Apple Cultivar Responses to Fungal Diseases and Insect Pests Under Variable Orchard Conditions: A Multisite Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area and Local Climatic Conditions
2.2. Biological Material and Experimental Conditions
2.3. Assessment of Major Diseases and Pests
2.4. Statistical Analysis
3. Results
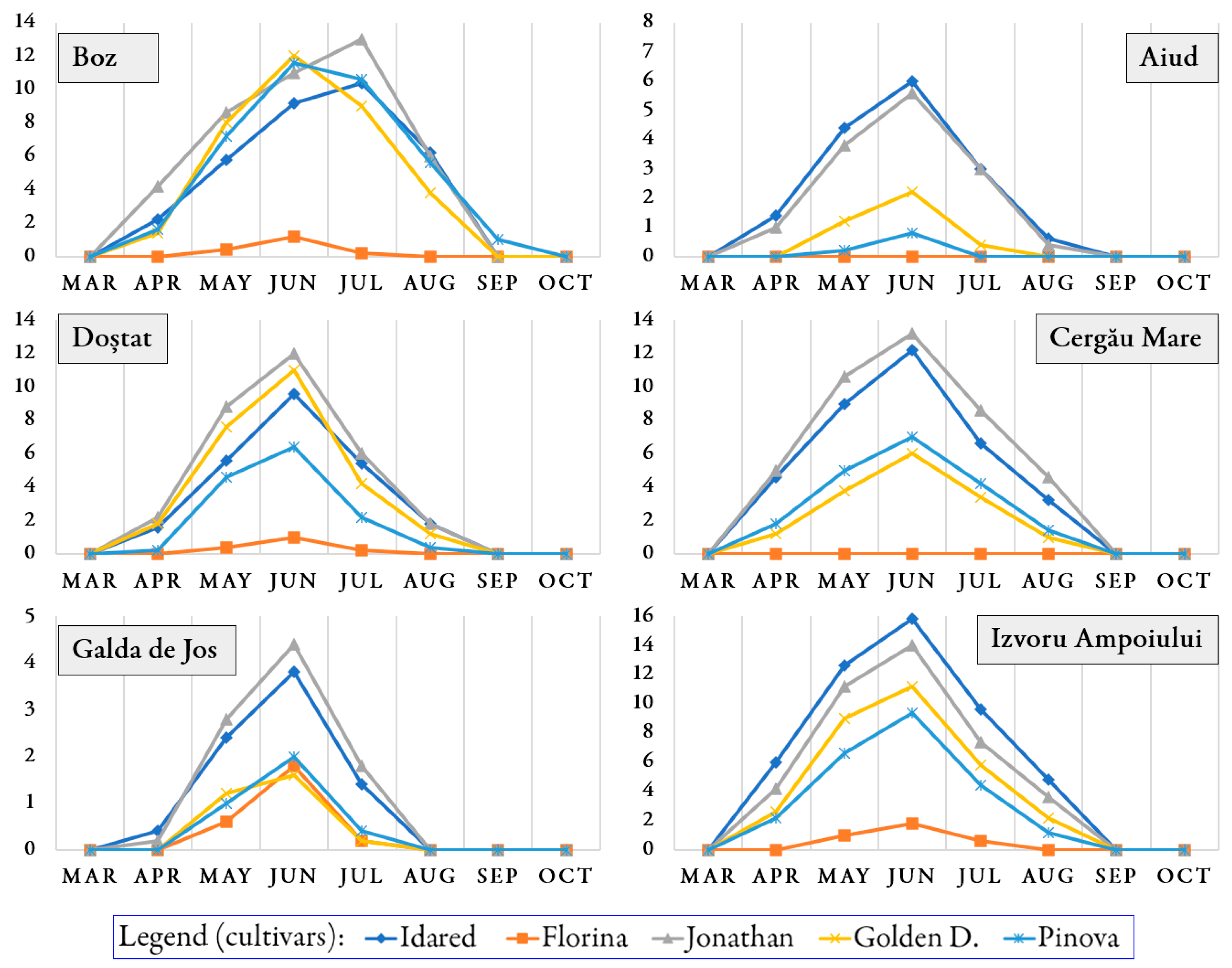
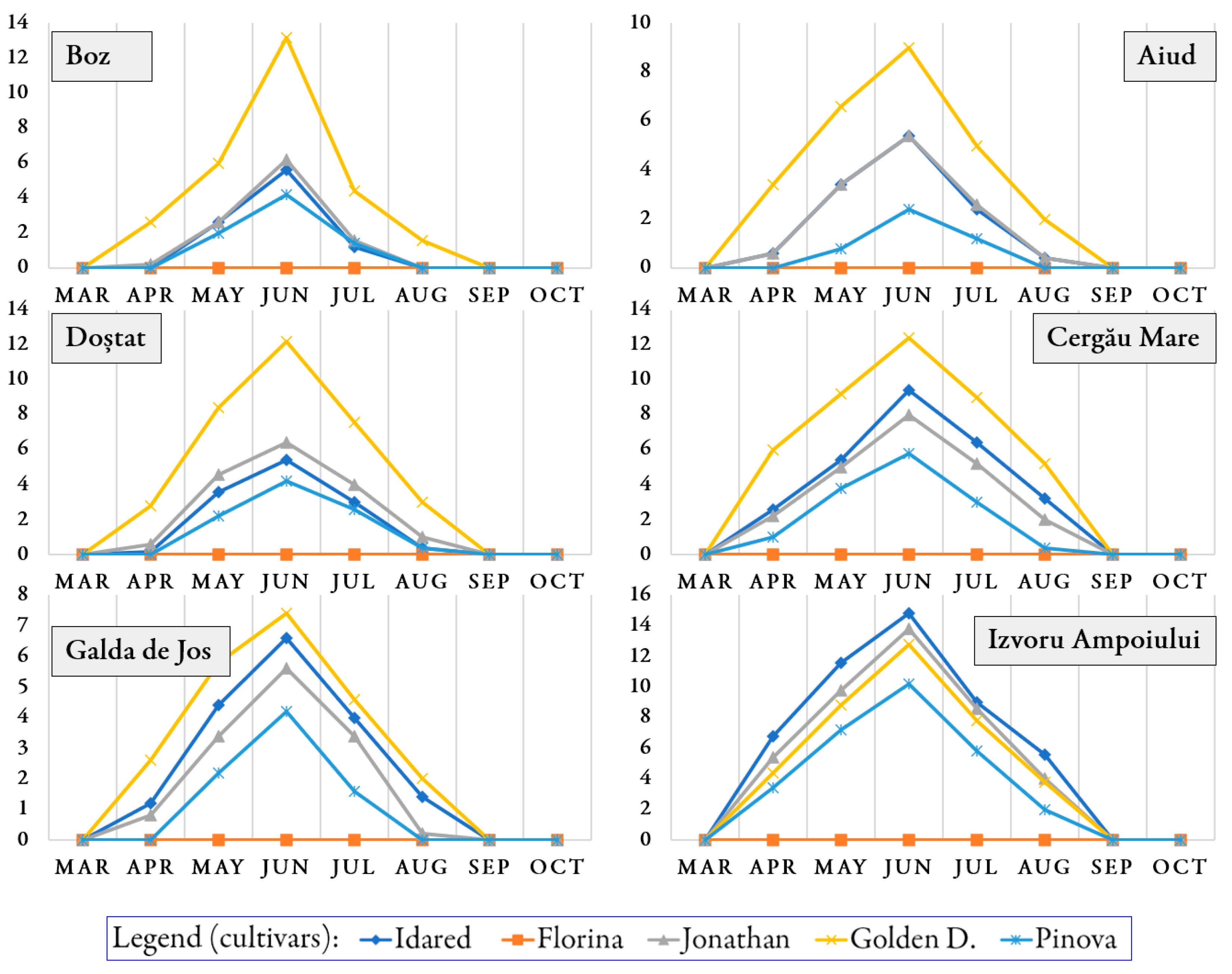
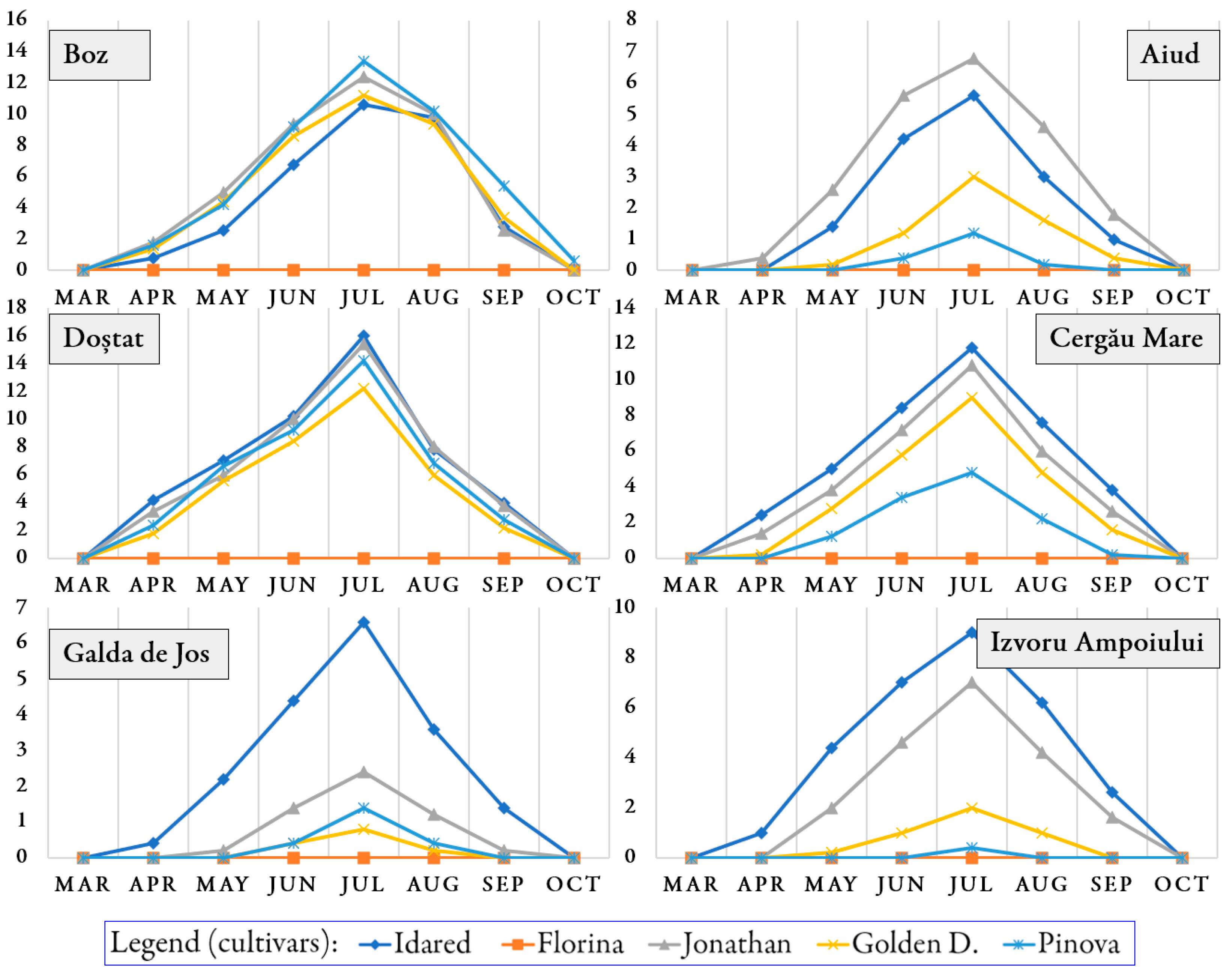
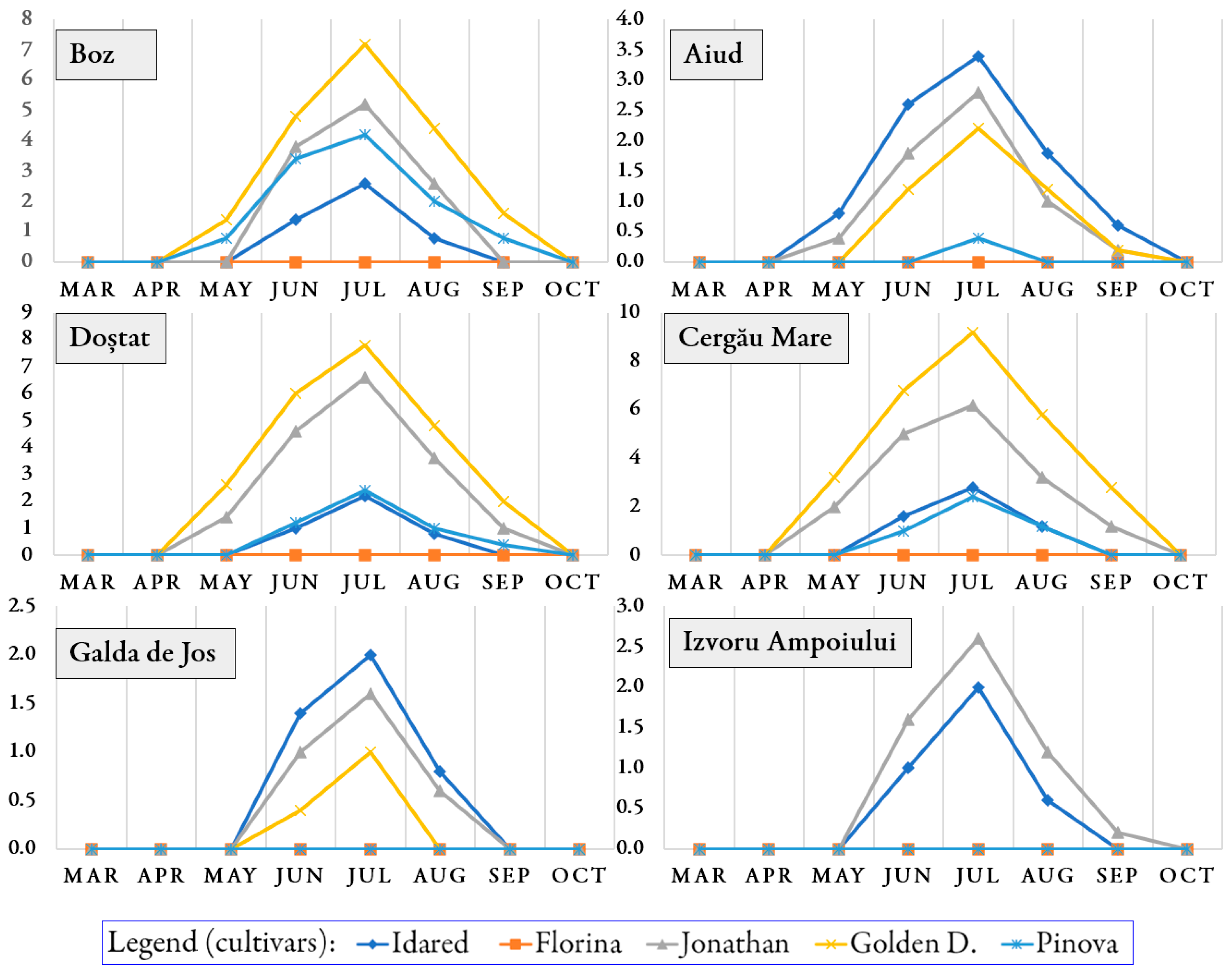
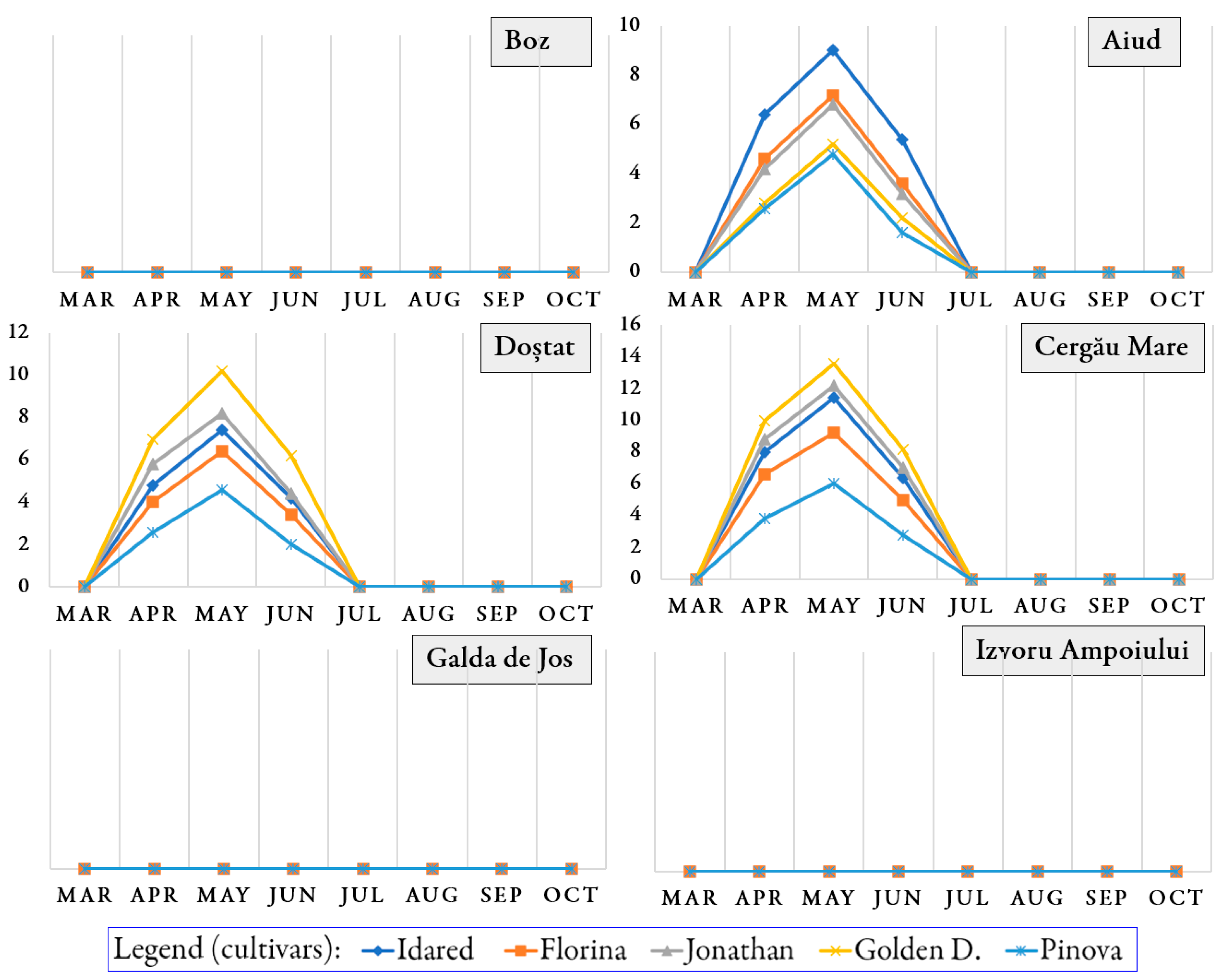
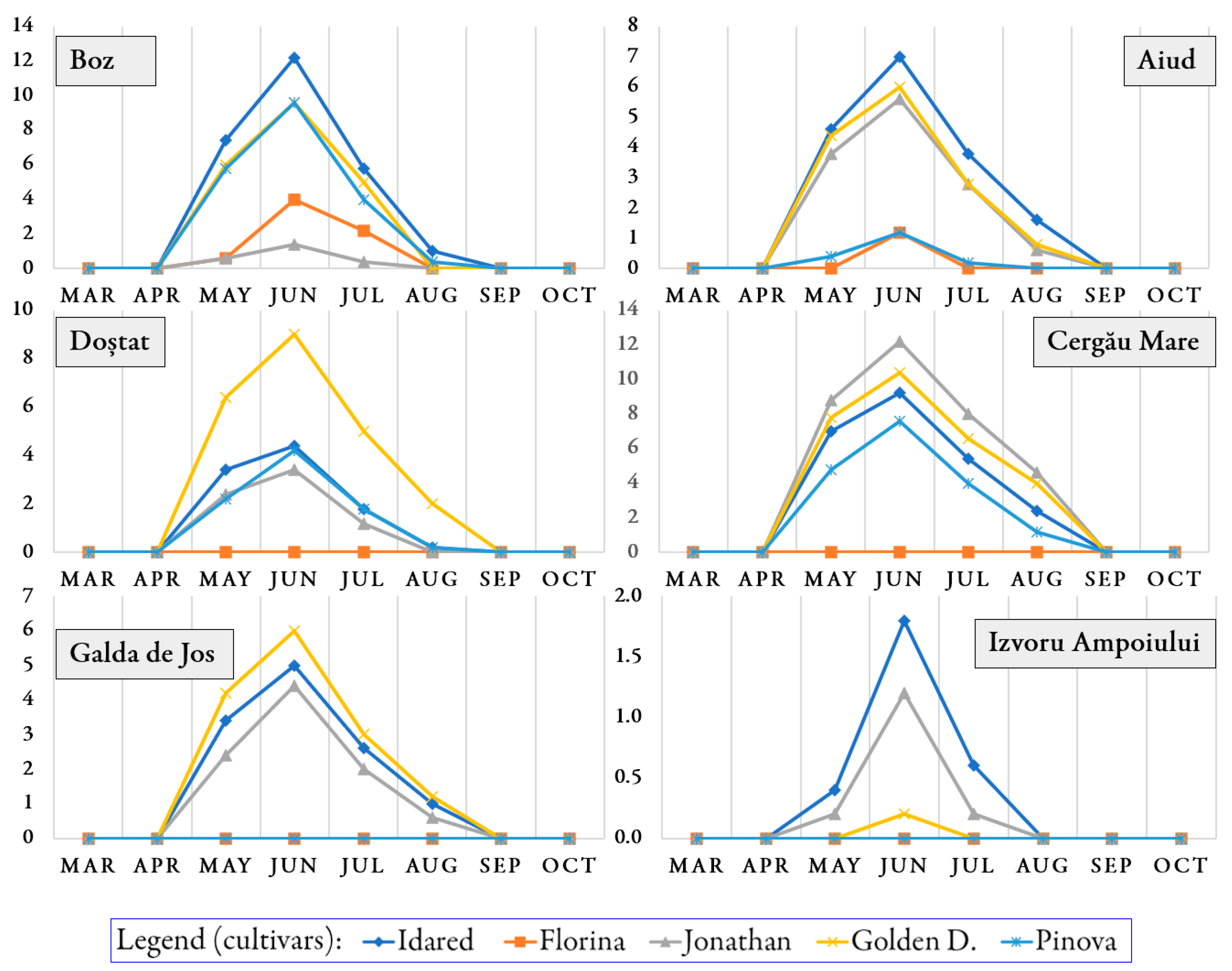
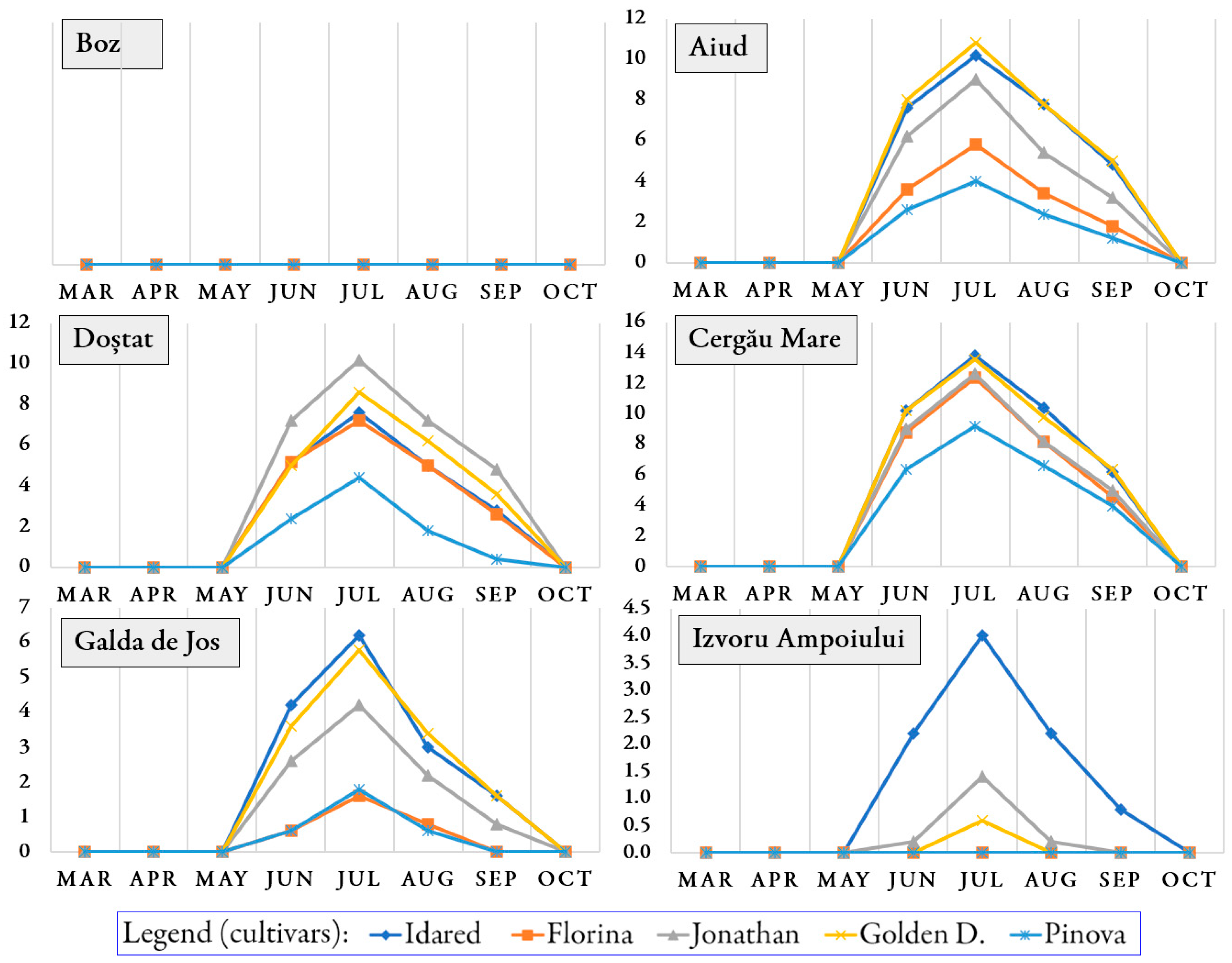
3.1. Occurrence of Pathogens and Pests on Trees During the Growing Season
3.2. Infection Rate and Infestation Level Depending on Orchards and Cultivars
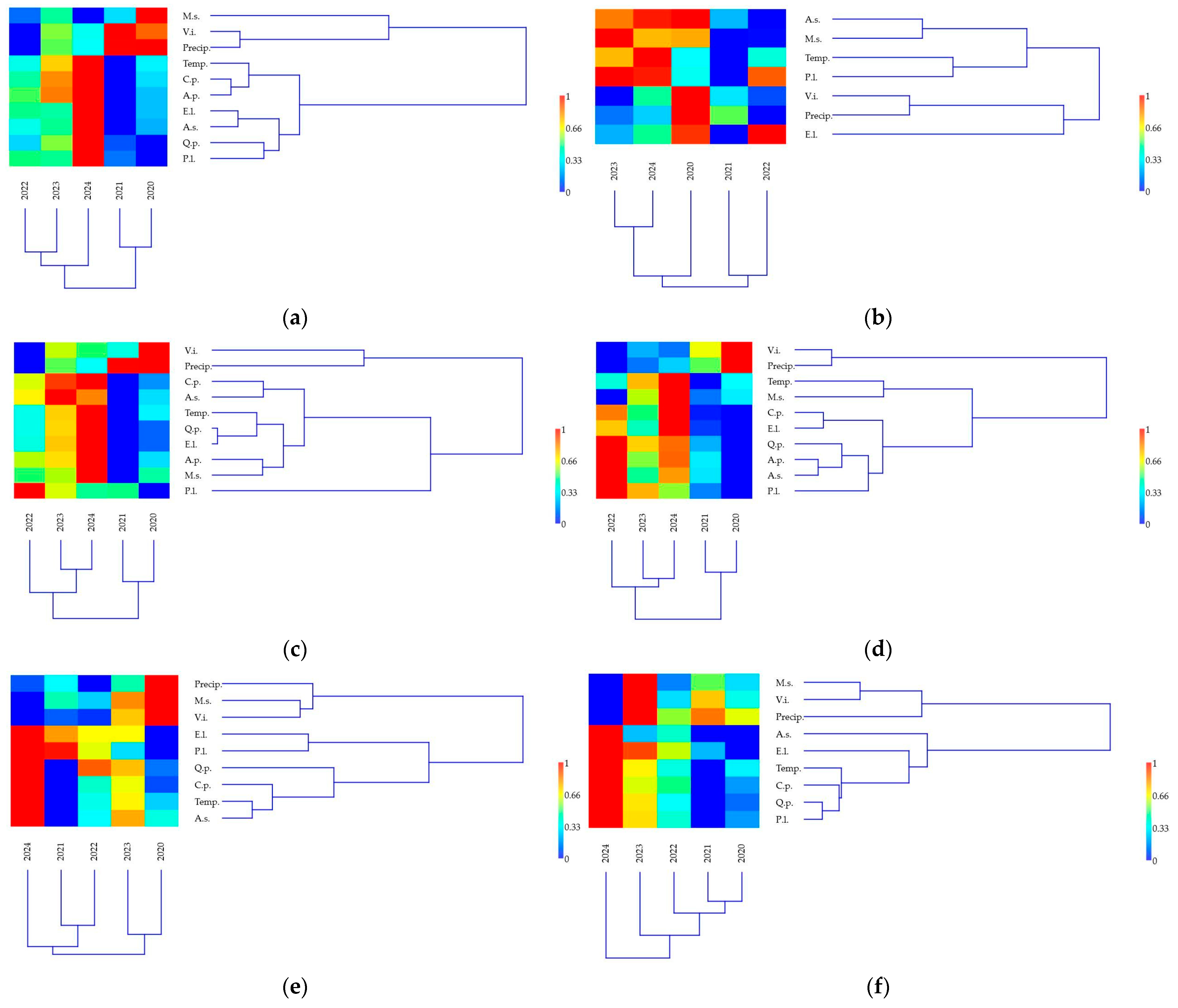
3.3. Relationships Between Biotic Stressors and Environmental and Management Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Brown, S. Apple. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; pp. 329–367. [Google Scholar]
- Janick, J.; Cummins, J.N.; Brown, S.K.; Hemmat, M. Apples. In Fruit Breeding: Volume 1, Tree and Tropical Fruits; Janick, J., Moore, J.N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 1, pp. 1–78. [Google Scholar]
- FAO. Faostat. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 18 April 2025).
- Morariu, P.A.; Mureșan, A.E.; Sestras, A.F.; Tanislav, A.E.; Dan, C.; Mareși, E.; Militaru, M.; Mureșan, V.; Sestras, R.E. A comprehensive morphological, biochemical, and sensory study of traditional and modern apple cultivars. Horticulturae 2025, 11, 264. [Google Scholar] [CrossRef]
- Zanetti, M.; Samoggia, A.; Young, J. Fruit sector strategic management: An exploration of agro-food chain actors’ perception of market sustainability of apple innovation. Sustainability 2020, 12, 6542. [Google Scholar] [CrossRef]
- Morariu, P.A.; Mureșan, A.E.; Sestras, A.F.; Dan, C.; Andrecan, A.F.; Borsai, O.; Militaru, M.; Mureșan, V.; Sestras, R.E. The impact of cultivar and production conditions on apple quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2025, 53, 14046. [Google Scholar] [CrossRef]
- Korban, S.S. The Apple Genome; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
- Way, R.; Aldwinckle, H.S.; Lamb, R.; Rejman, A.; Sansavini, S.; Shen, T.; Watkins, R.; Westwood, M.; Yoshida, Y. Apples (Malus). Acta Hortic. 1991, 290, 3–46. [Google Scholar] [CrossRef]
- Strohm, K. Of the 30,000 Apple Varieties Found All over the World Only 30 Are Used and Traded Commercially. Available online: http://www.agribenchmark.org/agri-benchmark/did-you-know/einzelansicht/artikel//only-5500-wi.html (accessed on 11 January 2023).
- Sestras, R.E.; Sestras, A.F. Quantitative traits of interest in apple breeding and their implications for selection. Plants 2023, 12, 903. [Google Scholar] [CrossRef]
- Devi, C.A.; Pandey, A.K.; Mika, K. Genetic improvement of apple. In Genetic Engineering of Crop Plants for Food and Health Security: Volume 1; Tiwari, S., Koul, B., Eds.; Springer Nature: Singapore, 2023; pp. 39–55. [Google Scholar]
- Sansavini, S.; Donati, F.; Costa, F.; Tartarini, S. Advances in apple breeding for enhanced fruit quality and resistance to biotic stresses: New varieties for the European market. J. Fruit Ornam. Plant Res. 2004, 12, 13–52. [Google Scholar]
- Bramel, P.; Volk, G. A Global Strategy for the Conservation and Use of Apple Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2019. [Google Scholar]
- Pereira-Lorenzo, S.; Fischer, M.; Ramos-Cabrer, A.M.; Castro, I. Apple (Malus spp.) Breeding: Present and Future. In Advances in Plant Breeding Strategies: Fruits: Volume 3; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–29. [Google Scholar]
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; McFerson, J.; Zhong, G.-Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Peil, A.; Kellerhals, M.; Höfer, M.; Flachowsky, H. Apple breeding—From the origin to genetic engineering. Fruit Veg. Cer. Sci. Biotechn. 2011, 5, 118–138. [Google Scholar]
- Liu, W.; Chen, Z.; Jiang, S.; Wang, Y.; Fang, H.; Zhang, Z.; Chen, X.; Wang, N. Research progress on genetic basis of fruit quality traits in apple (Malus × domestica). Front. Plant Sci. 2022, 13, 918202. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Denancé, C.; Ravon, E.; Guyader, A.; Guisnel, R.; Feugey, L.; Poncet, C.; Lateur, M.; Houben, P.; Ordidge, M.; et al. Analysis of the genetic diversity and structure across a wide range of germplasm reveals prominent gene flow in apple at the European level. BMC Plant Biol. 2016, 16, 130. [Google Scholar] [CrossRef]
- Kellerhals, M.; Szalatnay, D.; Hunziker, K.; Duffy, B.; Nybom, H.; Ahmadi-Afzadi, M.; Höfer, M.; Richter, K.; Lateur, M. European pome fruit genetic resources evaluated for disease resistance. Trees 2012, 26, 179–189. [Google Scholar] [CrossRef]
- MacHardy, W.E. Apple Scab: Biology, Epidemiology, and Management; American Phytopathological Society: St. Paul, MN, USA, 1996. [Google Scholar]
- Turechek, W.W. Apple diseases and their management. In Diseases of Fruits Vegetables Volume I; Naqvi, S., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 1–108. [Google Scholar]
- Forsline, P.L.; Aldwinckle, H.S.; Dickson, E.E.; Luby, J.J.; Hokanson, S.C. Collection, maintenance, characterization, and utilization of wild apples of Central Asia. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 1–61. [Google Scholar]
- Fazio, G.; Aldwinckle, H.S.; Volk, G.M.; Richards, C.M.; Janisiewicz, W.J.; Forsline, P.L. Progress in evaluating Malus sieversii for disease resistance and horticultural traits. Acta Hortic. 2009, 814, 59–66. [Google Scholar] [CrossRef]
- Sestras, A.F.; Pamfil, D.; Dan, C.; Bolboaca, S.D.; Jäntschi, L.; Sestras, R.E. Possibilities to improve apple scab (Venturia inaequalis (Cke.) Wint.) and powdery mildew [Podosphaera leucotricha (Ell. et Everh.) Salm.] resistance on apple by increasing genetic diversity using potentials of wild species. Aust. J. Crop Sci. 2011, 5, 748–755. [Google Scholar]
- Papp, D.; Gao, L.; Thapa, R.; Olmstead, D.; Khan, A. Field apple scab susceptibility of a diverse Malus germplasm collection identifies potential sources of resistance for apple breeding. CABI Agric. Biosci. 2020, 1, 16. [Google Scholar] [CrossRef]
- Aldwinckle, H.S.; Forsline, P.L.; Gustafson, H.L.; Hokanson, S.C. Evaluation of apple scab resistance of Malus sieversii populations from Central Asia. HortSci. 1997, 32, 440A–440. [Google Scholar] [CrossRef]
- Dan, C.; Sestras, A.; Bozdog, C.; Sestras, R. Investigation of wild species potential to increase genetic diversity useful for apple breeding. Genetika 2015, 47, 993–1011. [Google Scholar] [CrossRef]
- Forsline, P.L.; Aldwinckle, H.S. Evaluation of Malus sieversii seedling populations for disease resistance and horticultural traits. Acta Hortic. 2004, 663, 529–534. [Google Scholar] [CrossRef]
- Hancock, J.F.; Luby, J.J.; Brown, S.K.; Lobos, G.A. Apples. In Temperate Fruit Crop Breeding: Germplasm to Genomics; Hancock, J.F., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–38. [Google Scholar]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Fischer, M. Breeding apple (Malus x domestica Borkh). In Breeding Plantation Tree Crops: Temperate Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp. 33–81. [Google Scholar]
- Patocchi, A.; Frei, A.; Frey, J.E.; Kellerhals, M. Towards improvement of marker assisted selection of apple scab resistant cultivars: Venturia inaequalis virulence surveys and standardization of molecular marker alleles associated with resistance genes. Mol. Breed. 2009, 24, 337–347. [Google Scholar] [CrossRef]
- Papp, D.; Singh, J.; Gadoury, D.; Khan, A. New North American isolates of Venturia inaequalis can overcome apple scab resistance of Malus floribunda 821. Plant Dis. 2020, 104, 649–655. [Google Scholar] [CrossRef]
- Volz, R.K.; Rikkerink, E.; Austin, P.; Lawrence, T.; Bus, V.G.M. “Fast-breeding” in apple: A strategy to accelerate introgression of new traits into elite germplasm. Acta Hortic. 2009, 814, 163–168. [Google Scholar] [CrossRef]
- Gessler, C.; Patocchi, A.; Sansavini, S.; Tartarini, S.; Gianfranceschi, L. Venturia inaequalis resistance in apple. Crit. Rev. Plant Sci. 2006, 25, 473–503. [Google Scholar] [CrossRef]
- Khan, A.; Korban, S.S. Breeding and genetics of disease resistance in temperate fruit trees: Challenges and new opportunities. Theor. Appl. Genet. 2022, 135, 3961–3985. [Google Scholar] [CrossRef]
- Sedov, E.N. Apple breeding programs and methods, their development and improvement. Russ. J. Genet. Appl. Res. 2014, 4, 43–51. [Google Scholar] [CrossRef]
- Harshman, J.M.; Evans, K.M.; Hardner, C.M. Cost and accuracy of advanced breeding trial designs in apple. Hortic. Res. 2016, 3, 16008. [Google Scholar] [CrossRef] [PubMed]
- Wannemuehler, S.D.; Luby, J.J.; Yue, C.; Bedford, D.S.; Gallardo, R.K.; McCracken, V.A. A cost–benefit analysis of DNA informed apple breeding. HortScience 2019, 54, 1998–2004. [Google Scholar] [CrossRef]
- Khan, M.A.; Korban, S.S. Association mapping in forest trees and fruit crops. J. Exp. Bot. 2012, 63, 4045–4060. [Google Scholar] [CrossRef]
- Sestras, R.E.; Pamfil, D.; Ardelean, M.; Botez, C.; Sestras, A.F.; Mitre, I.; Dan, C.; Mihalte, L. Use of phenotypic and MAS selection based on bulk segregant analysis to reveal the genetic variability induced by artificial hybridization in apple. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 273–277. [Google Scholar] [CrossRef]
- Peace, C.P.; Bianco, L.; Troggio, M.; van de Weg, E.; Howard, N.P.; Cornille, A.; Durel, C.-E.; Myles, S.; Migicovsky, Z.; Schaffer, R.J.; et al. Apple whole genome sequences: Recent advances and new prospects. Hortic. Res. 2019, 6, 59. [Google Scholar] [CrossRef]
- Gardner, K.M.; Brown, P.; Cooke, T.F.; Cann, S.; Costa, F.; Bustamante, C.; Velasco, R.; Troggio, M.; Myles, S. Fast and cost-effective genetic mapping in apple using next-generation sequencing. G3 Genes Genomes Genet. 2014, 4, 1681–1687. [Google Scholar] [CrossRef]
- Evans, K.; Peace, C. Advances in marker-assisted breeding of apples. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing Limited: London, UK, 2017; Volume 18, pp. 189–216. [Google Scholar]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Gessler, C.; Patocchi, A. Recombinant DNA Technology in Apple. In Green Gene Technology: Research in an Area of Social Conflict; Fiechter, A., Sautter, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 113–132. [Google Scholar]
- Khajuria, Y.P.; Kaul, S.; Wani, A.A.; Dhar, M.K. Genetics of resistance in apple against Venturia inaequalis (Wint.) Cke. Tree Genet. Genom. 2018, 14, 16. [Google Scholar] [CrossRef]
- Hanke, M.-V.; Flachowsky, H.; Peil, A.; Emeriewen, O.F. Malus × domestica apple. In Biotechnology of Fruit and Nut Crops; CAB International: Wallingford, UK, 2020; pp. 440–473. [Google Scholar]
- James, C.M.; Clarke, J.B.; Evans, K.M. Identification of molecular markers linked to the mildew resistance gene Pl-d in apple. Theor. Appl. Genet. 2004, 110, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, I.O.; Patocchi, A.; Frey, J.E.; Peil, A.; Kellerhals, M. Breeding elite lines of apple carrying pyramided homozygous resistance genes against apple scab and resistance against powdery mildew and fire blight. Plant Mol. Biol. Rep. 2015, 33, 1573–1583. [Google Scholar] [CrossRef]
- Pajač, I.; Pejić, I.; Barić, B. Codling moth, Cydia pomonella (Lepidoptera: Tortricidae)–major pest in apple production: An overview of its biology, resistance, genetic structure and control strategies. Agric. Consp. Sci. 2011, 76, 87–92. [Google Scholar]
- Frechette, B.; Cormier, D.; Chouinard, G.; Vanoosthuyse, F.; Lucas, E. Apple aphid, Aphis spp.(Hemiptera: Aphididae), and predator populations in an apple orchard at the non-bearing stage: The impact of ground cover and cultivar. Eur. J. Entomol. 2008, 105, 521–529. [Google Scholar] [CrossRef]
- Rai, R.; Joshi, S.; Roy, S.; Singh, O.; Samir, M.; Chandra, A. Implications of changing climate on productivity of temperate fruit crops with special reference to apple. J. Hortic. 2015, 2, 135. [Google Scholar] [CrossRef]
- Pârvu, M. Ghid Practic de Fitopatologie; Presa Universitară Clujeană: Cluj-Napoca, Romania, 2010. [Google Scholar]
- Simionca Mărcășan, L.I.; Oltean, I.; Popa, S.; Plazas, M.; Vilanova, S.; Gramazio, P.; Sestras, A.F.; Prohens, J.; Sestras, R.E. Comparative analysis of phenotypic and molecular data on response to main pear diseases and pest attack in a germplasm collection. Int. J. Mol. Sci. 2023, 24, 6239. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Bos, I.; Caligari, P. Selection Methods in Plant Breeding; Springer Science & Business Media: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Titirică, I.; Roman, I.A.; Nicola, C.; Sturzeanu, M.; Iurea, E.; Botu, M.; Sestras, R.E.; Pop, R.; Militaru, M.; Ercisli, S.; et al. The main morphological characteristics and chemical components of fruits and the possibilities of their improvement in raspberry breeding. Horticulturae 2023, 9, 50. [Google Scholar] [CrossRef]
- Salcă Roman, G.M.; Sestras, A.F.; Stoian-Dod, R.L.; Dan, C.; Mircea, D.-M.; Boscaiu, M.; Sestras, R.E. Comparative assessment of different rose cultivars under environmental conditions in central Transylvania, Romania. Nova Geod. 2024, 4, 205. [Google Scholar] [CrossRef]
- Brun, L.; Didelot, F.; Parisi, L. Effects of apple cultivar susceptibility to Venturia inaequalis on scab epidemics in apple orchards. Crop Prot. 2008, 27, 1009–1019. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Stampar, F.; Veberic, R. Parameters of inner quality of the apple scab resistant and susceptible apple cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Beckerman, J. Disease Susceptibility of Common Apple Cultivars; Purdue University: West Lafayette, IN, USA, 2006; pp. 1–4. [Google Scholar]
- Fischer, M.; Fischer, C. Pinova apple cultivar. Compact. Fruit Tree 2002, 35, 19–20. [Google Scholar]
- Orosz-Tóth, M.; Kincses, S. The examination of flesh firmness in different apple varieties. Acta Agrar. Debreceniensis 2019, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Jamar, L.; Cavelier, M.; Lateur, M. Primary scab control using a “during-infection” spray timing and the effect on fruit quality and yield in organic apple production. Biotechnol. Agron. Soc. Environ. 2010, 14, 423–439. [Google Scholar]
- Švara, A.; De Storme, N.; Carpentier, S.; Keulemans, W.; De Coninck, B. Phenotyping, genetics, and “-omics” approaches to unravel and introgress enhanced resistance against apple scab (Venturia inaequalis) in apple cultivars (Malus × domestica). Hortic. Res. 2024, 11, uhae002. [Google Scholar] [CrossRef]
- Ellis, M.A.; Ferree, D.C.; Funt, R.C.; Madden, L.V. Effects of an apple scab-resistant cultivar on use patterns of inorganic and organic fungicides and economics of disease control. Plant Dis. 1998, 82, 428–433. [Google Scholar] [CrossRef]
- Webber, S.M.; Bailey, A.P.; Huxley, T.; Potts, S.G.; Lukac, M. Traditional and cover crop-derived mulches enhance soil ecosystem services in apple orchards. Appl. Soil Ecol. 2022, 178, 104569. [Google Scholar] [CrossRef]
- Zucoloto, M.; Ku, K.-M.; Kim, M.J.; Kushad, M.M. Influence of 1-Methylcyclopropene treatment on postharvest quality of four scab (Venturia inaequalis)-resistant apple cultivars. J. Food Qual. 2017, 2017, 5951041. [Google Scholar] [CrossRef]
- Bus, V.G.; Rikkerink, E.H.; Caffier, V.; Durel, C.-E.; Plummer, K.M. Revision of the nomenclature of the differential host-pathogen interactions of Venturia inaequalis and Malus. Annu. Rev. Phytopathol. 2011, 49, 391–413. [Google Scholar] [CrossRef]
- Garofalo, E.W. Apple Disease Forecasting Models: When Climate Changes the Rules; University of Massachusetts Amherst: Amherst, MA, USA, 2019. [Google Scholar]
- Singh, N.; Sharma, D.; Chand, H. Impact of climate change on apple production in India: A review. Curr. World Environ. 2016, 11, 251. [Google Scholar] [CrossRef]
- Hirschi, M.; Stoeckli, S.; Dubrovsky, M.; Spirig, C.; Calanca, P.; Rotach, M.W.; Fischer, A.M.; Duffy, B.; Samietz, J. Downscaling climate change scenarios for apple pest and disease modeling in Switzerland. Earth Syst. Dynam. 2012, 3, 33–47. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ghalhari, G.F.; Baaghideh, M. Impacts of climate change on apple tree cultivation areas in Iran. Clim. Change 2019, 153, 91–103. [Google Scholar] [CrossRef]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungău, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef] [PubMed]
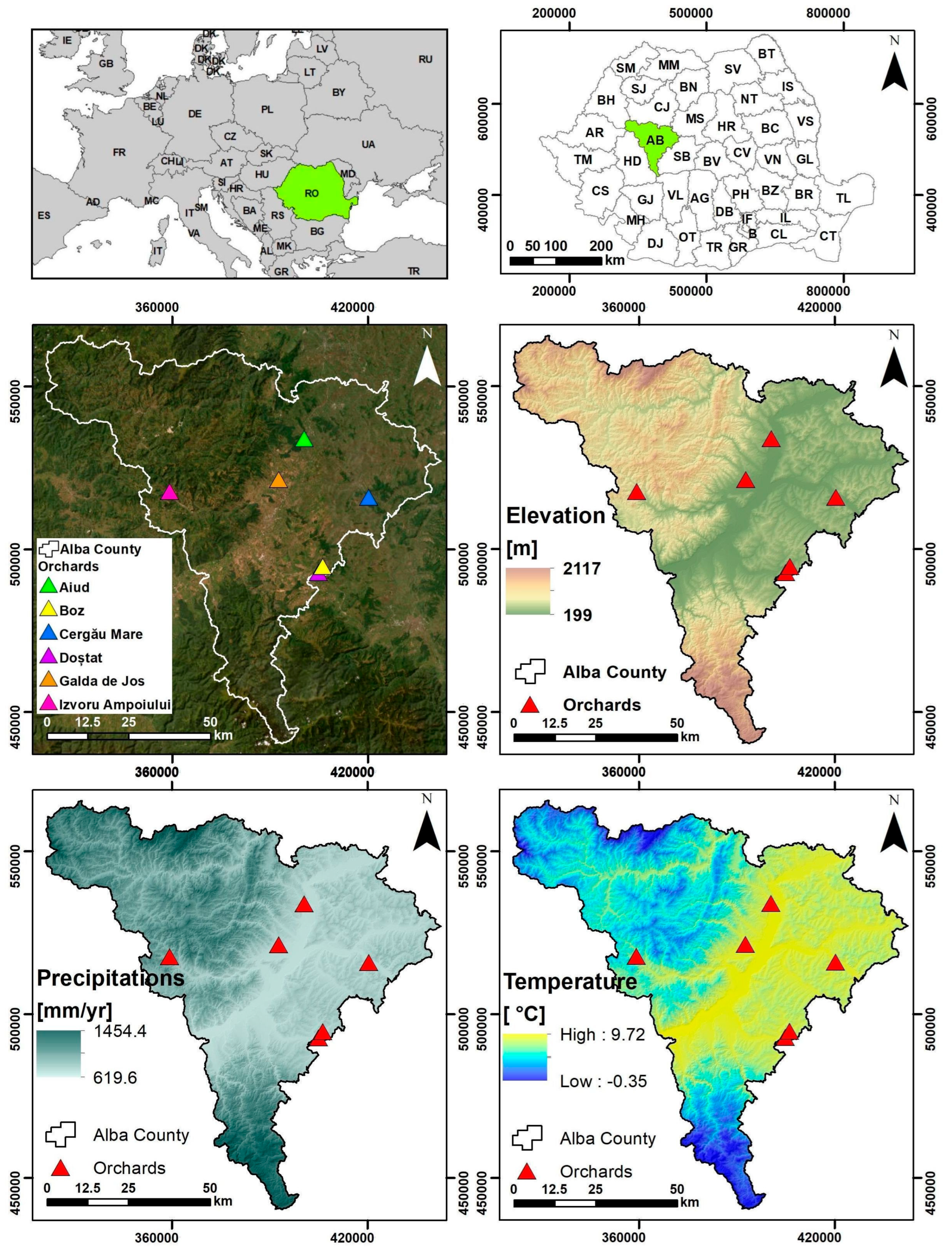


| Average Annual Temperature (°C) | Annual Amount of Precipitation (mm) | Average Annual Temperature (°C) | Annual Amount of Precipitation (mm) | |
|---|---|---|---|---|
| Year | Blaj (Aiud—347 m, and Cergău Mare—303 m) | Alba Iulia (Galda de Jos—236 m) | ||
| 2020 | 10.6 | 709.2 | 11.3 | 746.4 |
| 2021 | 9.9 | 708.0 | 10.7 | 558.0 |
| 2022 | 10.7 | 475.2 | 11.5 | 464.4 |
| 2023 | 11.5 | 602.4 | 12.2 | 589.2 |
| 2024 | 12.1 | 555.6 | 12.9 | 496.8 |
| Year | Sebeș-Alba (Boz—335 m, and Doștat—455 m) | Câmpeni (Izvoru Ampoiului—503 m) | ||
| 2020 | 11.1 | 681.6 | 8.7 | 855.6 |
| 2021 | 10.4 | 573.6 | 8.1 | 920.4 |
| 2022 | 11.2 | 446.4 | 8.8 | 832.8 |
| 2023 | 12.0 | 481.2 | 9.4 | 964.8 |
| 2024 | 12.5 | 510.0 | 10.0 | 658.8 |
| No | Orchard | Cultivar | Podosphaera leucotricha | Venturia inaequalis | Monilinia ssp. |
|---|---|---|---|---|---|
| 1 | Aiud | ‘Idared’ | 1.93 ± 0.32 a | 1.53 ± 0.29 b | 2.25 ± 0.96 a |
| 2 | ‘Florina’ | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.98 ± 0.46 c | |
| 3 | ‘Jonathan’ | 1.73 ± 0.42 a | 1.55 ± 0.25 b | 0.70 ± 0.34 d | |
| 4 | Golden D. | 0.48 ± 0.12 b | 3.25 ± 0.45 a | 1.28 ± 0.62 b | |
| 5 | ‘Pinova’ | 0.13 ± 0.04 c | 0.55 ± 0.08 c | 0.45 ± 0.23 e | |
| 1 | Boz | ‘Idared’ | 4.23 ± 0.32 c | 1.18 ± 0.11 c | 4.53 ± 1.31 a |
| 2 | ‘Florina’ | 0.23 ± 0.03 d | 0.00 ± 0.00 e | 2.83 ± 0.74 b | |
| 3 | ‘Jonathan’ | 5.35 ± 0.42 a | 1.33 ± 0.16 b | 2.88 ± 0.77 b | |
| 4 | Golden D. | 4.28 ± 0.56 bc | 3.48 ± 0.11 a | 4.48 ± 1.13 a | |
| 5 | ‘Pinova’ | 4.70 ± 0.69 b | 0.95 ± 0.08 d | 4.08 ± 1.07 a | |
| 1 | Cergău Mare | ‘Idared’ | 4.45 ± 0.41 b | 3.38 ± 0.46 b | 2.55 ± 0.82 c |
| 2 | ‘Florina’ | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 1.65 ± 0.56 d | |
| 3 | ‘Jonathan’ | 5.25 ± 0.63 a | 2.80 ± 0.44 c | 2.68 ± 0.99 bc | |
| 4 | Golden D. | 1.93 ± 0.39 d | 5.23 ± 0.71 a | 2.83 ± 0.93 b | |
| 5 | ‘Pinova’ | 2.43 ± 0.44 c | 1.75 ± 0.30 d | 3.65 ± 1.11 a | |
| 1 | Doștat | ‘Idared’ | 3.00 ± 0.59 b | 1.58 ± 0.36 c | 4.08 ± 1.07 c |
| 2 | ‘Florina’ | 0.20 ± 0.12 d | 0.00 ± 0.00 e | 3.68 ± 0.95 c | |
| 3 | ‘Jonathan’ | 3.85 ± 0.73 a | 2.08 ± 0.41 b | 3.28 ± 0.86 d | |
| 4 | Golden D. | 3.23 ± 0.70 b | 4.25 ± 0.92 a | 4.85 ± 1.28 b | |
| 5 | ‘Pinova’ | 1.73 ± 0.36 c | 1.18 ± 0.27 d | 5.88 ± 1.49 a | |
| 1 | Galda de Jos | ‘Idared’ | 1.00 ± 0.23 b | 2.20 ± 0.59 b | 1.10 ± 0.57 a |
| 2 | ‘Florina’ | 0.33 ± 0.09 e | 0.00 ± 0.00 e | 0.63 ± 0.36 c | |
| 3 | ‘Jonathan’ | 1.15 ± 0.27 a | 1.68 ± 0.45 c | 0.50 ± 0.31 d | |
| 4 | Golden D. | 0.38 ± 0.06 d | 2.80 ± 0.88 a | 0.95 ± 0.68 b | |
| 5 | ‘Pinova’ | 0.43 ± 0.10 c | 1.00 ± 0.22 d | 0.28 ± 0.21 e | |
| 1 | Izvoru Ampoiului | ‘Idared’ | 6.10 ± 0.97 a | 5.98 ± 0.91 a | 1.23 ± 0.74 c |
| 2 | ‘Florina’ | 0.43 ± 0.13 e | 0.00 ± 0.00 e | 1.53 ± 0.86 b | |
| 3 | ‘Jonathan’ | 5.05 ± 0.85 b | 5.20 ± 0.83 b | 1.08 ± 0.57 d | |
| 4 | Golden D. | 3.85 ± 0.65 c | 4.70 ± 0.70 c | 2.08 ± 1.13 a | |
| 5 | ‘Pinova’ | 2.98 ± 0.53 d | 3.58 ± 0.58 d | 0.88 ± 0.46 e |
| No | Orchard | Cultivar | E. lanigerum | Q. perniciosus | A. pomorum | Aphis spp. | C. pomonella |
|---|---|---|---|---|---|---|---|
| 1 | Aiud | ‘Idared’ | 1.15 ± 0.24 a | 1.13 ± 0.18 b | 2.60 ± 0.46 a | 2.13 ± 0.41 a | 2.23 ± 0.46 d |
| 2 | ‘Florina’ | 0.00 ± 0.00 e | 0.05 ± 0.03 d | 1.93 ± 0.35 b | 0.15 ± 0.03 e | 1.55 ± 0.36 e | |
| 3 | ‘Jonathan’ | 0.78 ± 0.16 b | 1.23 ± 0.22 b | 1.78 ± 0.28 b | 1.60 ± 0.29 c | 2.63 ± 0.66 c | |
| 4 | Golden D. | 0.60 ± 0.21 c | 1.65 ± 0.26 a | 1.28 ± 0.20 c | 1.75 ± 0.31 b | 3.50 ± 0.63 b | |
| 5 | ‘Pinova’ | 0.05 ± 0.03 d | 0.23 ± 0.12 c | 1.13 ± 0.21 d | 0.23 ± 0.14 d | 3.93 ± 0.60 a | |
| 1 | Boz | ‘Idared’ | 0.60 ± 0.14 c | - | - | 3.30 ± 0.44 a | - |
| 2 | ‘Florina’ | 0.00 ± 0.00 d | - | - | 0.85 ± 0.16 c | - | |
| 3 | ‘Jonathan’ | 1.45 ± 0.19 b | - | - | 0.30 ± 0.03 d | - | |
| 4 | Golden D. | 2.43 ± 0.25 a | - | - | 2.58 ± 0.45 b | - | |
| 5 | ‘Pinova’ | 1.40 ± 0.25 b | - | - | 2.48 ± 0.26 b | - | |
| 1 | Cergău Mare | ‘Idared’ | 0.70 ± 0.12 c | 2.08 ± 0.29 b | 2.40 ± 0.35 c | 3.00 ± 0.43 c | 3.08 ± 0.22 c |
| 2 | ‘Florina’ | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 1.70 ± 0.26 d | 0.00 ± 0.00 e | 2.38 ± 0.19 d | |
| 3 | ‘Jonathan’ | 2.20 ± 0.32 b | 2.50 ± 0.41 b | 3.18 ± 0.52 b | 4.20 ± 0.57 a | 4.70 ± 0.42 b | |
| 4 | Golden D. | 3.48 ± 0.52 a | 2.93 ± 0.47 a | 3.45 ± 0.42 b | 3.60 ± 0.48 b | 5.75 ± 0.42 a | |
| 5 | ‘Pinova’ | 0.58 ± 0.15 c | 1.25 ± 0.25 c | 4.15 ± 0.53 a | 2.20 ± 0.30 d | 6.05 ± 0.52 a | |
| 1 | Doștat | ‘Idared’ | 0.50 ± 0.12 d | 1.43 ± 0.21 b | 2.05 ± 0.32 c | 1.23 ± 0.19 b | 1.35 ± 0.21 c |
| 2 | ‘Florina’ | 0.00 ± 0.00 e | 0.15 ± 0.09 d | 1.73 ± 0.30 d | 0.00 ± 0.00 e | 1.45 ± 0.25 c | |
| 3 | ‘Jonathan’ | 2.15 ± 0.42 b | 1.95 ± 0.25 a | 2.30 ± 0.33 b | 0.88 ± 0.13 d | 3.48 ± 0.58 a | |
| 4 | Golden D. | 2.90 ± 0.49 a | 1.88 ± 0.42 a | 2.93 ± 0.45 a | 2.80 ± 0.43 a | 2.65 ± 0.54 b | |
| 5 | ‘Pinova’ | 0.63 ± 0.21 c | 0.73 ± 0.21 c | 1.15 ± 0.20 e | 1.05 ± 0.23 c | 3.88 ± 0.55 a | |
| 1 | Galda de Jos | ‘Idared’ | 0.53 ± 0.11 a | 1.15 ± 0.29 a | - | 1.50 ± 0.32 b | 0.80 ± 0.17 d |
| 2 | ‘Florina’ | 0.00 ± 0.00 d | 0.00 ± 0.00 d | - | 0.00 ± 0.00 d | 0.70 ± 0.23 e | |
| 3 | ‘Jonathan’ | 0.40 ± 0.06 b | 1.28 ± 0.35 a | - | 1.18 ± 0.25 c | 1.10 ± 0.43 c | |
| 4 | Golden D. | 0.18 ± 0.03 c | 0.85 ± 0.28 b | - | 1.80 ± 0.28 a | 1.35 ± 0.35 b | |
| 5 | ‘Pinova’ | 0.00 ± 0.00 d | 0.45 ± 0.14 c | - | 0.00 ± 0.00 d | 1.70 ± 0.50 a | |
| 1 | Izvoru Ampoiului | ‘Idared’ | 0.45 ± 0.14 b | 1.28 ± 0.21 a | - | 0.35 ± 0.10 a | 0.20 ± 0.17 d |
| 2 | ‘Florina’ | 0.00 ± 0.00 c | 0.00 ± 0.00 d | - | 0.00 ± 0.00 d | 0.13 ± 0.10 e | |
| 3 | ‘Jonathan’ | 0.70 ± 0.18 a | 0.80 ± 0.14 b | - | 0.20 ± 0.08 b | 0.30 ± 0.24 c | |
| 4 | Golden D. | 0.00 ± 0.00 c | 0.40 ± 0.11 c | - | 0.03 ± 0.03 c | 0.35 ± 0.26 b | |
| 5 | ‘Pinova’ | 0.00 ± 0.00 c | 0.00 ± 0.00 d | - | 0.00 ± 0.00 d | 0.48 ± 0.33 a |
| Cultivar | Orchard | Diseases | Pests | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P.l. | V.i. | M.s. | E.l. | Q.p. | A.p. | A.s. | C.p. | ||
| ‘Idared’ | Aiud | 0.828 | 0.042 | −0.348 | 0.951 * | 0.892 * | 0.942 * | 0.917 * | 0.985 ** |
| Boz | 0.973 ** | −0.169 | 0.890 * | 0.240 | − | − | 0.541 | − | |
| Cergău Mare | 0.402 | −0.656 | 0.936 * | 0.921 * | 0.980 ** | 0.946 * | 0.889 * | 0.993 *** | |
| Doștat | 0.419 | −0.497 | 0.926 * | 0.532 | 0.679 | 0.529 | 0.102 | 0.685 | |
| Galda de Jos | 0.094 | −0.232 | −0.191 | 0.364 | 0.832 | − | 0.975 ** | 0.864 | |
| Izvoru Ampoiului | 0.972 ** | −0.422 | −0.156 | 0.880 * | 0.963 ** | − | 0.810 | 0.963 ** | |
| ‘Florina’ | Aiud | − | − | − | − | 0.899 * | 0.927 * | 0.747 | 0.977 ** |
| Boz | 0.709 | − | − | − | − | − | 0.696 | − | |
| Cergău Mare | − | − | − | − | − | 0.904 * | − | 0.934 * | |
| Doștat | 0.144 | − | − | − | 0.122 | 0.605 | − | 0.793 | |
| Galda de Jos | 0.394 | − | − | − | − | − | − | 0.562 | |
| Izvoru Ampoiului | 0.749 | − | − | − | − | − | − | − | |
| ‘Jonathan’ | Aiud | 0.901 * | 0.239 | −0.352 | 0.812 | 0.907 * | 0.963 ** | 0.955 * | 0.879 * |
| Boz | 0.900 * | −0.245 | 0.762 | 0.134 | − | − | 0.401 | − | |
| Cergău Mare | 0.418 | −0.684 | 0.890 * | 0.981 ** | 0.976 ** | 0.932 * | 0.835 | 0.899 * | |
| Doștat | 0.569 | −0.527 | 0.812 | 0.749 | 0.640 | 0.432 | −0.039 | 0.741 | |
| Galda de Jos | −0.106 | −0.186 | −0.496 | 0.344 | 0.760 | − | 0.814 | 0.884 * | |
| Izvoru Ampoiului | 0.969 ** | −0.356 | −0.545 | 0.763 | 0.976 ** | − | 0.772 | 0.900 * | |
| ‘Golden Delicious’ | Aiud | 0.454 | 0.265 | −0.289 | 0.883 * | 0.883 * | 0.973 ** | 0.838 | 0.974 ** |
| Boz | 0.507 | −0.009 | 0.521 | 0.320 | − | − | 0.610 | − | |
| Cergău Mare | −0.357 | −0.312 | 0.950 * | 0.930 * | 0.978 ** | 0.954 * | 0.842 | 0.922 * | |
| Doștat | 0.614 | −0.567 | 0.848 | 0.841 | 0.581 | 0.589 | 0.597 | 0.719 | |
| Galda de Jos | −0.382 | −0.036 | −0.306 | −0.344 | 0.854 | − | 0.990 ** | 0.924 * | |
| Izvoru Ampoiului | 0.994 *** | −0.382 | 0.445 | − | 0.987 ** | − | 0.772 | 0.756 | |
| ‘Pinova’ | Aiud | 0.622 | −0.719 | −0.481 | 0.417 | 0.954 * | 0.985 ** | 0.901 * | 0.960 ** |
| Boz | 0.840 | −0.104 | 0.253 | 0.183 | − | − | 0.598 | − | |
| Cergău Mare | −0.358 | −0.151 | 0.722 | 0.991 *** | 0.945 * | 0.990 ** | 0.880 * | 0.956 * | |
| Doștat | −0.017 | −0.477 | 0.830 | 0.408 | 0.560 | 0.081 | 0.485 | 0.586 | |
| Galda de Jos | 0.787 | −0.120 | −0.774 | − | 0.896 * | − | − | 0.771 | |
| Izvoru Ampoiului | 0.989 ** | −0.311 | −0.441 | − | − | − | − | − | |
| Cultivar | Orchard | Diseases | Pests | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P.l. | V.i. | M.s. | E.l. | Q.p. | A.p. | A.s. | C.p. | ||
| ‘Idared’ | Aiud | −0.685 | 0.687 | 0.709 | −0.725 | −0.382 | −0.692 | −0.769 | −0.627 |
| Boz | −0.462 | 0.938 * | −0.247 | 0.766 | − | − | 0.488 | − | |
| Cergău Mare | −0.915 * | 0.979 ** | −0.628 | −0.506 | −0.514 | −0.746 | −0.554 | −0.774 | |
| Doștat | −0.957 * | 0.982 ** | −0.140 | −0.898 * | −0.922 * | 0.749 | −0.775 | −0.870 | |
| Galda de Jos | −0.718 | 0.946 * | 0.753 | −0.941 * | −0.658 | − | −0.335 | −0.636 | |
| Izvoru Ampoiului | −0.464 | 0.945 * | 0.865 | −0.332 | −0.501 | − | −0.676 | −0.617 | |
| ‘Florina’ | Aiud | − | − | − | − | 0.282 | −0.700 | 0.302 | −0.353 |
| Boz | −0.212 | − | − | − | − | − | 0.362 | − | |
| Cergău Mare | − | − | − | − | − | −0.806 | − | −0.677 | |
| Doștat | −0.786 | − | − | − | −0.747 | −0.880 * | − | −0.633 | |
| Galda de Jos | −0.730 | − | − | − | − | − | − | 0.557 | |
| Izvoru Ampoiului | −0.478 | − | − | − | − | − | − | − | |
| ‘Jonathan’ | Aiud | −0.634 | 0.576 | 0.663 | −0.848 | −0.557 | −0.683 | −0.342 | −0.721 |
| Boz | −0.748 | 0.957 * | −0.064 | 0.764 | − | − | 0.564 | − | |
| Cergău Mare | −0.891 * | 0.970 ** | −0.683 | −0.601 | −0.591 | −0.763 | −0.726 | −0.744 | |
| Doștat | −0.965 ** | 0.970 ** | −0.105 | −0.802 | −0.920 * | −0.910 * | −0.453 | −0.841 | |
| Galda de Jos | −0.724 | 0.914 * | 0.762 | −0.287 | −0.625 | − | 0.250 | −0.560 | |
| Izvoru Ampoiului | −0.520 | 0.921 * | 0.988 ** | −0.347 | −0.542 | − | −0.880 * | −0.709 | |
| ‘Golden Delicious’ | Aiud | −0.877 | 0.506 | 0.615 | −0.452 | −0.632 | −0.577 | −0.417 | −0.639 |
| Boz | −0.724 | 0.624 | 0.472 | 0.625 | − | − | 0.405 | − | |
| Cergău Mare | −0.544 | 0.953 * | −0.707 | −0.564 | −0.533 | −0.729 | −0.727 | −0.722 | |
| Doștat | −0.940 * | 0.980 ** | −0.150 | −0.736 | −0.945 * | −0.965 ** | −0.924 * | −0.867 | |
| Galda de Jos | −0.714 | 0.852 | 0.988 ** | −0.287 | −0.666 | − | −0.367 | −0.619 | |
| Izvoru Ampoiului | −0.611 | 0.925 * | 0.355 | − | −0.662 | − | −0.880 * | −0.341 | |
| ‘Pinova’ | Aiud | −0.539 | 0.954 * | 0.752 | 0.202 | −0.492 | −0.401 | −0.475 | −0.348 |
| Boz | −0.605 | 0.815 | 0.520 | 0.758 | − | − | 0.429 | − | |
| Cergău Mare | −0.561 | 0.840 | 0.088 | −0.501 | −0.651 | −0.416 | −0.785 | −0.601 | |
| Doștat | −0.793 | 0.935 * | 0.015 | −0.387 | −0.898 * | −0.862 | −0.827 | −0.510 | |
| Galda de Jos | −0.696 | 0.905 * | 0.676 | − | −0.544 | − | − | 0.346 | |
| Izvoru Ampoiului | −0.695 | 0.913 * | 0.674 | − | − | − | − | − | |
| V.i. | M.s. | E.l. | Q.p. | A.p. | A.s. | C.p. | |
|---|---|---|---|---|---|---|---|
| P.l. | 0.598 *** | 0.375 * | 0.467 ** | 0.512 ** | 0.552 ** | 0.361 * | 0.018 |
| V.i. | 0.008 | 0.452 ** | 0.546 ** | 0.519 ** | 0.302 | 0.003 | |
| M.s. | 0.485 ** | 0.365 * | 0.256 | 0.463 ** | 0.439 * | ||
| E.l. | 0.839 *** | 0.591 *** | 0.690 *** | 0.573 *** | |||
| Q.p. | 0.592 *** | 0.877 *** | 0.585 *** | ||||
| A.p. | 0.693 *** | 0.549 ** | |||||
| A.s. | 0.662 *** |
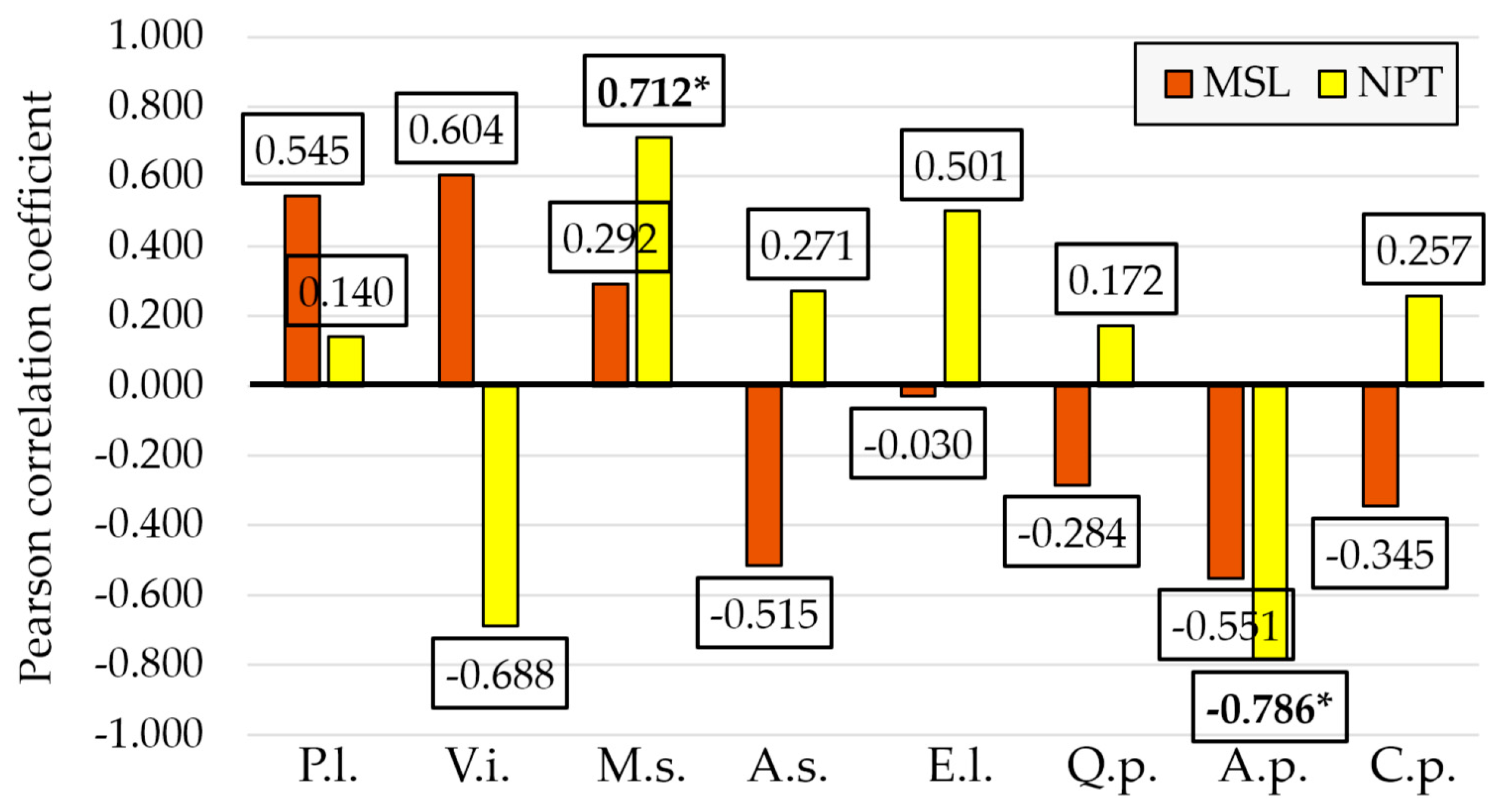
| P.l. | V.i. | M.s. | E.l. | Q.p. | A.p. | A.s. | C.p. | |
|---|---|---|---|---|---|---|---|---|
| MSL | 0.545 | 0.604 | 0.292 | −0.030 | −0.108 | −0.032 | −0.515 | −0.212 |
| NPT | 0.140 | −0.688 | 0.712 | 0.501 | −0.383 | −0.050 | 0.271 | −0.215 |
| P.l. | 0.547 | 0.548 | 0.409 | −0.263 | −0.138 | 0.129 | −0.320 | |
| V.i. | −0.181 | −0.214 | 0.133 | −0.047 | −0.356 | −0.070 | ||
| M.s. | 0.863 * | 0.080 | 0.313 | 0.475 | 0.099 | |||
| E.l. | 0.398 | 0.631 | 0.832 * | 0.482 | ||||
| Q.p. | 0.892 * | 0.420 | 0.940 ** | |||||
| A.p. | 0.602 | 0.966 ** | ||||||
| A.s. | 0.575 |
| Orchard | Diseases | Pests | ||||||
|---|---|---|---|---|---|---|---|---|
| P.l. | V.i. | M.s. | A.s. | E.l. | Q.p. | A.p. | C.p. | |
| Aiud | 0.933 | 0.956 | 0.591 | 0.919 | 0.904 | 0.935 | 0.774 | 0.751 |
| Boz | 0.951 | 0.933 | 0.404 | 0.943 | 0.960 | - | - | - |
| Cergău Mare | 0.960 | 0.949 | 0.385 | 0.941 | 0.961 | 0.925 | 0.830 | 0.949 |
| Doștat | 0.875 | 0.910 | 0.446 | 0.946 | 0.940 | 0.897 | 0.799 | 0.863 |
| Galda de Jos | 0.839 | 0.810 | 0.345 | 0.937 | 0.945 | 0.819 | - | 0.566 |
| Izvoru Ampoiului | 0.908 | 0.921 | 0.260 | 0.882 | 0.912 | 0.951 | - | 0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morariu, P.A.; Sestras, A.F.; Andrecan, A.F.; Borsai, O.; Bunea, C.I.; Militaru, M.; Dan, C.; Sestras, R.E. Apple Cultivar Responses to Fungal Diseases and Insect Pests Under Variable Orchard Conditions: A Multisite Study. Crops 2025, 5, 30. https://doi.org/10.3390/crops5030030
Morariu PA, Sestras AF, Andrecan AF, Borsai O, Bunea CI, Militaru M, Dan C, Sestras RE. Apple Cultivar Responses to Fungal Diseases and Insect Pests Under Variable Orchard Conditions: A Multisite Study. Crops. 2025; 5(3):30. https://doi.org/10.3390/crops5030030
Chicago/Turabian StyleMorariu, Paula A., Adriana F. Sestras, Andreea F. Andrecan, Orsolya Borsai, Claudiu Ioan Bunea, Mădălina Militaru, Catalina Dan, and Radu E. Sestras. 2025. "Apple Cultivar Responses to Fungal Diseases and Insect Pests Under Variable Orchard Conditions: A Multisite Study" Crops 5, no. 3: 30. https://doi.org/10.3390/crops5030030
APA StyleMorariu, P. A., Sestras, A. F., Andrecan, A. F., Borsai, O., Bunea, C. I., Militaru, M., Dan, C., & Sestras, R. E. (2025). Apple Cultivar Responses to Fungal Diseases and Insect Pests Under Variable Orchard Conditions: A Multisite Study. Crops, 5(3), 30. https://doi.org/10.3390/crops5030030










