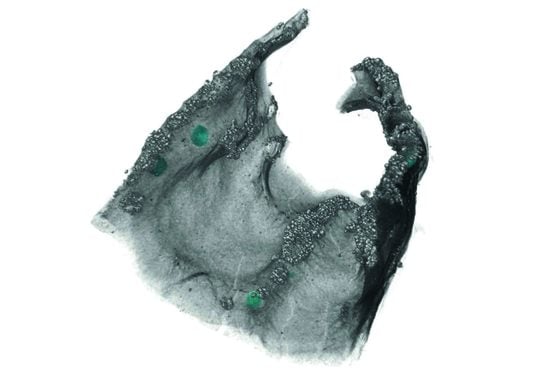
MicroCT as a Useful Tool for Analysing the 3D Structure of Lichens and Quantifying Internal Cephalodia in Lobaria pulmonaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. CT Acquisition
2.3. Image Processing Using Amira Software
2.3.1. Visualisation of Lichen Structure Performed by Volume Rendering and Clipping Tools
2.3.2. Segmentation
2.3.3. Measurements of Segmented Structures Performed by Label Analysis
3. Results
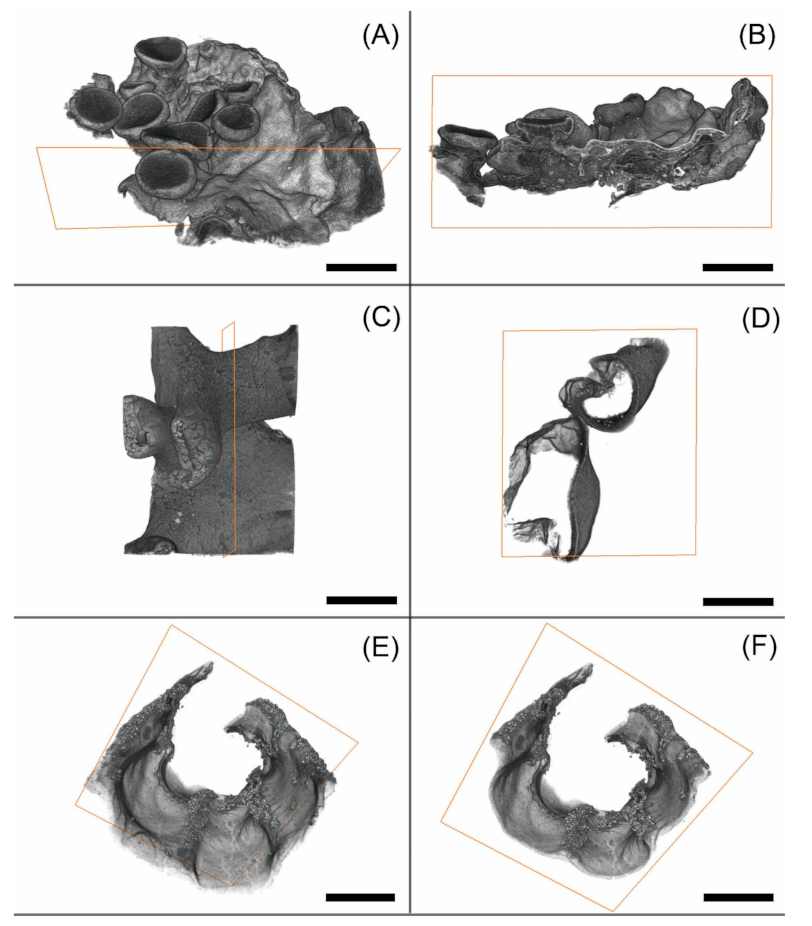
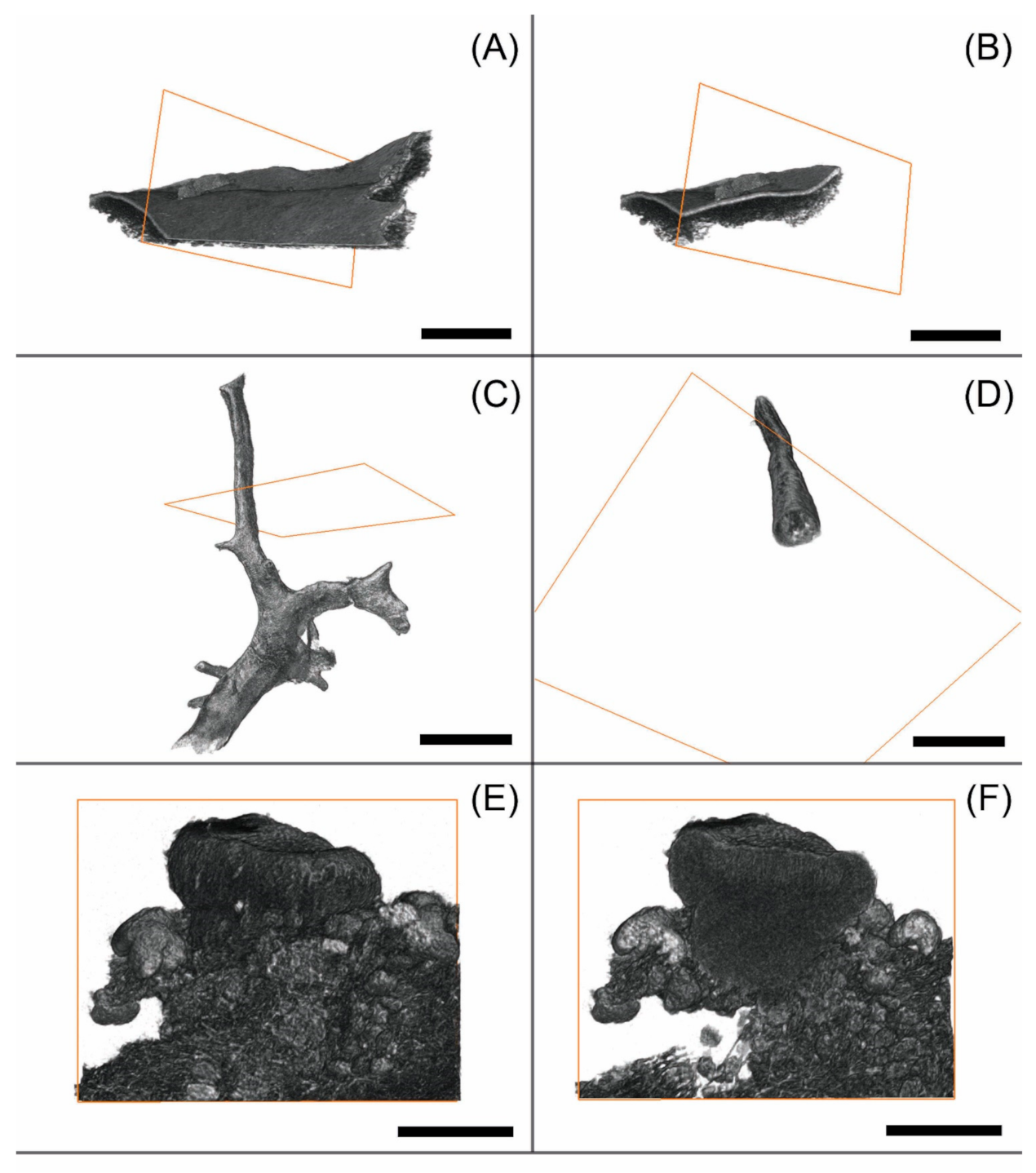
3.1. Morphological Observation
3.2. Observation of Microstructures
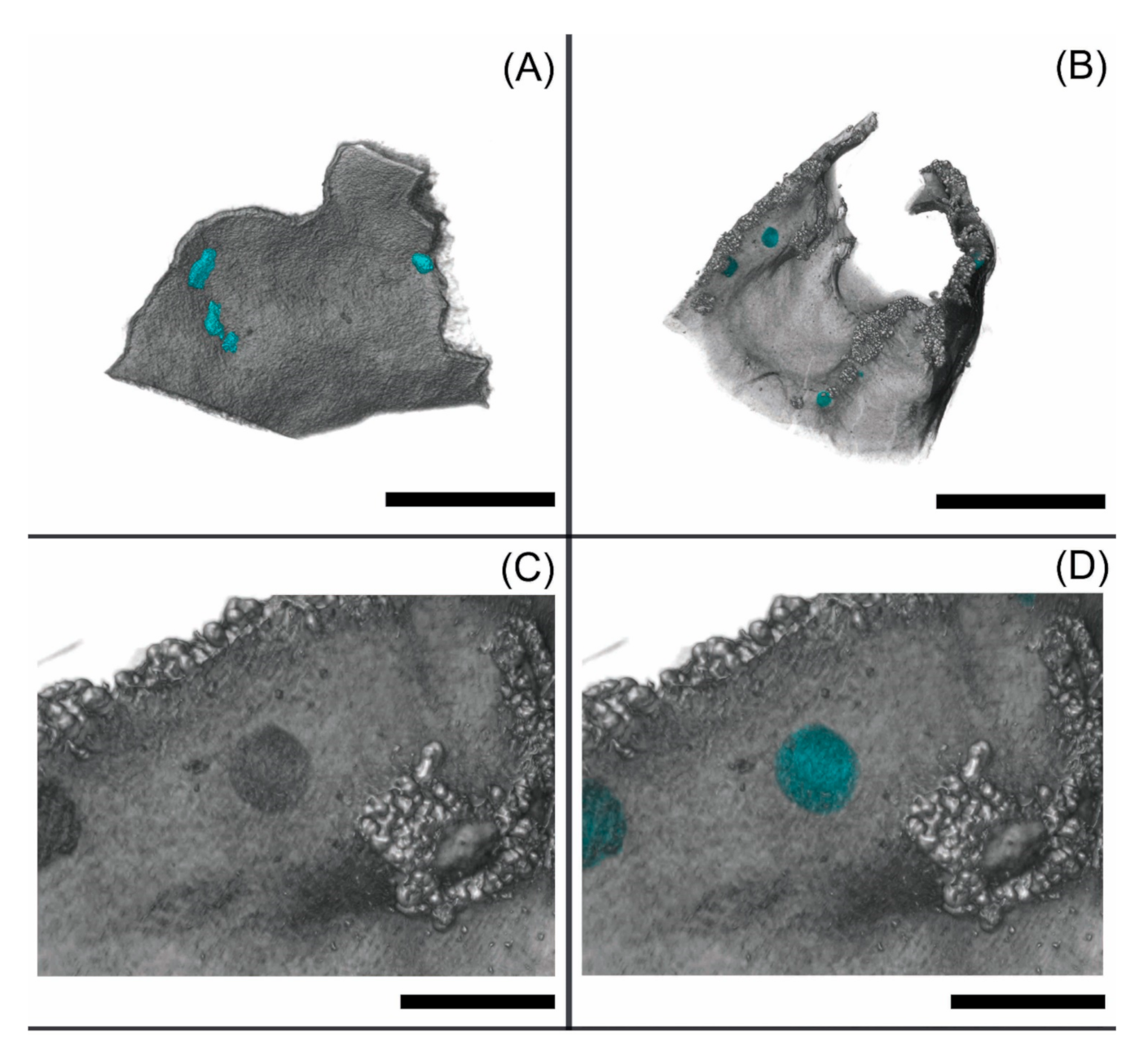
3.3. Segmentation Results and Label Analysis
4. Discussion
4.1. Pros and Cons in the Visualisation of Lichens Using MicroCT
4.2. Segmentation and Statistical Analysis of External and Internal Cephalodia
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statements
References
- Ruthensteiner, B. Soft Part 3D visualisation by serial sectioning and computer reconstruction. Zoosymposia 2008, 1, 61–100. [Google Scholar] [CrossRef]
- Handschuh, S.; Baeumler, N.; Schwaha, T.; Ruthensteiner, B. A correlative approach for combining microCT, light and transmission electron microscopy in a single 3D scenario. Front. Zool. 2013, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.F. The lichen as an ecosystem: Observation and experiment. In Lichenology: Progress and Problems; Brown, D.H., Hawksworth, D.L., Bailey, R.H., Eds.; Academic Press: London, UK, 1976; pp. 385–406. [Google Scholar]
- Werth, S. How does the lichen symbiosis work? Journey into the unknown. Fritschiana 2017, 85, 48–49. [Google Scholar]
- Mark, K.; Laanisto, L.; Bueno, C.G.; Niinemets, Ü.; Keller, C.; Scheidegger, C. Contrasting co-occurrence patterns of photobiont and cystobasidiomycete yeast associated with common epiphytic lichen species. New Phytol. 2020, 227, 1362–1375. [Google Scholar] [CrossRef]
- Tzovaras, B.G.; Segers, F.H.I.D.; Bicker, A.; Dal Grande, F.; Otte, J.; Anvar, S.Y.; Hankeln, T.; Schmitt, I.; Ebersberger, I. What is in Umbilicaria pustulata? A metagenomic approach to reconstruct the holo-genome of a lichen. Genome Biol. Evol. 2020, 12, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Grube, M. Lichens growing greenhouses en miniature. Microbial Cell 2021, 8, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R. The Symbiotic Phenotype of Lichen-Forming Ascomycetes and Their Endo- and Epibionts. In The Mycota IX—Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 287–339. [Google Scholar]
- Büdel, B.; Scheidegger, C. Thallus morphology and anatomy. In Lichen Biology; Nash, T.H., III, Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 24–36. [Google Scholar]
- Eisenreich, W.; Knispel, N.; Beck, A. Advanced methods for the study of the chemistry and the metabolism of lichens. Phytochem. Rev. 2011, 10, 445–456. [Google Scholar] [CrossRef]
- Kuhn, V.; Geisberger, T.; Huber, C.; Beck, A.; Eisenreich, W. A facile in vivo procedure to analyse metabolic pathways in intact lichens. New Phytol. 2019, 224, 1657–1667. [Google Scholar] [CrossRef]
- Singh, G.; Grande, F.D.; Divakar, P.K.; Otte, J.; Leavitt, S.D.; Szczepańska, K.; Crespo, A.; Rico, V.J.; Aptroot, A.; Cáceres, M.E.D.S.; et al. Coalescent–based species delimitation approach uncovers high cryptic diversity in the cosmopolitan lichen–forming fungal genus Protoparmelia (Lecanorales, Ascomycota). PLoS ONE 2015, 10, e0124625. [Google Scholar] [CrossRef]
- Altermann, S.; Leavitt, S.D.; Goward, T.; Nelsen, M.P.; Lumbsch, H.T. How do you solve a problem like Letharia? A new look at cryptic species in lichen-forming fungi using Bayesian clustering and SNPs from multilocus sequence data. PLoS ONE 2014, 9, e97556. [Google Scholar] [CrossRef]
- Kraichak, E.; Lücking, R.; Aptroot, A.; Beck, A.; Dornes, P.; John, V.; Lendemer, J.C.; Nelsen, M.P.; Neuwirth, G.; Nutakki, A.; et al. Hidden diversity in the morphologically variable script lichen (Graphis scripta) complex (Ascomycota, Ostropales, Graphidaceae). Org. Divers. Evol. 2015, 15, 447–458. [Google Scholar] [CrossRef]
- Frolov, I.; Vondrák, J.; Fernández-Mendoza, F.; Wilk, K.; Khodosovtsev, A.; Halici, H.G. Three new, seemingly–cryptic species in the lichen genus Caloplaca (Teloschistaceae) distinguished in two–phase phenotype evaluation. Ann. Bot. Fenn. 2016, 53, 243–262. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Esslinger, T.L.; Divakar, P.K.; Crespo, A.; Lumbsch, H.T. Hidden diversity before our eyes: Delimiting and describing cryptic lichen-forming fungal species in camouflage lichens (Parmeliaceae, Ascomycota). Fungal Biol. 2016, 120, 1374–1391. [Google Scholar] [CrossRef]
- Boluda, C.G.; Rico, V.J.; Divakar, P.K.; Nadyeina, O.; Myllys, L.; McMullin, R.T.; Zamora, J.C.; Scheidegger, C.; Hawksworth, D.L. Evaluating methodologies for species delimitation: The mismatch between phenotypes and genotypes in lichenised fungi (Bryoria sect. Implexae, Parmeliaceae). Persoonia 2019, 42, 75–100. [Google Scholar] [CrossRef]
- Del-Prado, R.; Buaruang, K.; Lumbsch, H.T.; Crespo, A.; Divakar, P.K. DNA sequence-based identification and barcoding of a morphologically highly plastic lichen forming fungal genus (Parmotrema, Parmeliaceae) from the tropics. Bryologist 2019, 122, 281–291. [Google Scholar] [CrossRef]
- Nash, T.H. (Ed.) Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Lawrey, J.D. Biological role of lichen substances. Bryologist 1986, 89, 111–122. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Nash, T.H., III; Boucher, V.L.; Schlesinger, W.H. Mineral cycling and epiphytic lichens: Implications at the ecosystem level. Lichenologist 1991, 23, 309–321. [Google Scholar] [CrossRef]
- Knowles, R.D.; Pastor, J.; Biesboer, D.D. Increased soil nitrogen associated with dinitrogen-fixing, terricolous lichens of the genus Peltigera in northern Minnesota. Oikos 2006, 114, 37–48. [Google Scholar] [CrossRef]
- Asplund, J.; Wardle, D.A. How lichens impact on terrestrial community and ecosystem properties. Biol. Rev. 2017, 92, 1720–1738. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.; Purvis, O.W. Lobaria (Schreb.) Hoffm. 1796. In Lichens of Great Britain and Ireland; Smith, C.W., Aptroot, A., Coppins, B.J., Fletcher, A., Gilbert, O.L., James, P.W., Wolseley, P.A., Eds.; MPG Books Group: London, UK, 2009; pp. 560–562. [Google Scholar]
- Cornejo, C.; Scheidegger, C. New morphological aspects of cephalodium formation in the lichen Lobaria pulmonaria (Lecanorales, Ascomycota). Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef]
- Škaloud, P.; Friedl, T.; Hallmann, C.; Beck, A.; Dal Grande, F. Taxonomic revision and species delimitation of coccoid green algae currently assigned to the genus Dictyochloropsis (Trebouxiophyceae, Chlorophyta). J. Phycol. 2016, 52, 599–617. [Google Scholar] [CrossRef]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The Lichens’ Microbiota, Still a Mystery? Front. Microbiol. 2021, 12, 623839. [Google Scholar] [CrossRef] [PubMed]
- Metscher, B.D. MicroCT for developmental biology: A versatile tool for high-contrast 3D imaging at histological resolutions. Dev. Dyn. 2009, 238, 632–640. [Google Scholar] [CrossRef]
- Thermo Scientific. User’s Guide Amira Software 2019. Available online: https://assets.thermofisher.com/TFS-Assets/MSD/Product-Guides/users-guide-amira-software-2019.pdf (accessed on 9 March 2021).
- Handschuh, S.; Beisser, C.J.; Ruthensteiner, B.; Metscher, B.D. Microscopic dual-energy CT (microDECT): A flexible tool for multichannel ex vivo 3D imaging of biological specimens. J. Microsc. 2017, 67, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Karreman, M.A.; Ruthensteiner, B.; Mercier, L.; Schieber, N.L.; Solecki, G.; Winkler, F.; Goetz, J.G.; Schwab, Y. Find your way with X-Ray: Using microCT to correlate in vivo imaging with 3D electron microscopy. Methods Cell Biol. 2017, 140, 277–301. [Google Scholar] [PubMed]
- Jacob, D.E.; Ruthensteiner, B.; Trimby, P.; Henry, H.; Martha, S.O.; Leitner, J.; Otter, L.M.; Scholz, J. Architecture of Anoteropora latirostris (Bryozoa, Cheilostomata) and implications for their biomineralisation. Sci. Rep. 2019, 9, 11439. [Google Scholar] [CrossRef]
- Schwaha, T.; Ruthensteiner, B.; Melzer, R.R.; Asami, T.; Páll-Gergely, B. Three phyla—Two type specimens—One shell: History of a snail shell revealed by modern imaging technology. J. Zool. Syst. Evol. Res. 2019, 57, 527–533. [Google Scholar] [CrossRef]
- Orhan, K. (Ed.) Micro-Computed Tomography (micro-CT) in Medicine and Engineering; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kosugi, M.; Shizuma, R.; Moriyama, Y.; Koike, H.; Fukunaga, Y.; Takeuchi, A.; Uesugi, K.; Suzuki, Y.; Imura, S.; Kudoh, S.; et al. Ideal osmotic spaces for chlorobionts or cyanobionts are differentially realized by lichenized fungi. Plant Physiol. 2014, 166, 337–348. [Google Scholar] [CrossRef][Green Version]
- Brandt, A.; de Vera, J.P.; Onofri, S.; Ott, S. Viability of the lichen Xanthoria elegans and its symbionts after 18 months of space exposure and simulated mars conditions on the ISS. Int. J. Astrobiol. 2015, 14, 411–425. [Google Scholar] [CrossRef]
- Goward, T.; Goffinet, B.; Vitikainen, O. Synopsis of the genus Peltigera (lichenized Ascomycetes) in British Columbia, with a key to the North American species. Canad. J. Bot. 1995, 73, 91–111. [Google Scholar] [CrossRef]

| Name | Herbarium Number | Growth Form | Voxel Size (µm) | X, Y, and Z Voxel Dimensions of 8-Bit Datasets | Number of Projections | Duration of CT Acquisition |
|---|---|---|---|---|---|---|
| Bacidia rubella | M-0304302 | crustose | 1.24992 | 2085 × 1425 × 2262 | 1800 | 76 min |
| Evernia divaricata | M-0304299 | fruticose | 1.40618 | 1107 × 1332 × 2193 | 1700 | 71 min |
| Hypogymnia physodes | M-0304295 | foliose | 1.29594 | 2931 × 2875 × 2234 | 1800 | 90 min |
| Xanthoria parietina | M-0304310 | foliose | 1.65375 | 1104 × 2451 × 2298 | 1800 | 76 min |
| Lobaria pulmonaria | M-0304308 | foliose | 1.60658 | 2080 × 2290 × 2200 | 1800 | 76 min |
| Peltigera leucophlebia | M-0304309 | foliose | 1.61011 | 1901 × 2439 × 1820 | 1800 | 76 min |
| Material | Volume 3D (10−3 mm3) |
|---|---|
| Whole sample (thallus) | 2091 |
| Cephalodium 1 | 4.525 |
| Cephalodium 2 | 3.527 |
| Cephalodium 3 | 3.202 |
| Cephalodium 4 | 2.913 |
| Cephalodium 5 | 0.722 |
| Cephalodium 6 | 0.249 |
| Cephalodium 7 | 0.059 |
| Cephalodium 8 | 0.054 |
| Cephalodium 9 | 0.046 |
| Sum cephalodia | 15.30 (0.73%) |
| Material | Volume 3D (10−3 mm3) |
|---|---|
| Whole sample (thallus) | 200 |
| Cephalodium 1 | 0.873 |
| Cephalodium 2 | 0.507 |
| Cephalodium 3 | 0.366 |
| Cephalodium 4 | 0.201 |
| Sum cephalodia | 1.947 (0.97%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, J.; Ruthensteiner, B.; Beck, A. MicroCT as a Useful Tool for Analysing the 3D Structure of Lichens and Quantifying Internal Cephalodia in Lobaria pulmonaria. Appl. Microbiol. 2021, 1, 189-200. https://doi.org/10.3390/applmicrobiol1020015
Gerasimova J, Ruthensteiner B, Beck A. MicroCT as a Useful Tool for Analysing the 3D Structure of Lichens and Quantifying Internal Cephalodia in Lobaria pulmonaria. Applied Microbiology. 2021; 1(2):189-200. https://doi.org/10.3390/applmicrobiol1020015
Chicago/Turabian StyleGerasimova, Julia, Bernhard Ruthensteiner, and Andreas Beck. 2021. "MicroCT as a Useful Tool for Analysing the 3D Structure of Lichens and Quantifying Internal Cephalodia in Lobaria pulmonaria" Applied Microbiology 1, no. 2: 189-200. https://doi.org/10.3390/applmicrobiol1020015
APA StyleGerasimova, J., Ruthensteiner, B., & Beck, A. (2021). MicroCT as a Useful Tool for Analysing the 3D Structure of Lichens and Quantifying Internal Cephalodia in Lobaria pulmonaria. Applied Microbiology, 1(2), 189-200. https://doi.org/10.3390/applmicrobiol1020015