Comparison of Sodium Nitrite and ‘Natural’ Nitrite on the Inhibition of Spore Germination and Outgrowth of Clostridium sporogenes in Low- and High-Fat Frankfurters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures and Media
2.2. Antibiotic Assay of Clostridium sporogenes
2.3. Sporulation and Spore Crop Isolation
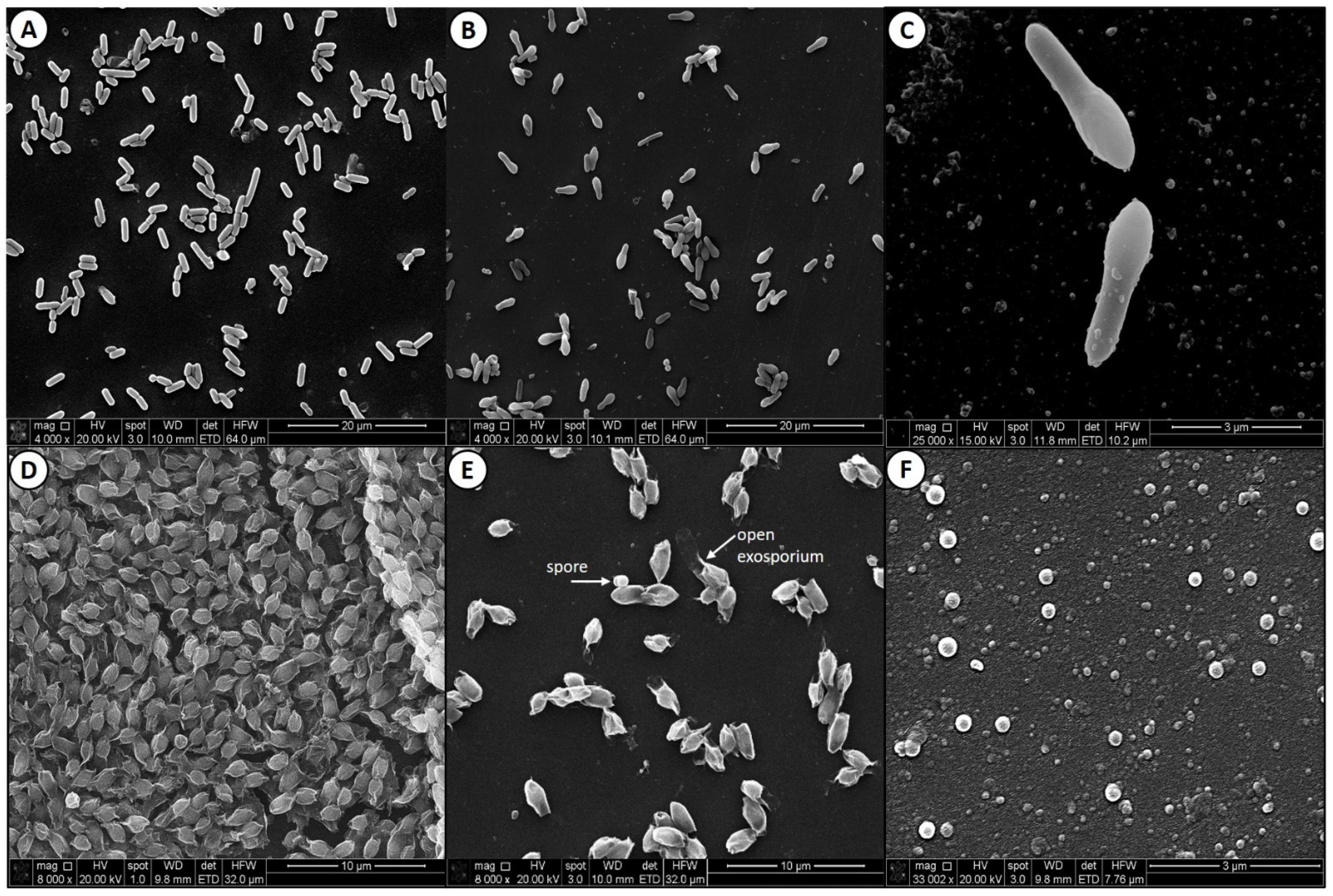
2.4. Electron Microscopy of Clostridium sporogenes Cells and Spores
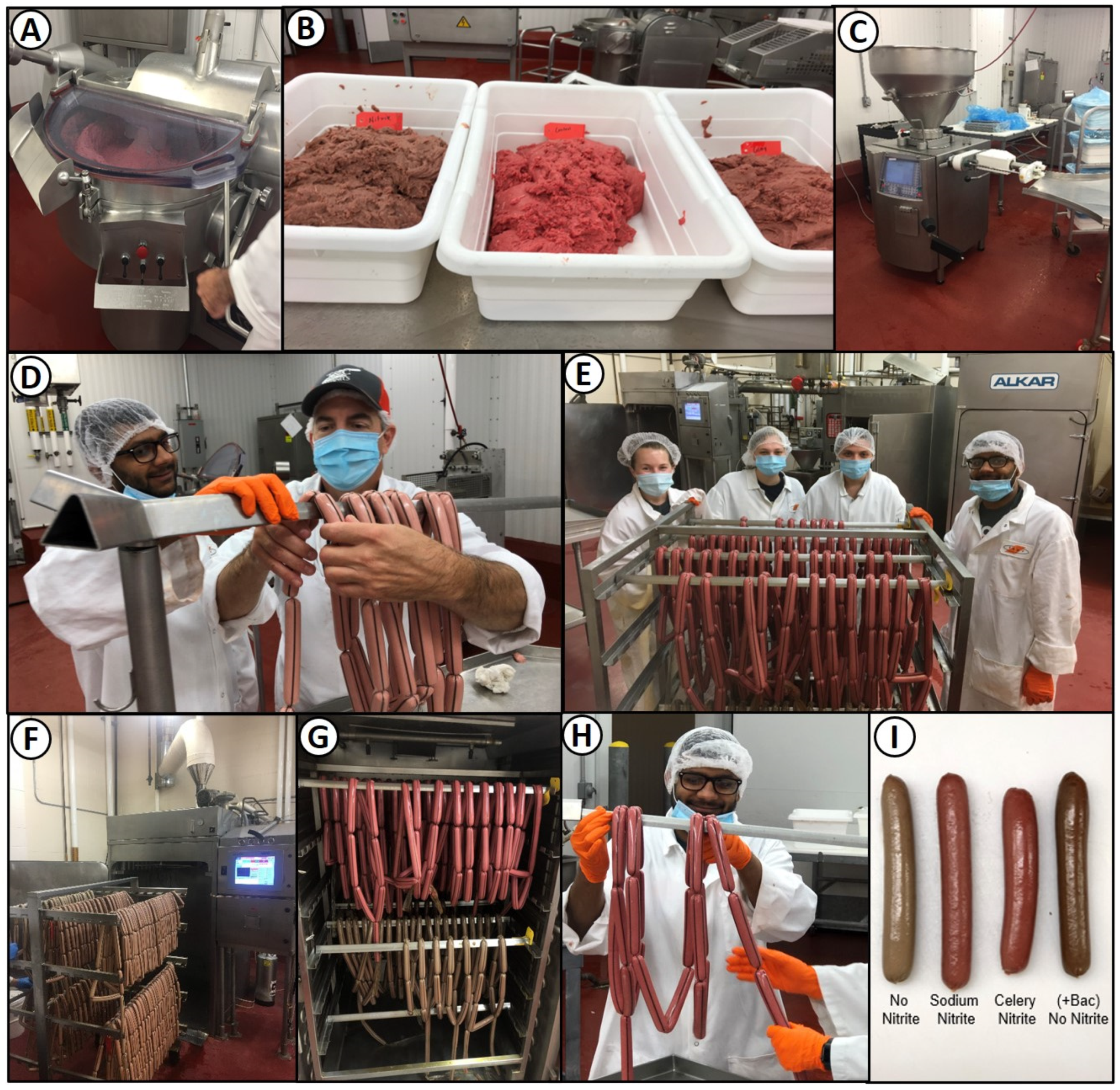
2.5. Meat Formulation and Hotdog Manufacture
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antibiotic Resistance of Clostridium sporogenes ATCC 3584, ATCC 19404, and ATCC BAA-2695
3.2. Scanning Electron Microscopy of Clostridium sporogenes Cells and Spores
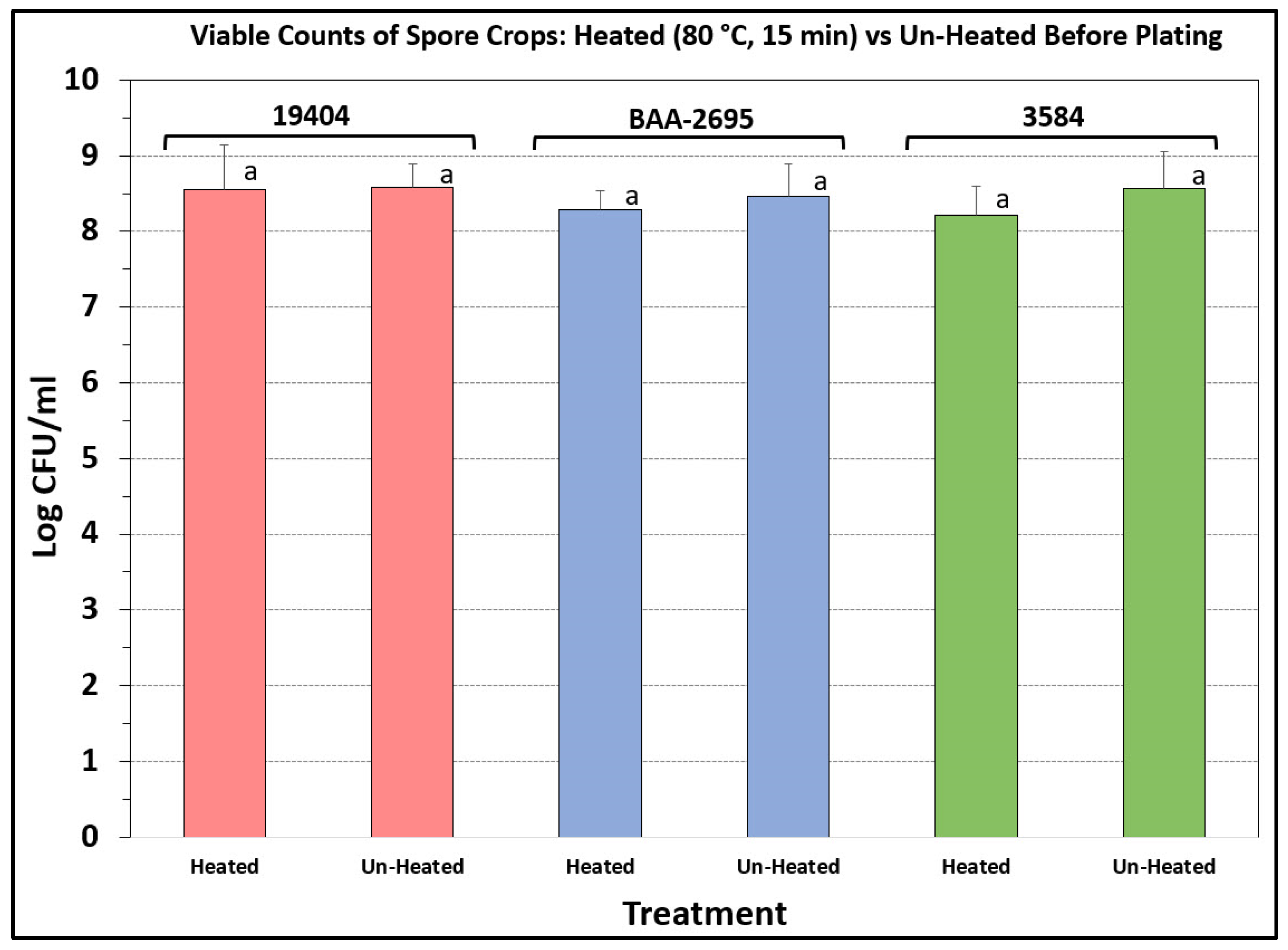
3.3. Heated vs. Unheated Spore Crops
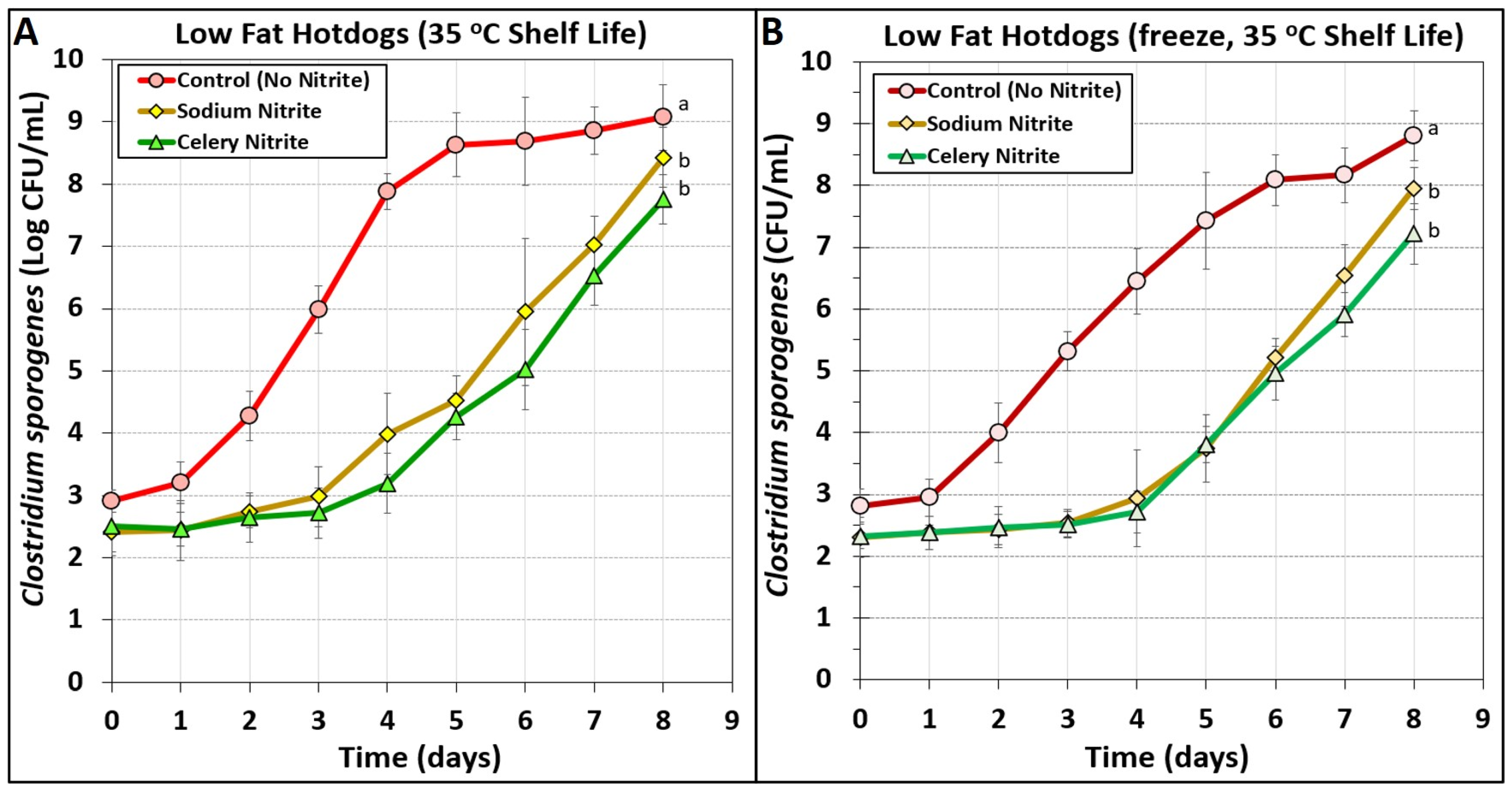
3.4. Low-Fat Hotdogs Challenged with Spores from Clostridium sporogenes
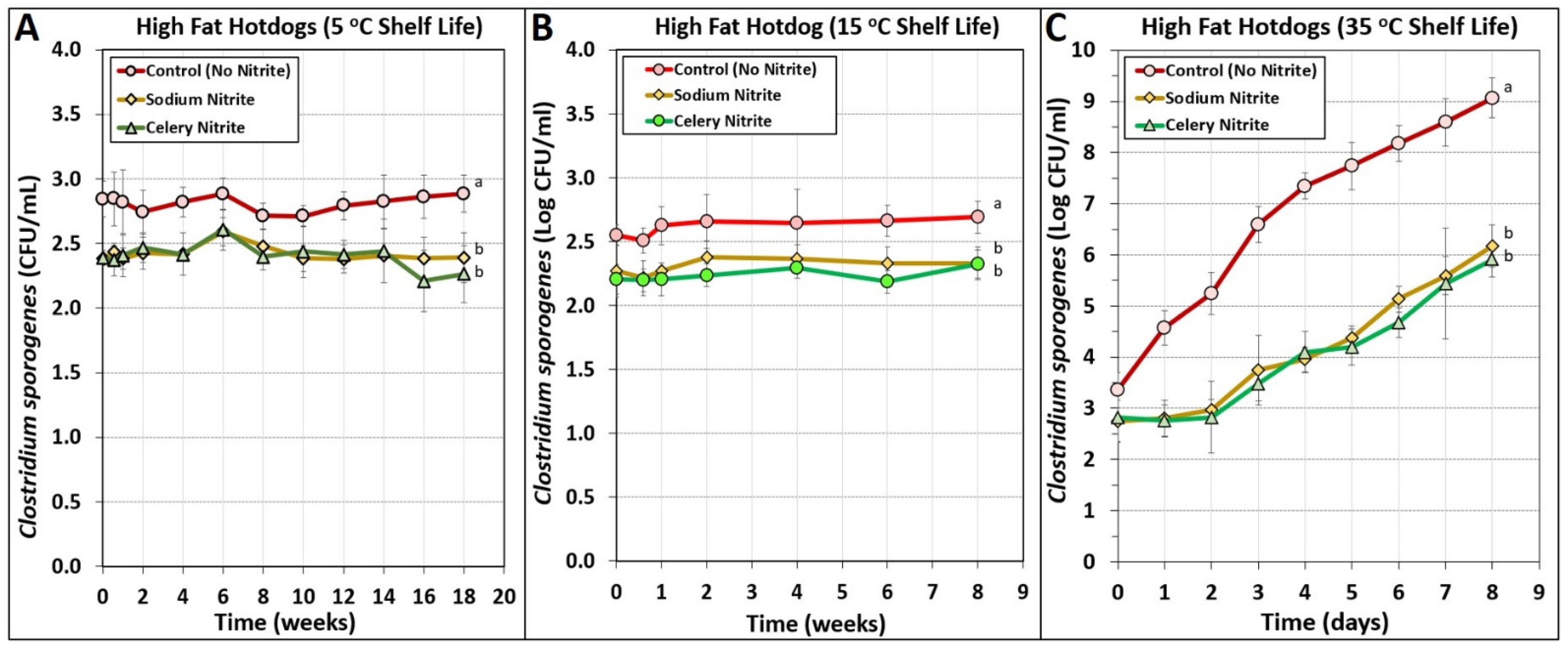
3.5. Manufacturing of High-Fat Hotdogs Challenged with Spores from C. sporogenes
3.6. Manufacturing of Low Fat Hotdogs Containing Bacteriocins Challenged with Spores from C. sporogenes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatheway, C.L. Toxigenic clostridia. Clin. Microbiol. Rev. 1990, 3, 66–98. [Google Scholar] [CrossRef]
- Al-Hinai, M.A.; Jones, S.W.; Papoutsakis, E.T. Sporulation programs: Diversity and preservation of endospore differentiation. Microbiol. Mol. Biol. Rev. 2015, 79, 19. [Google Scholar] [CrossRef] [Green Version]
- Broussolle, V.; Alberto, F.; Shearman, C.A.; Mason, D.R.; Botella, L.; Nguyen-The, C.; Peck, M.W.; Carlin, F. Molecular and Physiological Characterisation of Spore Germination in Clostridium botulinum and C. sporogenes. Anaerobe 2002, 8, 89–100. [Google Scholar] [CrossRef]
- Long, S.C.; Tauscher, T. Watershed issues associated with Clostridium botulinum: A literature review. J. Water Health 2006, 4, 277–288. [Google Scholar] [CrossRef]
- García, S.; Heredia, N. Clostridium perfringens: A dynamic foodborne pathogen. Food Bioprocess Technol. 2011, 4, 624–630. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Processed meat consumption and stomach cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2006, 98, 1078–1087. [Google Scholar] [CrossRef] [Green Version]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products—Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, A.; Muriana, P.M. Isolation and characterization of nitrate reducing bacteria for conversion of vegetable-derived nitrate to ‘natural nitrite’. Appl. Microbiol. 2021, 1, 11–23. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J. Food Compos. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Sullivan, G.A.; Jackson-Davis, A.L.; Niebuhr, S.E.; Xi, Y.; Schrader, K.D.; Sebranek, J.G.; Dickson, J.S. Inhibition of Listeria monocytogenes using natural antimicrobials in no-nitrate-or-nitrite-added ham. J. Food Prot. 2012, 75, 1071–1076. [Google Scholar] [CrossRef]
- Jackson, A.L.; Kulchaiyawat, C.; Sullivan, G.A.; Sebranek, J.G.; Dickson, J.S. Use of natural ingredients to control growth of Clostridium perfringens in naturally cured frankfurters and hams. J. Food Prot. 2011, 74, 417–424. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Glass, K.A.; Milkowski, A.L.; Sindelar, J.J. Impact of clean-label antimicrobials and nitrite derived from natural sources on the outgrowth of Clostridium perfringens during cooling of deli-style turkey breast. J. Food Prot. 2015, 78, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Olszewska, M.A.; Diez-Gonzalez, F. Selection and application of natural antimicrobials to control Clostridium perfringens in sous-vide chicken breasts: Inhibition of C. perfringens in sous-vide chicken. Int. J. Food Microbiol. 2021, 347, 109193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M.; Jung, D.Y.-G.; Jin, D.Y.-Y.; Jayabalan, D.R.; Yang, D.S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767. [Google Scholar] [CrossRef]
- Muriana, P.M. Bacteriocins for control of Listeria spp in food. J. Food Prot. 1996, 59, 54–63. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Joly, C.; Oulahal, N.; Degraeve, P. Nisin as a Food Preservative: Part 2: Antimicrobial polymer materials containing nisin. Crit. Rev. Food Sci. Nutr. 2016, 56, 1275–1289. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 2017, 15, e05063. [Google Scholar]
- Burke, D.G.; Cotter, P.D.; Ross, R.P.; Hill, C. 14—Microbial production of bacteriocins for use in foods. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 353–384. [Google Scholar]
- Harlander, S.K. Chapter 11—Regulatory aspects of bacteriocin use. In Bacteriocins of Lactic Acid Bacteria; Hoover, D.G., Steenson, L.R., Eds.; Academic Press: Cambridge, MA, USA, 1993; pp. 233–247. [Google Scholar]
- Diao, M.M.; André, S.; Membré, J.-M. Meta-analysis of D-values of proteolytic Clostridium botulinum and its surrogate strain Clostridium sporogenes PA 3679. Int. J. Food Microbiol. 2014, 174, 23–30. [Google Scholar] [CrossRef]
- Brown, J.L.; Tran-Dinh, N.; Chapman, B. Clostridium sporogenes PA 3679 and its uses in the derivation of thermal processing schedules for low-acid shelf-stable foods and as a research model for proteolytic Clostridium botulinum. J. Food Prot. 2012, 75, 779–792. [Google Scholar] [CrossRef]
- Bradbury, M.; Greenfield, P.; Midgley, D.; Li, D.; Tran-Dinh, N.; Vriesekoop, F.; Brown, J.L. Draft genome sequence of Clostridium sporogenes PA 3679, the common nontoxigenic surrogate for proteolytic Clostridium botulinum. J. Bact. 2012, 194, 1631. [Google Scholar] [CrossRef] [Green Version]
- Khanipour, E.; Flint, S.H.; Mccarthy, O.J.; Golding, M.; Palmer, J.; Ratkowsky, D.A.; Ross, T.; Tamplin, M. Modelling the combined effects of salt, sorbic acid and nisin on the probability of growth of Clostridium sporogenes in a controlled environment (nutrient broth). Food Control 2016, 62, 32–43. [Google Scholar] [CrossRef]
- Taylor, R.H.; Dunn, M.L.; Ogden, L.V.; Jefferies, L.K.; Eggett, D.L.; Steele, F.M. Conditions associated with Clostridium sporogenes growth as a surrogate for Clostridium botulinum in nonthermally processed canned butter. J. Dairy Sci. 2013, 96, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Gonzalez, M.; Sarker, M.R.; Torres, J.A. Combined effects of hydrostatic pressure, temperature, and pH on the inactivation of spores of Clostridium perfringens Type A and Clostridium sporogenes in buffer solutions. J. Food Sci. 2007, 72, M202–M206. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Yemmireddy, V.K.; Costello, M.J.; Gray, P.M.; Salvadalena, R.; Rasco, B.; Killinger, K. Effect of storage time and temperature on the viability of E. coli O157:H7, Salmonella spp., Listeria innocua, Staphylococcus aureus, and Clostridium sporogenes vegetative cells and spores in vacuum-packed canned pasteurized milk cheese. Int. J. Food Microbiol. 2018, 286, 148–154. [Google Scholar] [CrossRef]
- Valero, A.; Olague, E.; Medina-Pradas, E.; Garrido-Fernández, A.; Romero-Gil, V.; Cantalejo, M.J.; García-Gimeno, R.M.; Pérez-Rodríguez, F.; Posada-Izquierdo, G.D.; Arroyo-López, F.N. Influence of acid adaptation on the probability of germination of Clostridium sporogenes spores against pH, NaCl and time. Foods 2020, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Enfors, S.-O.; Molin, G. The influence of high concentrations of carbon dioxide on the germination of bacterial spores. J. Appl. Bacteriol. 1978, 45, 279–285. [Google Scholar] [CrossRef]
- Garver, K.I.; Muriana, P.M. Detection, identification and characterization of bacteriocin-producing lactic acid bacteria from retail food products. Int. J. Food Microbiol. 1993, 20, 241–258. [Google Scholar] [CrossRef]
- Henning, C.; Vijayakumar, P.; Adhikari, R.; Jagannathan, B.; Gautam, D.; Muriana, P.M. Isolation and taxonomic identity of bacteriocin-producing lactic acid bacteria from retail foods and animal sources. Microorganisms 2015, 3, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, C.; Gautam, D.; Muriana, P. Identification of multiple bacteriocins in Enterococcus spp. using an Enterococcus-specific bacteriocin PCR array. Microorganisms 2015, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sandle, T. 14—Antibiotics and preservatives. In Pharmaceutical Microbiology; Sandle, T., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 171–183. [Google Scholar]
- Karolenko, C.E.; Bhusal, A.; Gautam, D.; Muriana, P.M. Selenite cystine agar for enumeration of inoculated Salmonella serovars recovered from stressful conditions during antimicrobial validation studies. Microorganisms 2020, 8, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Abel Zur Wiesch, P.; Busa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Bonhoeffer, S. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Yeh, P.J. Suppressive drug combinations and their potential to combat antibiotic resistance. J. Antibiot. 2017, 70, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Perkins, W.E. Production of clostridial spores. J. Appl. Bacteriol. 1965, 28, 1–16. [Google Scholar]
- Yang, W.W.; Crow-Willard, E.N.; Ponce, A. Production and characterization of pure Clostridium spore suspensions. J. Appl. Microbiol. 2009, 106, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, J.J. Conventional specimen preparation techniques for scanning electron microscopy of biological specimens. In Electron Microscopy: Methods and Protocols; Kuo, J., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 449–466. [Google Scholar]
- Bray, D.F.; Bagu, J.; Koegler, P. Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens. Microsc. Res. Tech. 1993, 26, 489–495. [Google Scholar] [CrossRef]
- Pettinati, J.D.; Swift, C.E. Rapid determination of fat in meat and meat products by Foss-let solvent extraction and density measurement. J. Assoc. Off. Anal. Chem. 2020, 58, 1182–1187. [Google Scholar] [CrossRef]
- Su, Y.K.; Bowers, J.A.; Zayas, J.F. Physical characteristics and microstructure of reduced-fat frankfurters as affected by salt and emulsified fats stabilized with nonmeat proteins. J. Food Sci. 2000, 65, 123–128. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Muriana, P. Inhibition of Listeria monocytogenes on ready-to-eat meats using bacteriocin mixtures based on mode-of-action. Foods 2017, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Macwana, S.; Muriana, P.M. Spontaneous bacteriocin resistance in Listeria monocytogenes as a susceptibility screen for identifying different mechanisms of resistance and modes of action by bacteriocins of lactic acid bacteria. J. Microbiol. Methods 2012, 88, 7–13. [Google Scholar] [CrossRef] [PubMed]
- National Advisory Committee on the Microbiological Criteria for Foods. Parameters for determining inoculated pack/challenge study protocols. J. Food Prot. 2010, 73, 140–202. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS. FSIS Compliance Guideline HACCP Systems Validation; USDA-FSIA: Washington, DC, USA, 2015; pp. 1–68.
- Janganan, T.K.; Mullin, N.; Tzokov, S.B.; Stringer, S.; Fagan, R.P.; Hobbs, J.K.; Moir, A.; Bullough, P.A. Characterization of the spore surface and exosporium proteins of Clostridium sporogenes; implications for Clostridium botulinum group I strains. Food Microbiol. 2016, 59, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Romero, P.; Castro-Córdova, P.; Reyes-Ramírez, R.; Milano-Céspedes, M.; Guerrero-Araya, E.; Pizarro-Guajardo, M.; Olguín-Araneda, V.; Gil, F.; Paredes-Sabja, D. Clostridium difficile exosporium cysteine-rich proteins are essential for the morphogenesis of the exosporium layer, spore resistance, and affect C. difficile pathogenesis. PLoS Pathog. 2018, 14, e1007199. [Google Scholar] [CrossRef]
- Gøtterup, J.; Olsen, K.; Knöchel, S.; Tjener, K.; Stahnke, L.H.; Møller, J.K. Relationship between nitrate/nitrite reductase activities in meat associated staphylococci and nitrosylmyoglobin formation in a cured meat model system. Int. J. Food Microbiol. 2007, 120, 303–310. [Google Scholar] [CrossRef]
- Gøtterup, J.; Olsen, K.; Knøchel, S.; Tjener, K.; Stahnke, L.H.; Møller, J.K. Colour formation in fermented sausages by meat-associated staphylococci with different nitrite- and nitrate-reductase activities. Meat Sci. 2008, 78, 492–501. [Google Scholar] [CrossRef]
- Vareltzis, K.; Buck, E.M.; Labbe, R.G. Effectiveness of a betalains/potassium sorbate system versus sodium nitrite for color development and control of total aerobes, Clostridium perfringens and Clostridium sporogenes in chicken frankfurters. J. Food Prot. 1984, 47, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Sullivan, G.A.; Kulchaiyawat, C.; Sebranek, J.G.; Dickson, J.S. Survival and growth of Clostridium perfringens in commercial no-nitrate-or-nitrite-added (natural and organic) frankfurters, hams, and bacon. J. Food Prot. 2011, 74, 410–416. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Huang, L.; Yoon, W.B. Mathematical modeling and growth kinetics of Clostridium sporogenes in cooked beef. Food Control 2016, 60, 471–477. [Google Scholar] [CrossRef]
- Rayman, M.K.; Aris, B.; Hurst, A. Nisin: A possible alternative or adjunct to nitrite in the preservation of meats. Appl. Environ. Microbiol. 1981, 41, 375. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.L.; Somers, E.B.; Krueger, L.A. Antibotulinal effectiveness of nisin-nitrite combinations in culture medium and chicken frankfurter emulsions. J. Food Prot. 1985, 48, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, A.S.; Crandall, A.D.; Montville, T.J. Nisin resistance in Clostridium botulinum spores and vegetative cells. Appl. Environ. Microbiol. 1997, 63, 2654–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, A. Nisin. In Advances Applied Microbiology; Perlman, D., Laskin, A.I., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 27, pp. 85–123. [Google Scholar]
- Han, S.-K.; Shin, M.-S.; Park, H.-E.; Kim, S.-Y.; Lee, W.-K. Screening of bacteriocin-producing Enterococcus faecalis strains for antagonistic activities against Clostridium perfringens. Korean J. Food Sci. Anim. Resour. 2014, 34, 614–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA-FSIS. Verification Procedures for Lethality and Stabilization; USDA-FSIS: Washington, DC, USA, 2017; Volume 7111.1, pp. 1–21.


| Ingredient | Formulation % | Low-Fat Formulation | High-Fat Formulation | ||||
|---|---|---|---|---|---|---|---|
| Beef | 72.69% | (Beef 90, beef 50) | (Beef 80, beef 50) | ||||
| Ice/Water | 24.92% | (80% + 20% portions) * | (80% + 20% portions) * | ||||
| AC Legg Bologna/Frank Seasoning w/o Erythorbate Salt, dextrose, MSG (2.91%), onion powder, garlic powder, spice extracts, tricalcium phosphate | 2.14% | ||||||
| CTL | Nitrite | Celery | CTL | Nitrite | Celery | ||
| Prague Powder (6.25% sodium nitrite) | -- | 0.18% | -- | -- | 0.18% | -- | |
| Sodium Erythorbate | -- | 0.04% | -- | -- | 0.04% | -- | |
| Celery Powder (2.34% sodium nitrite) | -- | -- | 0.48% | -- | -- | 0.48% | |
| Cherry Powder (17% ascorbic acid) | -- | -- | 0.20% | -- | -- | 0.20% | |
| Alkaline Phosphate | 0.25% | ||||||
| Total: | 100% | ||||||
| Restricted ingredients (maximum permitted levels): | |||||||
| Sodium nitrite (max = 156 ppm) | |||||||
| Sodium erythorbate (max = 547 ppm) | |||||||
| Ascorbic acid (max = 468 ppm) | |||||||
| Alkaline phosphate (max = 0.5%) | 0.34% | ||||||
| Sensi-Disc Designation: | OX/1 | CL/10 | AN/30 | NN/10 | INH/5 | EA/25 | S/50 | VA/30 | RA/5 | NA/30 | NB/5 |
| Sensi-Disc Dose (μg): | 1 | 10 | 30 | 10 | 5 | 25 | 50 | 30 | 5 | 30 | 5 |
| C. sporogenes 3584 | R | R | R | R | R | R | VS | VS | VS | VS | S |
| C. sporogenes 19404 | R | R | R | R | R | R | S | VS | ES | VS | S |
| C. sporogenes BAA-2695 | R | R | R | R | R | S | S | VS | VS | VS | S |
| Sensi-Disc Designation: | FX/100 | F/M /300 | CF/30 | GM/10 | E/15 | C/5 | CZ/30 | FOX/30 | TE/30 | AM/10 | |
| Sensi-Disc Dose (μg): | 100 | 300 | 30 | 10 | 15 | 5 | 30 | 30 | 30 | 10 | |
| C. sporogenes 3584 | ES | ES | ES | S | VS | VS | ES | VS | ES | ES | |
| C. sporogenes 19404 | ES | ES | ES | S | VS | VS | VS | VS | ES | ES | |
| C. sporogenes BAA-2695 | ES | ES | ES | S | VS | VS | ES | VS | ES | ES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhusal, A.; Nelson, J.; Pletcher, D.; Muriana, P.M. Comparison of Sodium Nitrite and ‘Natural’ Nitrite on the Inhibition of Spore Germination and Outgrowth of Clostridium sporogenes in Low- and High-Fat Frankfurters. Appl. Microbiol. 2021, 1, 104-122. https://doi.org/10.3390/applmicrobiol1010009
Bhusal A, Nelson J, Pletcher D, Muriana PM. Comparison of Sodium Nitrite and ‘Natural’ Nitrite on the Inhibition of Spore Germination and Outgrowth of Clostridium sporogenes in Low- and High-Fat Frankfurters. Applied Microbiology. 2021; 1(1):104-122. https://doi.org/10.3390/applmicrobiol1010009
Chicago/Turabian StyleBhusal, Arjun, Jacob Nelson, Dennis Pletcher, and Peter M. Muriana. 2021. "Comparison of Sodium Nitrite and ‘Natural’ Nitrite on the Inhibition of Spore Germination and Outgrowth of Clostridium sporogenes in Low- and High-Fat Frankfurters" Applied Microbiology 1, no. 1: 104-122. https://doi.org/10.3390/applmicrobiol1010009
APA StyleBhusal, A., Nelson, J., Pletcher, D., & Muriana, P. M. (2021). Comparison of Sodium Nitrite and ‘Natural’ Nitrite on the Inhibition of Spore Germination and Outgrowth of Clostridium sporogenes in Low- and High-Fat Frankfurters. Applied Microbiology, 1(1), 104-122. https://doi.org/10.3390/applmicrobiol1010009







