Abstract
Ocimum basilicum is an herbaceous plant, rich in essential oils. This research represents a groundbreaking exploration of the cultivation of Ocimum basilicum in Greece, a Mediterranean nation. It emphasizes the impact of biostimulants on various basil varieties, assessing both quantitative aspects and qualitative features. This study was conducted through a field trial at the University of Thessaly’s experimental farm located in the Velestino region. This study examined different testing varieties (V1: Lemon, V2: Siam Queen, V3: Salat, V4: Bascuro, and V5: Genovese), under different biostimulant applications (B1: control, B2: seaweed extracts, amino acids, vitamins, trace elements, polyphenols, antioxidants and mannitol; B3: plant amino acids, glutamic and aspartic acid, vitamins and other nutrients, B4: B1 and B2 combination in a 1:1 ratio). The findings highlight the significant differences in both fresh and dry yields across various basil cultivars, with Lemon basil demonstrating the most substantial yields. Specifically, the Lemon variety attained the highest dry yield, surpassing the lowest-performing cultivar by more than two times. Additionally, this research evaluated the production of essential oil per hectare, emphasizing the relationship between essential oil content and the crop’s dry yield. The results revealed considerable variability among the examined varieties, with the Lemon variety yielding nearly 65 kg ha−1, the highest among them. Biostimulant treatments (B2) led to the greatest total yields of essential oils, while the control treatments yielded the least. The chemical composition of essential oils derived from O. basilicum shows significant variability, often associated with the plants’ nutritional conditions. The application of biostimulants has led to considerable alterations in the volatile profile of sweet basil, supporting this study’s conclusions.
1. Introduction
Basil originates from India and thrives in the tropical and subtropical regions of Africa and Asia [1]. Its cultivation has extended to Greece and throughout the Mediterranean, as well as to California [2,3,4].
It is an herbaceous plant belonging to the Lamiaceae family [5], which includes both annual and perennial species. Linnaeus initially classified the genus Ocimum in 1753, proposing that it encompassed five species, amidst the various types of basil and the challenges associated with their classification [6]. Currently, it is recognized that Ocimum genus comprises over 30 species, with certain varieties also being grown in temperate regions [7].
The Ocimum genus exhibits significant variability in both morphology and chemo-types, primarily due to its propensity for cross-pollination. This variability has resulted in a multitude of interspecific hybrids, subspecies, and varieties, each displaying markedly different essential oil compositions [8]. Consequently, this diversity has created consider-able challenges and confusion regarding naming and classification, as morphological traits and phenotypic characteristics, along with sensory attributes, can intersect in unpredictable manners [9,10,11].
This plant exhibits remarkable versatility, showcasing various phenotypes among its cultivars, which can be distinguished by characteristics such as leaf size, shape, and color, as well as plant height, weight, branching, foliage density, etc. Currently, commercial basil cultivars demonstrate significant diversity in their growth habits, as well as in the colors of their flowers, leaves, and stems [7], along with their aromatic profiles.
Currently, Ocimum is extensively grown not only for its culinary applications but also as a favored ornamental plant. The primary components utilized from this dense, bushy species, which may be categorized as either annual or perennial [12], are its leaves and terminal shoots. In certain cases, the seeds may also be ingested; this distinguishes Ocimum from various other herbs, where the roots, seeds, fruits, and/or flowers are also consumed [13,14].
Basil, known scientifically as Herba basilici, is an herb rich in essential oils [15] and is widely employed in culinary practices [16]. Its applications extend beyond the kitchen, finding utility in the pharmaceutical sector [17], as well as in the production of confections and perfumes [18]. This herb is acknowledged as the most economically important species within its genus [19].
The taste, scent, and overall characteristics of basil, akin to numerous other herbs, are shaped by the environmental conditions during its cultivation [1,20,21] as well as the timing of its harvest [22]. Furthermore, the essential oil’s composition can differ depending on the specific geographical region in which the basil is cultivated [23,24,25,26,27,28]. The notable chemical variability observed in the essential oils of various basil cultivars is also attributed to interspecific hybridization. Finally, cultivation practices influence both the quantity and quality of aromatic and medicinal plants.
In the past ten years, extensive research has been conducted on the application of biostimulants across different crops to evaluate their impact on both yield and quality. Biostimulants represent sustainable approaches to managing abiotic stresses, thereby improving both crop yield and quality [29,30]. In the agricultural sector, these substances are categorized as fertilizers that facilitate plant nutritional processes, bolster resilience against various stresses, and enhance the quality of the final products [31]. Biostimulants are sourced from natural materials, including byproducts from animals and plants, such as hydrolyzed proteins, amino acids, extracts from microalgae and seaweed, humic substances, and microorganisms [32].
Research indicates that biostimulants derived from seaweed and plant extracts positively influence plant growth, especially in terms of root development and branching [33]. Hydrolyzed proteins, along with extracts from seaweed and microalgae, improve plant tolerance to drought and temperature fluctuations. Additionally, betaines and cytokinins are significant compounds linked to the stress-relieving effects found in microalgae and seaweed extracts [29,34,35,36], although the exact mechanisms by which they operate are not yet fully understood [37].
The classification of chemotypes in Ocimum basilicum L. has been conducted not only based on morphological characteristics but also through the analysis of the chemical pro-files of their essential oils [38]. This analysis is performed using gas chromatography (GC), which identifies roughly 1% of the volatile compounds present [39,40].
The global output of basil essential oil is estimated to be between 50 and 100 tons [41]. Reports suggest that the yield of Indian basil essential oil ranges from 132.0 to 162.5 kg per hectare [42]. This essential oil is recognized for its antifungal and antibacterial properties, rendering it a valuable component in the food, fragrance, and pharmaceutical industries [43,44]. The improvement of both the qualitative and quantitative dimensions of essential oil production is an area of considerable commercial interest [45].
Numerous factors contribute to the composition of essential oil in specific basil varieties, including cultivation methods [46,47,48,49], genetic makeup, developmental stages, morphological characteristics, environmental conditions, the extraction technique employed for essential oils, drying methods, and storage practices [8].
There is a growing interest in the cultivation of aromatic and medicinal plants in Greece and the broader Mediterranean region, with basil being a prominent example. This interest is particularly heightened when these crops are produced organically. Consequently, there is a significant focus on exploring the factors that can enhance both the quantity and quality of basil. This study aims to examine the impact of various biostimulants on the yield and essential oil content of five different varieties of Ocimum basilicum. Therefore, a thorough investigation was carried out to evaluate the impact of various biostimulants on multiple basil varieties. This research aimed to analyze both the quantitative aspects, such as biomass and essential oil yields, and the qualitative characteristics, specifically the composition of essential oils, in different Ocimum basilicum varieties.
2. Materials and Methods
2.1. Experimental Design
This study was conducted in the spring–summer growing season of 2023 at the experimental farm of the University of Thessaly, located in the Thessaly plain of Greece (coordinates: 39°30′45″ N, 22°28′03.1″ E, altitude 170 m). This region is noted for its Mediterranean climate, characterized by hot, arid summers and mild, rainy winters, which are conducive to the growth of basil.
The local soil is predominantly clayey, and the cultivation took place on the field (outdoors), allowing for the influence of natural environmental conditions. Throughout the experiment, climatic factors, including temperature and precipitation, were systematically recorded to facilitate a comprehensive analysis of the findings.
A completely randomized two-factor experimental design was utilized, with three replicates (blocks) and 20 plots per replication. The study factors were the five distinct varieties of Ocimum basilicum species (V1: Lemon, V2: Siam Queen, V3: Salat, V4: Bascuro, and V5: Genovese) and the use of different biostimulants (B1: control, B2: seaweed ex-tracts, amino acids, vitamins, trace elements, polyphenols, antioxidants and mannitol; application rate of 1.5 L per hectare per sampling, B3: plant amino acids, glutamic and aspartic acid, vitamins and other nutrients; 1.5 L per hectare per sampling, and B4: Combination of B1 and B2 in a 1:1 ratio; 1.5 L in total per hectare per sampling; the selected biostimulants represent the two most significant categories available in both the international and Greek markets). Each plot comprised three rows, and each one contained four plants (twelve plants per plot). The spacing between the rows was set at 50 cm, while the intra-row distance was maintained at 30 cm. During the harvesting phase, samples were taken from the two central plants within each plot, with all other plants being removed subsequently. Following this, irrigation was administered to facilitate the preparation for the next harvest cycle.
The seedlings were cultivated at the Laboratory of Agriculture and Applied Crop Physiology at the University of Thessaly. On 22 March 2023, seeds were sown in trays de-signed to accommodate 144 seedlings (arranged in a 9 × 16 configuration). After approximately two months, the seedlings were deemed ready for transplantation, which occurred manually on May 16 (Figure 1).

Figure 1.
Seedling production in the lab (upper figures), seedling transplantation (bottom left figure), and drip irrigation system (bottom right figure).
Throughout the duration of the experiment, a drip irrigation system was employed to provide water to the plants. This system utilized self-regulating drippers with a flow rate of 4 L per hour. The irrigation pipes were arranged in two rows corresponding to the plants, with one dripper allocated for each pair of plants (Figure 1). The irrigation schedule was developed utilizing the Class A evaporation basin method [50] in conjunction with the reference evapotranspiration (ETo). This approach was implemented on a weekly basis to effectively represent the real conditions encountered by farmers during irrigation practices, ensuring that the crops received the full 100% of the ETo.
The application of biostimulants occurred throughout the growing season, utilizing a precision battery-operated sprayer. The biostimulants were administered in two separate spray events. The initial application took place at the end of June, prior to the first harvest, while the second application was conducted at the end of July, just before the second harvest.
2.2. Plants Yield
Total yield was assessed at the optimal harvest phase, which coincides with the full flowering period, a stage recognized for its potential to enhance essential oil concentration. To mitigate any marginal variations, samples were collected from the inner rows of each plot, as previously indicated. Two harvests were carried out during the experiment. The initial harvest took place on 2 July, followed by the second on 1 August. The harvested samples were weighed promptly and subsequently transported for additional laboratory analyses. Thereafter, the samples were air-dried at temperatures not exceeding 59 °C to ensure accurate measurements until a stable weight was attained. The dry weight for each plot was used to calculate the dry biomass yield per hectare, and the distillation process was subsequently conducted.
Hand weeding was performed biweekly to control weed competition, and there were no significant occurrences of pests or diseases.
2.3. Essential Oil
Essential oil yield was calculated by multiplying the essential oil concentration by the dry biomass yield of basil per hectare, while the evaluation of essential oil content was performed utilizing a Clevenger-type distillation apparatus (Figure 2).

Figure 2.
Hydro-distillation process using Clevenger-type distillation apparatus.
Specifically, 25 g of dried plant material was subjected to hydro distillation with 500 mL of distilled water for a duration of 1.5 h. This procedure was repeated three times for each sample, and the essential oil yield was determined relative to the dry weight of the plant material.
The essential oils obtained were quantified volumetrically and stored in glass vials at a temperature of 4 °C in a refrigerator until further analysis [51,52]. After storage, the essential oils were analyzed using gas chromatography–mass spectrometry (GC-MS), utilizing a fused silica DB-5 column. The relative content of each compound was calculated as a percentage of the total chromatographic area, with the findings reported as the mean percentage derived from three replicates [51,52,53]. The identification of compounds was accomplished by correlating retention indices (RI) in relation to n-alkanes (C7–C22) in existing studies and by comparing the obtained spectra with those in mass spectrometry databases (NIST 98, Willey; [54]).
2.4. Statistical Analysis and Meteorological Data
The collected data underwent analysis of variance (ANOVA) for all measured and derived variables at specified time intervals, utilizing the statistical software GenStat (7th Edition). The assessment of mean differences for the treatments effects was conducted using the LSD0.05 (Least Significant Difference at 5%) test criteria [55]. This rigorous statistical analysis facilitated an in-depth examination of the data, ensuring that any identified differences among the variables under investigation were statistically significant and not attributable to random variation.
Comprehensive meteorological data, including air temperature, radiation, humidity, wind speed, precipitation, and class-A pan evaporation rate, were collected on an hourly basis from an automatic weather station located next to the experimental field.
The Mediterranean climate prevalent in the study region is distinguished by its warm summers and temperate winters. Throughout the growth period of basil, the average air temperature fluctuated between 16.8 and 29.7 °C, with a mean temperature of 24.5 °C. The extremes of air temperature were recorded from the time of transplanting until the initial sampling, yielding an average of 23.1 °C (Figure 3). In contrast, during the re-emergence of basil and the second sampling, the average air temperature increased significantly to 28.3 °C (refer to Figure 3), representing an elevation of nearly 5 degrees Celsius.
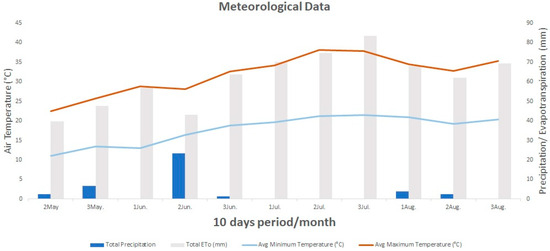
Figure 3.
Mean minimum and maximum air temperature (°C), total precipitation and total ETo (mm) from May (basil transplanting) to (second basil sampling) August 2023 (10-day intervals).
Precipitation serves as a critical environmental factor that significantly affects both crop growth and productivity. Throughout the experimental phase, the area received a total of 38.5 mm of precipitation, which coincided with the basil transplanting and the second sampling. Specifically, during the initial sampling period from 16 May to 2 July, precipitation was 34.2 mm, while for the interval between the first and second sampling was recorded only 3.8 mm of precipitation. The above data highlight the occurrence of drought conditions during the summer months (Figure 3).
3. Results
3.1. Basil Fresh and Dry Yield
The information in Table 1 indicates that there are statistically significant differences in both fresh and dry yields across the varieties examined. The variety labeled V1 (Lemon basil) produced the highest yield, while V5 (Genovese basil) had the lowest. Specifically, V1 recorded a total fresh yield of 29,499 kg per hectare, corresponding to a dry yield of 4509 kg ha−1. In comparison, V5 showed significantly lower yields, with fresh and dry yields of 16,189 kg ha−1 and 1942 kg ha−1, respectively, as detailed in Table 1. Although the yields of varieties V2, V3, and V4 did not show statistically significant differences among themselves, they were distinct from those of V1 and V5 (Table 1).

Table 1.
The effect of various varieties (V1: Lemon, V2: Siam Queen, V3: Salat, V4: Bascuro, and V5: Genovese) and biostimulants (B1: control, B2: seaweed extracts, amino acids, vitamins, trace elements, polyphenols, antioxidants and mannitol, B3: plant amino acids, glutamic and aspartic acid, vitamins and other nutrients, and B4: is a combination of B1 and B2 in a 1:1 ratio), along with their interactions, on fresh and dry yield (kg ha−1) was assessed during the first and second sampling periods, as well as in the overall summary of the samplings.
Moreover, in the case of the biostimulants factor, statistically significant differences were identified. All treatments that included biostimulants showed higher yields when compared to control. Notably, the treatment B2, which comprised seaweed extracts, amino acids, vitamins, trace elements, polyphenols, antioxidants, and mannitol, resulted in the highest total fresh and dry biomass yields (22,764 kg ha−1 and 2917 kg ha−1, respectively; Table 1). In contrast, the control treatments yielded the lowest results across all varieties, with an average fresh biomass of 18,871 kg ha−1 and an average dry biomass of approximately 2433 kg ha−1 (Table 1).
Since no statistically significant differences were observed regarding interactions, the data are not presented. It is important to highlight that the variety V1 exhibited greater fresh and dry biomass yields when treated with the biostimulant B2, reaching up to 34,737 kg ha−1 and 5149 kg ha−1, respectively. Additionally, the variety V2 recorded the lowest fresh yield in the control treatment, measuring 14,329 kg ha−1.
3.2. Basil Essential Oil Yield
The data presented in Figure 4, Figure 5 and Figure 6 indicate that the yield of essential oil exhibited statistically considerable variation across the various tested varieties (Figure 4) of Ocimum basilicum. Additionally, this yield was affected by the application of biostimulant treatments (Figure 5) and the interactions (Figure 6) between these treatments and the plant varieties.
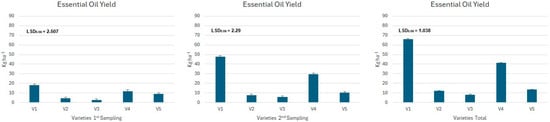
Figure 4.
Mean essential oil yield (kg ha−1) for the different basil varieties (V1: Lemon, V2: Siam Queen, V3: Salat, V4: Bascuro, and V5: Genovese) where significant differences were found.
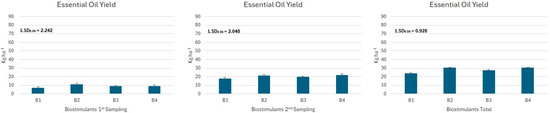
Figure 5.
Mean essential oil yield (kg ha−1) for the different biostimulants treatments (B1: control, B2: seaweed extracts, amino acids, vitamins, trace elements, polyphenols, antioxidants and mannitol, B3: plant amino acids, glutamic and aspartic acid, vitamins and other nutrients, and B4: is a combination of B1 and B2 in a 1:1 ratio) where significant differences were found.
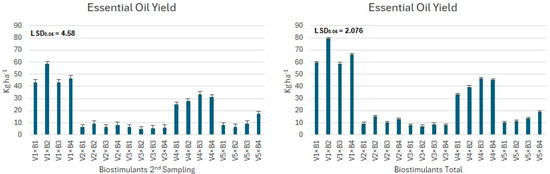
Figure 6.
Mean essential oil yield (kg ha−1) for the interactions of the tested factors (varieties and biostimulants treatments), where significant differences were found.
All tested varieties showed statistically significant variations in total essential oil yield. The V1 variety (Lemon) produced the highest yield, nearing 65 kg ha−1, while the V3 variety (Salat) had the lowest yield, amounting to just 8 kg ha−1 (Figure 4).
The use of biostimulants revealed that the treatments with B2 and B4 produced the highest total essential oil yield among all varieties, each achieving about 30.5 kg ha−1. The B3 treatment showed moderate performance, with an average yield of approximately 28 kg ha−1, while the control group recorded the lowest yield of around 24 kg ha−1, regardless of the variety (Figure 5).
In the case of interactions, the Lemon variety (V1) treated with biostimulant B2, demonstrated a statistically significant increase in essential oil yield, achieving an impressive yield of nearly 80 kg ha−1. For the V1 (Lemon variety), all treatments, where biostimulants were used, resulted in the highest essential oil production. Notably, in the case of the Lemon variety, even the control treatments showed statistically significant superiority over other combinations within the examined varieties. This observation has been attributed to the distinct advantages of the Lemon variety compared to others. Conversely, the V3 (Salat) variety yielded the lowest essential oil per hectare, with no statistically significant differences noted among the treatments for this specific variety (Figure 6).
3.3. Basil Essential Oil Composition
The analysis of the essential oil obtained from the experimental treatments, performed using gas chromatography coupled with mass spectrometry (GC–MS), not only revealed the primary constituents of basil but also identified a total of 42 unique compounds. Of these, 28 were detected in concentrations greater than 1% across the various tested varieties and treatments. The compounds identified include 1,8-cineole, cis-Sabinene hydrate, cis-Linalool oxide, trans-Linalool oxide, Terpinolene, Linalool, Camphor, Terpinen-4-ol, α-Terpineol, Methyl chavicol, Nerol, Neral, Geranial, Bornyl acetate, Eugenol, Neryl acetate, β-Elemene, Methyl eugenol, Ε-Caryophyllene, α-trans-Bergamotene, Germacrene D, α-Bulnesene, γ-Cadinene, trans-α-Bisabolene, Spathulenol, Caryophyllene oxide, 1,10-di-epicubenol, and epi-α-Cadinol (see Table 2).

Table 2.
Essential oil compounds found to the tested treatments (average values for tested varieties V1: Lemon, V2: Siam Queen, V3: Salat, V4: Bascuro, and V5: Genovese, and treatments B1: control, B2: seaweed extracts, amino acids, vitamins, trace elements, polyphenols, antioxidants and mannitol, B3: plant amino acids, glutamic and aspartic acid, vitamins and other nutrients, and B4: a combination of B1 and B2 in a 1:1 ratio).
The anticipated variation in the components and their respective contents among the studied varieties is illustrated in Table 2. As is presented, the application of various biostimulants resulted, in most cases, in a differentiation of components within the same variety. This differentiation is particularly significant, as it often results in a 50% reduction or tenfold increase, highlighting a crucial finding that may influence the utilization of the resulting product.
The key compounds that demonstrated the highest concentrations, as shown in Table 2, are Methyl chavicol (up to 81.7% in the V2B1 treatment), linalool (up to 54.85% in the V5B4 treatment), 1,8-Cineole (21.1% in the V4B4 treatment), Geranial (18.5% in the V1B1 treatment), Eugenol (13.7% in the V4B1 treatment), α-trans-Bergamotene (12.8% in the V3B1 treatment), and γ-Cadinene (also 12.8% in the V3B1 treatment). Table 2 highlights notable differences in the concentrations of these individual components across the various treatments compared to the control group.
The essential oils show considerable differences among the tested varieties concerning their primary compounds, especially regarding Methyl chavicol. This particular compound is found in varieties V2 and V3 but is absent in the other varieties. Variety V2, in particular, exhibited the highest concentration of Methyl chavicol at 81.7%, while it also had a very low concentration of Linalool at 0.5%. In contrast, the other varieties displayed significantly higher levels of Linalool, which ranged from 32.95% to 55.45%. Additionally, Geranial was exclusively identified in variety 1, regardless of the biostimulant treatment applied.
Additionally, as shown in Table 2, the use of biostimulant 2 may lead to a decrease in specific compounds in variety V1, notably Neral, Geranial, and α-trans-Bergamotene, which are present in lower amounts. On the other hand, this biostimulant can also encourage the production of compounds such as Camphor in variety V1, and lead to an increase to the concentrations of Nerol, Neryl acetate, and E-Caryophyllene within the same variety. Ultimately, the effects of the other biostimulants produce similar results, as outlined in Table 2.
4. Discussion
The dry yield observed in earlier investigations examining various sweet basil genotypes varied between 600 and 6200 kg ha−1 [38,39,56,57,58]. This range in variation can be attributed to a combination of genetic, agronomic, and environmental factors, and it aligns with the yield variations documented in the current study.
Numerous studies have indicated that an increase in nitrogen availability significantly enhances plant biomass [21,59,60,61,62,63]. This observation is consistent with the findings of the current research, which demonstrates that the application of biostimulants, which promote better nutrient uptake from the soil, resulted in an increase in basil weight, regardless of the variety. Additionally, it has been noted that a novel agronomic approach involves the use of biostimulants, which can reduce fertilizer application [64] and enhance water productivity [65]. Lastly, Consentino et al. [66] found that the application of biostimulants led to an increase in sweet basil yield, corroborating the results of this study.
The essential oil yield serves as the primary metric for evaluating the quality of aromatic and medicinal plants. Research indicates that the application of various biostimulants significantly affects essential oil yield, regardless of the specific plant variety. Notable statistical differences were identified among the tested varieties, the biostimulants utilized, and their interactions (see Figure 4).
Furthermore, an increase in essential oil yield was observed between the initial and subsequent harvests. This improvement during the summer months can be linked to the influence of higher air temperatures and UVB radiation, a trend that has been documented in several aromatic species within the Lamiaceae family [67,68].
It is important to highlight that the essential oil yields recorded in this investigation align with the typical ranges reported in previous studies, which have shown that the essential oil yield for various sweet basil genotypes spans from 3.1 kg ha−1 to 58.8 kg ha−1 [38,39,58,69,70]. This range includes the essential oil yields observed in the present study, with the exception of the exceptionally high yield noted for the Lemon variety (V1) treated with biostimulant B2, as well as for the Lemon variety overall.
The analysis of the essential oil revealed a total of 42 unique compounds (see Table 2). Of these, 28 compounds were detected in concentrations greater than 1% across the various tested varieties and treatments. The compounds identified include 1,8-cineole, cis-Sabinene hydrate, cis-Linalool oxide, trans-Linalool oxide, Terpinolene, Linalool, Camphor, Terpinen-4-ol, α-Terpineol, Methyl chavicol, Nerol, Neral, Geranial, Bornyl acetate, Eugenol, Neryl acetate, β-Elemene, Methyl eugenol, Ε-Caryophyllene, α-trans-Bergamotene, Germacrene D, α-Bulnesene, γ-Cadinene, trans-α-Bisabolene, Spathulenol, Caryophyllene oxide, 1,10-di-epicubenol, and epi-α-Cadinol (refer to Table 2).
Previous research has examined the chemical composition of essential oils derived from O. basilicum, revealing considerable variability [71,72]. This variability in the qualitative characteristics of the plant’s aromatic profile is often attributed to the effects of plant nutrition [73]. The current study corroborates these observations, highlighting differences among the tested varieties and the various applications of biostimulants. Furthermore, it has been documented that distinct genotypes of O. basilicum yield varying levels of specific compounds, including linalool [74,75,76,77,78], and methyl chavicol [79], which aligns with the results of the present study.
Additionally, it has been noted that biostimulants have a significant impact on the overall aromatic profile of basil plants. Specifically, the application of biostimulants containing seaweed extract resulted in an increased linalool content when compared to control plants, while simultaneously decreasing the levels of trans-2-hexanal, eucalyptol, and α-bergamotene [66]. Given that mineral availability influences the concentration of volatile oils, as indicated by Aktsoglou et al. [80], and considering that seaweed and plant extracts can significantly alter mineral uptake, the use of biostimulants has led to notable changes in the volatile profile of sweet basil. These findings are consistent with the results of the current investigation.
Linalool, an oxygenated monoterpene, is identified as the key compound contributing to the fruity-flowery flavor profile [81]. The biosynthesis of linalool is facilitated by enzymes, whose activities are notably influenced by environmental factors. In sweet basil, this biosynthesis may be affected by the interaction between genotype and growing conditions [82]. Research by Simon et al. [83] and Khalid [84] indicates that linalool concentration in O. basilicum plants increases under moderate drought stress. Additionally, Phippen and Simon [85] observed that one variety grown under controlled greenhouse conditions exhibited a 28% increase in linalool content alongside a 70% re-duction in methyl chavicol.
Previous studies have identified eugenol as the second predominant component [86,87], with its concentration varying between 0 and 13.7% in this investigation. Eugenol is recognized for its antioxidant properties and its contribution to a spicy flavor [88,89].
Lastly, earlier research has documented that the essential oil of O. basilicum contains significant levels of methyl chavicol, ranging from 45% to 86% [90,91,92,93], which aligns with the results observed in the current study for the V1 (Lemon variety).
5. Conclusions
The research indicated that different basil cultivars produced varying levels of yield, with Lemon and Genovese basil varieties achieving the highest and lowest output, respectively. The application of biostimulants, such as seaweed extracts and vitamins, contributed to enhanced yields. Specifically, the V1 variety (Lemon) exhibited significantly higher fresh and dry yields when treated with the B2 biostimulant, leading to a 19% increase in total dry weight. The differences in yield were linked to genetic, agronomic, and environmental influences.
Additionally, this study evaluated the production of essential oils per hectare in Ocimum basilicum, highlighting considerable differences among the various cultivars. The Lemon variety produced the highest yield, while biostimulant treatments B2 and B4 resulted in the greatest overall essential oil yields.
Moreover, it was found that biostimulants significantly impact the yield of essential oils, regardless of plant variety, which may be attributed to elevated air temperatures and enhanced UVB radiation exposure.
This research identified 42 essential oils derived from O. basilicum, with 28 of these present in concentrations greater than 1% across different varieties and treatments. The application of biostimulants notably transformed the aromatic profile of basil, as seaweed extract was found to enhance linalool levels while decreasing the concentrations of trans-2-hexanal, eucalyptol, and α-bergamotene. This indicates that biostimulants play a significant role in altering the volatile profile of sweet basil.
The findings discussed in this study originate from a field study focused on the growth of Ocimum basilicum in a Mediterranean climate. Further investigation is necessary, especially concerning the use of a wider variety of biostimulants at different concentrations. Additionally, it is crucial to examine the effects of irrigation and other factors, such as diverse microenvironments, which may affect both the crop yield and the quality characteristics, including the essential oil profile of Ocimum basilicum.
Author Contributions
Conceptualization, K.D.G., E.W.-K. and N.G.D.; data curation, K.A., A.-Z.B., P.-K.M., K.D.G., E.Z. and D.B.; formal analysis, K.D.G. and D.B.; investigation, K.A., A.-Z.B., P.-K.M., K.D.G., E.W.-K. and N.G.D.; methodology, K.D.G. and N.G.D.; resources, K.A., A.-Z.B., P.-K.M., E.Z. and E.W.-K.; writing—original draft preparation, K.D.G.; writing—review and editing, K.D.G. and N.G.D.; supervision, K.D.G. and N.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klimánková, E.; Holadová, K.; Hajšlová, J.; Cájka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.R.; Vila, R.; Tomi, F.; Canigueral, S.; Casanova, J.; Proença da Cunha, A.; Adzet, T. Composition of the essential oils of Ocimum canum, O. gratissimum and O. minimum. Planta Med. 1999, 65, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Sanda, K.; Koba, K.; Nambo, P.; Gaset, A. Chemical investigation of Ocimum species growing in Togo. Flavour Fragr. J. 1998, 13, 226–232. [Google Scholar] [CrossRef]
- Heath, H.B. Source Book of Flavour. Avi Publishers, Westport. Hemphill, I., Cobiac, L., 2006. The historical and cultural use of herbs and spices. Med. J. Aust. 1981, 185, S5. [Google Scholar]
- Grayer, R.G.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemtypes in basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Bast, F.; Rani, P.; Meena, D. Chloroplast DNA phylogeography of holy basil (Ocimum tenuiflorum) in Indian subcontinent. Sci. World J. 2014, 2014, 847482. [Google Scholar] [CrossRef]
- Lal, R.K.; Gupta, P.; Chanotiya, C.S.; Sarkar, S. Traditional plant breeding in Ocimum. In The Ocimum Genome, Compendium of Plant Genomes; Shasany, A.K., Kole, C., Eds.; Springer Nature AG: Cham, Switzerland, 2018; pp. 89–98. [Google Scholar] [CrossRef]
- Bernhardt, B.; Szabo, K.; Bernath, J. Sources of variability in essential oil composition of Ocimum americanum and Ocimum tenuiflorum. Acta Aliment. 2015, 44, 111–118. [Google Scholar] [CrossRef]
- Paton, A.J.; Springate, D.; Suddee, S.; Otieno, D.; Grayer, R.J.; Harley, M.M.; Willis, F.; Simmonds, M.S.J.; Powell, M.P.; Savolainen, V. Phylogeny and evolution of basils and allies (Ocimeae, Labiatae) based on three plastid DNA regions. Mol. Phylogenet. Evol. 2004, 31, 277–299. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem. 1996, 44, 3926–3929. [Google Scholar] [CrossRef]
- Paton, A.; Putievsky, E. Taxonomic problems and cytotaxonomic relationships between varieties of Ocimum basilicum and related species (Labiatae). Kew Bull. 1996, 5, 509–524. [Google Scholar] [CrossRef]
- Darrah, H. Investigations of the cultivars of basils (Ocimum). Econ. Bot. 1974, 28, 63–67. Available online: https://www.jstor.org/stable/4253469 (accessed on 1 January 2025). [CrossRef]
- Spence, C. Coriander: A most divisive herb? Int. J. Gastron. Food Sci. 2023, 33, 100779. [Google Scholar]
- Spence, C. Lovage: A neglected culinary herb? Int. J. Gastron. Food Sci. 2023, 33, 100764. [Google Scholar]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Ozcan, M.M. Comparative essential oil composition of flowers, leaves and stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 2008, 110, 501–503. [Google Scholar] [PubMed]
- Ahmed, E.A.; Hassan, E.A.; Tobgy, K.M.; Ramadan, E.M. Evaluation of rhizobacteria of some medicinal plants for plant growth promotion and biological control. Ann. Agric. Sci. 2014, 59, 273–280. [Google Scholar]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar]
- Bravo, E.; Amrani, S.; Aziz, M.; Harnafi, H.; Napolitano, M. Ocimum basilicum ethanolic extract decreases cholesterol synthesis and lipid accumulation in human macrophages. Fitoterapia 2008, 79, 515–523. [Google Scholar] [PubMed]
- Wang, Z.F.; Chen, P.; Yu, L.L.; Harrington, P.D. Authentication of organically and conventionally grown basils by gas chromatography/mass spectrometry chemical profiles. Anal. Chem. 2013, 85, 2945–2953. [Google Scholar]
- Kandil, M.A.M.; Khatab, M.E.; Ahmed, S.S.; Schnug, E. Herbal and essential oil yield of Genovese basil (Ocimum basilicum L.) grown with mineral and organic fertilizer sources in Egypt. J. Kulturpflanzen. 2009, 61, 443–449. [Google Scholar]
- Miele, M.; Dondero, R.; Ciarallo, G.; Mazzei, M. Methyl eugenol in Ocimum basilicum L. Cv. Genovese gigante. J. Agric. Food Chem. 2001, 49, 517–521. [Google Scholar]
- Woliso, W.G.; Abuwey, D.; Fikadu, D.; Bansa, A.; Alemu, A.; Melka, B.; Mokonin, M. Performance of Ethiopian sweet basil (Ocimum bacilicum L) genotypes for agronomic and chemical traits in Ethiopia. Adv. Crop Sci. Technol. 2022, 10, 527. [Google Scholar] [CrossRef]
- Gebrehiwot, H.; Bachetti, R.K.; Dekebo, A. Chemical composition and antimicrobial activities of leaves of sweet basil (Ocimum basilicum L.) herb. Int. J. Basic Clin. Pharmacol. 2015, 4, 869–875. [Google Scholar] [CrossRef]
- Telci, I.; Bayram, E.; Yilmaz, G.; Avci, B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem. Syst. Ecol. 2006, 34, 489–497. [Google Scholar] [CrossRef]
- Vina, A.; Murillo, E. Essential oil composition from twelve varieties of basil (Ocimum spp.) grown in Colombia. J. Braz. Chem. Soc. 2003, 14, 744–749. [Google Scholar]
- Ozcan, M.; Chalchat, J.C. Essential oil composition of Ocimum basilicum L. and Ocimum minimum L. in Turkey. Czech J. Food Sci. 2002, 20, 223–228. [Google Scholar]
- Keita, S.M.; Vincent, C.; Schmit, J.P.; Belanger, A. Essential oil composition of Ocimum basilicum L., O. gratissimum L and O. suave L in the Republic of Guinea. Flavour Fragr. J. 2000, 15, 339–341. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A New Trend towards Solving an Old Problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant Applications in Low Input Horticultural Cultivation Systems. Italus Hortus 2018, 25, 27–36. [Google Scholar]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021, 16, 149–171. [Google Scholar]
- La Torre, A.; Battaglia, V.; Caradonia, F. An Overview of the Current Plant Biostimulant Legislations in Different European Member States: Plant Biostimulants. J. Sci. Food Agric. 2016, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Wise, K.; Gill, H.; Selby-Pham, J. Willow Bark Extract and the Biostimulant Complex Root Nectar Increase Propagation Efficiency in Chrysanthemum and Lavender Cuttings. Sci. Hortic. 2020, 263, 109108. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Mian, G.; Cantone, P.; Golinelli, F. First Evidence of the Effect of a New Biostimulant Made by Fabaceae Tissue on Ripening Dynamics and Must Technological Main Parameters in Vitis vinifera ‘Ribolla Gialla’. Acta Hortic. 2022, 1333, 317–322. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar]
- Κhan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Bowes, K.M.; Zheljazkov, V.D. Factors affecting yields and essential oil quality of Ocimum sanctum L. and Ocimum basilicum L. cultivars. J. Am. Soc. Hortic. Sci. 2004, 129, 789–794. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2007, 56, 241–245. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil Ocimum basilicum L. and thyme leaves Thymus vulgaris L. and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Singh, A.K.; Kalra, A.; Yadav, A.; Patra, D.D. Enhancing productivity of Indian basil (Ocimum basilicum L.) through harvest management under rainfed conditions of subtropical north Indian plains. Ind. Crops Prod. 2010, 32, 601–606. [Google Scholar] [CrossRef]
- Varban, D.L.; Duda, M.M.; Varban, R. The study of the Ocimum basilicum L. species cultivated in organic system. Pro Environ. 2010, 3, 284–288. [Google Scholar]
- Wannissorn, B.; Jarikasem, S.; Siriwangchai, T.; Thubthimthed, S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 2005, 76, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, K.; Platis, I.; Gougoulias, N.; Wogiatzi, E. Influence of Trichoderma harzianum and Glomus mycorrhiza on biomass and essential oil yield of organic Ocimum basilicum cultivation. Agric. For. 2020, 66, 139–154. [Google Scholar] [CrossRef]
- Kandeel, A.M.; Naglaa, S.A.T.; Sadek, A.A. Effect of biofertilizers on the growth, volatile oil yield and chemical composition of Ocimum basilicum L. plant. Ann. Agric. Sci. Cairo 2002, 1, 351–371. [Google Scholar]
- Rao, B.R.R. Biomass and essential oil yields of rainfed palmarosa (Cymbopogon martinii (Roxb) wats var. motia Burk) supplied with different levels of organic manure and fertilizer nitrogen in semi-arid tropical climate. Ind. Crop Prod. 2001, 14, 171–178. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- FAO. The International Support Programme for Irrigation Water Management Land and Water Development Division; FAO Via delle Terme di Caracalla: Rome, Italy, 1986; pp. 1–74. [Google Scholar]
- Tegou, A.; Giannoulis, K.D.; Zournatzis, E.; Papadopoulos, S.; Bartzialis, D.; Danalatos, N.G.; Wogiatzi-Kamvoukou, E. Assessing the Impact of Irrigation and Biostimulants on the Yield and Quality Characteristics of Two Different St. John’s Wort Cultivars in Their Second Growing Season. Plants 2024, 13, 3573. [Google Scholar] [CrossRef]
- Tsivelika, N.; Sarrou, E.; Gusheva, K.; Pankou, C.; Koutsos, T.; Chatzopoulou, P.; Mavromatis, A. Phenotypic variation of wild Chamomile (Matricaria chamomilla L.) populations and their evaluation for medicinally important essential oil. Biochem. Syst. Ecol. 2018, 80, 21–28. [Google Scholar] [CrossRef]
- Sarrou, E.; Tsivelika, N.; Chatzopoulou, P.; Tsakalidis, G.; Menexes, G.; Mavromatis, A. Conventional breeding of Greek oregano (Origanum vulgare ssp. Hirtum) and development of improved cultivars for yield potential and essential oil quality. Euphytica 2017, 213, 104. [Google Scholar]
- Adams, R.P. Identification of Volatile Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Co.: Carol Stream, IL, USA, 1995. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics. A Biometrical Approach, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1982; p. 633. [Google Scholar]
- Blank, A.F.; Rosa, Y.R.S.; Carvalho Filho, J.L.S.d.; Santos, C.A.d.; Arrigoni-Blank, M.d.F.; Niculau, E.d.S.; Alves, P.B. A diallel study of yield components and essential oil constituents in basil (Ocimum basilicum L.). Ind. Crops Prod. 2012, 38, 93–98. [Google Scholar]
- Zheljazkov, V.D.; Cantrell, C.L.; Evans, W.B.; Ebelhar, M.W.; Coker, C. Yield and composition of Ocimum basilicum L. and Ocimum sanctum L. grown at four locations. HortScience 2008, 43, 737–741. [Google Scholar]
- Anwar, M.; Patra, D.D.; Chand, S.; Kumar, A.; Naqvi, A.A.; Khanuja, S.P.S. Effect of organic manures and inorganic fertilizer on growth, herb and oil yield, nutrient accumulation, and oil quality of French basil. Commun. Soil Sci. Plant Anal. 2005, 36, 1737–1746. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Kamvoukou, C.A.; Gougoulias, N.; Wogiatzi, E. Irrigation and nitrogen application affect Greek oregano (Origanum vulgare ssp. hirtum) dry biomass, essential oil yield and composition. Ind. Crops Prod. 2020, 150, 112392. [Google Scholar]
- Smitha, G.R.; Basak, B.B.; Thondaiman, V.; Saha, A. Nutrient Management through Organics, Bio-Fertilizers and Crop Residues Improves Growth, Yield and Quality of Sacred Basil (Ocimum sanctum Linn). Ind. Crop. Prod. 2019, 128, 599–606. [Google Scholar]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Sifola, M.I.; Barbieri, G. Growth, yield and essential oil content of three cultivars of basil grown under different levels of nitrogen in the field. Sci. Hortic. 2006, 108, 408–413. [Google Scholar]
- Ram, M.; Ram, D.; Roy, S.K. Influence of an organic mulching on fertilizer nitrogen use efficiency and herb and essential oil yields in geranium (Pelargonium graveolens). Biores. Technol. 2003, 87, 273–278. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant Application under Different Nitrogen Fertilization Levels: Assessment of Yield, Leaf Quality, and Nitrogen Metabolism of Tunnel-Grown Lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Ntatsi, G.; La Bella, S.; Baldassano, S.; Rouphael, Y. Stand-Alone or Combinatorial Effects of Grafting and Microbial and Non-Microbial Derived Compounds on Vigour, Yield and Nutritive and Functional Quality of Greenhouse Eggplant. Plants 2022, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- Consentino, B.B.; Vultaggio, L.; Sabatino, L.; Ntatsi, G.; Rouphael, Y.; Bondì, C.; De Pasquale, C.; Guarino, V.; Iacuzzi, N.; Capodici, G. Combined effects of biostimulants, N level and drought stress on yield, quality and physiology of greenhouse-grown basil. Plant Stress 2023, 10, 100268. [Google Scholar] [CrossRef]
- Ioannidis, D.; Bonner, L.; Johnson, C.B. UN-B is required for normal development of oil glands in Ocimum basilicum L. (sweet basil). Ann. Bot. 2002, 90, 453–460. [Google Scholar] [CrossRef]
- Fahlen, A.; Welander, M.; Wennersten, R. Effects of light-temperature regimes on plant growth and essential oil yield of selected aromatic plants. J. Sci. Food Agric. 1997, 73, 111–119. [Google Scholar] [CrossRef]
- Wetzeil, S.B.; Kruger, H.; Hammer, K.; Bachmann, K. Investigations on morphological and molecular variability of Ocimum L. species. J. Herbs Spices Med. Plants 2002, 9, 8183–8187. [Google Scholar]
- Pino, J.A.; Roncal, E.; Rosado, A.; Goire, I. The essential oil of Ocimum basilicum L. from Cuba. J. Essent. Oil Res. 1994, 6, 89–90. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Antimicrobial activity of basil (Ocimum basilicum) oil against salmonella enteritidis in vitro and in food. Biosci. Biotechnol. Biochem. 2010, 74, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Farrag, E.S. Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Food/Nahrung 2003, 47, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Onofrei, V.; Benchennouf, A.; Jancheva, M.; Loupassaki, S.; Ouaret, W.; Burducea, M.; Lobiuc, A.; Teliban, G.C.; Robu, T. Ecological foliar fertilization effects on essential oil composition of sweet basil (Ocimum basilicum L.) cultivated in a field system. Sci. Hortic. 2018, 239, 104–113. [Google Scholar] [CrossRef]
- Rao, B.R.R.; Kotharia, S.K.; Rajput, D.K.; Patel, R.P.; Darokar, M.P. Chemical and biological diversity in fourteen selections of four Ocimum species. Nat. Prod. Commun. 2011, 6, 1705–1710. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar]
- Pozzatti, P.; Scheid, L.A.; Spader, T.B.; Atayde, M.L.; Santurio, J.M.; Alves, S.H. In vitro activity of essential oils extracted from plants used as spices against fluconazole resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef]
- Nardoni, S.; Giovanelli, S.; Pistelli, L.; Mugnaini, L.; Profili, G.; Pisseri, F.; Mancianti, F. In vitro activity of twenty commercially available, plant-derived essential oils against selected dermatophyte species. Nat. Prod. Commun. 2015, 10, 1473–1478. [Google Scholar]
- Cardoso, N.N.R.; Alviano, C.S.; Blank, A.F.; Romanos, M.T.V.; Fonseca, B.B.; Rozental, S.; Rodrigues, I.A.; Alviano, D.S. Synergism effect of the essential oil from Ocimum basilicum var. Maria Bonita and its major components with fluconazole and its influence on ergosterol biosynthesis. Evid. Based. Complement. Alternat. Med. 2016, 2016, 5647182. [Google Scholar] [PubMed]
- Opalchenova, G.; Obreshkova, D. Comparative studies on the activity of basil—An essential oil from Ocimum basilicum L.—Against multidrug resistant clinical isolates of the genera Staphylococcus, Enterococcus and Pseudomonas by using different test methods. J. Microbiol. Methods 2003, 54, 105–110. [Google Scholar] [PubMed]
- Aktsoglou, D.C.; Kasampalis, D.S.; Sarrou, E.; Tsouvaltzis, P.; Chatzopoulou, P.; Martens, S.; Siomos, A.S. Protein Hydrolysates Supplement in the Nutrient Solution of Soilless Grown Fresh Peppermint and Spearmint as a Tool for Improving Product Quality. Agronomy 2021, 11, 317. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Volatile ester-synthesising capacity throughout on-tree maturation of ‘Golden Reinders’ apples. Sci. Hortic. 2011, 131, 6–14. [Google Scholar] [CrossRef]
- Pinto, J.; Blank, A.; Nogueira, P.C.; Arrigoni-Blank, M.d.F.; Andrade, T.; Sampaio, T.S.; Pereira, K. Chemical characterization of the essential oil from leaves of basil genotypes cultivated in different seasons. Bol. Latinoam. Caribe Plantas Med. Aromat. 2019, 18, 58–70. [Google Scholar]
- Simon, J.E.; Reiss-Buhenheinra, D.; Joly, R.J.; Charles, D.J. Water stress induced alterations in essential oil content and composition of sweet basil. J. Essen. Oil Res. 1992, 4, 71–75. [Google Scholar] [CrossRef]
- Khalid, K.A. Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp.). Int. Agrophys. 2006, 20, 289–296. [Google Scholar]
- Phippen, W.B.; Simon, J.E. Anthocyanin inheritance and instability in purple basil (Ocimum basilicum L.). J. Hered. 2000, 91, 289–296. [Google Scholar] [PubMed]
- Smitha, G.R.; Tripathy, V. Seasonal variation in the essential oils extracted from leaves and inflorescence of different Ocimum species grown in Western plains of India. Ind. Crop Prod. 2016, 94, 52–64. [Google Scholar]
- Saran, P.L.; Tripathy, V.; Saha, A.; Kalariya, K.A.; Suthar, M.K.; Kumar, J. Selection of superior Ocimum sanctum L. Accessions for industrial application. Ind. Crop Prod. 2017, 108, 700–707. [Google Scholar]
- Jordan, M.J.; Quilez, M.; Luna, M.C.; Bekhradi, F.; Sotomayor, J.; Sanchez-Gomez, P.; Gil, M.I. Influence of Water Stress and Storage Time on Preservation of the Fresh Volatile Profile of Three Basil Genotypes. Food Chem. 2017, 221, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R. Sweet basil essential oil composition: Relationship between cultivar, foliar feeding with nitrogen and oil content. J. Essent. Oil Res. 2012, 24, 217–227. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar]
- Císarová, M.; Tančinová, D.; Medo, J.; Kačániová, M. The in vitro effect of selected essential oils on the growth and mycotoxin production of Aspergillus species. J. Environ. Sci. Health Part B 2016, 51, 668–674. [Google Scholar]
- Shirazi, M.T.; Gholami, H.; Kavoosi, G.; Rowshan, V.; Tafsiry, A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Sci. Nutr. 2014, 2, 146–155. [Google Scholar]
- Bozin, B.; Mimca-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).