Physio-Biochemical Responses and Cadmium Partitioning Associated with Stress Tolerance in Hulless Barley Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Conditions, Treatment, and Sampling
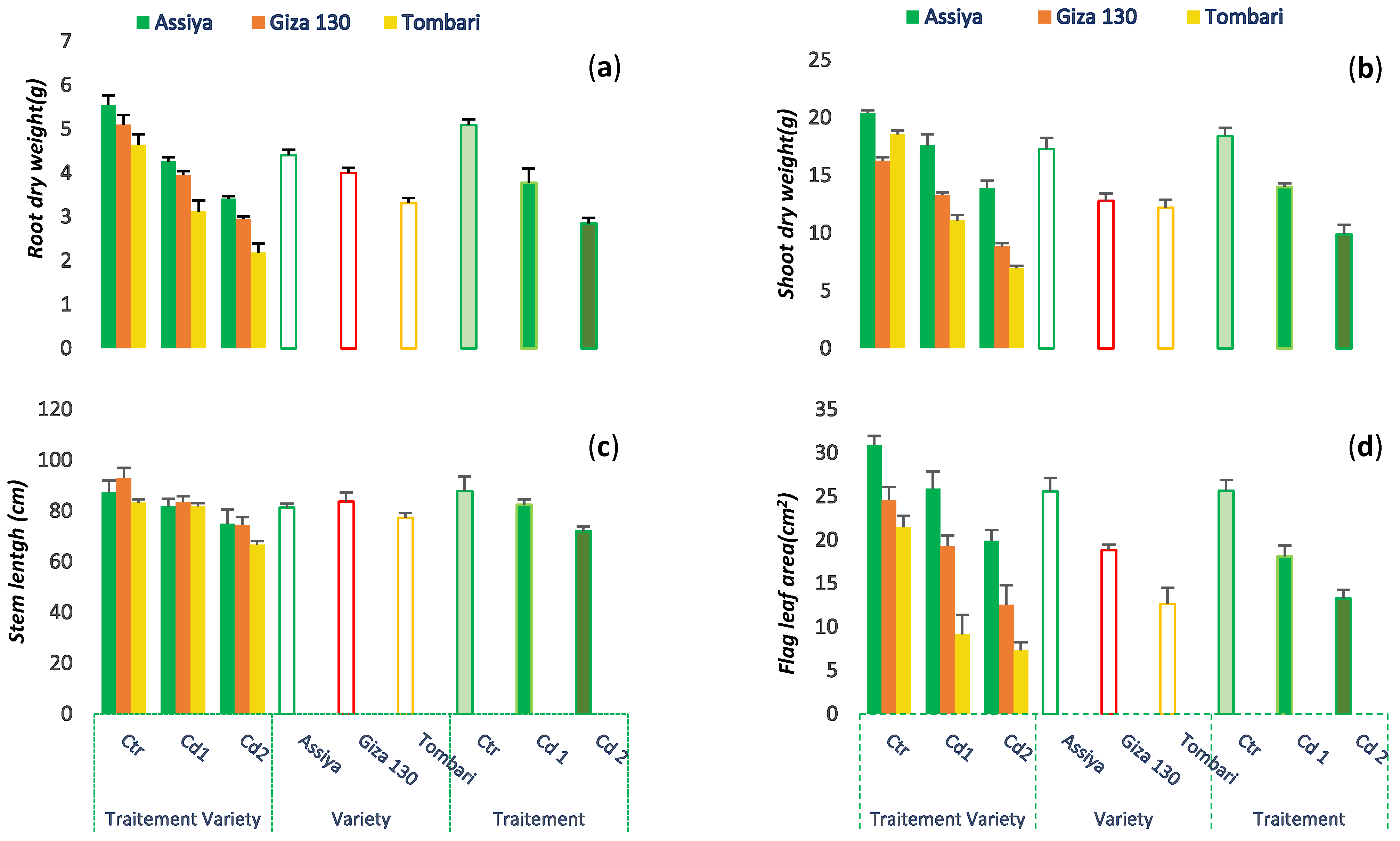
2.2. Morphological Measurements
2.3. Determination of Cd Accumulation Using ICP-AES
2.4. Estimation of Fag Leaf Relative Water Content
2.5. Flag Leaf Photosynthetic Parameters and Transpiration Measurement
2.6. Determination of Leaf Proline Content
2.7. Determination of Lipid Peroxidation, Hydrogen Peroxide (H2O2) Contents, and Enzymatic Activities in Leaves
2.8. Statistical Analysis
3. Results
3.1. Data Variability
3.2. Cadmium Effect on Plant Growth
| Source | DF | RDW | SDW | Stem Length | FLA | RWC | E | SPAD | Fv/Fm | Proline | MDA | H2O2 | CAT | APX | POD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety (VAR) | 2 | 2.732 *** | 69.801 *** | 92.68 *** | 375.59 *** | 559.855 *** | 2.6842 *** | 15.134 *** | 0.0016 *** | 8.5164 *** | 176.80 *** | 5.9331 *** | 0.0136 *** | 1.536 *** | 180.236 *** |
| Treatment (TRT) | 2 | 11.48 *** | 162.21 *** | 582.49 *** | 350.85 *** | 449.832 *** | 27.920 *** | 199.57 *** | 0.00401 *** | 22.807 *** | 134.02 *** | 23.397 *** | 0.0329 *** | 9.233 *** | 399.568 *** |
| Replicates | 2 | 0.0325 | 1.601 | 0.823 | 0.326 | 0.05 | 0.0093 | 0.42 | 0.00004 | 0.0649 | 0.03 | 0.0607 | 0.0002 | 0.0125 | 0.043 |
| VAR*TRT | 4 | 0.037 *** | 7.312 *** | 21.997 *** | 13.24 *** | 41.898 *** | 1.2295 *** | 8.833 *** | 0.00044 *** | 4.0231 *** | 42.29 *** | 5.8488 *** | 0.0111 *** | 2.434 *** | 122.363 *** |
| Error | 16 | 0.092 | 0.394 | 1.999 | 0.51 | 4.306 | 0.1377 | 0.819 | 0.000054 | 0.1334 | 0.484 | 0.0459 | 0.0002 | 0.0336 | 1.455 |
| Total | 26 |
3.3. Accumulation Levels of Cadmium in Roots, Stems, and Leaves
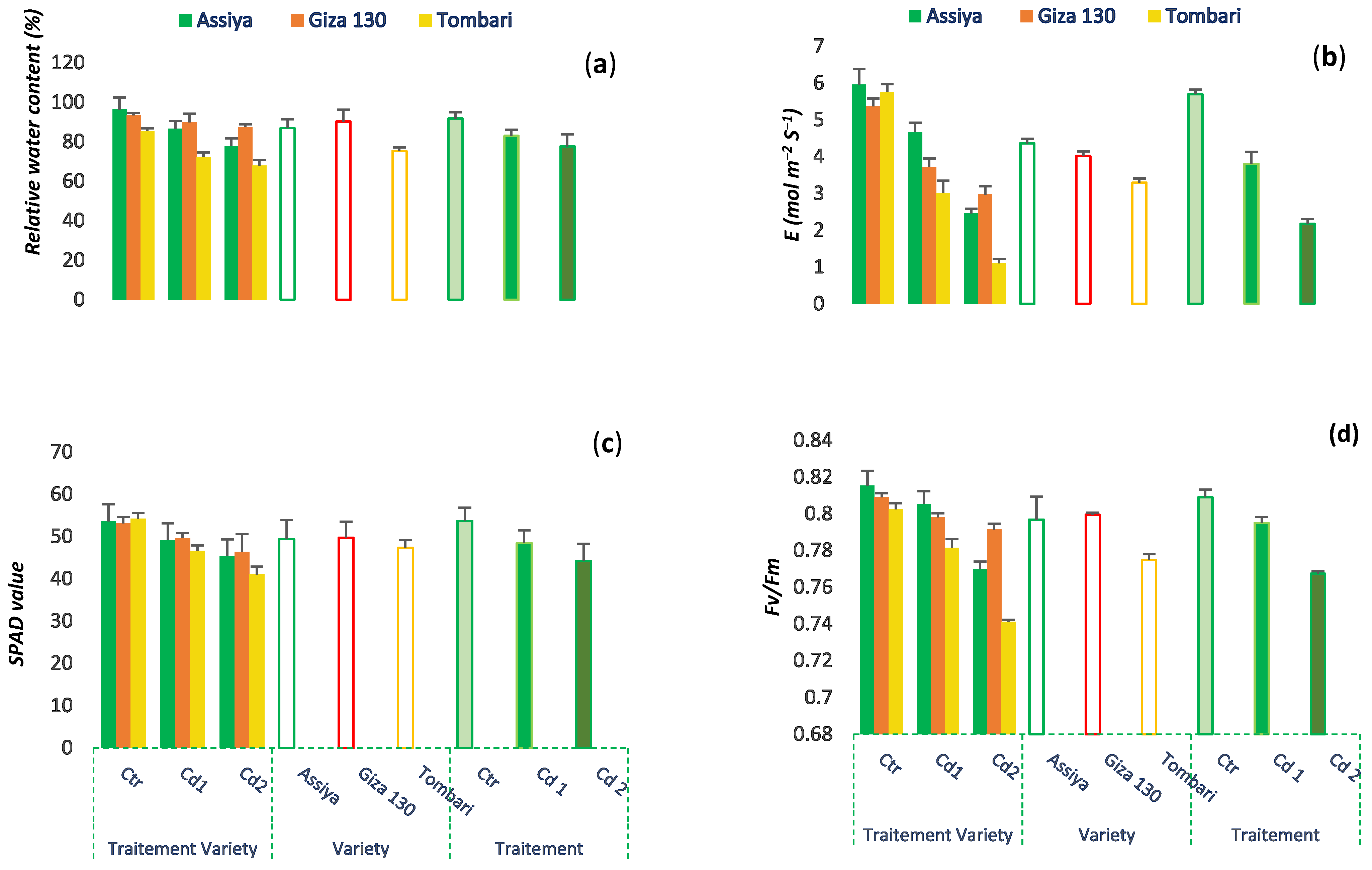
3.4. Cadmium Effects on SPAD Index, Chlorophyll Fluorescence and Transpiration
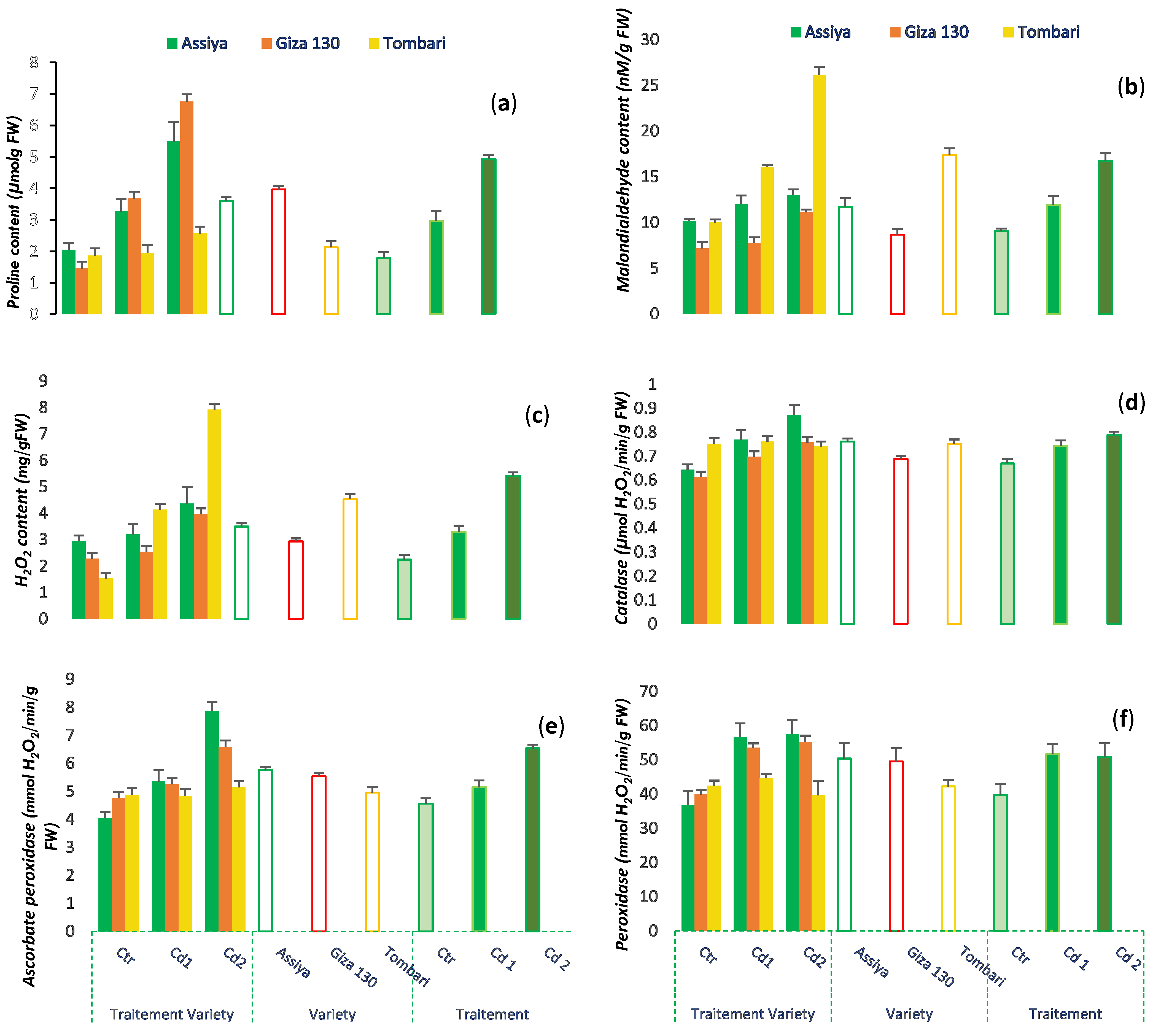
3.5. Effects of Cadmium on Relative Water Content, Proline Content, and Oxidative Damage
3.6. Effect of Cadmium on the Activity of Antioxidant Enzymes
3.7. Relationship Between the Studied Parameters
3.8. Principal Component Analysis (PCA)
| Variables | SDW | Stem Length | FLA | RWC | E | SPAD | ProC | MDA | H2O2 | Fv/Fm | CAT | APX | POD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RDW | 0.938 *** | 0.887 ** | 0.926 *** | 0.843 ** | 0.958 *** | 0.945 *** | −0.466 | −0.751 * | −0.803 ** | 0.879 ** | −0.589 | −0.489 | −0.323 |
| SDW | 0.753 * | 0.934 *** | 0.696 * | 0.913 *** | 0.875 ** | −0.426 | −0.654 | −0.765 * | 0.797 ** | −0.315 | −0.374 | −0.188 | |
| LP | 0.712 * | 0.752 * | 0.866 ** | 0.890 ** | −0.530 | −0.792 ** | −0.835 ** | 0.867 ** | −0.659 | −0.530 | −0.294 | ||
| FLA | 0.821 ** | 0.842 ** | 0.796 * | −0.239 | −0.698 | −0.690 * | 0.796 ** | −0.381 | −0.254 | −0.072 | |||
| RWC | 0.819 ** | 0.822 ** | −0.047 | −0.869 ** | −0.780 * | 0.907 *** | −0.610 | −0.287 | −0.044 | ||||
| E | 0.982 *** | −0.465 | −0.755 * | −0.866 | 0.924 *** | −0.555 | −0.539 | −0.308 | |||||
| SPAD | −0.452 | −0.810 | −0.911 | 0.911 *** | −0.566 | −0.508 | −0.309 | ||||||
| ProC | −0.047 | 0.183 | −0.216 | 0.580 | 0.848 ** | 0.789 * | |||||||
| MDA | 0.937 | −0.886 ** | 0.338 | 0.051 | −0.204 | ||||||||
| H2O2 | −0.909 *** | 0.321 | 0.231 | −0.061 | |||||||||
| Fv/Fm | −0.522 | −0.402 | −0.044 | ||||||||||
| CAT | 0.789 * | 0.666 * | |||||||||||
| APX | 0.771 * |

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Fv/Fm | Chlorophyll fluorescence |
| E | Transpiration rates |
| Cd | Cadmium |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| POD | Guaiacol peroxidase |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| RWC | Relative water content |
References
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a Major Facilitator Superfamily Gene Contributes to Differential Cadmium Accumulation between Rice Subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef]
- Borjac, J.; El Joumaa, M.; Kawach, R.; Youssef, L.; Blake, D.A. Heavy Metals and Organic Compounds Contamination in Leachates Collected from Deir Kanoun Ras El Ain Dump and Its Adjacent Canal in South Lebanon. Heliyon 2019, 5, e02212. [Google Scholar] [CrossRef]
- Abdikulov, Z.; Ergashev, M. Effect of Cadmium Metal on Barley (Hordeum Vulgare) Growth and Development. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 304, p. 03017. [Google Scholar]
- Ai-Qasi, B.A.; Sharqi, M.M.; Faiath, S.E. Effect of Nitrogen Fertilizer Sources, Lead and Cadmium Pollution on Some Properties of Barley (Hordeum Vulgare). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Ramadi, Iraq, 17–18 November 2021; Volume 904, p. 012057. [Google Scholar]
- González, A.; Gil-Díaz, M.; Lobo, M.C. Response of two barley cultivars to increasing concentrations of cadmium or chromium in soil during the growing period. Biol. Trace Elem. Res. 2015, 163, 235–243. [Google Scholar] [PubMed]
- Saxena, G.; Chandra, R.; Bharagava, R.N. Environmental Pollution, Toxicity Profile and Treatment Approaches for Tannery Wastewater and Its Chemical Pollutants; Impact Factor: 7.00 @ JCR Reports 2018 Environmental Pollution; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Haddioui, A. Human and Animal Health Risk Assessment of Metal Contamination in Soil and Plants from Ait Ammar Abandoned Iron Mine, Morocco. Environ. Monit. Assess. 2016, 188, 6. [Google Scholar] [CrossRef]
- Liang, C.; Xiao, H.; Hu, Z.; Zhang, X.; Hu, J. Uptake, Transportation, and Accumulation of C60 Fullerene and Heavy Metal Ions (Cd, Cu, and Pb) in Rice Plants Grown in an Agricultural Soil. Environ. Pollut. 2018, 235, 330–338. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Bouhraoua, S.; Ferioun, M.; Boussakouran, A.U.; Boussakouran, A.; Belahcen, D.; Srhiouar, N.; Louahlia, S. BIO Web of Conferences. BIO Web Conf. 2024, 98, 1–5. [Google Scholar]
- Tran, T.A.; Popova, L.P. Functions and Toxicity of Cadmium in Plants: Recent Advances and Future Prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, M.M.; Qiu, C.W.; Cao, F.; Chen, Z.H.; Zhang, G.; Wu, F. Response of Tibetan Wild Barley Genotypes to Drought Stress and Identification of Quantitative Trait Loci by Genome-Wide Association Analysis. Int. J. Mol. Sci. 2019, 20, 791. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of Biochar on Alleviation of Cadmium Toxicity in Wheat (Triticum Aestivum L.) Grown on Cd-Contaminated Saline Soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Rochayati, S.; Du Laing, G.; Rinklebe, J.; Meissner, R.; Verloo, M. Use of Reactive Phosphate Rocks as Fertilizer on Acid Upland Soils in Indonesia: Accumulation of Cadmium and Zinc in Soils and Shoots of Maize Plants. J. Plant Nutr. Soil Sci. 2011, 174, 186–194. [Google Scholar] [CrossRef]
- Vassilev, A.; Perez-Sanz, A.; Semane, B.; Carleer, R.; Vangronsveld, J. Cadmium Accumulation and Tolerance of Two Salix Genotypes Hydroponically Grown in Presence of Cadmium. J. Plant Nutr. 2005, 28, 2159–2177. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar Enhances the Cadmium Tolerance in Spinach (Spinacia oleracea) through Modification of Cd Uptake and Physiological and Biochemical Attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Hasan, S.A.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium-Induced Changes in the Growth and Carbonic Anhydrase Activity of Chickpea. Turk. J. Biol. 2007, 31, 1–5. [Google Scholar]
- Farooq, M.A.; Ali, S.; Hameed, A.; Bharwana, S.A.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium Stress in Cotton Seedlings: Physiological, Photosynthesis and Oxidative Damages Alleviated by Glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium Toxicity in Maize (Zea mays L.): Consequences on Antioxidative Systems, Reactive Oxygen Species and Cadmium Accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Syed, R.; Kapoor, D.; Bhat, A.A. Heavy Metal Toxicity in Plants: A Review. Plant Arch. 2018, 18, 1229–1238. [Google Scholar]
- Heyno, E.; Klose, C.; Krieger-Liszkay, A. Origin of Cadmium-Induced Reactive Oxygen Species Production: Mitochondrial Electron Transfer versus Plasma Membrane NADPH Oxidase. New Phytol. 2008, 179, 687–699. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling Cadmium Toxicity and Tolerance in Plants: Insight into Regulatory Mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Newton, A.C.; Flavell, A.J.; George, T.S.; Leat, P.; Mullholland, B.; Ramsay, L.; Revoredo-Giha, C.; Russell, J.; Steffenson, B.J.; Swanston, J.S.; et al. Crops That Feed the World 4. Barley: A Resilient Crop? Strengths and Weaknesses in the Context of Food Security. Food Secur. 2011, 3, 141–178. [Google Scholar] [CrossRef]
- Bouhraoua, S.; Ferioun, M.; Nassira, S.; Boussakouran, A.; Akhazzane, M.; Belahcen, D.; Hammani, K.; Louahlia, S. Biomass Partitioning and Physiological Responses of Four Moroccan Barley Varieties Subjected to Salt Stress in a Hydroponic System. J. Plant Biotechnol. 2023, 50, 115–126. [Google Scholar]
- Ferioun, M.; Zouitane, I.; Bouhraoua, S.; Elouattassi, Y.; Belahcen, D.; Errabbani, A.; Louahlia, S.; Sayyed, R.; El Ghachtouli, N. Applying Microbial Biostimulants and Drought-Tolerant Genotypes to Enhance Barley Growth and Yield under Drought Stress. Front. Plant Sci. 2025, 15, 1494987. [Google Scholar]
- Salih, G.; Jilal, A.; Garcia, M.S.; Visioni, A. L’orge Alimentaire En Afrique Du Nord: Etat Des Connaissances et Opportunités de Développement. Afr. Mediterr. Agric. J. Al Awamia 2022, 137, 41–64. [Google Scholar]
- Ferioun, M.; Bouhraoua, S.; Srhiouar, N.; Boussakouran, A.; Belahcen, D.; El Ghachtouli, N.; Sayyed, R.Z.; Louahlia, S. Advanced Multivariate Approaches for Selecting Moroccan Drought-Tolerant Barley (Hordeum vulgare L.) Cultivars. Ecol. Front. 2024, 44, 820–828. [Google Scholar]
- FAO (2016) FAOSTAT. Online Statistical Database—Recherche Google. Available online: https://www.google.com/search?q=FAO+%282016%29+FAOSTAT.+Online+statistical+datab (accessed on 5 February 2025).
- Ferioun, M.; Zouitane, I.; Bouhraoua, S.; Belahcen, D.; Srhiouar, N.; Louahlia, S.; El Ghachtouli, N. PGPR Consortia Promote Soil Quality and Functioning in Barley Rhizosphere under Different Levels of Drought Stress. Ecol. Front. 2024, 45, 444–454. [Google Scholar] [CrossRef]
- Cop 2017 Recherche Google. Available online: https://www.google.com/search?q=cop+2017+Morocco+is+know (accessed on 11 March 2025).
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Valorization of Phosphate Waste Rocks and Sludge from the Moroccan Phosphate Mines: Challenges and Perspectives. Procedia Eng. 2016, 138, 110–118. [Google Scholar] [CrossRef]
- Forster, B.P.; Ellis, R.P.; Moir, J.; Talamè, V.; Sanguineti, M.C.; Tuberosa, R.; This, D.; Teulat-Merah, B.; Ahmed, I.; Mariy, S.A.E.E.; et al. Genotype and Phenotype Associations with Drought Tolerance in Barley Tested in North Africa. Ann. Appl. Biol. 2004, 144, 157–168. [Google Scholar] [CrossRef]
- Nouri, M.; El Rasafi, T.; Haddioui, A. Responses of Two Barley Subspecies to in Vitro-Induced Heavy Metal Stress: Seeds Germination, Seedlings Growth and Cytotoxicity Assay. Agriculture 2019, 65, 107–118. [Google Scholar] [CrossRef]
- ICARDA 2017. Barley Is an Adaptable Crop That Can Thrive in Adverse Conditions, Promoting Typically Constant Yields with Little Labor—Recherche Google. Available online: https://www.google.com/search?q=ICARDA+2017+Barley+is+an+adapta (accessed on 11 March 2025).
- Dawson, I.K.; Russell, J.; Powell, W.; Steffenson, B.; Thomas, W.T.B.; Waugh, R. Barley: A Translational Model for Adaptation to Climate Change. New Phytol. 2015, 206, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Amatriaín, M.; Cuesta-Marcos, A.; Hayes, P.M.; Muehlbauer, G.J. Barley Genetic Variation: Implications for Crop Improvement. Brief. Funct. Genom. Proteom. 2014, 13, 341–350. [Google Scholar] [CrossRef]
- Ryan, J.; Sommer, R. Soil Fertility and Crop Nutrition Research at an International Center in the Mediterranean Region: Achievements and Future Perspective. Arch. Agron. Soil Sci. 2012, 58, 37–41. [Google Scholar] [CrossRef]
- Ayachi, I.; Ghabriche, R.; Zineb, A.B.; Hanana, M.; Abdelly, C.; Ghnaya, T. NaCl Effect on Cd Accumulation and Cell Compartmentalization in Barley. Environ. Sci. Pollut. Res. 2023, 30, 49215–49225. [Google Scholar] [CrossRef]
- Stephania, G.; Macpherson, H.G. Food Barley: Importance, Uses and Local Knowledge. In Barley-Based Food in Southern Morocco; ICARDA: Beriut, Lebanon, 2005; pp. 53–82. [Google Scholar]
- Bouhraoua, S.; Ferioun, M.; Boussakouran, A.; Belahcen, D.; Srhiouar, N.; Hammani, K.; Louahlia, S. Physio-Biochemical Responses and Yield Performance of North African Barley Genotypes Submitted to Moderate and Severe Salinity. Cereal Res. Commun. 2024, 53, 337–353. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Chen, W. Manganese, Zinc, and pH Affect Cadmium Accumulation in Rice Grain under Field Conditions in Southern China. J. Environ. Qual. 2018, 47, 306–311. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Stepien, P.; Johnson, G.N. Sorghum (Sorghum bicolor) Varieties Adopt Strongly Contrasting Strategies in Response to Drought. Physiol. Plant. 2014, 152, 389–401. [Google Scholar] [CrossRef]
- Bates, L.S. Short Communication Rapid Determination of Free Proline for water-stress studies. Plant Soil 1973, 207, 205–207. [Google Scholar]
- Stewart, R.R.C.; Bewley, J.D. Lipid Peroxidation Associated with Accelerated Aging of Soybean Axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Lipkin, H.J. Interference, Mixing, and Angular Correlations in Decays of Boson Resonances. Phys. Rev. 1968, 176, 1709–1714. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, Y.; Dai, Y.; Cui, N.; Anderson, B.; Cheng, S. Biological Mechanisms Associated with Triazophos (TAP) Removal by Horizontal Subsurface Flow Constructed Wetlands (HSFCW). Sci. Total Environ. 2016, 553, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. APX Nakano & Asada 1981.Pdf. Plant Cell Physiol. 2018, 22, 867–880. [Google Scholar]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Ali, S.; Wen, X.; Khan, K.A.; Ghramh, H.A.; Zhang, Z.; Zhang, D. Impact of Cadmium Stress on Growth and Physio-Biochemical Attributes of Eruca Sativa Mill. Plants 2022, 11, 2981. [Google Scholar] [CrossRef]
- Gonçalves, J.F.; Antes, F.G.; Maldaner, J.; Pereira, L.B.; Tabaldi, L.A.; Rauber, R.; Rossato, L.V.; Bisognin, D.A.; Dressler, V.L.; de Moraes Flores, É.M.; et al. Cadmium and Mineral Nutrient Accumulation in Potato Plantlets Grown under Cadmium Stress in Two Different Experimental Culture Conditions. Plant Physiol. Biochem. 2009, 47, 814–821. [Google Scholar] [CrossRef]
- Hasan, S.; Sehar, Z.; Khan, N.A. Gibberellic Acid and Sulfur-Mediated Reversal of Cadmium-Inhibited Photosynthetic Performance in Mungbean (Vigna radiata L.) Involves Nitric Oxide. J. Plant Growth Regul. 2020, 39, 1605–1615. [Google Scholar] [CrossRef]
- Daud, M.K.; Sun, Y.; Dawood, M.; Hayat, Y.; Variath, M.T.; Wu, Y.X.; Raziuddin; Mishkat, U.; Salahuddin; Najeeb, U.; et al. Cadmium-Induced Functional and Ultrastructural Alterations in Roots of Two Transgenic Cotton Cultivars. J. Hazard. Mater. 2009, 161, 463–473. [Google Scholar] [CrossRef]
- An, M.J.; Wang, H.J.; Fan, H.; Ippolito, J.A.; Meng, C.; Yulian, E.; Li, Y.; Wang, K.; Wei, C. Effects of Modifiers on the Growth, Photosynthesis, and Antioxidant Enzymes of Cotton Under Cadmium Toxicity. J. Plant Growth Regul. 2019, 38, 1196–1205. [Google Scholar] [CrossRef]
- Saidi, I.; Ayouni, M.; Dhieb, A.; Chtourou, Y.; Chaïbi, W.; Djebali, W. Oxidative Damages Induced by Short-Term Exposure to Cadmium in Bean Plants: Protective Role of Salicylic Acid. S. Afr. J. Bot. 2013, 85, 32–38. [Google Scholar] [CrossRef]
- Ali, B.; Deng, X.; Hu, X.; Gill, R.A.; Ali, S.; Wang, S.; Zhou, W. Deteriorative Effects of Cadmium Stress on Antioxidant System and Cellular Structure in Germinating Seeds of Brassica napus L. J. Agric. Sci. Technol. 2015, 17, 63–74. [Google Scholar]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) Alleviates Cotton (Gossypium hirsutum L.) from Zinc (Zn) Toxicity Stress by Limiting Zn Uptake and Oxidative Damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at High Dose Perturbs Growth, Photosynthesis and Nitrogen Metabolism While at Low Dose It up Regulates Sulfur Assimilation and Antioxidant Machinery in Garden Cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Shah, A.A.; Khan, W.U.; Yasin, N.A.; Akram, W.; Ahmad, A.; Abbas, M.; Ali, A.; Safdar, M.N. Butanolide Alleviated Cadmium Stress by Improving Plant Growth, Photosynthetic Parameters and Antioxidant Defense System of Brassica Oleracea. Chemosphere 2020, 261, 127728. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Exogenously Supplied Silicon (Si) Improves Cadmium Tolerance in Pepper (Capsicum annuum L.) by up-Regulating the Synthesis of Nitric Oxide and Hydrogen Sulfide. J. Biotechnol. 2020, 316, 35–45. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of Cadmium on Antioxidant Enzyme and Photosynthetic Activities in Leaves of Two Maize Cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossain, M.S.; Fujita, M. Maleic Acid Assisted Improvement of Metal Chelation and Antioxidant Metabolism Confers Chromium Tolerance in Brassica Juncea L. Ecotoxicol. Environ. Saf. 2017, 144, 216–226. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Y.; Wei, S.; Gu, W. Exogenous Hemin Confers Cadmium Tolerance by Decreasing Cadmium Accumulation and Modulating Water Status and Matter Accumulation in Maize Seedlings. Agronomy 2021, 11, 739. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Wang, J.; Zhou, C.; Feng, H.; Mahajan, M.D.; Han, X. Influence and Interaction of Iron and Cadmium on Photosynthesis and Antioxidative Enzymes in Two Rice Cultivars. Chemosphere 2017, 171, 240–247. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Meney, K.; Pantelic, L.; Tomanovic, R. Polynuclear Aromatic Hydrocarbons (PAHs) Mediate Cadmium Toxicity to an Emergent Wetland Species. J. Hazard. Mater. 2011, 189, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ferioun, M.; Bouhraoua, S.; Belahcen, D.; Zouitane, I.; Srhiouar, N.; Louahlia, S.; El Ghachtouli, N. PGPR Consortia Enhance Growth and Yield in Barley Cultivars Subjected to Severe Drought Stress and Subsequent Recovery. Rhizosphere 2024, 31, 100926. [Google Scholar]
- Chu, J.; Zhu, F.; Chen, X.; Liang, H.; Wang, R.; Wang, X.; Huang, X. Effects of Cadmium on Photosynthesis of Schima Superba Young Plant Detected by Chlorophyll Fluorescence. Environ. Sci. Pollut. Res. 2018, 25, 10679–10687. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and Functional Alterations in Photosynthetic Apparatus of Plants under Cadmium Stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.; Shi, Z. Influence of Selenium on Root Morphology and Photosynthetic Characteristics of Winter Wheat under Cadmium Stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Jain, M.; Pal, M.; Gupta, P.; Gadre, R. Effect of Cadmium on Chlorophyll Biosynthesis and Enzymes of Nitrogen Assimilation in Greening Maize Leaf Segments: Role of 2-Oxoglutarate. Indian J. Exp. Biol. 2007, 45, 385–389. [Google Scholar] [PubMed]
- López-Millán, A.F.; Sagardoy, R.; Solanas, M.; Abadía, A.; Abadía, J. Cadmium Toxicity in Tomato (Lycopersicon esculentum) Plants Grown in Hydroponics. Environ. Exp. Bot. 2009, 65, 376–385. [Google Scholar] [CrossRef]
- Khan, K.Y.; Ali, B.; Stoffella, P.J.; Cui, X.; Yang, X.; Guo, Y. Study Amino Acid Contents, Plant Growth Variables and Cell Ultrastructural Changes Induced by Cadmium Stress between Two Contrasting Cadmium Accumulating Cultivars of Brassica rapa ssp. Chinensis L. (Pak choi). Ecotoxicol. Environ. Saf. 2020, 200, 110748. [Google Scholar] [CrossRef]
- Bloomfield, K.J.; Farquhar, G.D.; Lloyd, J. Photosynthesis-Nitrogen Relationships in Tropical Forest Tree Species as Affected by Soil Phosphorus Availability: A Controlled Environment Study. Funct. Plant Biol. 2014, 41, 820–832. [Google Scholar] [CrossRef]
- Liu, L.; Shang, Y.K.; Li, L.; Chen, Y.H.; Qin, Z.Z.; Zhou, L.J.; Yuan, M.; Ding, C.B.; Liu, J.; Huang, Y.; et al. Cadmium Stress in Dongying Wild Soybean Seedlings: Growth, Cd Accumulation, and Photosynthesis. Photosynthetica 2018, 56, 1346–1352. [Google Scholar] [CrossRef]
- Daud, M.K.; Quiling, H.; Lei, M.; Ali, B.; Zhu, S.J. Ultrastructural, Metabolic and Proteomic Changes in Leaves of Upland Cotton in Response to Cadmium Stress. Chemosphere 2015, 120, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in Rice: Transport Mechanisms, Influencing Factors, and Minimizing Measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.K. The effect of copper and cadmium on oxygen consumption of the juvenile common carp, Cyprinus carpio (L.). Mesopotamian J. Mar. Sci. 2011, 26, 25–34. [Google Scholar]
- Zouari, M.; Ben Ahmed, C.; Zorrig, W.; Elloumi, N.; Rabhi, M.; Delmail, D.; Ben Rouina, B.; Labrousse, P.; Ben Abdallah, F. Exogenous Proline Mediates Alleviation of Cadmium Stress by Promoting Photosynthetic Activity, Water Status and Antioxidative Enzymes Activities of Young Date Palm (Phoenix dactylifera L.). Ecotoxicol. Environ. Saf. 2016, 128, 100–108. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Li, Z.; Fan, F.; Liang, Y. Silicon Ameliorates Manganese Toxicity by Regulating Both Physiological Processes and Expression of Genes Associated with Photosynthesis in Rice (Oryza sativa L.). Plant Soil 2015, 397, 289–301. [Google Scholar] [CrossRef]
- Li, X.; Bu, N.; Li, Y.; Ma, L.; Xin, S.; Zhang, L. Growth, Photosynthesis and Antioxidant Responses of Endophyte Infected and Non-Infected Rice under Lead Stress Conditions. J. Hazard. Mater. 2012, 213, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of Cadmium Stress on Growth and Physiological Characteristics of Sassafras Seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Jha, A.B.; Dubey, R.S. Carbohydrate Metabolism in Growing Rice Seedlings under Arsenic Toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and Sulfur Influence Ethylene Formation and Alleviate Cadmium-Induced Oxidative Stress by Improving Proline and Glutathione Production in Wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Hemida, K.A. Modulation of Cadmium Toxicity and Enhancing Cadmium-Tolerance in Wheat Seedlings by Exogenous Application of Polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Cadmium Tolerance in Mustard Cultivars: Dependence on Proline Accumulation and Nitrogen Assimilation. J. Funct. Environ. Bot. 2012, 3, 30. [Google Scholar] [CrossRef]
- Deng, X.; Walker, R.G.; Morris, J.; Davidson, W.S.; Thompson, T.B. Role of Conserved Proline Residues in Human Apolipoprotein A-IV Structure and Function. J. Biol. Chem. 2015, 290, 10689–10702. [Google Scholar] [CrossRef]
- Sharmila, P.; Pardha Saradhi, P. Proline Accumulation in Heavy Metal Stressed Plants: An Adaptive Strategy. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2002; pp. 179–199. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline Accumulation and Its Defensive Role under Diverse Stress Condition in Plants: An Overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Shahid, M. Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 232, ISBN 9783319067452. [Google Scholar]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X. Enhanced Brassinosteroid Signaling Intensity via SlBRI1 Overexpression Negatively Regulates Drought Resistance in a Manner Opposite of That via Exogenous BR Application in Tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef]
- Sairam, R.K.; Chandrasekhar, V.; Srivastava, G.C. Sairam et al 2000.PDF. Biol. Plant. 2001, 44, 89–94. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Bahtiyar, M.; Kucukoduk, M. The Humic Acid-Induced Changes in the Water Status, Chlorophyll Fluorescence and Antioxidant Defense Systems of Wheat Leaves with Cadmium Stress. Ecotoxicol. Environ. Saf. 2018, 155, 66–75. [Google Scholar] [CrossRef]
- Chaâbene, Z.; Hakim, I.R.; Rorat, A.; Elleuch, A.; Mejdoub, H.; Vandenbulcke, F. Copper Toxicity and Date Palm (Phoenix dactylifera) Seedling Tolerance: Monitoring of Related Biomarkers. Environ. Toxicol. Chem. 2018, 37, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Vetukuri, R.R.; Xu, W.; Xu, X. Transcriptomic Responses of Dove Tree (Davidia involucrata Baill.) to Heat Stress at the Seedling Stage. Forests 2019, 10, 656. [Google Scholar] [CrossRef]

| Official Name | Country of Origin | Row Type | Hulled/Hulless |
|---|---|---|---|
| Assiya | Morocco | 6 rows | Hulless |
| Tombari | Tunisia | 6 rows | Hulless |
| Giza 130 | Egypt | 6 rows | Hulless |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhraoua, S.; Ferioun, M.; Boussakouran, A.; Belahcen, D.; Benali, T.; El Hachlafi, N.; Akhazzane, M.; Khabbach, A.; Hammani, K.; Louahlia, S. Physio-Biochemical Responses and Cadmium Partitioning Associated with Stress Tolerance in Hulless Barley Genotypes. Crops 2025, 5, 15. https://doi.org/10.3390/crops5020015
Bouhraoua S, Ferioun M, Boussakouran A, Belahcen D, Benali T, El Hachlafi N, Akhazzane M, Khabbach A, Hammani K, Louahlia S. Physio-Biochemical Responses and Cadmium Partitioning Associated with Stress Tolerance in Hulless Barley Genotypes. Crops. 2025; 5(2):15. https://doi.org/10.3390/crops5020015
Chicago/Turabian StyleBouhraoua, Said, Mohamed Ferioun, Abdelali Boussakouran, Douae Belahcen, Taoufiq Benali, Naoufal El Hachlafi, Mohamed Akhazzane, Abdelmajid Khabbach, Khalil Hammani, and Said Louahlia. 2025. "Physio-Biochemical Responses and Cadmium Partitioning Associated with Stress Tolerance in Hulless Barley Genotypes" Crops 5, no. 2: 15. https://doi.org/10.3390/crops5020015
APA StyleBouhraoua, S., Ferioun, M., Boussakouran, A., Belahcen, D., Benali, T., El Hachlafi, N., Akhazzane, M., Khabbach, A., Hammani, K., & Louahlia, S. (2025). Physio-Biochemical Responses and Cadmium Partitioning Associated with Stress Tolerance in Hulless Barley Genotypes. Crops, 5(2), 15. https://doi.org/10.3390/crops5020015








