Comparison of the Nutritional, Physicochemical, Technological–Functional, and Structural Properties and Antioxidant Compounds of Corn Kernel Flours from Native Mexican Maize Cultivated in Jalisco Highlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize Collection
2.2. Nutritional Composition
2.3. Physicochemical Parameters
2.4. Technological–Functional Properties
2.5. Bioactive Compounds
2.5.1. Soluble Phenols
2.5.2. Flavonoids
2.5.3. Total Anthocyanins
2.5.4. Condensed Tannins
2.5.5. Total Carotenoids
2.6. Antioxidant Activity
2.6.1. Radical DPPH Scavenging Assay
2.6.2. ABTS• + Radical Scavenging Assay
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.7. FTIR Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Nutritional Composition of Corn Kernel Flour Samples
3.2. Physicochemical Properties of Corn Kernel Flour Samples
3.3. Technological–Functional Properties of Corn Kernel Flour Samples
3.4. Bioactive Compounds of Corn Kernel Flour Samples
3.5. Antioxidant Activity of Corn Kernel Flour Samples
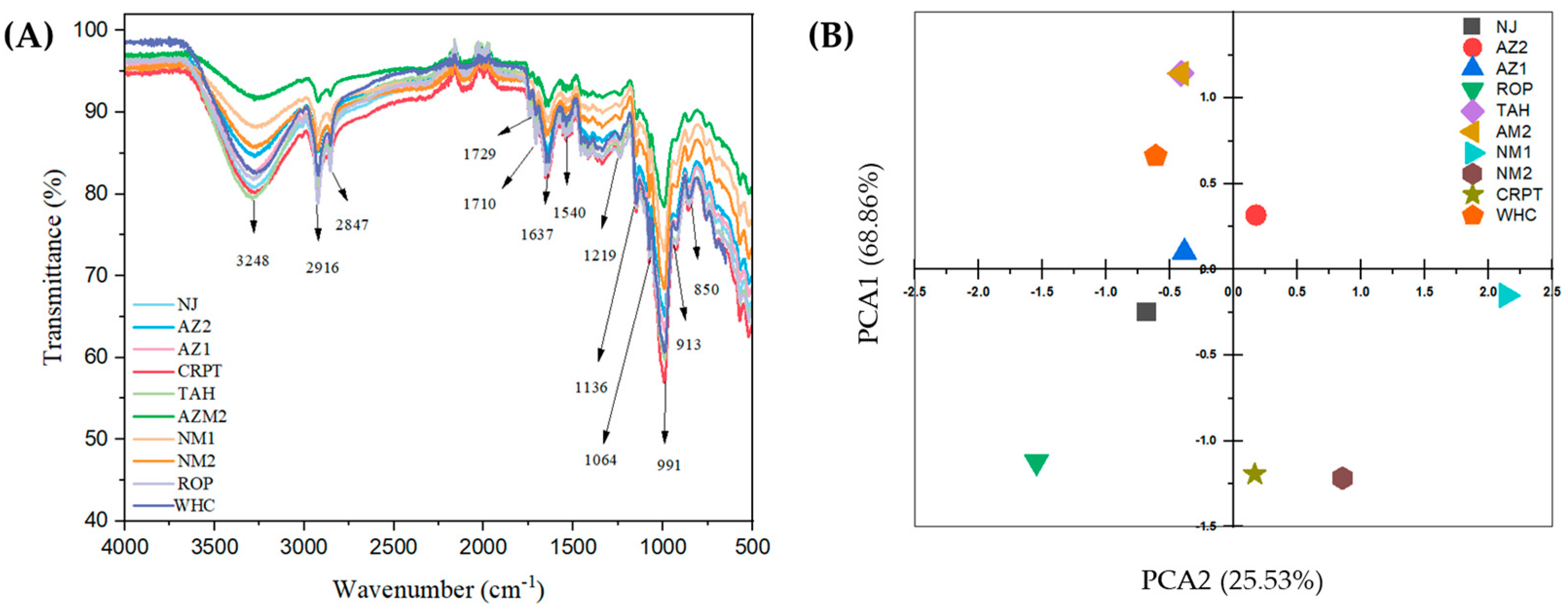
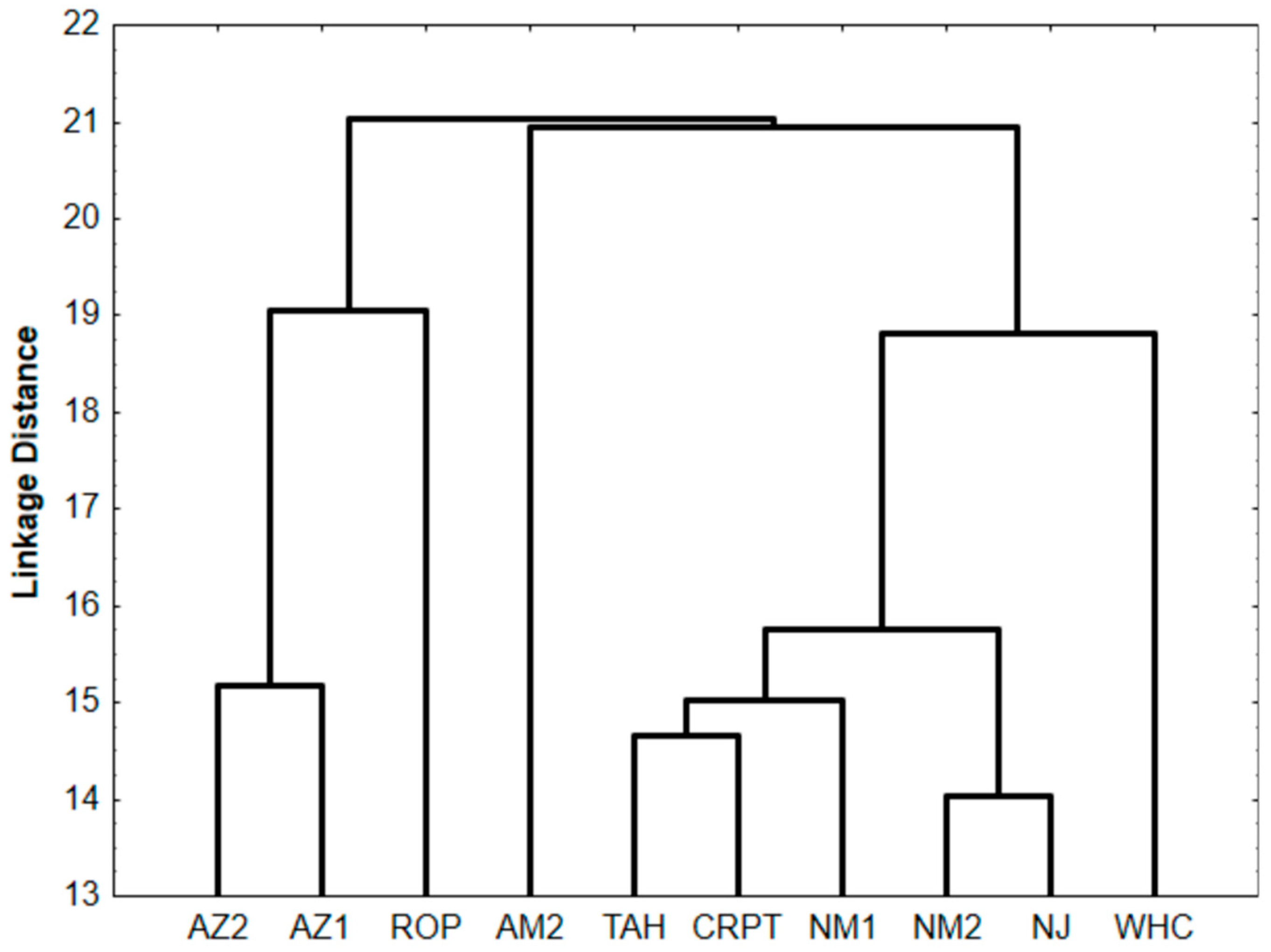
3.6. Structural Properties via FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revilla, P.; Alves, M.L.; Andelković, V.; Balconi, C.; Dinis, I.; Mendes-Moreira, P.; Redaelli, R.; De Galarreta, J.I.R.; Patto, M.C.V.; Žilić, S.; et al. Traditional Foods from Maize (Zea mays L.) in Europe. Fnut 2022, 8, 683399. [Google Scholar]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Production, Consumption And Trade: Trends And R&D Implications. Food Segurity 2022, 14, 1295–1319. [Google Scholar]
- Jayaprakash, S.; Raja, S.; He, J.; Paramannil, M. Functional Relevance of Bioactive Compounds in Purple Maize: A Contemporary Extraction Progressions and Prospective Applications. Cereal Res. Commun. 2023, 51, 263–282. [Google Scholar] [CrossRef]
- Medina-Hernández, J.; Caamal-Cauich, I.; Pat-Fernández, V.G.; Ávila-Dorantes, J.A. Current Challenges and Forecasts in Maize Grain Production and Consumption in Mexico. Agro Prod. 2024, 17, 165–172. [Google Scholar]
- USDA. Northwest Africa Wheat: Varying Weather Conditions; Mixed Results for MY 2024/25. United States Department of Agriculture. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 17 February 2025).
- Servicio de Información Agroalimentaria y Pesquera. Producción Agrícola. 2024. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 17 February 2025).
- Padilla-Gutiérrez, H.; Pérez-Esparza, A.; navarro-Pantoja, C.A. Jalisco Gigante Agroalimentario; Desarrollo Económico y Bienestar: Guadalajara, Mexico, 2019. [Google Scholar]
- Serna-Saldivar, S.O. Understanding the Functionality and Manufacturing of Nixtamalized Maize Products. J. Cereal Sci. 2021, 99, 103205. [Google Scholar] [CrossRef]
- Rivera, B.E.; Morales, D.; Gómez, M.G.; Nevárez, G.V. Consumo Responsable de la Tortilla de Maíz, una Herencia que Debemos Cuidar. Tema de Cienc. Y Tecnol. 2021, 25, 9–14. [Google Scholar]
- Portillo-Vázquez, M.; Sangermán-Jarquín, D.M.; Pérez-Robles, K. Creación de Regiones Agrícolas Altamente Especializadas en el Cultivo Maíz. Estudio de caso: Sinaloa, Sonora, Nayarit y Jalisco. Rev. Mex. De Cienc. Agrícolas 2023, 14, 303–310. [Google Scholar]
- SIAP. Producción Agrícola de Maíz. 2022. Available online: https://nube.siap.gob.mx/avance_agricola/ (accessed on 19 February 2025).
- CONABIO. Biodiversidad Mexicana. Razas de Maíz de México. 2020. Available online: https://www.biodiversidad.gob.mx/diversidad/alimentos/maices/razas-de-maiz (accessed on 19 February 2025).
- Ramírez-Vega, H.; Vázquez-Carrillo, G.; Muñóz-Rosales, G.M.; Martínez-Loperena, R.; Heredia-Nava, D.; Martínez-Sifuentes, J.Á.; Anaya-Esparza, L.M.; Gómez-Rodríguez, V.M. Physical and Chemical Characteristics of Native Maize from the Jalisco Highlands and their Influence on the Nixtamalization Process. Agriculture 2022, 12, 1293. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Esquivel-Esquivel, G.; Ramírez-Díaz, J.L.; La Torre, I.A.; Bautista-Ramírez, E.; Santillán-Fernández, A. Selección de Germoplasma de Maíz Morado (Zea mays L.) con Potencial para Extracción de Pigmentos. Rev. Fitotec. Mex. 2021, 44, 309–321. [Google Scholar]
- Vázquez-Carrillo, M.G.; Toledo-Aguilar, R.; Aragón-Cuevas, F.; Salinas-Moreno, Y.; Palacios-Rojas, N.; Santiago-Ramos, D. From Maize to Tlayuda, a Traditional Big-flat Leathery Tortilla. Effect of Two Nixtamalization Processes on Some Physicochemical and Nutraceutical Properties. Int. J. Gastron. Food Sci. 2023, 31, 100661. [Google Scholar]
- Diario Oficial de la Federación. Ley Federal Para El Fomento y Protección Del Maíz Nativo; Gobierno Federal Mexicano: Mexico, Mexico, 2020; Available online: https://www.diputados.gob.mx/LeyesBiblio/ref/lffpmn/LFFPMN_orig_13abr20.pdf (accessed on 19 February 2025).
- Paulsmeyer, M.N.; Vermillion, K.E.; Juvik, J.A. Assessing the Diversity of Anthocyanin Composition in Various Tissues of Purple Corn (Zea mays L.). Phytochemistry 2022, 201, 113263. [Google Scholar] [CrossRef]
- Zhang, Q.; De Mejia, E.G.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of Phenolic Composition of Selected Purple Maize (Zea mays L.) Genotypes with their Anti-Inflammatory, Anti-Adipogenic and Anti-Diabetic Potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Filipčev, B.; Đalović, I.; Nježić, Z.; Šimurina, O.; Bekavac, G.; Pojić, M. Physical and Milling Properties of Maize; CRC Press eBooks: Boca Raton, FL, USA, 2022; 38p. [Google Scholar]
- Hasmadi, M.; Noorfarahzilah, M.; Noraidah, H.; Zainol, M.; Jahurul, M. Functional Properties of Composite Flour: A Review. Food Res. 2020, 4, 1820–1831. [Google Scholar]
- Hernández-Santos, B.; Lerdo-Reyes, A.A.; Téllez-Morales, J.A.; Rodríguez-Miranda, J. Chemical Composition, Techno-functional Properties, and Bioactive Components of Blends of Blue Corn/Purple Sweet Potato for its Possible Application in the Food Industry. J. Food Meas. Charact. 2022, 17, 1909–1920. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Z.; Xing, X. Study on the Pyrolysis Properties of Corn Straw by TG–FTIR and TG–GC/MS. J. Therm. Anal. Calorim. 2021, 143, 3783–3791. [Google Scholar] [CrossRef]
- Colombo, R.; Ferron, L.; Papetti, A. Colored Corn: An Up-Date on Metabolites Extraction, Health Implication, and Potential Use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyedeji, A.B.; Adebiyi, J.A.; Chinma, C.E.; Oyeyinka, S.A.; Olatunde, O.O.; Green, E.; Njobeh, P.B.; Kondiah, K. Kinetics of Phenolic Compounds Modification during Maize Flour Fermentation. Molecules 2021, 26, 6702. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L.L.; Cruz-Gracida, M.; Barriada-Bernal, L.G.; Rodríguez-Méndez, L.I. Profile of Phenolic Acids, Antioxidant Activity and Total Phenolic Compounds During Blue Corn Tortilla Processing and its Bioaccessibility. J. Food Sci. Technol. 2020, 57, 4688–4696. [Google Scholar] [CrossRef]
- Salvador-Reyes, R.; Rebellato, A.P.; Pallone, J.A.L.; Ferrari, R.A.; Clerici, M.T.P.S. Kernel Characterization and Starch Morphology in Five Varieties of Peruvian Andean Maize. Food Res. Int. 2021, 140, 110044. [Google Scholar] [CrossRef]
- Vázquez-Carrillo, M.G.; Aparicio-Eusebio, L.A.; Salinas-Moreno, Y.; Buendía-Gonzalez, M.O.; Santiago-Ramos, D. Nutraceutical, Physicochemical, and Sensory Properties of Blue Corn Polvorones, a Traditional Flour-Based Confectionery. Plant Foods Hum. Nutr. 2018, 73, 321–327. [Google Scholar] [CrossRef]
- IIEG. Tepatitlán de Morelos. Diagnóstico del Municipio. Recuperado de. 2021. Available online: https://iieg.gob.mx/ns/wp-content/uploads/2023/02/Tepatitl%C3%A1n-de-Morelos-1.pdf (accessed on 20 February 2025).
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Functional Study of Raw and Cooked Blue Maize Flour: Starch Digestibility, Total Phenolic Content and Antioxidant Activity. J. Cereal Sci. 2017, 76, 179–185. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of Association of Official Analytical Chemists International; AOAC International Gaitherburg: Gaithersburg, MD, USA, 2016; Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 20 February 2025).
- Dongmo, H.; Tambo, S.T.; Teboukeu, G.B.; Mboukap, A.N.; Fotso, B.S.; Djuidje, M.C.T.; Klang, J.M. Effect of process and variety on physico-chemical and rheological properties of two corn flour varieties (Atp and Kassaï). J. Agric. Food Res. 2020, 2, 100075. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, H.F.; Peplinski, A.J. Gelatinization of Corn Grits by Roll Cooking, Extrusion Cooking and Steaming. Starch-Stärke 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-Chemical Characterization of Culled Plantain Pulp Starch, Peel Starch, and Flour. Int. J. Food Prop. 2015, 18, 165–177. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Amante, E.R.; Demiate, I.M.; Vieira, F.; Delgadillo, I.; Maraschin, M. Physicochemical, Thermal, and Pasting Properties of Flours and Starches of Eight Brazilian Maize Landraces (Zea mays L.). Food Hydrocoll. 2013, 30, 614–624. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Agric. Biol. Chem. 1972, 36, 719–727. [Google Scholar] [CrossRef]
- Piornos, J.A.; Burgos, C.; Morales, E.; Rubilar, M.; Acevedo, F. Highly efficent encapsulation of linseed oil into alginate/lupin protein beads: Optimizatation of the emulsión formulation. Food Hydrocoll. 2017, 63, 139–148. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Montreau, F.R. Sur Le Dosage Des Composés Phénoliques Totaux Dans Les Vins Par La Méthode Folin-Ciocalteu. OENO One 1972, 6, 397–404. [Google Scholar] [CrossRef]
- Esmaeili, A.K.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from in Vivo and in Vitro Grown Trifolium pratense L. (Red Clover). BioMed Res. Int. 2015, 1, 643285. [Google Scholar]
- Barba, F.J.; Rajha, H.N.; Debs, E.; Abi-Khattar, A.-M.; Khabbaz, S.; Dar, B.N.; Simirgiotis, M.J.; Castagnini, J.M.; Maroun, R.G.; Louka, N. Optimization of Polyphenols’ Recovery from Purple Corn Cobs Assisted by Infrared Technology and Use of Extracted Anthocyanins as a Natural Colorant in Pickled Turnip. Molecules 2022, 27, 5222. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, R.B.; Jones, W.T. Analysis of Condensed Tannins Using Acidified Vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Ortega, V.G.; Ramírez, J.A.; Velázquez, G.; Tovar, B.; Mata, M.; Montalvo, E. Effect of High Hydrostatic Pressure on Antioxidant Content of ‘Ataulfo’ Mango During Postharvest Maturation. Food Sci. Technol. Int. 2013, 33, 561–568. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation de colorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of "Antioxidant Power": The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bharat, B.; Bhushan, B.; Ajay Pal, M.; Kumar, M.; Manesh, C.D.; Yathish, K.R.; Bahadur, S.J.; Pardeep, K.; Sumit, K.A.; Alla, S.; et al. FTIR Spectra, Antioxidant Capacity and Degradation Kinetics of Maize Anthocyanin Extract Under Variable Process Conditions. Appl. Food Res. 2023, 3, 100282. [Google Scholar]
- Trehan, S.; Singh, N.; Kaur, A. Diversity and Relationship Among Grain, Flour and Starch Characteristics of Indian Himalayan Colored Corn Accessions. J. Food Sci. Technol. 2020, 57, 3801–3813. [Google Scholar] [CrossRef]
- Patil, S.; Kaur, C.; Puniya, M.K.; Mahapatra, A.; Dhakane-Lad, J.; Jalgaonkar, K.; Mahawar, M.K. Functional Properties of Extruded Corn Flour. Turk. J. Agric. Eng. Res. 2021, 2, 167–174. [Google Scholar] [CrossRef]
- Barbosa-Pinto, A.T.B.; Pereira, J.; de Oliveira, T.R.; Prestes, R.A.; Mattielo, R.R.; Demiate, I.M. Characterization of Corn Landraces Planted Grown in the Campos Gerais Region (Paraná, Brazil) for Industrial Utilization. Braz. Arch. Biol. Technol. 2009, 52, 17–28. [Google Scholar] [CrossRef]
- Yaqoob, S.; Cai, D.; Liu, M.; Zheng, M.; Zhao, C.B.; Liu, J.S. Characterization of Microstructure, Physicochemical and Functional Properties of Corn Varieties Using Different Analytical Techniques. Int. J. Food Prop. 2019, 22, 572–582. [Google Scholar] [CrossRef]
- Oas, S.E.; Adams, K.R. The Nutritional Content of Five Southwestern US Indigenous Maize (Zea mays L.) Landraces of Varying Endosperm Type. Am. Antiq. 2022, 87, 284–302. [Google Scholar] [CrossRef]
- Mutlu, C.; Arslan-Tontul, S.; Candal, C.; Kilic, O.; Erbas, M. Physicochemical, Thermal, and Sensory Properties of Blue Corn (Zea mays L.). J. Food Sci. 2018, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Žilić, S.; Simic, M.; Vasić, M. Wholegrain Flours of Differently Colored Maize Kernels as Macro-and Micronutrient-Rich Food Ingredients Integralno Brašno od Kukuruza Različite Boje Zrna Kao Izvor Makro-i Mikro-NutriJenata. J. Process. Energy Agric. 2022, 26, 64–67. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. Effects of Baking Conditions and Dough Formulations on Phenolic Compound Stability, Antioxidant Capacity and Color of Cookies Made From Anthocyanin-Rich Corn Flour. Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Shi, L.; Li, W.; Sun, J.; Qiu, Y.; Wei, X.; Luan, G.; Hu, Y.; Tatsumi, E. Grinding of Maize: The Effects of Fine Grinding on Compositional, Functional and Physicochemical Properties of Maize Flour. J. Cereal Sci. 2016, 68, 25–30. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Alimentarius. Normas Internationals de los Alimentos. 2013. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/codes-of-practice/es/ (accessed on 20 February 2025).
- Akaffou, F.; Koffi, D.; Cisse, M.; Niamké, S. Physicochemical and Functional Properties of Flours from Three Purple Maize Varieties Named “Violet de Katiola” in Côte d’Ivoire. Asian Food Sci. J. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Martínez-Trejo, G.I.; Anaya-Esparza, L.M.; Vargas-Torres, A.; Hernández-Uribe, J.P.; Viñas-Bravo, O. Dual Modification of Chayotextle Starch: Effect on Physicochemical, Functional, and Structural Properties. Starch 2023, 75, 9–10. [Google Scholar]
- Uriarte-Aceves, P.; Cuevas-Rodriguez, E.O.; Gutiérrez-Dorado, R.; Mora-Rochin, S.; Reyes-Moreno, C.; Puangpraphant, S.; Milán-Carrillo, J. Physical, Compositional, and Wet-Milling Characteristics of Mexican Blue Maize (Zea mays L.) Landrace. Cereal J. Chem. 2015, 92, 491–496. [Google Scholar] [CrossRef]
- An, N.; Li, D.; Wang, L.; Wang, Y. Microwave irradiation of corn kernels: Effects on Structural, Thermal, Functional and Rheological Properties of Corn Flour. Food Hydrocoll. 2023, 143, 108939. [Google Scholar] [CrossRef]
- Milán-Carrillo, J.; Gutiérrez-Dorado, R.; Cuevas-Rodríguez, E.; Garzón-Tiznado, J.; Reyes-Moreno, C. Nixtamalized Flour From Quality Protein Maize (Zea mays L.) Optimization of Alkaline Processing. Plant Foods Hum. Nutr. 2004, 59, 35–44. [Google Scholar] [CrossRef]
- Balseiro, G.; Taron, A.; Garcia-Zapateiro, L.A. Nutritional Properties of Different Composite Flours from Maize (Zea mays) Variety ica v109 and Pearl Millet (Pennisetum glaucum) Malted with Calcium Chloride and Gibberellic Acid. Eur. Food Res. Technol. 2014, 240, 471–475. [Google Scholar] [CrossRef]
- Machado-Filho, G.; Nascimento, I.; Sakai, T.; Rocha, W.; Santos, M. Brix analysis and green corn cob productivy by nitrogen fertilization time. Appl. Res. Agrotechnol. 2018, 11, 33–41. [Google Scholar]
- García-Campos, A.U.; Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; Jiménez-Guzmán, J.; Fabela-Morón, M.F.; De la Paz Salgado-Cruz, M.; De Jesús Cortés-Sánchez, A.; Villanueva-Carvajal, A.; Díaz-Ramírez, M. Caracterización Físico-Química de Maíz (Zea mays L.) Criollo (Azul y Rojo) del Estado de México. Agro Product. 2020, 13, 95–100. [Google Scholar] [CrossRef]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, Nutritional and Nutraceutical Properties of Pigmented Native Maize (Zea mays L.) Genotypes From the Northeast of Mexico. Arab. J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Savlak, N.; Türker, B.; Yeşilkanat, N. Effects of Particle Size Distribution on Some Physical, Chemical and Functional Properties of Unripe Banana Flour. Food Chem. 2016, 213, 180–186. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Mejía-Terán, A.; Blanco-Lizarazo, C.M.; Mateus, E.L.; Sotelo-Díaz, I. Techno-Functional and Physicochemical Properties of Corn Flours as Potential Food Ingredients. Appl. Food Res. 2024, 4, 100427. [Google Scholar] [CrossRef]
- Khan, A.; Siddiqui, S.; Rahman, U.U.; Ali, H.; Saba, M.; Azhar, F.A.; Rehman, M.M.U.; Shah, A.A.; Badshah, M.; Hasan, F.; et al. Physicochemical Properties of Enzymatically Prepared Resistant Starch from Maize Flour and its Use in Cookies Formulation. Int. J. Food Prop. 2020, 23, 549–569. [Google Scholar] [CrossRef]
- Shad, M.; Nawaz, H.; Noor, M.; Badar, H.; Hussain, M.; Choudhry, M. Functional Properties of Maize Flour and its Blends with Wheat Flour: Optimization of Preparation Conditions by response Surface Methodology. Pak. J. Bot. 2013, 45, 2027–2035. [Google Scholar]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of Aqueous solutions: Role of surfactant type and concentration. Adv. Colloid Interface Sci. 2020, 276, 102084. [Google Scholar] [CrossRef]
- Keramaris, A.; Papadopoulos, V.; Kasapidou, E.; Mitlianga, P. A comprehensive analysis on nutritional and antioxidant characteristics of a traditional roasted maize flour (Furniko) of ponti greeks: Comparative study to related flour products. Plant Foods Hum. Nutr. 2023, 58, 476–482. [Google Scholar] [CrossRef]
- Montilla, E.C.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and Bound Phenolic Compounds in Different Bolivian Purple Corn (Zea mays L.) Cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar]
- Žilić, S.; Mogol, B.A.; Akıllıoğlu, G.; Serpen, A.; Babić, M.; Gökmen, V. Effects of Infrared Heating on Phenolic Compounds and Maillard Reaction Products in Maize Flour. J. Cereal Sci. 2013, 58, 1–7. [Google Scholar]
- Oladapo, A.S.; Adepeju, A.B.; Akinyele, A.A.; Adepeju, D.M. The Proximate, Functional and Anti-Nutritional Properties of Three Selected Varieties of Maize (Yellow, White and Pop Corn) flour. Int. J. Sci. Eng. Sci. 2017, 1, 23–26. [Google Scholar]
- Inyang, U.E.; Akindolu, B.E.; Elijah, A.I. Nutrient Composition, Amino Acid Profile and Anti-Nutritional Factors of Nixtamalized Maize Flour Supplemented With Sprouted Soybean Flour. Eur. J. Nutr. Food Saf. 2019, 9, 41–51. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of Total Polyphenols, Profile Anthocyanins, Color Analysis, Carotenoids and Tocols in Pigmented Maize. Lwt 2021, 144, 111257. [Google Scholar] [CrossRef]
- Hu, Q.P.; Xu, J.G. Profiles of Carotenoids, Anthocyanins, Phenolics, and Antioxidant Activity of Selected Color Waxy Corn Grains during Maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Beta, T.; Hwang, T. Influence of heat and Moisture Treatment on Carotenoids, Phenoliccontent, and Antioxidant Capacity of Orange Maize Flour. Food Chem. 2017, 246, 58–64. [Google Scholar] [CrossRef]
- Awolu, O.O.; Odoro, J.W.; Adeloye, J.B.; Lawal, O.M. Physicochemical Evaluation and Fourier Transform Infrared SpectrosCopy Characterization of Quality Protein Maize Starch Subjected to Different Modifications. J. Food Sci. 2020, 85, 3052–3060. [Google Scholar] [CrossRef]
- Cueto, M.; Farroni, A.; Rodríguez, S.D.; Schoenlechner, R.; Schleining, G.; Del Pilar Buera, M. Assessing Changes in Enriched Maize Flour Formulations After Extrusion by Means of FTIR, XRD, and Chemometric Analysis. Food Bioprocess Technol. 2018, 11, 1586–1595. [Google Scholar]
- Ying, D.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR Analysis of Rice-Oat Flour and Maize-Oat Flour Based Extruded Food Products Containing Olive Pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar]
- Kuhnen, S.; Ogliari, J.; Dias, P.; Boffo, E.; Correia, I.; Ferreira, A.; Delgadillo, I.; Maraschin, M. ATR-FTIR Spectroscopy and Chemometric Analysis Applied to Discrimination of Landrace Maize Flours Produced in Southern Brazil. Int. J. Food Sci. Technol. 2010, 45, 1673–1681. [Google Scholar] [CrossRef]

| Name of Corn Sample | Code | Visual Kernel Color | Cob Sample (Pieces) | Corn Kernel (g) |
|---|---|---|---|---|
| White hybrid (Control) | WHC | White | 1000 | |
| Negro Jalisco | NJ | Dark red | 10 | 1000 |
| Rojo occidente pozolero | ROP | Purple | 10 | 1000 |
| Amarillo Zamorano 1 | AZ1 | Yellow | 10 | 1000 |
| Amarillo Zamorano 2 | AZ2 | Yellow | 10 | 1000 |
| Negro Michoacan 1 | NM1 | Dark blue | 10 | 1000 |
| Negro Michoacan 2 | NM2 | Dark gray | 9 | 1000 |
| Criollo Rojo Pozolero Tepatitlán | CRPT | Light purple | 11 | 1000 |
| Tabloncillo Ahumado | TAH | Light brown | 10 | 1000 |
| Azúl México 2 | AM2 | Black | 23 | 1000 |
| Sample | Moisture (%) | Protein (%) | Fat (%) | Carbohydrates (%) | Starch (%) | Ash (%) | Energy Value (Kcal/100 g) |
|---|---|---|---|---|---|---|---|
| WHC | 9.78 ± 0.04 b | 8.65 ± 0.03 e | 3.78 ± 0.03 e | 76.39 ± 0.07 a | 63.07 ± 0.17 a | 2.29 ± 0.04 f | 374.18 |
| NJ | 8.70 ± 0.04 e | 9.79 ± 0.13 c | 3.91 ± 0.01 d | 75.94 ± 0.17 b | 62.29 ± 0.27 b | 2.48 ± 0.01 d | 378.11 |
| ROP | 8.68 ± 0.01 e | 10.16 ± 0.13 b | 4.37 ± 0.06 a | 75.43 ± 0.19 c | 57.85 ± 0.69 e | 2.71 ± 0.08 b | 381.69 |
| AZ1 | 8.50 ± 0.12 f | 10.96 ± 0.11 a | 3.76 ± 0.03 e | 75.37 ± 0.01 c | 60.52 ± 0.14 c | 2.68 ± 0.02 b | 379.16 |
| AZ2 | 8.01 ± 0.05 h | 9.67 ± 0.12 c | 4.33 ± 0.03 a | 76.56 ± 0.20 a | 63.19 ± 0.25 a | 2.47 ± 0.03 d | 383.89 |
| NM1 | 8.51 ± 0.05 f | 9.72 ± 0.19 c | 3.74 ± 0.03 e | 76.39 ± 0.17 a | 61.54 ± 0.63 b | 2.61 ± 0.01 c | 378.10 |
| NM2 | 9.30 ± 0.08 c | 9.20 ± 0.07 d | 4.03 ± 0.05 c | 75.97 ± 0.03 b | 63.07 ± 0.02 a | 2.37 ± 0.02 e | 376.95 |
| CRPT | 8.94 ± 0.09 d | 9.74 ± 0.01 c | 3.98 ± 0.04 c | 75.75 ± 0.03 b | 60.18 ± 0.03 cd | 2.43 ± 0.03 de | 377.78 |
| TAH | 8.28 ± 0.01 g | 10.23 ± 0.04 b | 4.16 ± 0.04 b | 75.90 ± 0.02 b | 59.68 ± 0.01 d | 2.69 ± 0.03 b | 381.84 |
| AM2 | 10.52 ± 0.09 a | 10.19 ± 0.12 b | 3.38 ± 0.03 f | 74.71 ± 0.18 d | 51.41 ± 0.12 f | 3.48 ± 0.07 a | 370.02 |
| Sample | pH | Titratable Acidity (% Malic Acid) | Total Soluble Solids (°Brix) | Water Activity |
|---|---|---|---|---|
| WHC | 6.13 ± 0.03 f | 0.43 ± 0.02 d | 0.77 ± 0.12 e | 0.56 ± 0.01 a |
| NJ | 6.13 ± 0.06 de | 0.26± 0.07 ef | 1.57 ± 0.15 c | 0.42 ± 0.01 e |
| ROP | 6.38 ± 0.04 a | 0.23 ± 0.01 ef | 1.40 ± 0.17 cd | 0.41 ± 0.01 e |
| AZ1 | 6.25 ± 0.06 bcd | 0.28 ± 0.06 e | 2.00 ± 0.10 ab | 0.42 ± 0.01 e |
| AZ2 | 6.20 ± 0.01 cde | 0.60 ± 0.03 b | 0.60 ± 0.20 e | 0.46 ± 0.01 c |
| NM1 | 6.11 ± 0.01 ef | 0.54 ± 0.01 c | 0.57 ± 0.15 e | 0.47 ± 0.01 bc |
| NM2 | 6.14 ± 0.01 ef | 0.68 ±0.02 a | 1.17 ± 0.21 d | 0.46 ± 0.01 cd |
| CRPT | 6.28 ± 0.06 abc | 0.23 ± 0.02 ef | 1.23 ± 0.06 d | 0.45 ± 0.01 d |
| TAH | 6.16 ± 0.10 def | 0.22 ± 0.03 f | 2.17 ± 0.21 a | 0.41 ± 0.01 e |
| AM2 | 6.31 ± 0.02 ab | 0.63 ± 0.03 ab | 1.90 ± 0.10 b | 0.48 ± 0.02 b |
| Sample | Luminosity | a* | b* | Color |
|---|---|---|---|---|
| WHC | 86.26 ± 0.93 bcd | −0.47 ± 0.44 c | 9.86 ± 0.81 e |  |
| NJ | 87.85 ± 3.08 abc | 2.66 ± 0.35 a | 4.95± 0.89 f |  |
| ROP | 78.33 ± 1.43 e | 2.96 ± 0.33 a | 23.03 ± 1.18 c |  |
| AZ1 | 90.80 ± 0.77 a | −0.57 ± 0.58 c | 26.82 ± 1.58 b |  |
| AZ2 | 90.09 ± 0.88 ab | −0.27 ± 0.43 c | 32.79 ± 0.48 a |  |
| NM1 | 86.43 ± 2.38 bcd | 2.88 ± 0.73 a | 6.11 ± 0.74 f |  |
| NM2 | 86.13 ± 3.24 cd | 1.49 ± 0.30 b | 2.47 ± 0.98 h |  |
| CRPT | 82.72 ± 2.49 d | 3.31 ± 0.18 a | 2.75 ± 0.86 gh |  |
| TAH | 84.65 ± 3.52 cd | −0.65 ± 0.16 c | 12.05 ± 1.79 d |  |
| AM2 | 74.57 ± 0.62 e | 0.47 ± 0.14 c | 2.89 ± 0.03 h |  |
| Sample | WSI (%) | WAI (g g−1) | Swelling Power (%) | OAI (%) | Emulsifying Capacity (%) | Foaming Capacity (%) |
|---|---|---|---|---|---|---|
| WHC | 7.41 ± 0.10 a | 2.40 ± 0.07 c | 5.03 ± 0.32 ef | 5.52 ± 0.09 a | 2.97 ± 0.11 b | 6.31 ± 0.23 ef |
| NJ | 5.81 ± 0.12 bc | 3.29 ± 0.02 a | 9.33 ± 0.57 a | 4.70 ± 0.08 c | 1.91 ± 0.16 c | 5.19 ± 0.68 fg |
| ROP | 6.58 ± 1.61 ab | 2.63 ± 0.32 c | 8.42 ± 0.30 b | 4.70 ± 0.07 c | 3.67 ± 0.60 a | 10.03 ± 0.21 c |
| AZ1 | 5.95 ± 0.61 bc | 2.55 ± 0.04 b | 6.83 ± 0.67 d | 4.80 ± 0.04 c | 3.11 ± 0.34 b | 7.99 ± 1.34 de |
| AZ2 | 6.45 ± 0.20 abc | 2.41 ± 0.08 c | 6.82 ± 0.42 d | 5.41 ± 0.13 a | 1.98 ± 0.07 c | 12.13 ± 1.46 b |
| NM1 | 5.51 ± 0.06 c | 2.09 ± 0.04 f | 5.49 ± 0.26 e | 5.17 ± 0.16 b | 1.44 ± 0.08 d | 16.70 ± 1.91 a |
| NM2 | 6.16 ± 0.36 bc | 2.16 ± 0.03 e | 3.58 ± 0.09 g | 5.03 ±0.25 b | 2.02 ± 0.17 c | 9.77 ± 1.22 cd |
| CRPT | 6.21 ± 0.34 bc | 2.13 ± 0.03 ef | 4.69 ± 0.31 f | 4.74 ± 0.04 c | 2.84 ± 0.42 b | 7.42 ± 1.70 e |
| TAH | 5.48 ± 0.40 c | 2.54 ± 0.02 b | 7.56 ± 0.64 c | 4.79 ± 0.08 c | 2.95 ± 0.11 b | 7.03 ± 0.63 ef |
| AM2 | 5.43 ± 0.39 c | 2.24 ± 0.01 d | 3.96 ± 0.18 g | 5.17 ± 0.12 b | 3.12 ± 0.16 b | 3.52 ± 0.94 h |
| Sample | TSP (mg GAE/g) | CT (mg CE/g) | FLA (mg CE/g) | TA (mg C3G/g) | TC (mg βCE/g) |
|---|---|---|---|---|---|
| WHC | 10.02 ± 1.24 d | 1.33 ± 0.14 b | 2.56 ± 0.77 e | ˂LOD | ˂LOQ |
| NJ | 23.23 ± 0.57 e | 0.30 ± 0.08 d | 19.96 ± 0.77 a | ˂LOD | ˂LOQ |
| ROP | 27.49 ± 2.29 c | 0.38 ± 0.08 d | 20.86 ± 0.17 a | ˂LOD | ˂LOQ |
| AZ1 | 19.01 ± 0.18 fg | 0.90 ± 0.10 c | 14.42 ± 0.83 c | ˂LOD | 0.42 ± 0.02 a |
| AZ2 | 15.66 ± 1.47 g | 0.22 ± 0.08 d | 14.64 ± 0.05 c | ˂LOD | 0.29 ± 0.05 b |
| NM1 | 22.26 ± 0.26 ef | 0.99 ± 0.34 bc | 16.60 ± 0.50 b | ˂LOD | 0.14 ± 0.01 c |
| NM2 | 33.03 ± 1.95 b | 0.47 ± 0.08 d | 17.83 ± 0.01 b | 0.04 ± 0.001 b | ˂LOQ |
| CRPT | 17.18 ± 0.83 g | 2.83 ± 0.17 a | 14.67 ± 0.13 c | ˂LOD | 0.29 ± 0.02 b |
| TAH | 17.18 ± 0.18 g | 0.34 ± 0.01 d | 10.65 ± 0.56 d | ˂LOD | 0.08 ± 0.01 d |
| AM2 | 38.76 ± 1.05 a | 0.93 ± 0.17 c | 16.89 ± 0.18 b | 0.10 ± 0.01 a | ˂LOQ |
| Sample | DPPH (mmol TE/g) | ABTS (mmol TE/g) | FRAP (mmol TE/g) |
|---|---|---|---|
| WHC | 26.46 ± 0.37 a | 12.79 ± 0.23 g | 31.16 ± 1.87 f |
| NJ | 24.44 ± 0.33 bc | 22.45 ± 0.23 c | 46.46 ± 5.12 a |
| ROP | 24.78 ± 0.23 b | 16.86 ± 0.24 ef | 33.30 ± 2.54 ef |
| AZ1 | 23.86 ± 0.29 c | 15.83 ± 0.24 f | 26.80 ± 1.41 g |
| AZ2 | 24.31 ± 0.46 bc | 17.40 ± 0.24 e | 37.99 ± 1.24 c |
| NM1 | 24.06 ± 0.03 c | 24.34 ± 0.38 b | 37.33 ± 4.07 cd |
| NM2 | 20.44 ± 0.26 d | 21.37 ± 1.50 d | 43.29 ± 3.17 b |
| CRPT | 24.31 ± 0.29 bc | 19.36 ± 0.19 e | 30.24 ± 2.46 f |
| TAH | 23.87 ± 0.12 c | 25.64 ± 0.29 a | 34.66 ± 0.98 de |
| AM2 | 19.86 ± 0.35 e | 26.15 ± 0.29 a | 31.55 ± 2.01 ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Villaseñor, L.A.; Hernández-Estrada, S.; Gómez-Rodríguez, V.M.; Ramírez-Vega, H.; Villagrán, Z.; Ortega-Martínez, A.; Montalvo-González, E.; Ruvalcaba-Gómez, J.M.; González-Silva, N.; Anaya-Esparza, L.M. Comparison of the Nutritional, Physicochemical, Technological–Functional, and Structural Properties and Antioxidant Compounds of Corn Kernel Flours from Native Mexican Maize Cultivated in Jalisco Highlands. Crops 2025, 5, 26. https://doi.org/10.3390/crops5030026
Hernández-Villaseñor LA, Hernández-Estrada S, Gómez-Rodríguez VM, Ramírez-Vega H, Villagrán Z, Ortega-Martínez A, Montalvo-González E, Ruvalcaba-Gómez JM, González-Silva N, Anaya-Esparza LM. Comparison of the Nutritional, Physicochemical, Technological–Functional, and Structural Properties and Antioxidant Compounds of Corn Kernel Flours from Native Mexican Maize Cultivated in Jalisco Highlands. Crops. 2025; 5(3):26. https://doi.org/10.3390/crops5030026
Chicago/Turabian StyleHernández-Villaseñor, Luis Alfonso, Salvador Hernández-Estrada, Víctor Manuel Gómez-Rodríguez, Humberto Ramírez-Vega, Zuamí Villagrán, Araceli Ortega-Martínez, Efigenia Montalvo-González, José Martín Ruvalcaba-Gómez, Napoleón González-Silva, and Luis Miguel Anaya-Esparza. 2025. "Comparison of the Nutritional, Physicochemical, Technological–Functional, and Structural Properties and Antioxidant Compounds of Corn Kernel Flours from Native Mexican Maize Cultivated in Jalisco Highlands" Crops 5, no. 3: 26. https://doi.org/10.3390/crops5030026
APA StyleHernández-Villaseñor, L. A., Hernández-Estrada, S., Gómez-Rodríguez, V. M., Ramírez-Vega, H., Villagrán, Z., Ortega-Martínez, A., Montalvo-González, E., Ruvalcaba-Gómez, J. M., González-Silva, N., & Anaya-Esparza, L. M. (2025). Comparison of the Nutritional, Physicochemical, Technological–Functional, and Structural Properties and Antioxidant Compounds of Corn Kernel Flours from Native Mexican Maize Cultivated in Jalisco Highlands. Crops, 5(3), 26. https://doi.org/10.3390/crops5030026









