1. Introduction
The common bean (
Phaseolus vulgaris) is one of the most important grain legumes globally and serves as a staple food in many regions, particularly among vulnerable populations, such as smallholder farmers and low-income urban communities [
1,
2,
3]. Its high nutritional value—characterized by elevated levels of protein, slowly digested starch, dietary fiber, antioxidants, B complex vitamins, and essential minerals, including calcium, potassium, phosphorus, magnesium, iron, zinc, manganese, and copper—makes it a cornerstone of food and nutrition security [
4,
5]. Moreover, these nutrients remain stable during postharvest storage, enabling year-round consumption as a non-perishable food [
6]. For these reasons, beans have been referred to as the “bean of hope” for economically disadvantaged populations [
7].
One of the key characteristics of beans is their ability to establish symbiotic associations with atmospheric nitrogen-fixing bacteria. In the case of the common bean, this interaction with rhizobia is called symbiotic nitrogen fixation (SNF), which plays a critical role in plant growth, particularly in low-fertility soils and under adverse environmental conditions [
8,
9,
10]. The efficiency of SNF varies depending on plant species, bacterial strain, soil, and climatic conditions. However, common beans have been reported to exhibit relatively low SNF efficiency, often prompting farmers to apply high doses of nitrogen fertilizers to achieve desirable yields [
6,
11]. Paradoxically, the overuse of synthetic fertilizers not only suppresses the bean’s natural nitrogen-fixing capacity but also contributes to environmental degradation, including soil acidification, contamination of water bodies, and increased greenhouse gas emissions [
12].
Small-scale bean production systems, typical of the Andean region of Peru, are not exempt from the challenges associated with low yields and nitrogen dependency. In the department of Cajamarca—Peru’s leading bean-producing region—these systems are characterized by cultivating diverse genotypes with varying growth habits and vegetative periods. This diversification strategy helps farmers mitigate risks related to water scarcity and sanitary problems while enabling staggered harvests. A notable group of beans in this region, locally known as “tiachos”, are consumed either as dry beans or fresh green beans [
13]. Despite the region’s prominence in national bean production [
14], yields often fall below the national average [
13]. This highlights the urgent need to explore alternative strategies to enhance crop productivity and yield. Simultaneously, it is essential to evaluate technologies that improve the efficiency of SNF in bean cultivation, thereby reducing reliance on synthetic nitrogen fertilizers and mitigating their associated environmental impacts [
6,
12].
A promising strategy to enhance SNF in legumes is inoculating nitrogen-fixing bacteria, which stimulates the formation of root nodules—specialized structures where atmospheric nitrogen is converted into available forms for plant uptake [
15]. This technology not only reduces the dependence on chemical fertilizers but also has the potential to increase bean productivity and improve soil quality, contributing to a more sustainable model of agricultural production. Among the bacterial species used as inoculants,
Rhizobium phaseoli has demonstrated particularly promising results in common bean production [
16]. Its use is especially relevant in the Andean region, where native bean genotypes represent a vital resource for smallholder farmers, playing a key role in local economies, food security, and agro-biodiversity conservation.
In this context, the present study aimed to evaluate the effect of Rhizobium phaseoli inoculation combined with varying levels of phosphorus (P) and potassium (K) fertilization on the yield, development, and nutritional profile of the native bean genotype ‘Tiachos bayo’ in the Peruvian Andes. It was hypothesized that integrating biological and P and K chemical fertilization would enhance nitrogen fixation efficiency, increase crop yield, and improve grain quality. The goal is to generate practical knowledge for the sustainable management of this valuable genetic resource, promoting reduced synthetic nitrogen fertilizer doses and contributing to food security in rural communities.
2. Materials and Methods
2.1. Characteristics of the Study Area
The study was conducted under field conditions in the Pampa Grande annex on an experimental plot belonging to the National Institute for Agrarian Innovation (INIA; 8°23′38.89″ S, 91°57′59.3″ W; altitude: 2635 m a.s.l.), located in the province of Cajabamba, department of Cajamarca, Peru (
Figure 1), during the period from February to July 2024. The region has a temperate–humid climate, with average annual temperatures between 12 and 18 °C and annual precipitation between 800 and 1200 mm. These climatic conditions and a concentrated rainfall regime from November to March are optimal for cultivating
P. vulgaris. Before crop establishment, a comprehensive soil characterization was conducted to schedule the application of relevant amendments. The results of the soil sample analysis were as follows: pH 6.6, electrical conductivity (EC) 19.1 mS·m
−1, organic matter (OM) 3.4%, total nitrogen 1.5 mg·g
−1, available phosphorus 3.4 mg·kg
−1, and available potassium 598.9 mg·kg
−1.
2.2. Biological Material and Inoculum Preparation
The plant material evaluated in this study was the common bean (P. vulgaris L.) ‘Tiachos bayo’ genotype, a native variety traditionally cultivated in the Andean region. It is characterized by medium- to small-sized seeds exhibiting a wide range of colors, including yellow, berry, red, or black with white mottling. The growth habit is determinate and bushy, with a relatively short vegetative cycle ranging from 3 to 6 months, depending on the environmental conditions.
The bacterial strains used for inoculation were Rhizobium phaseoli UNC-1 and R. phaseoli CIAT-2, supplied by the Rhizobiology Laboratory of the National University of Cajamarca (UNC). The CIAT-2 strain was originally provided by the International Center for Tropical Agriculture (CIAT), while the UNC-1 strain was isolated from the Cajamarca region. Both strains were isolated from the roots of common bean (P. vulgaris). They were selected as promising candidates following a screening and selection process of rhizobacteria, which involved biochemical tests and plant assays under controlled conditions conducted by UNC (unpublished results).
The inoculum for each bacterial strain (UNC-1 and CIAT-2) was prepared in liquid suspension using a mixture of solid inoculum and distilled water. The production of the solid inoculum and quality control followed the modified procedure of Condori et al. [
17]. Bacterial strains were propagated in standard mannitol-yeast extract broth (YEM) with pH adjusted to 7 using HCl. The solid inoculum was prepared by mixing 40 mL of broth with 100 g of chopped and sterilized peat with 43% moisture. The inoculated peat was left to mature at room temperature until use. The bacterial concentration of
R. phaseoli in the solid formulation, prior to liquid suspension preparation, was 1.4 × 10
8 CFU/g (35 days after preparation). The solid formulation had a pH of 6.8 ± 0.1, and the absence of contaminants was verified by colony counts on successive dilutions up to 1:10
3. Then, 200 g of powdered solid inoculum was weighed per kilogram of native bean seed. A mixture of 0.5 L of distilled water and 0.5 kg of molasses was pre-homogenized and used as an adhesive agent. The solid inoculum was then incorporated into this solution, and the bean seeds were added. The mixture was stirred thoroughly to ensure uniform adhesion of the inoculant to the seed surface.
2.3. Experimental Design
The experiment was established using a factorial design arranged in a completely randomized block design (CRBD), incorporating two factors with three levels each (3 × 3), and replicated across three blocks (
Table 1). The first factor corresponded to fertilizer dose, with treatments comprising 100%, 50%, and 0% of the recommended application dose, as detailed in the following section. The second factor was the inoculum strain: CIAT-2, UNC-1, and a non-inoculated control. A total of 27 experimental units were evaluated, each corresponding to a plot measuring 4 m in length by 3 m in width. Plots were separated by 1 m between rows and columns.
2.4. Crop Management
The total area of the experimental trial was 592 m
2. Based on the soil analysis results, liming was conducted to correct soil acidity before establishing the experiment. The lime requirement was calculated using the methodology proposed by Cochrane et al. [
18], considering factors such as clay texture and high organic matter levels. The following formula was applied:
Estimated values for calculation:
LR = lime requirement (cmolc·kg−1),
lf (lime factor) = 2 (for clayey, highly damped soils),
ASi (initial acidity saturation %) = 30% (estimated from pH and CECe),
TAS (target acidity saturation %) = 10% (for bean cultivation),
CECe (effective cation exchange capacity of soil) = 20 cmolc·kg−1.
Based on the calculation, the lime requirement was estimated at 8 cmolc·kg−1, where 1 cmolc·kg−1 equals 0.5 tons of lime per hectare. Accordingly, a total application dose of 4 t·ha−1 was used to correct the soil pH.
Following the liming process and before crop establishment, a new soil characterization analysis was conducted to determine the updated properties of the soil where the beans were sown (
Table 2).
The experimental area was then delineated into blocks and plots to ensure homogeneity of trial conditions, as outlined in the previous section. Seeds were sown manually in furrows, adhering to the planting density recommended by Horacio et al. [
19], with a spacing of 45 cm between plants and 80 cm between furrows. Irrigation was conducted every 15 days, while phytosanitary control involved the application of insecticides based on abamectin.
The phosphorus and potassium fertilization doses were determined based on soil analysis and conventional crop management practices in the study area [
20]. It was established that the native bean required 69 kg·ha
−1 of phosphorus (P) and 45 kg·ha
−1 of potassium (K). Consequently, a fertilization dose of 150 kg·ha
−1 of diammonium phosphate and 90 kg·ha
−1 of potassium sulfate was calculated. To promote natural infection by nitrogen-fixing bacteria, the application of urea was excluded, thereby creating nitrogen-deficient conditions.
Base fertilization was applied in two fractions at 10 cm from the plants, following the recommendations of the National Institute for Agrarian Innovation [
21]. The first application was made 10 days after sowing (DAS), following seedling emergence, with half the total treatment dose incorporated. The second application was performed at 40 DAS, applying the remaining amount of fertilizer.
Hilling was carried out 45 DAS, by accumulating soil around the base of the plants to provide support and stability, reducing the risk of lodging due to wind or heavy rains. At 48 DAS, trellising was implemented by installing wooden supports at the ends of the furrows connected with galvanized wire. The plants were vertically guided using raffia twine, which promoted their development and optimal structuring.
2.5. Characterization of Nodulation
To quantify the total number of nodules, these were counted on ten randomly selected plants from each plot, following the methodology described by Mpongwana et al. [
22]. Roots were carefully washed with ample water to remove soil particles and facilitate accurate identification and counting of nodules [
19]. Nodule color was assessed through direct observation using a magnifying glass and microscope. Additionally, the Munsell color chart (or color scale) was employed to classify nodule color [
23].
2.6. Characterization of Growth and Development in Common Bean
The developmental stages of the crop were determined through visual assessments. The following phenological events were recorded: number of days to 50% flower bud emergence, number of days to 50% flowering, number of days to 50% physiological maturity, and number of days to harvest maturity. Physiological maturity was defined as the stage at which grains were fully filled, and the leaves began to yellow, while harvest maturity was defined as the point at which pods were completely dry. Ten plants were randomly selected per treatment to assess plant height, and the distance from the stem’s base to the main stem’s apex was measured using a tape measure.
2.7. Determination of Yield
Harvesting occurred shortly after each treatment reached harvest maturity. For the F0I0 treatment, harvesting took place at 141 days, for F1I0 at 145 days, for F2I0 and F0I2 at 146 days, for F1I2 at 148 days, for F2I2 at 147 days, for F0I1 at 144 days, for F1I1 at 152 days, and for F2I1 at 158 days. Pods were harvested manually and then air-dried at room temperature. Following this, a manual aeration process was performed to remove impurities and damaged grains.
The dry grain yield (kg·ha
−1) was determined by weighing all the harvested grains from each experimental unit. The grains were dried at room temperature, and residues and impurities were removed until clean grains were obtained. The dry grains were then weighed using a precision digital scale, and the weight for each treatment was recorded. The dry weight was subsequently converted to yield per hectare using the following formula:
2.8. Proximal Composition Analysis
Samples of dry bean grains were sent to the Soil, Water, and Foliar Laboratory facilities at the Baños del Inca Experimental Station of INIA for proximal composition analysis. The analyzed parameters included humidity, dry matter, ash, protein, fat, and crude fiber. The Weende reference standard for proximal analysis [
24] was followed. Humidity and dry matter content were determined by drying the samples at a controlled temperature until a constant weight was achieved. Ash content was quantified through muffle ashing. Total protein was measured using the Kjeldahl method following the ISO 11261 standard [
25], with protein content calculated based on nitrogen content according to proximal analysis. Crude fiber was determined through acid and alkaline digestion. Nitrogen-free extract (NFE) was calculated as the residual fraction from the proximal analysis. Total metal content was analyzed using inductively coupled plasma atomic emission spectrometry (MP-AES 4210).
2.9. Statistical Analysis
The data were analyzed using the R programming language on the R Studio v.4.1.2 platform. The assumptions of normality and homogeneity of variances for each variable were assessed using the Shapiro–Wilk and Levene tests, respectively. After confirming the validity of these assumptions, an analysis of variance (ANOVA) was conducted to evaluate the main effects and the interaction between the two factors. Variables showing significant differences were further analyzed using post hoc multiple comparisons to identify significant differences between treatments, applying the Student–Newman–Keuls test from the
agricolae package [
26].
Additionally, a Pearson correlation analysis was conducted between all the evaluated response variables, with a significance level of α = 0.05. This analysis was assessed using the R environment’s
corrplot package [
27].
Finally, a principal component analysis (PCA) was performed using the complete dataset and variables from the study. Data were centered and scaled (mean = 0 and standard deviation = 1) using the
prcomp function from
stats package [
28]. The PCA plot was generated with the
factoextra package [
29].
3. Results
3.1. Number of Nodules and Nodule Color Frequencies
Table 3 presents the analysis of variance results for nodule number, while
Table 4 shows the frequency distribution of nodule colors.
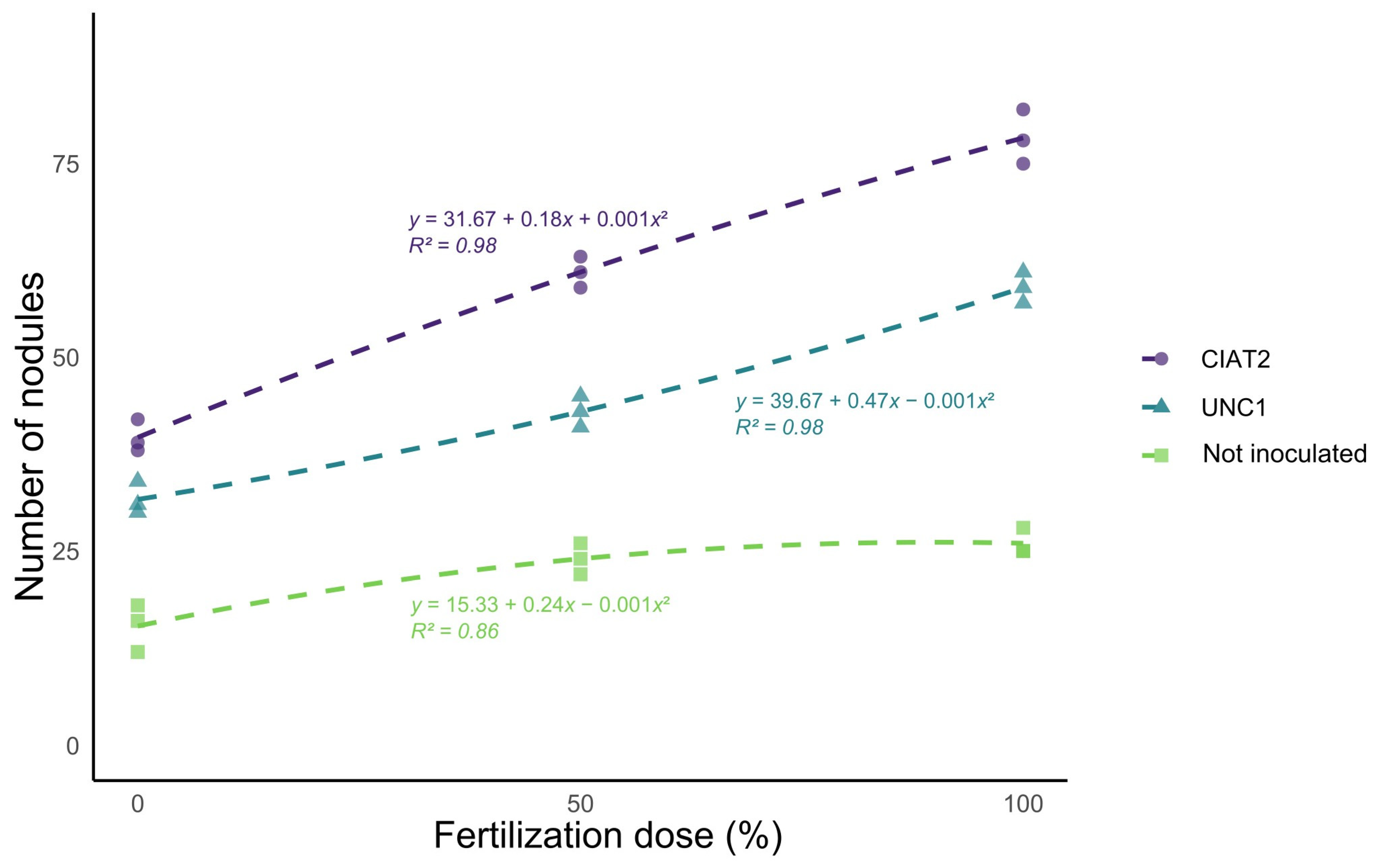
Figure 2 illustrates the quadratic effects of the factors. A significant interaction between fertilizer dose and
Rhizobium strain and highly significant individual effects for both factors were observed.
Inoculation with Rhizobium strains significantly increased the number of root nodules. Significant differences were also observed among the strains, with CIAT-2 producing the highest nodule count. Additionally, the applied fertilization dose positively improved this parameter, with treatments receiving 100% of the recommended dose yielding the highest number of nodules.
Treatment F2I1 produced the highest number of nodules (an average of 78.33), with 56% exhibiting an intense pink coloration—an indicator of high symbiotic activity. Similarly, treatments F1I2 (61 nodules) and F2I1 (59 nodules) demonstrated that combining inoculation and fertilization optimizes symbiotic efficiency.
Phosphorus and potassium fertilization significantly increased nodule formation in treatments without inoculation, suggesting that it promoted natural infection by native nitrogen-fixing bacterial strains. This was evident from the results of treatment F1I0, which produced 24 nodules, compared to 15.33 nodules in treatment F0I0—a statistically significant difference. Nevertheless, the number of nodules remained considerably lower than in the inoculated treatments.
3.2. Duration of Phenological Stages and Plant Height
Table 5 presents the results of the analysis of variance for the phenological stage duration and plant height of the ‘Tiachos bayo’ bean, while the treatment means are shown in
Table 6. A highly significant response was observed for the individual effects of the fertilizer dose, inoculum strain, and their interaction on the native bean’s phenological stage duration and plant height.
It was observed that the full fertilizer dose extended the bean development cycle by two to three days compared to the 50% dose and by six to eight days compared to treatments without fertilization. Notably, this extension in phenological duration does not constitute a significant prolongation in agronomic terms nor represent a detriment to bean productivity. Furthermore, the longer duration of the vegetative cycle and grain-filling period of bean crops is likely associated with increased dry matter and photosynthate accumulation, as will be discussed later. Inoculation with Rhizobium strains also prolonged beans’ phenological stages by six to eight days with the CIAT-2 strain and by three to five days with the UNC-1 strain. The increase in the duration of each phenological stage observed with inoculation is similar to that caused by fertilizer application and is not expected to affect bean agronomic management significantly.
For the flower bud emergence stage, treatments with the CIAT-2 strain combined with 100% fertilization (F2I2) required the most time (81 days), followed by F1I2 (76 days). A similar trend was observed during the flowering stage, with F2I2 treatment reaching this stage in 93.67 days and F1I2 in 89 days. Likewise, during physiological maturity and harvest stages, the F2I2 treatment exhibited the longest durations (142 and 156 days, respectively), followed by F1I2 (137 and 151 days) and F2I1 (133 and 146.67 days).
In summary, the applied fertilizer doses and Rhizobium strains had a statistically significant effect in slightly extending the phenological stages of the ‘Tiachos’ native bean. However, this modest prolongation of the bean cycle likely contributed to increased biomass production, resulting in significantly higher crop yields.
3.3. Yield of P. vulgaris ‘Tiachos Bayo’
The results of the variance analysis for the yield of the ‘Tiachos bayo’ bean are presented in
Table 7, with treatment means shown in
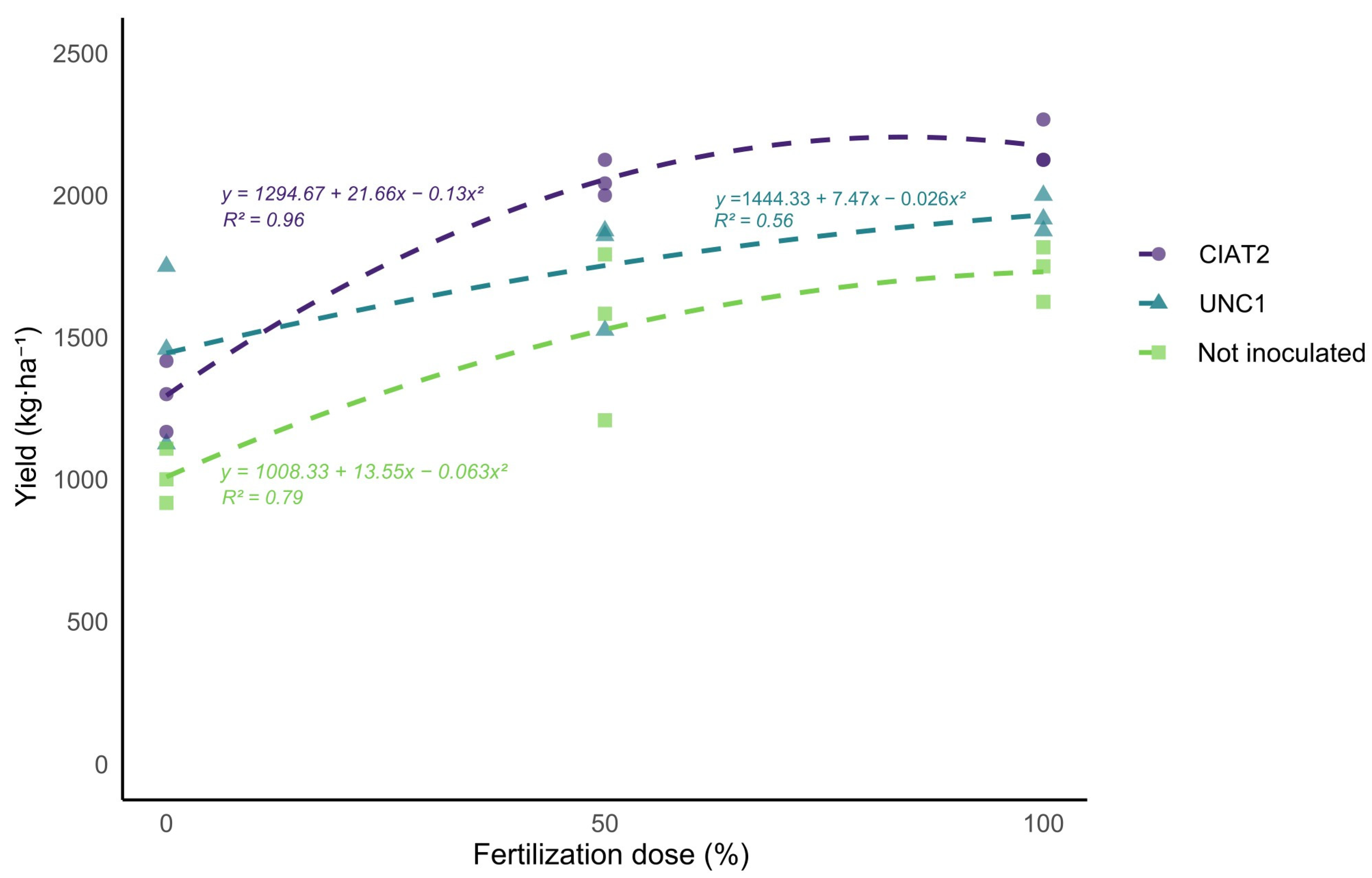
Table 8. A highly significant response was observed for the individual effects of the fertilization dose and inoculum strain on the native bean yield. However, the interaction between these two factors did not produce a statistically significant effect on this parameter. The responses of both factors on bean yield are illustrated in
Figure 3.
Regarding the fertilization dose, the treatment with 100% of the recommended dose achieved the highest average yield of 1944.56 kg·ha
−1, significantly higher than the 50% dose level and the unfertilized control. It is important to note that the average bean yield in Peru is 1.78 t·ha
−1 [
14]. Concerning the inoculum strain factor, the CIAT-2 and UNC-1 strains did not differ significantly from each other, but both strains showed significant differences when compared to the control without inoculum. Notable differences were observed between treatments with varying fertilization and inoculum strain levels. The F2I2 treatment yielded 2172 kg·ha
−1, representing an increase of 115.48% compared to F0I0 (without inoculation and no fertilization, with a 1008 kg·ha
−1 yield).
It is important to note that in treatments with 0% fertilization, the average yield was below the national average, with significant differences observed between treatments without inoculum or fertilization (F0I0) and those with inoculation (F0I1 and F0I2). Consequently, the inoculation of Rhizobium strains enhanced bean yields in treatments with at least 50% of the recommended phosphorus and potassium doses. However, treatments without inoculum consistently yielded below-average results, with the 100% fertilization treatment only marginally reaching the expected national yield for this crop.
The results suggest that the interaction between inoculation with R. phaseoli and appropriate fertilization significantly enhanced yield, with the R. phaseoli CIAT-2 strain proving the most effective. This strategy helps optimize production while reducing reliance on chemical fertilizers, promoting more sustainable agricultural management.
3.4. Proximal Composition of Dry Bean Grain
The results of the variance analysis for the nutritional profile parameters of the ‘Tiachos’ bean are presented in
Table 9, with treatment means shown in
Table 10. The analysis showed a low response to the effects of the factors on most of the nutritional variables. Regarding the fertilizer dose factor, only protein content exhibited a marginally significant response (
p < 0.01). In contrast, for the inoculum strain factor, significant differences were observed for humidity, dry matter (
p < 0.01), and fiber (
p < 0.05). Furthermore, no significant interaction effects were found.
Treatment F2I1 (R. phaseoli CIAT-2 with 100% PK) exhibited the highest protein concentration (27.35%) along with lower fat (5.34%) and humidity (7.93%) content, demonstrating an optimal balance between nutritional quality and composition. In treatments with R. phaseoli UNC-1, F2I2 (100% PK) achieved 27.10% protein, with moderate fat (7.38%) and humidity (9.65%), indicating adequate nutritional performance, though lower than the treatments with CIAT-2. In contrast, in the absence of inoculation, F2I0 (100% PK) attained the highest percentage of protein (28.45%) and fat (9.33%) but with lower crude fiber content (1.19%), suggesting that chemical fertilization can enhance nutritional quality, albeit with lower sustainability compared to R. phaseoli inoculation.
In general, Rhizobium CIAT-2, when combined with high fertilization levels, optimized dry matter and fiber content in beans while reducing humidity levels. However, no statistically significant differences were observed in protein and nitrogen content.
3.5. Correlation
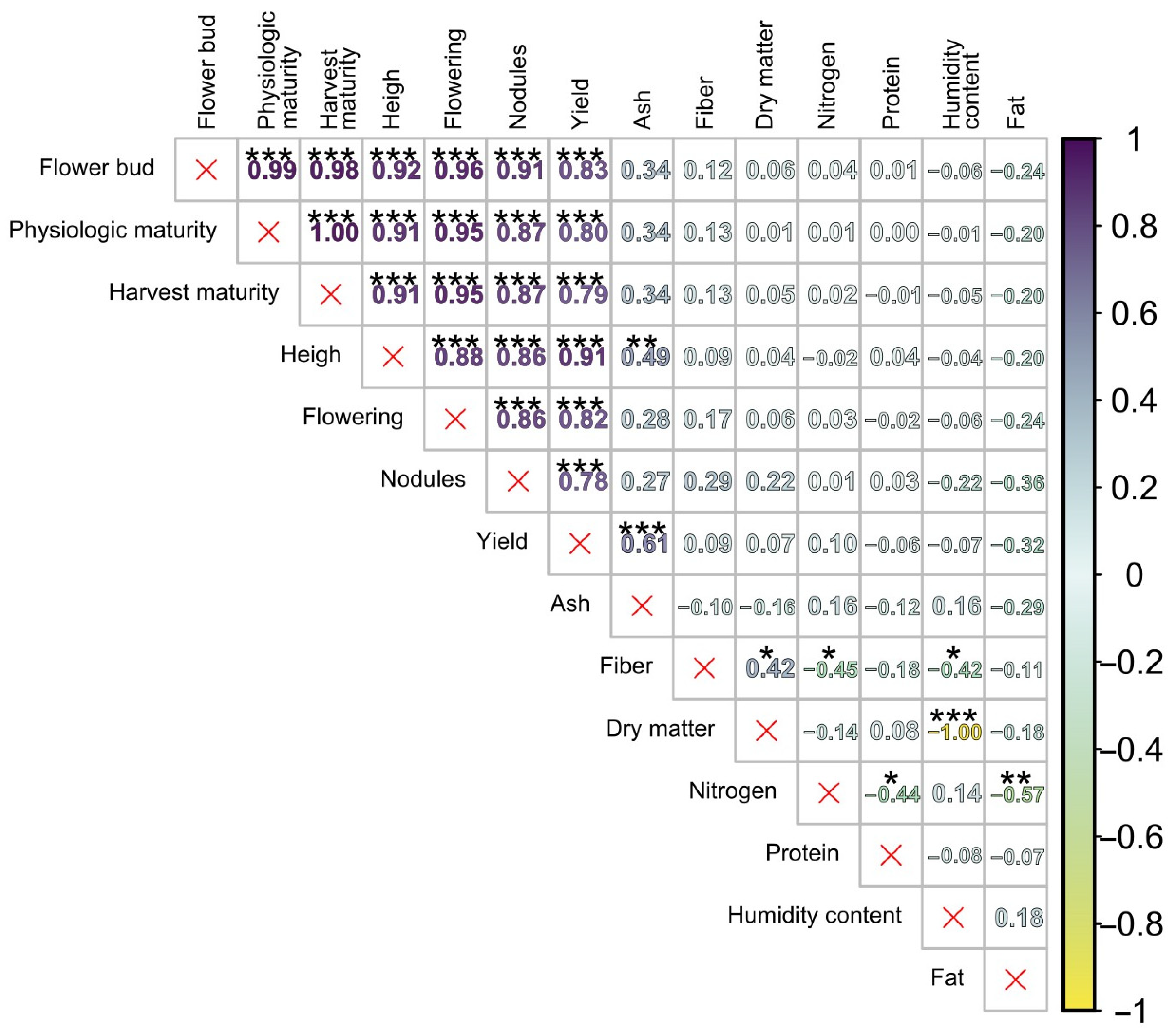
Pearson’s correlation analysis revealed associations among yield, nodulation, phenological stage duration, and the proximal composition of native beans (
Figure 4). A strong and statistically significant correlation was observed between phenological variables and crop yield, notably a positive correlation between yield and days to flowering (R
2 = 0.82). This suggests that extended vegetative development is associated with higher bean grain production, likely due to increased photosynthetic capacity and a more significant accumulation of photosynthates. In contrast, the nutritional profile variables exhibited low correlation coefficients and limited statistical significance with the other variables. Only ash content significantly correlated with yield and plant height at a significance level of α = 0.01.
3.6. Principal Component Analysis
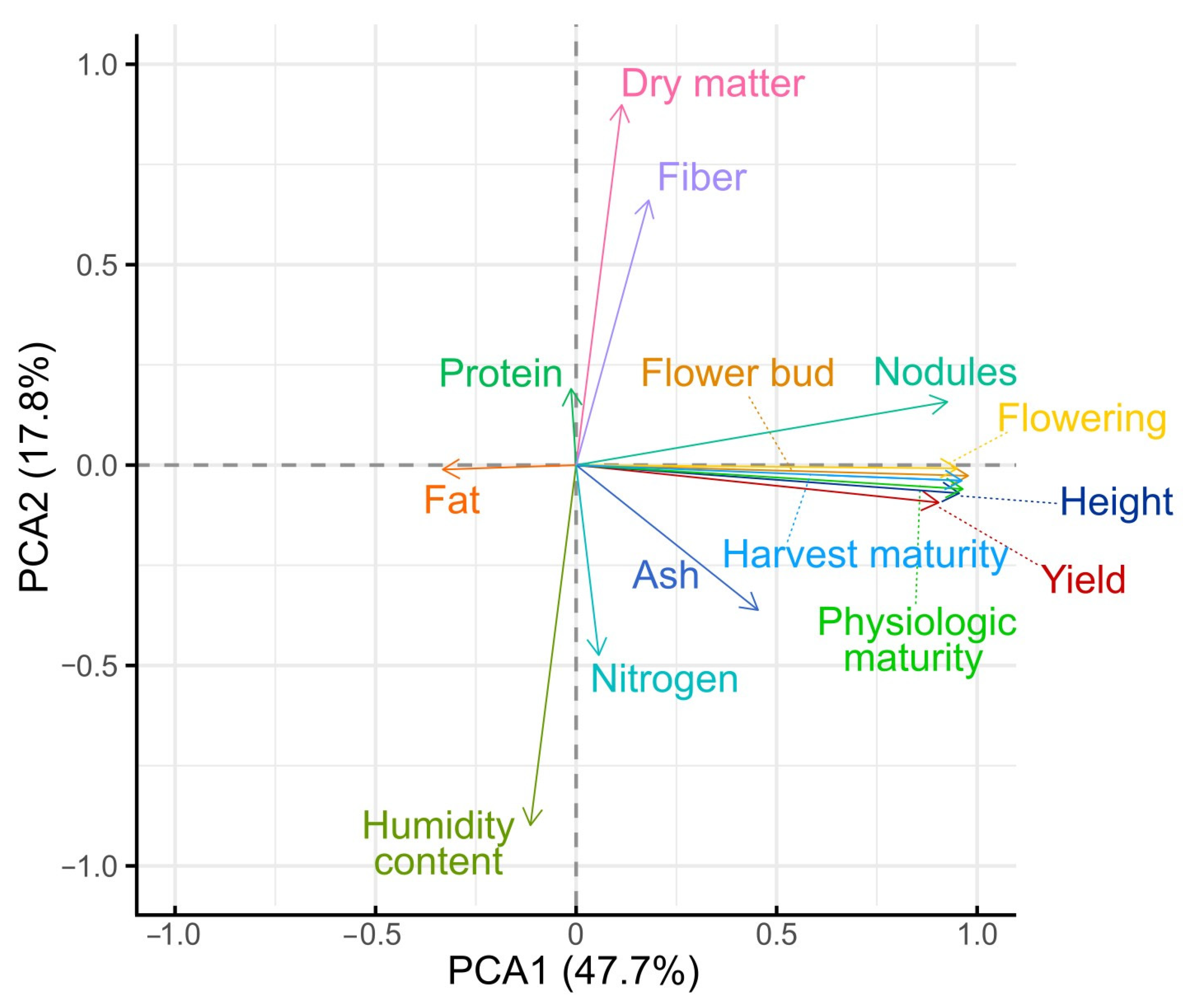
The PCA plot of the first two principal components is shown in
Figure 5. Based on this analysis, the first two components together explained 65.47% of the total variability. Phenological variables made a strong contribution to the variance and showed high loading values on PC1: days to flower bud appearance (contribution: 14.31%), flowering (13.52%), physiological maturity (13.92%), and harvest maturity (13.85%). Similarly, plant height (13.66%), number of nodules (12.82%), and yield (12.23%) also made a strong contribution to this component. These variables exhibited similar and positive loadings, indicating that they cluster together and jointly contribute to the overall variation pattern in crop productivity and development, as well as showing a strong association among them.
In contrast, for the second component (PC2), the variables that contributed most to the variance were moisture content (32.46%) and dry matter content (32.46%), which, as expected, were inversely related. Fiber content (17.54%) and nitrogen content (9.04%) also contributed, although to a lesser extent. Yield was strongly associated with PC1 and showed a very low loading on PC2, suggesting that its variability is mainly influenced by agronomic and phenological factors rather than nutritional characteristics. The variables with the weakest association with yield were fat, protein, fiber, and nitrogen, which showed low contributions to PC1, as also confirmed by Pearson correlation analysis.
This differentiation suggests that PC1 and PC2 capture distinct dimensions of plant behavior: one agronomic-productive and the other compositional-nutritional. Therefore, PC1 could serve as a useful synthetic index for selecting treatments with higher yield.
4. Discussion
In our study, inoculation with
Rhizobium phaseoli enhanced the number of root nodules and their symbiotic activity; however, the effectiveness varied depending on the bacterial strain. Similarly, phosphorus (P) and potassium (K) fertilization supported nodulation in naturally occurring symbiosis and inoculated treatments. Comparable findings were reported by Cantaro-Segura et al. [
30], who observed a 70% increase in the number of active nodules following
Rhizobium inoculation. Favero et al. [
31] also documented higher levels of nodulation with
Bradyrhizobium, reporting up to 133 nodules per plant with strain BR 14440, while other strains produced over 120 nodules. Likewise, Fiori et al. [
32] found that strain CIAT899 produced an average of 110.3 nodules per plant, while the mutant strains AzR18 and AzR19 yielded 117.2 and 117.3 nodules, respectively. In contrast, non-inoculated treatments resulted in significantly fewer nodules. Fiori et al. [
31] reported a maximum of 32 nodules in non-inoculated controls, consistent with our study’s findings.
Furthermore, de Souza et al. [
33] demonstrated that the combination of
Rhizobium with Fe
3O
4 increased the number of active nodules by 122%, suggesting that the application of specific chemical elements can enhance symbiotic efficiency, as also observed in the present study.
Regarding the nodulation phenotype, fertilization also influenced the detection of active nodules, as treatments with P and K showed a higher number of nodules with pink pigmentation inside. Since pink to reddish coloration indicates the presence of leghemoglobin—a protein involved in nitrogen fixation—it also signals that the nodules are in an active phase [
34,
35]. Therefore, it is assumed that the legumes are capable of fixing nitrogen in symbiosis with rhizobia. Similarly, the authors of [
16] reported that the presence of reddish-colored nodules during the symbiosis between
P. vulgaris and
R. phaseoli enhances biological nitrogen fixation (BNF) and promotes bean plant growth.
In relation to the phenological stages of beans in our trial, a slight increase in their duration was observed, attributed to
Rhizobium inoculation and fertilizer application. There is growing evidence that microorganisms influence plant developmental stages. Notably, the flowering stage has been identified as the most sensitive to microbial interactions, followed by maturity and senescence [
36]. However, in contrast to our findings, several studies have reported an accelerating effect of rhizobacteria on flowering and maturity stages. Granda-Mora et al. [
37] reported a reduction of approximately 24% in the crop cycle relative to the absolute control and 25.5% compared to treatment with synthetic fertilization alone. Singh et al. [
38] also observed decreased days to flowering and maturity in lentils (
Lens culinaris) inoculated with
Rhizobium leguminosarum. Although shorter crop cycles are generally considered agronomically desirable, in this study, the prolonged flowering and maturity stages were associated with increased plant vigor, evidenced by greater plant height (
Table 5) and, ultimately, higher yield (
Table 7). The extended vegetative development likely facilitated a more significant accumulation of photosynthates, benefiting bean production.
It was found that inoculation and fertilization were key factors influencing the yield of the ‘Tiachos bayo’ bean crop. This study’s results are consistent with previous research. For example, according to the results of [
39], inoculations with
R. phaseoli in common bean had a positive effect on grain yield, reaching 1500 ± 81 kg ha
−1. Similarly, the authors of [
16] reported that
R. phaseoli establishes an effective symbiosis with legumes, promoting healthy growth and profitable yields in
P. vulgaris. Furthermore, the potential of
R. phaseoli also represents an economical alternative to improve dry bean productivity, especially in rainfed areas where smallholder farmers often cannot afford the high cost of inorganic fertilizers [
40].
Likewise, other species of rhizobacteria also showed a positive effect on the yield of common bean. Da Silva Júnior et al. [
41] reported that inoculation with
Bradyrhizobium pachyrhizi strains increased yield from 850 to 1400 kg·ha
−1. Similarly, Ayalew et al. [
37] found that inoculation with
Bradyrhizobium strain CP-24 significantly enhanced caupí beans yield by 21%, from 2.50 t·ha
−1 in the control to 2.69 t·ha
−1. This was accompanied by a 16% increase in the number of pods per plant and a 13% increase in 100-seed weight, which confirmed the positive impact of inoculation on crop development and productivity. Coaquira et al. [
42] also demonstrated that combining
Rhizobium and
Trichoderma with biofertilizers increased bean yield by up to 26.62% compared to the control, reaching 2031 kg·ha
−1. In another study, Granda et al. [
37] evaluated the effect of inoculation with
R. leguminosarum bv.
viciae on Mantequilla var. beans and found that the treatment combining
R. leguminosarum,
P. fluorescens, and
B. subtilis yielded 1237 kg·ha
−1, a result comparable to that obtained with chemical fertilization (1185 kg·ha
−1). Finally, Cantaro-Segura et al. [
30] assessed the effectiveness of
Rhizobium inoculation in four bean varieties in Peru. They reported 2636 kg·ha
−1 yields in the Blanco Molinero variety and 2523 kg·ha
−1 in Canario Centenario, using
Rhizobium strains LMT10 and LMT15. These yields represented up to 90% of those achieved with nitrogen fertilization.
Other studies, such as that by Quddus et al. [
43], reported that the interaction between micronutrient application and
Rhizobium inoculation improved the nutritional quality of garden peas. Specifically, applying 3 kg of Zn, 2 kg of B, and 1 kg of organic matter per hectare, along with
Rhizobium inoculation (50 g·kg
−1 of seed), resulted in a higher protein content in seeds, although the underlying mechanism remains unclear. Similar results were reported by Khiangte et al. [
44], who found that the combination of
Rhizobium and 2 kg·ha
−1 of B was superior across most morphological and biochemical parameters, including chlorophyll and protein content. These findings are consistent with Raj et al. [
45] and Alam et al. [
46], who noted that balanced micro- and macro-nutrient nutrition in legumes enhances physiological performance and increases protein content.
Furthermore, Pearson’s correlation analysis revealed a strong positive relationship between growth and development variables, yield, and the number of nodules in the ‘Tiachos bayo’ bean. Similarly, PCA showed a strong association of these variables with principal component 1 (PC1). Both results align with previous studies that also reported significant positive correlations between days to flowering and bean yield, as well as other yield-related variables, such as bean weight per pod [
47,
48].
Treatments with the CIAT-2 strain showed a generally positive effect on the productive and developmental variables of the native bean. However, more detailed studies are needed to elucidate the physiological, biochemical, and biological mechanisms involved in this response. One possible mechanism is the production of plant hormones, such as indole-3-acetic acid (IAA) and the enzymatic activity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase [
49]. This may have been the main mechanism underlying the positive effect observed with the CIAT-2 strain, given that no significant increase in nitrogen uptake by the plant was detected at this treatment level (
Table 10). Another possible explanation, which is more difficult to evaluate, is the interaction with the local bacterial community, which may have synergistic effects under specific conditions. It has been shown that certain
Rhizobium strains exert greater influence on plants when combined with specific microbial consortia [
50]. Likewise, the authors of [
51] noted that successful agronomic performance depends not only on efficient nitrogen fixation but also on the genomic versatility of the strain, due to the wide range of genes that contribute to its fitness and competitiveness. The particular genetic structure of the CIAT-2 strain may be superior to that of UNC-1 under the conditions of this study, resulting in a better performance even though the latter strain was isolated from the local environment. These findings contrast with the general assumption that native strains adapted to local agroclimatic conditions and resident microbial populations are superior in terms of nodule infection and occupation efficiency [
52]. These findings underscore the importance of jointly evaluating the germplasm of bacterial strains and plants to optimize symbiosis and the efficiency of biological nitrogen fixation (BNF), particularly under local environmental conditions [
53].
5. Conclusions
The results of this trial demonstrated that inoculation with Rhizobium phaseoli strains CIAT2 and UNC1 had a significant positive effect on native bean yield and root nodule formation, which was associated with an extended duration of the crop’s phenological stages. Chemical fertilization with phosphorus (P) and potassium (K) further enhanced nodulation and yield, particularly in the inoculated treatments. Notably, inoculation with the CIAT2 strain increased native bean yield by 25%, reaching 2172 kg·ha−1, compared to 1730.67 kg·ha−1 in the non-inoculated treatment receiving the same fertilization dose.
These findings highlighted the importance of Rhizobium biofertilization as a key strategy for enhancing native bean productivity under field conditions. They also confirmed that adequate phosphorus and potassium fertilization is essential for promoting nodule formation and optimizing the efficiency of inoculation, both in inoculated and non-inoculated crops.
From a practical perspective, inoculation with efficient Rhizobium strains not only enhanced native bean yield but also reduced the reliance on nitrogen fertilizers, thereby lowering production costs and contributing to more sustainable agricultural practices. These results are particularly relevant for the Andean region of Peru, where optimizing nitrogen management is crucial for improving the profitability of smallholder farmers.
Future research should focus on evaluating the persistence and effectiveness of these Rhizobium strains across diverse soil types and agroclimatic conditions. It is also recommended to explore the integration of biofertilizers with other integrated crop management strategies. Additionally, further investigation into the effects of inoculation on grain nutritional quality and long-term biological nitrogen fixation would provide valuable insights into Rhizobium inoculation’s broader agronomic and environmental benefits.