83 B | 8.28 A | 0.10 | 0.007 |
| Streptococcus spp. (log10 gene copies/mL) | 8.61 | 8.43 | 8.57 | 0.12 | 0.600 |
| F. succinogenes (log10 gene copies/mL) | 6.90 | 7.24 | 7.33 | 0.13 | 0.074 |
| M. elsdenii (log10 gene copies/mL) | 5.88 A | 5.17 B | 5.33 B | 0.11 | 0.002 |
| Veillonella spp. (log10 gene copies/mL) | 5.94 | 5.82 | 5.92 | 0.10 | 0.662 |
| Total protozoa (log10 gene copies/mL) | 8.43 | 8.21 | 8.45 | 0.15 | 0.548 |
| Total SCFAs (mM) | 113.03 A | 103.68 B | 107.79 C | 0.99 | 0.000 |
| Acetic acid (mM) | 70.75 A | 66.00 B | 69.07 A | 0.54 | 0.000 |
| Propionic acid (mM) | 17.99 A | 15.73 B | 15.55 B | 0.30 | 0.000 |
| Butyric acid (mM) | 14.90 A | 13.94 B | 14.53 AB | 0.21 | 0.022 |
| Lactic acid (mM) | 3.57 A | 2.36 B | 3.18 A | 0.21 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinttilä, T.; Moran, C.A.; Apajalahti, J. DHA-Rich Aurantiochytrium Biomass, a Novel Dietary Supplement, Resists Degradation by Rumen Microbiota without Disrupting Microbial Activity. Appl. Microbiol. 2022, 2, 53-72. https://doi.org/10.3390/applmicrobiol2010004
Rinttilä T, Moran CA, Apajalahti J. DHA-Rich Aurantiochytrium Biomass, a Novel Dietary Supplement, Resists Degradation by Rumen Microbiota without Disrupting Microbial Activity. Applied Microbiology. 2022; 2(1):53-72. https://doi.org/10.3390/applmicrobiol2010004
Chicago/Turabian StyleRinttilä, Teemu, Colm A. Moran, and Juha Apajalahti. 2022. "DHA-Rich Aurantiochytrium Biomass, a Novel Dietary Supplement, Resists Degradation by Rumen Microbiota without Disrupting Microbial Activity" Applied Microbiology 2, no. 1: 53-72. https://doi.org/10.3390/applmicrobiol2010004
APA StyleRinttilä, T., Moran, C. A., & Apajalahti, J. (2022). DHA-Rich Aurantiochytrium Biomass, a Novel Dietary Supplement, Resists Degradation by Rumen Microbiota without Disrupting Microbial Activity. Applied Microbiology, 2(1), 53-72. https://doi.org/10.3390/applmicrobiol2010004