Effects of Biochar on Growth, Response to Water Stress, and Post-Stress Recovery in Underutilized Vegetable Hibiscus sabdariffa from Malawi
Abstract
1. Introduction
1.1. Hibiscus sabdariffa in Malawi
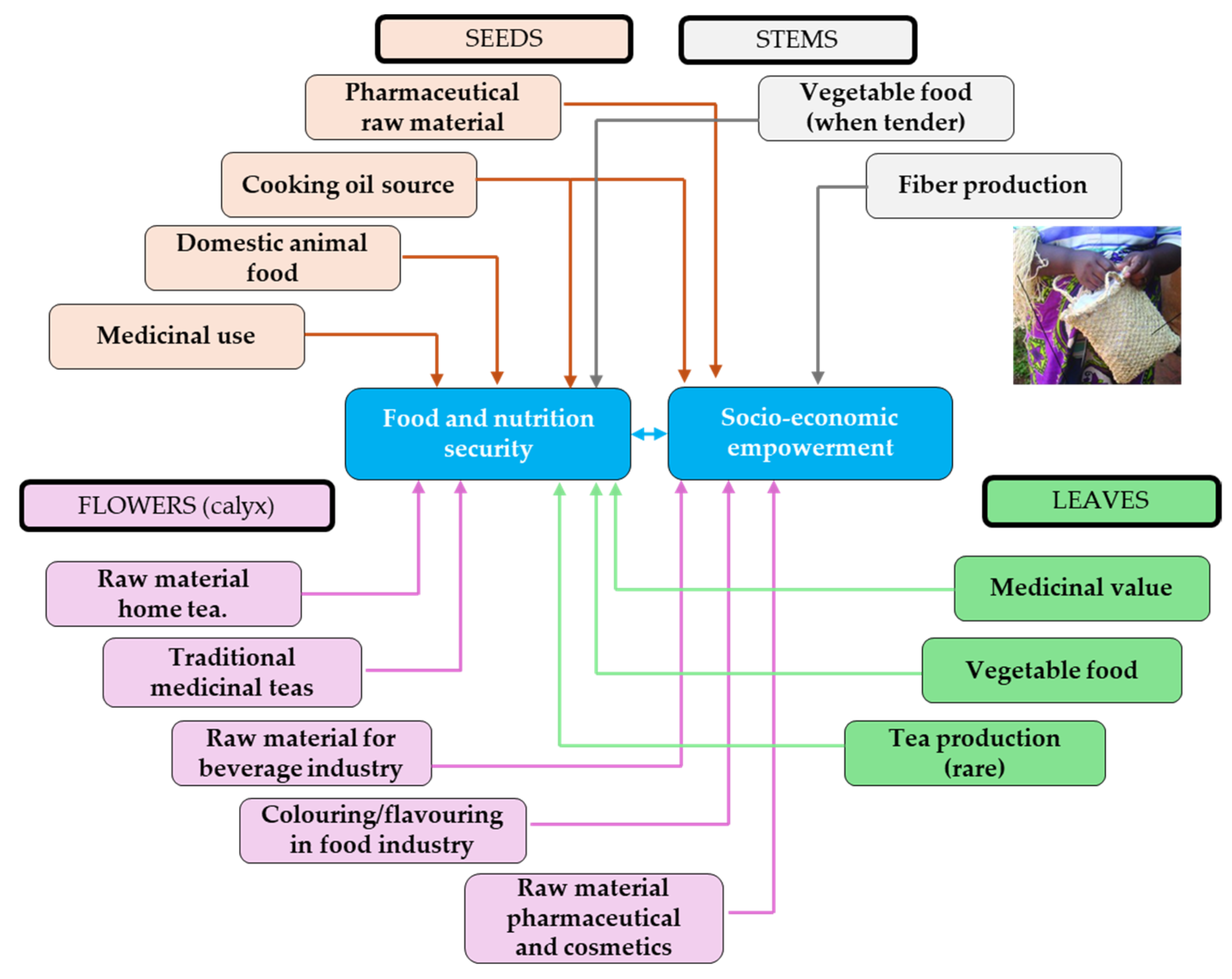
1.2. Important Uses of Hibiscus sabdariffa
1.3. Study Rationale and Objectives
2. Materials and Methods
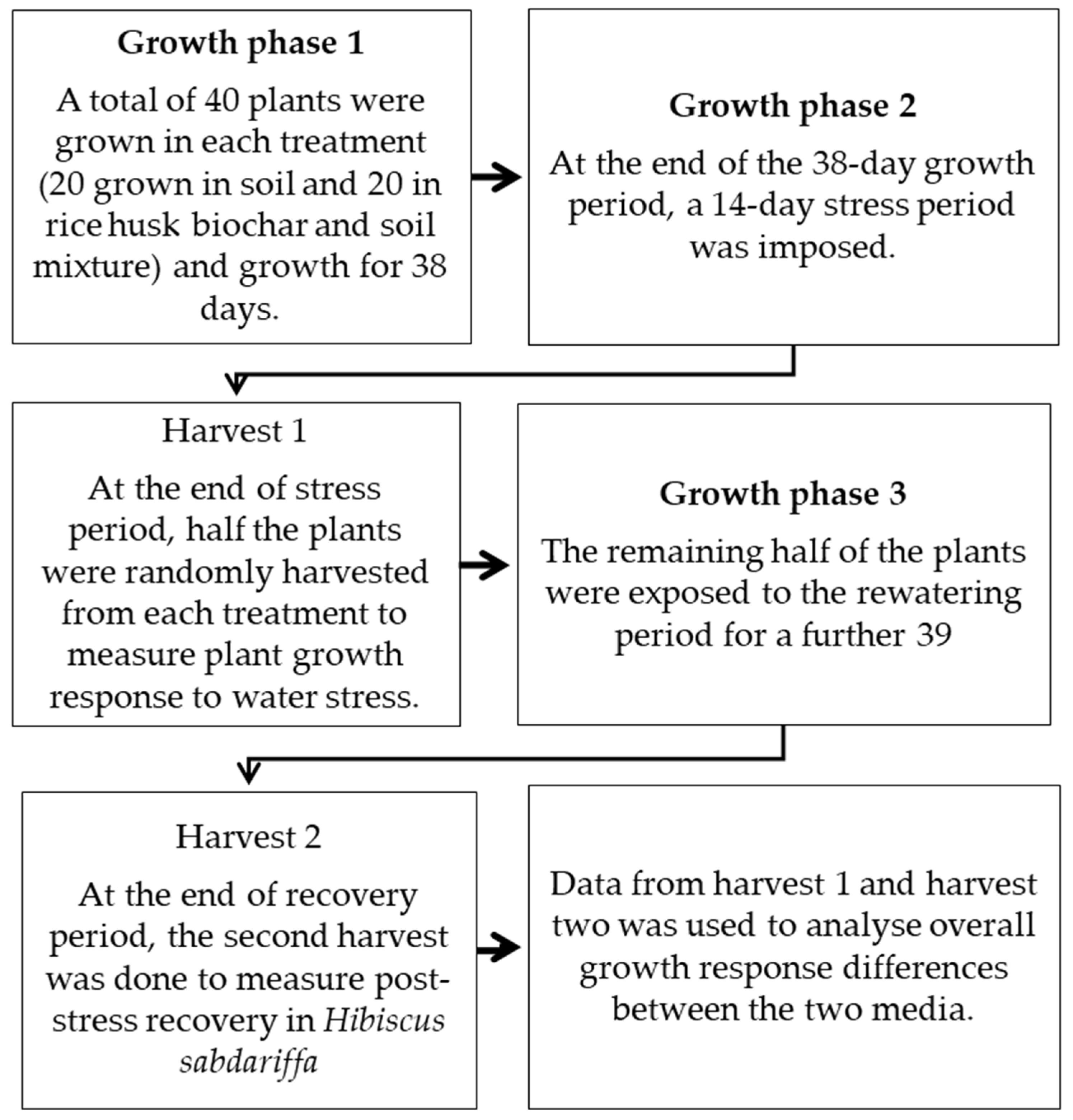
2.1. Experimental Setup and Design
2.2. Leaf Area Measurement
2.3. Chlorophyll and Biomass Determination
2.4. Determination of Relative Water Content
2.5. Data Analysis
3. Results
3.1. Effect of Rice Husk Biochar Media on Growth
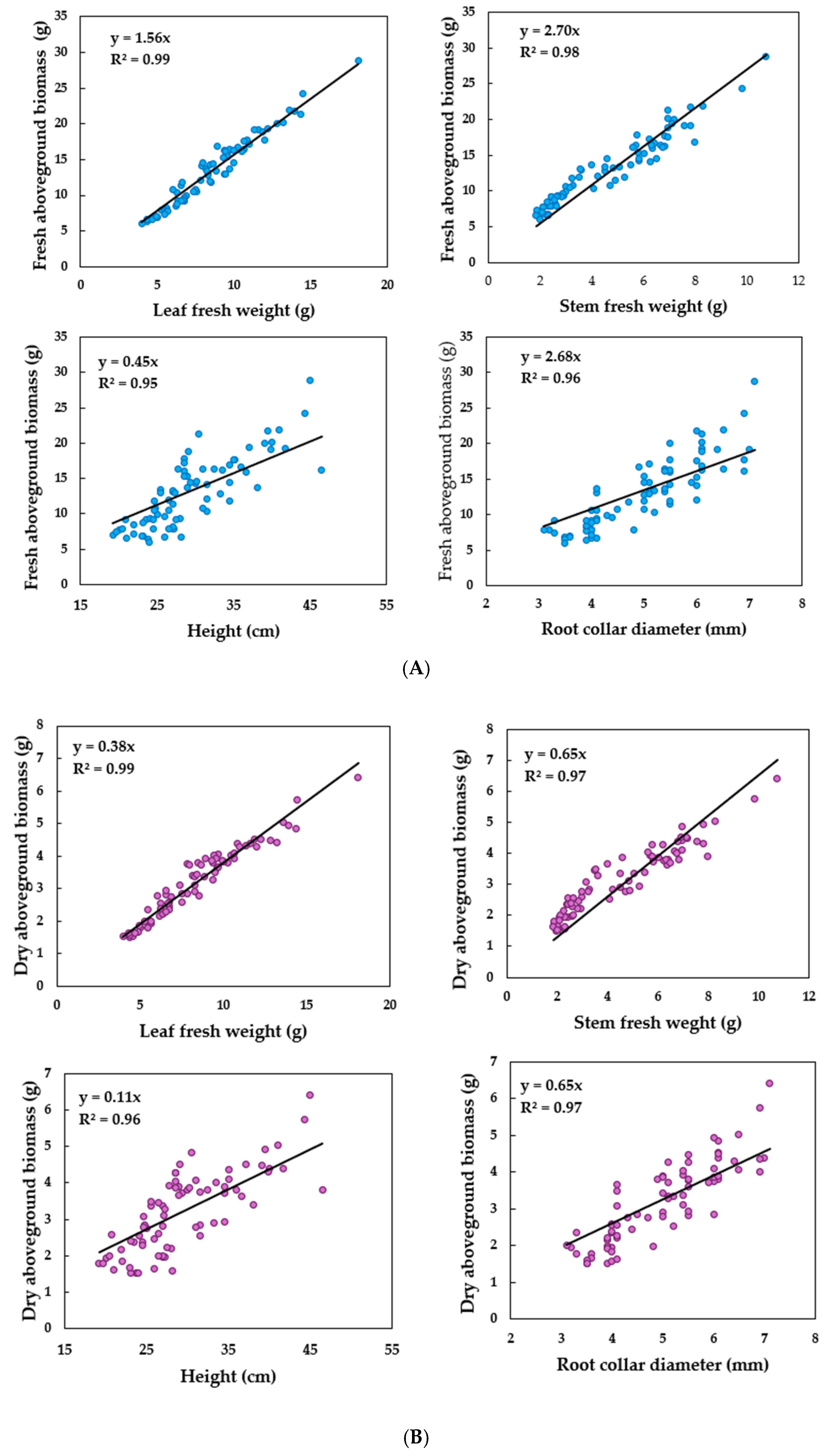
Correlation of Growth Attributes in Hibiscus sabdariffa
3.2. Effects of Stress on Growth in Hibiscus sabdariffa
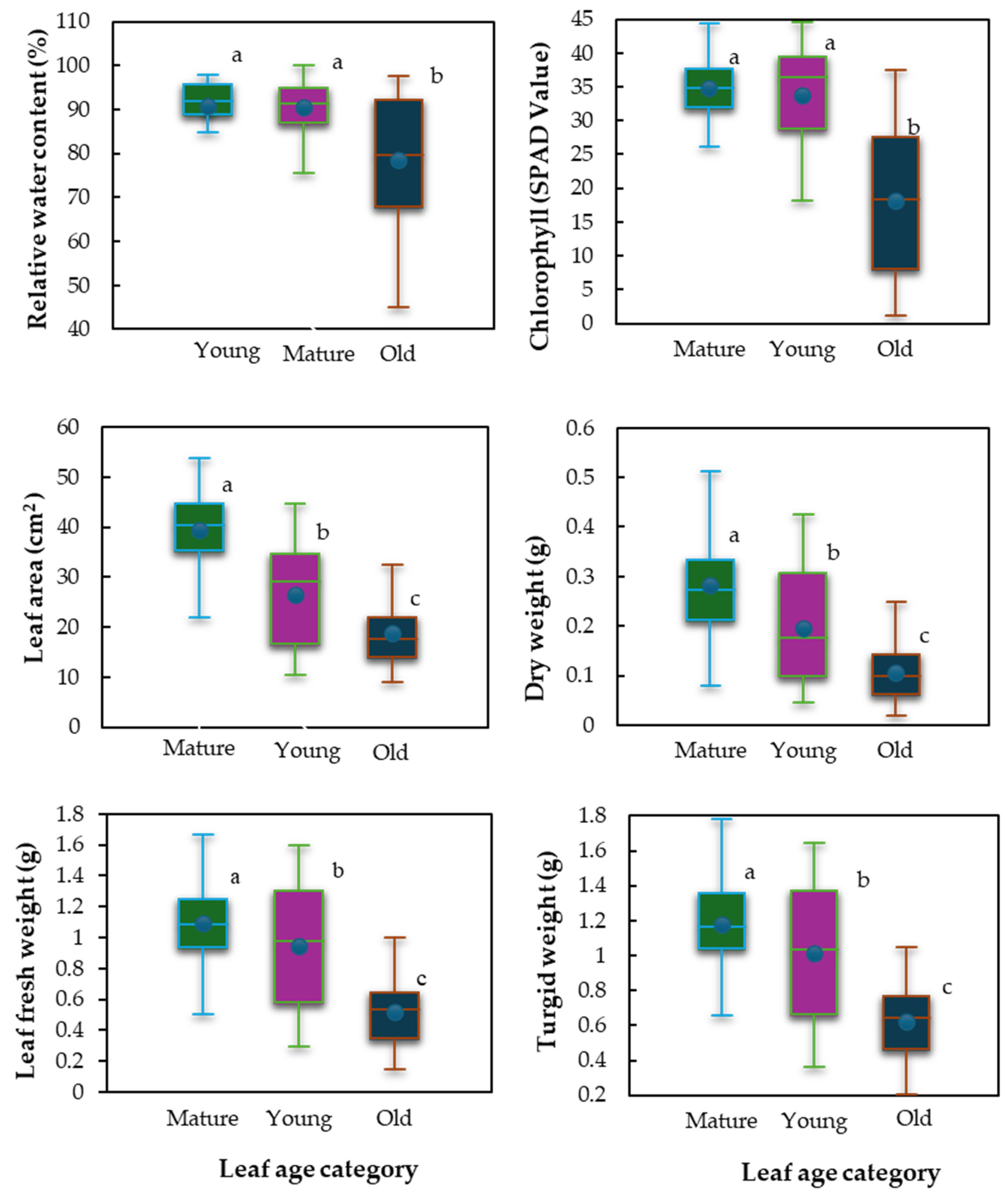
3.2.1. Leaf Response to Stress in Hibiscus sabdariffa
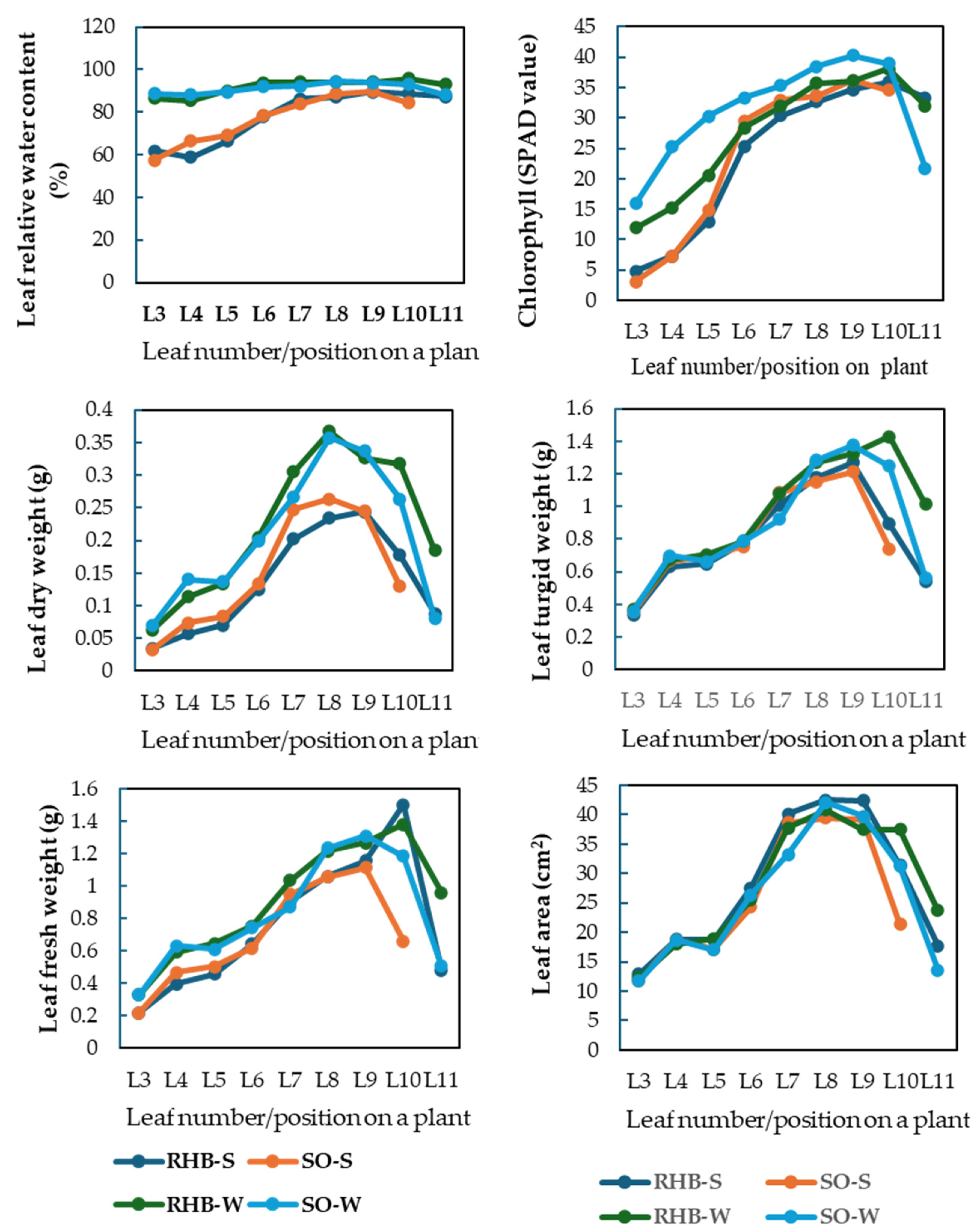
3.2.2. Leaf Age Variations in Relative Water Content
3.3. Effects of Biochar on Post-Stress Recovery
4. Discussion
4.1. Effects of RHB on Growth
4.2. Effects of Stress on Growth in Hibiscus sabdariffa
4.2.1. Water Stress Effects on Chlorophyll, Leaf Loss, and Area in Hibiscus sabdariffa
4.2.2. The Influence of Leaf Age on Response to Stress
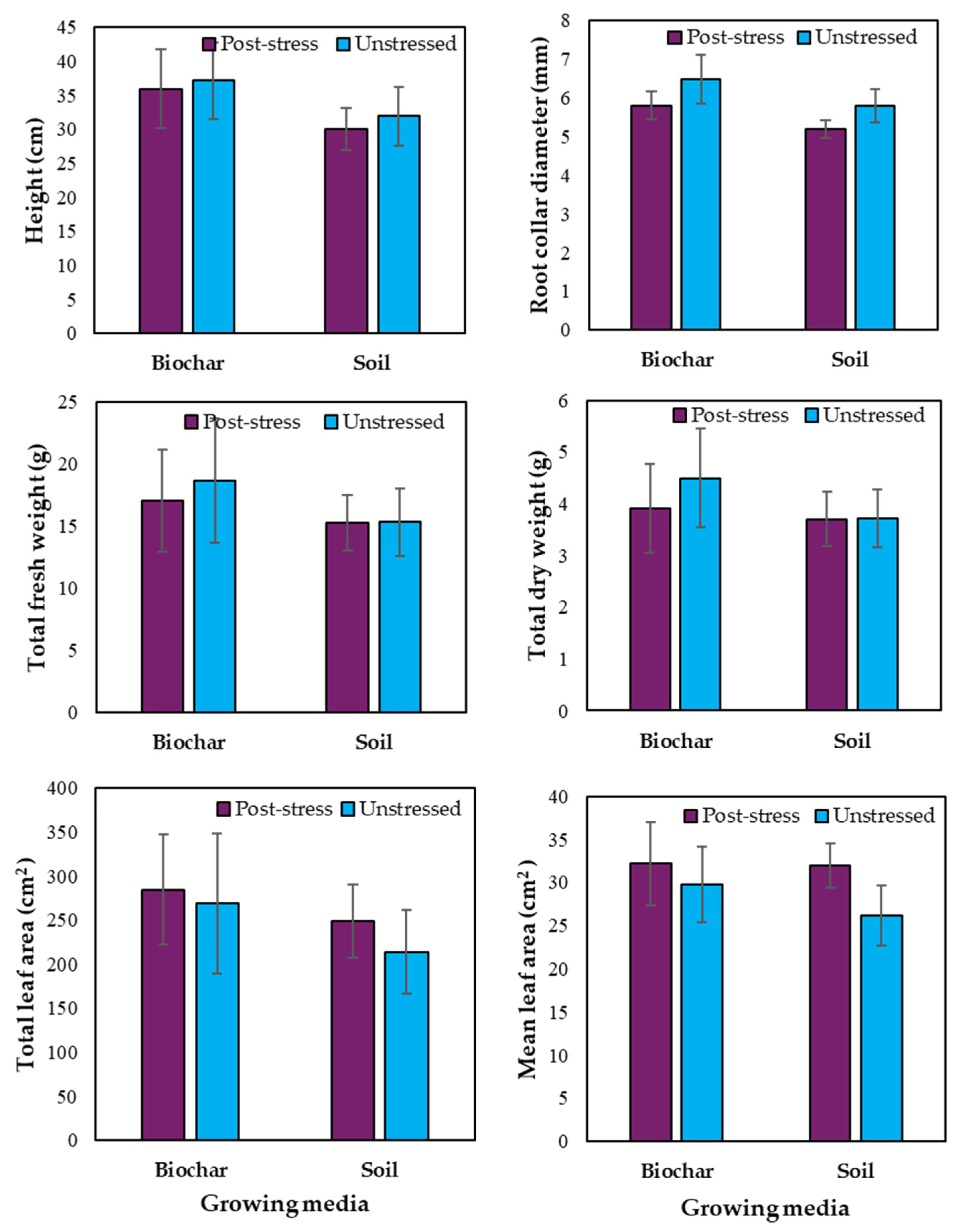
4.3. Biochar Effects of Post-Stress Recovery
Potential Post-Stress Recovery Mechanisms
4.4. Biochar Functionality Determinants, Trade-Offs and Implications for Farmers
4.4.1. Biochar Functionality Determinants: A Brief
4.4.2. Limitations and Trade-Offs of Biochar Application
4.4.3. Study Implications on Malawi’s Vegetables Farmers and Scientific Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salem, M.A.; Zayed, A.; Beshay, M.E.; Abdel Mesih, M.M.; Ben Khayal, R.F.; George, F.A.; Ezzat, S.M. Hibiscus sabdariffa L.: Phytoconstituents, Nutritive, and Pharmacological Applications. Adv. Tradit. Med. 2022, 22, 497–507. [Google Scholar] [CrossRef]
- Gheller, A.C.G.V.; Kerkhoff, J.; Vieira Júnior, G.M.; Campos, K.E.D.; Sugui, M.M. Antimutagenic Effect of Hibiscus sabdariffa L. Aqueous Extract on Rats Treated with Monosodium Glutamate. Sci. World J. 2017, 2017, 9392532. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Hegab, S.A.; Youssef, M.A.; Khalafalla, M.Y.; Almaroai, Y.A.; Ding, Z.; Eissa, M.A. Evaluation of Quality and Growth of Roselle (Hibiscus sabdariffa L.) as Affected by Bio-Fertilizers. J. Plant Nutr. 2020, 43, 1025–1035. [Google Scholar] [CrossRef]
- Mahunu, G.K. Breeding, Genetic Diversity, and Safe Production of Hibiscus Sabdariffa under Climate Change. In Roselle (Hibiscus sabdariffa); Mariod, A.A., Tahir, H.E., Mahunu, G.K., Eds.; Academic Press: New York, NY, USA, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Geja Woliso, W.; Kassahun Mengesha, B. Performance of Roselle (Hibiscus sabdariffa L.) in Different Agro-Ecologies of Ethiopia. Med. Aromat. Plants Los Angel. 2020, 9, 352. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=.+Performance+of+Roselle+%28Hibiscus+sabdariffa+L.%29+in+Different+Agro-Ecologies+of+Ethiopia.&btnG=. (accessed on 17 October 2024).
- Bakasso, Y.; Saadou, M.; Zongo, J.D.; Forso, J.; Toujan, A. Preliminary Assessment of the Influence of Environment on Growth Parameters and Yield in Some Genotypes of Hibiscus sabdariffa L. from Niger. Int. J. Biol. Chem. Sci. 2009, 3, 1133–1140. [Google Scholar] [CrossRef][Green Version]
- Montiel-González, C.; Montiel, C.; Ortega, A.; Pacheco, A.; Bautista, F. Development and Validation of Climatic Hazard Indicators for Roselle (Hibiscus sabdariffa L.) Crop in Dryland Agriculture. Ecol. Indic. 2021, 121, 107140. [Google Scholar] [CrossRef]
- Mazibuko, D.; Okazawa, H.; Gono, H.; Maskey, S. The Status of Vegetables Research in Malawi, Capacity, Progress, Gaps, and Way Forward—A Scoping Review. Agric. Sci. 2023, 14, 269–297. [Google Scholar] [CrossRef]
- Ngwira, T.N. Utilisation of local fruit in wine making in Malawi. In Domestication and Commercialisation of Non-Timber Forest Products in Agroforestry Systems; Leakey, R.R.B., Temu, A.B., Melnyk, M., Vantomme, P., Eds.; FAO: Rome, Italy, 1996; pp. 188–191. [Google Scholar]
- Mphande, B.C.; Pogrebnoi, A. Outdoor Photoelectrochemical Characterization of Dyes from Acalypha wilkesiana ‘Haleakala’ and Hibiscus sabdariffa as Dye Solar Cells Sensitizers. Br. J. Appl. Sci. Technol. 2015, 7, 195–204. [Google Scholar] [CrossRef]
- Chatepa, L.E.C.; Masamba, K.G.; Sanudi, T.; Ngwira, A.; Tanganyika, J.; Chamera, F. Effects of Aqueous and Methanolic Solvent Systems on Phytochemical and Antioxidant Extraction from Two Varieties of Roselle (Hibiscus sabdariffa L.) Var. Sabdariffa Plant from Central Malawi. Food Humanit. 2023, 1, 1172–1179. [Google Scholar] [CrossRef]
- Ngwira, A. Shared Geographic Spatial Risk of Childhood Undernutrition in Malawi: An Application of Joint Spatial Component Model. Public Health Pract. 2022, 3, 100224. [Google Scholar] [CrossRef]
- de Janvry, A.; Duquennois, C.; Sadoulet, E. Labor Calendars and Rural Poverty: A Case Study for Malawi. Food Policy 2022, 109, 102255. [Google Scholar] [CrossRef]
- Mataya, D.C.; Vincent, K.; Dougill, A.J. How Can We Effectively Build Capacity to Adapt to Climate Change? Insights from Malawi. Clim. Dev. 2020, 12, 781–790. [Google Scholar] [CrossRef]
- El Bilali, H. State and Contours of Research on Roselle (Hibiscus sabdariffa L.) in Africa. Open Agric. 2024, 9, 20220336. [Google Scholar] [CrossRef]
- Salami, S.O.; Afolayan, A.J. Evaluation of Nutritional and Elemental Compositions of Green and Red Cultivars of Roselle: Hibiscus sabdariffa L. Sci. Rep. 2021, 11, 1030. [Google Scholar] [CrossRef]
- Mohamed, B.B. Roselle (Hibiscus sabdariffa L.) in Sudan: Production and Uses. In Roselle; Elsevier: Amsterdam, The Netherlands, 2021; pp. 121–127. [Google Scholar] [CrossRef]
- Singh, D.; Batra, K.; Sharma, C.; Kaur, N.; Kaur, G.; Kapoor, M. The Traditional Uses, Phytochemistry and Pharmacology of Genus Hibiscus: A Review. Eur. J. Med. Plants 2021, 32, 1–37. [Google Scholar] [CrossRef]
- Montalvo-González, E.; Villagrán, Z.; González-Torres, S.; Iñiguez-Muñoz, L.E.; Isiordia-Espinoza, M.A.; Ruvalcaba-Gómez, J.M.; Arteaga-Garibay, R.I.; Acosta, J.L.; González-Silva, N.; Anaya-Esparza, L.M. Physiological Effects and Human Health Benefits of Hibiscus sabdariffa: A Review of Clinical Trials. Pharmaceuticals 2022, 15, 464. [Google Scholar] [CrossRef]
- Shruthi, V.H.; Ramachandra, C.T. Roselle (Hibiscus sabdariffa L.) Calyces: A Potential Source of Natural Color and Its Health Benefits. In Food Bioactives; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 169–190. [Google Scholar]
- Shruthi, V.H.; Ramachandra, C.T.; Udaykumar Nidoni, U.N.; Sharanagouda Hiregoudar, S.H.; Nagaraj Naik, N.N.; Kurubar, A.R. Roselle (Hibiscus sabdariffa L.) as a Source of Natural Colour: A Review. Plant Arch. 2016, 16, 515–522. Available online: http://www.plantarchives.org/PDF%20162/515-522.pdf (accessed on 21 November 2024).
- Keyata, E.O.; Tola, Y.B.; Bultosa, G.; Forsido, S.F. Proximate, Mineral, and Anti-Nutrient Compositions of Underutilized Plants of Ethiopia: Figl (Raphanus sativus L.), Girgir (Eruca sativa L.) and Karkade (Hibiscus sabdariffa): Implications for In-Vitro Mineral Bioavailability. Food Res. Int. 2020, 137, 109724. [Google Scholar] [CrossRef]
- Hughes, J. Just Famine Foods? What Contributions Can Underutilized Plants Make to Food Security? Acta Hortic. 2009, 806, 39–48. [Google Scholar] [CrossRef]
- Savita; Vimal, V. Underutilized Vegetables Introduction and Identification. In Production Technology of Underutilized Vegetable Crops; Savita Rawat, M., Vimal, V., Eds.; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant Responses to Drought Stress: Physiological, Biochemical and Molecular Basis. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Mazibuko, D.M.; Ndolo, V.; Akiyama, S.; Okazawa, H. Insights into the Journey towards Development of Dietary Guidelines: An Exploration of Synergy between Fruit and Vegetable Research with Food Composition Tables in Malawi. Afr. J. Food Sci. 2023, 17, 256–267. [Google Scholar] [CrossRef]
- Sun, C.X.; Chen, X.; Cao, M.M.; Li, M.Q.; Zhang, Y.L. Growth and metabolic responses of maize roots to straw biochar application at different rates. Plant Soil 2017, 416, 487–502. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C. High rates of gasified rice hull biochar affect geranium and tomato growth in a soilless substrate. J. Plant Nutr. 2017, 40, 1816–1828. [Google Scholar] [CrossRef]
- Bu, X.; Ma, W.; Ji, H.; Xue, J. Seed germination and early seedling growth of Rhododendron species in biochar-amended peat substrates. Commun. Soil Sci. Plant Anal. 2020, 51, 2310–2321. [Google Scholar] [CrossRef]
- Albert, L.P.; Wu, J.; Prohaska, N.; de Camargo, P.B.; Huxman, T.E.; Tribuzy, E.S.; Ivanov, V.Y.; Oliveira, R.S.; Garcia, S.; Smith, M.N. Age-Dependent Leaf Physiology and Consequences for Crown-Scale Carbon Uptake during the Dry Season in an Amazon Evergreen Forest. New Phytol. 2018, 219, 870–884. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-Based Leaf Nitrogen Estimation Is Impacted by Environmental Factors and Crop Leaf Characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.D. Determination of water deficit in plant tissues. In Water Deficit and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; pp. 236–368. [Google Scholar]
- Leuschner, C. Drought Response of European Beech (Fagus Sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Abdou, N.M.; El-Saadony, F.M.; Roby, M.H.; Mahdy, H.A.; El-Shehawi, A.M.; Elseehy, M.M.; El-Tahan, A.M.; Abdalla, H.; Saad, A.M.; AbouSreea, A.I.B. Foliar Spray of Potassium Silicate, Aloe Extract Composite and Their Effect on Growth and Yielding Capacity of Roselle (Hibiscus sabdariffa L.) under Water Deficit Stress Conditions. Saudi J. Biol. Sci. in Press. 2022. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Ali, A.M.; Mohamed, M.A.; Ibrahim, M.F. Combined Effect of Prickly Pear Waste Biochar and Azolla on Soil Fertility, Growth, and Yield of Roselle (Hibiscus sabdariffa L.) Plants. J. Soil Sci. Plant Nutr. 2022, 22, 3541–3552. [Google Scholar] [CrossRef]
- Liu, D.; Ding, Z.; Ali, E.F.; Kheir, A.M.; Eissa, M.A.; Ibrahim, O.H. Biochar and Compost Enhance Soil Quality and Growth of Roselle (Hibiscus sabdariffa L.) under Saline Conditions. Sci. Rep. 2021, 11, 8739. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: A meta-analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef] [PubMed]
- Albalsmeh, A.A.; Piri, H. Mitigating water stress effects on Roselle production: Effects of Conocarpus biochar and nitrogen fertilizer on soil nutrients and yield. Plant Soil 2024, 498, 617–635. [Google Scholar] [CrossRef]
- Salem, T.M.; Refaie, K.M.; Abd, A.E.H.E.G.; Sherif, E.L.; Eid, M.A.M. Biochar Application in Alkaline Soil and Its Effect on Soil and Plant. Acta Agric. Slov. 2019, 114, 85–96. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Singh, H.; Raghubanshi, A.S. Impact of Sole and Combined Application of Biochar, Organic and Chemical Fertilizers on Wheat Crop Yield and Water Productivity in a Dry Tropical Agro-Ecosystem. Biochar 2019, 1, 229–235. [Google Scholar] [CrossRef]
- Conner, J.K.; Cooper, I.A.; La Rosa, R.J.; Pérez, S.G.; Royer, A.M. Patterns of Phenotypic Correlations among Morphological Traits across Plants and Animals. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130246. [Google Scholar] [CrossRef]
- Reis, C.M.G.; Gazarini, L.C.; Fonseca, T.F.; Ribeiro, M.M. Above-Ground Biomass Estimation of Opuntia Ficus-indica (L.) Mill. for Forage Crop in a Mediterranean Environment by Using Non-Destructive Methods. Exp. Agric. 2018, 54, 227–242. [Google Scholar] [CrossRef]
- Moeckel, T.; Dayananda, S.; Nidamanuri, R.R.; Nautiyal, S.; Hanumaiah, N.; Buerkert, A.; Wachendorf, M. Estimation of Vegetable Crop Parameter by Multi-Temporal UAV-Borne Images. Remote Sens. 2018, 10, 805. [Google Scholar] [CrossRef]
- Besharati, J.; Shirmardi, M.; Meftahizadeh, H.; Ardakani, M.D.; Ghorbanpour, M. Changes in Growth and Quality Performance of Roselle (Hibiscus sabdariffa L.) in Response to Soil Amendments with Hydrogel and Compost under Drought Stress. S. Afr. J. Bot. 2022, 145, 334–347. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Dyderski, M.K.; Rawlik, K.; Kątna, B. Seasonal Variability of Biomass, Total Leaf Area and Specific Leaf Area of Forest Understory Herbs Reflects Their Life Strategies. For. Ecol. Manag. 2016, 374, 71–81. [Google Scholar] [CrossRef]
- Mohamed, B.B.; Sarwar, M.B.; Hassan, S.; Rashid, B.; Aftab, B.; Husnain, T. Tolerance of Roselle (Hibiscus sabdariffa L.) genotypes to drought stress at vegetative stage. Adv. Life sci. 2015, 2, 74–82. [Google Scholar] [CrossRef]
- Evans, D.; Al-Hamdani, S. Selected physiological responses of roselle (Hibiscus sabdariffa) to drought stress. J. Exp. Biol. Agric. 2015, 3, 500–507. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S. Water Stress Alleviation of Roselle Plant by Silicon Treatment through Some Physiological and Biochemical Responses. Annu. Res. Rev. Biol. 2017, 21, 1–17. [Google Scholar] [CrossRef]
- Ali, H.M.; Siddiqui, M.H.; Basalah, M.O.; Al-Whaibi, M.H.; Sakran, A.M.; Al-Amri, A. Effects of gibberellic acid on growth and photosynthetic pigments of Hibiscus sabdariffa L. under salt stress. Afr. J. Biotechnol. 2012, 11, 800–804. [Google Scholar] [CrossRef]
- Asghari, B.; Mafakheri, S.; Zarrabi, M.M. Effects of salicylic acid on physiological and phytochemical parameters of roselle (Hibiscus sabdariffa L.) under water shortage stress. IJMAPR 2023, 39, 367–386. [Google Scholar] [CrossRef]
- Akıncı, Ş.; Lösel, D.M. Plant water-stress response mechanisms. In Water Stress; Rahmnan, I.M.M., Hasegawa, H., Eds.; Intechopen: Rijeka, Croatia, 2012; pp. 15–42. [Google Scholar]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ responses under drought stress conditions: Effects of strategic management approaches—A review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Lyu, S.; Du, G.; Liu, Z.; Zhao, L.; Lyu, D. Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensis Maxim. under drought stress. Acta Physiol. Plant. 2016, 38, 220. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are key regulators of drought stress tolerance in tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Maseko, I.; Ncube, B.; Mabhaudhi, T.; Tesfay, S.; Chimonyo, V.G.P.; Araya, H.T.; Fessehazion, M.; Du Plooy, C.P. Moisture Stress on Physiology and Yield of Some Indigenous Leafy Vegetables under Field Conditions. S. Afr. J. Bot. 2019, 126, 85–91. [Google Scholar] [CrossRef]
- Guo, L.; Yu, H.; Kharbach, M.; Wang, J. The Response of Nutrient Uptake, Photosynthesis and Yield of Tomato to Biochar Addition under Reduced Nitrogen Application. Agronomy 2021, 11, 1598. [Google Scholar] [CrossRef]
- Aishwath, O.P.; Lal, R. Resilience of Spices, Medicinal and Aromatic Plants with Climate Change Induced Abiotic Stresses. Ann. Plant Soil Res. 2016, 18, 91–109. [Google Scholar]
- Nadal-Sala, D.; Grote, R.; Birami, B.; Knüver, T.; Rehschuh, R.; Schwarz, S.; Ruehr, N.K. Leaf Shedding and Non-Stomatal Limitations of Photosynthesis Mitigate Hydraulic Conductance Losses in Scots Pine Saplings during Severe Drought Stress. Front. Plant Sci. 2021, 12, 715127. [Google Scholar] [CrossRef]
- Wu, J.; Su, Y.; Chen, X.; Liu, L.; Yang, X.; Gong, F.; Zhang, H.; Xiong, X.; Zhang, D. Leaf Shedding of Pan-Asian Tropical Evergreen Forests Depends on the Synchrony of Seasonal Variations of Rainfall and Incoming Solar Radiation. Agric. For. Meteorol. 2021, 311, 108691. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, C.-Z.; Gao, J. Regulatory Mechanisms Underlying Activation of Organ Abscission. Annu. Plant Rev. Online 2021, 4, 27–56. [Google Scholar] [CrossRef]
- Mehar-un-Nisa Narejo, M.-N.N.; Puteri Edaroyati, M.W.; Siti Aishah Hassan, S.A.H.; Che Radziah Che, M.Z. Effects of Drought Stress on Growth and Physiological Characteristics of Roselle (Hibiscus sabdariffa L.). J. Trop. Plant Physiol. 2016, 8, 44–51. [Google Scholar]
- Zhao, Y.; Weng, Z.; Chen, H.; Yang, J. Analysis of the Evolution of Drought, Flood, and Drought-Flood Abrupt Alternation Events under Climate Change Using the Daily SWAP Index. Water 2020, 12, 1969. [Google Scholar] [CrossRef]
- Dambreville, A.; Lauri, P.E.; Normand, F.; Guédon, Y. Analysing Growth and Development of Plants Jointly Using Developmental Growth Stages. Ann. Bot. 2015, 115, 93–105. [Google Scholar] [CrossRef]
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of Water Stress on Gas Exchange, Water Relations and Leaf Structure in Two Ornamental Shrubs in the Mediterranean Area. Agronomy 2019, 9, 381. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Zhou, M. Environmental explanation of maize specific leaf area under varying water stress regimes. Environ. Exp. Bot. 2020, 171, 103932. [Google Scholar] [CrossRef]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of agricultural crops to free-air CO2 enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar] [CrossRef]
- Freschet, G.T.; Swart, E.M.; Cornelissen, J.H.C. Integrated plant phenotypic responses to contrasting above- and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef]
- Gao, J.; Wang, K.; Zhang, X. Patterns and drivers of community specific leaf area in China. Glob. Ecol. Conserv. 2022, 33, e01971. [Google Scholar] [CrossRef]
- Patakas, A.; Noitsakis, B. Leaf Age Effects on Solute Accumulation in Water-Stressed Grapevines. J. Plant Physiol. 2001, 158, 63–69. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating Physiological Responses of Plants to Salinity Stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.A.; Elsayed, S.; Hassan, W.M.; Dewir, Y.H.; Refay, Y.; Abdella, K.A. Potential of the Existing and Novel Spectral Reflectance Indices for Estimating the Leaf Water Status and Grain Yield of Spring Wheat Exposed to Different Irrigation Rates. Agric. Water Manag. 2019, 217, 356–373. [Google Scholar] [CrossRef]
- Vu, N.-T.; Park, J.-M.; Kim, S.; Tran, T.; Jang, D.-C. Effect of Abscisic Acid on Growth and Physiology of Arabica Coffee Seedlings under Water Deficit Condition. Sains Malays. 2020, 49, 1499–1508. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought Effect on Plant Biomass Allocation: A Meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Sanayei, S.; Barmaki, M.; Ebadi, A.; Torabi-Giglou, M. Amelioration of Water Deficiency Stress in Roselle (Hibiscus sabdariffa) by Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 11987. [Google Scholar] [CrossRef]
- Shala, A.Y.; Mahmoud, M.A. Influence of Glycinebetaine on Water Stress Tolerance of Hibiscus sabdariffa L. Plant. J. Plant Prod. 2018, 9, 981–988. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General Mechanisms of Drought Response and Their Application in Drought Resistance Improvement in Plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, R.; Volařík, D.; Houšková, K.; Matoušková, M.; Paschová, Z.; Štykar, J.; Vit´asek, R.; Urban, J.; Plichta, R. Sensitivity of physiological traits to different short-term drought events and subsequent recovery at the sapling stage in European white elm (Ulmus laevis Pall.). Environ. Exp. Bot. 2023, 214, 105469. [Google Scholar] [CrossRef]
- Zand-Silakhoor, A.; Madani, H.; Sharifabad, H.H.; Mahmoudi, M.; Nourmohammadi, G. Influence of Different Irrigation Regimes and Planting Times on the Quality and Quantity of Calyx, Seed Oil Content and Water Use Efficiency of Roselle (Hibiscus sabdariffa L.). Grasas Aceites 2022, 73, e472. [Google Scholar] [CrossRef]
- Skelton, R. Stem Diameter Fluctuations Provide a New Window into Plant Water Status and Function. Plant Physiol. 2020, 183, 1414–1415. [Google Scholar] [CrossRef]
- Rehschuh, R.; Cecilia, A.; Zuber, M.; Faragó, T.; Baumbach, T.; Hartmann, H.; Jansen, S.; Mayr, S.; Ruehr, N. Drought-Induced Xylem Embolism Limits the Recovery of Leaf Gas Exchange in Scots Pine. Plant Physiol. 2020, 184, 852–864. [Google Scholar] [CrossRef]
- Marron, N.; Dreyer, E.; Boudouresque, E.; Delay, D.; Petit, J.-M.; Delmotte, F.M.; Brignolas, F. Impact of Successive Drought and Re-Watering Cycles on Growth and Specific Leaf Area of Two Populus x canadensis (Moench) Clones,‘Dorskamp’ and ‘Luisa_Avanzo’. Tree Physiol. 2003, 23, 1225–1235. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The Relationship between Leaf Area Growth and Biomass Accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef]
- Sun, J.; Fan, R.; Niklas, K.J.; Zhong, Q.; Yang, F.; Li, M.; Chen, X.; Sun, M.; Cheng, D. “Diminishing Returns” in the Scaling of Leaf Area vs. Dry Mass in Wuyi Mountain Bamboos, Southeast China. Am. J. Bot. 2017, 104, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Ruehr, N.K.; Grote, R.; Mayr, S.; Arneth, A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 2019, 39, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Vanková, R.; Dobrá, J.; Štorchová, H. Recovery from drought stress in tobacco: An active process associated with the reversal of senescence in some plant parts and the sacrifice of others. Plant Signal. Behav. 2012, 7, 19–21. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J. The relations of stomatal closure and reopening to xylem ABA concentration and leaf water potential during soil drying and rewatering. Plant Growth Regul. 1999, 29, 77–86. [Google Scholar] [CrossRef]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F. Biochar modifies the content of primary metabolites in the rhizosphere of well-watered and drought-stressed Zea mays L.(maize). Biol. Fertil. Soils. 2022, 58, 633–647. [Google Scholar] [CrossRef]
- Akram, M.Z.; Rivelli, A.R.; Libutti, A.; Liu, F.; Andreasen, C. Mitigation of drought stress for Quinoa (Chenopodium quinoa Willd.) varieties using woodchip biochar-amended soil. Plants 2024, 13, 2279. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil pH-nutrient relationships: The diagram. Plant Soil. 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil. 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils. Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C: N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Idbella, M.; Baronti, S.; Giagnoni, L.; Renella, G.; Becagli, M.; Cardelli, R.; Maienza, A.; Vaccari, F.P.; Bonanomi, G. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 2024, 195, 105217. [Google Scholar] [CrossRef]
- Halmi, M.F.A.; Simarani, K. Effect of two contrasting biochars on soil microbiota in the humid tropics of Peninsular Malaysia. Geoderma 2021, 395, 115088. [Google Scholar] [CrossRef]
- Zeremski, T.; Randjelović, D.; Jakovljević, K.; Marjanović Jeromela, A.; Milić, S. Brassica Species in Phytoextractions: Real Potentials and Challenges. Plants 2021, 10, 2340. [Google Scholar] [CrossRef]
- Tisserant, A.; Morales, M.; Cavalett, O.; O’Toole, A.; Weldon, S.; Rasse, D.P.; Cherubini, F. Life-cycle assessment to unravel co-benefits and trade-offs of large-scale biochar deployment in Norwegian agriculture. Resour. Conserv. Recycl. 2022, 179, 106030. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Mukome, F.N.; Machado, S.; Nyamasoka, B. Biochar production and applications in sub-Saharan Africa: Opportunities, constraints, risks and uncertainties. J. Environ. Manag. 2015, 150, 250–261. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Motsi, H.; Manyevere, A.; Poswa, S.B. Assessing the potential of biochar as a viable alternative to synthetic fertilizers in sub-Saharan Africa smallholder farming: A review. Agronomy 2024, 14, 1215. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, limitations, co-benefits, and trade-offs of biochar applications to soils for climate change mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Lu, Y.; Gu, K.; Shi, B.; Zhou, Q. Does biochar mitigate rainfall-induced soil erosion? A review and meta-analysis. Biogeotechnics 2024, 2, 100096. [Google Scholar] [CrossRef]
- Gholamahmadi, B.; Jeffery, S.; Gonzalez-Pelayo, O.; Prats, S.A.; Bastos, A.C.; Keizer, J.J.; Verheijen, F.G. Biochar impacts on runoff and soil erosion by water: A systematic global scale meta-analysis. Sci. Total Environ. 2023, 871, 161860. [Google Scholar] [CrossRef] [PubMed]
- Chinseu, E.; Dougill, A.; Stringer, L. Why Do Smallholder Farmers Dis-Adopt Conservation Agriculture? Insights from Malawi. Land Degrad. Dev. 2019, 30, 533–543. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Molnar, J.J.; Fazio, R.A.; Sydnor, E.; Lowe, M.J. Barriers to Adoption of Sustainable Agriculture Practices: Change Agent Perspectives. Renew. Agric. Food Syst. 2009, 24, 60–71. [Google Scholar] [CrossRef]
- Dettling, M.; Indrawati, R.; Indriatmoko, I.; Adhiwibawa, M.A.S.; Brotosudarmo, T.H.P.; Limantara, L. Chlorophyll Values of Local Green Vegetables Common in Malang, East Java. Trans. Malays. Soc. Plant Physiol. 2015, 22, 305–309. [Google Scholar]
| Particle Size | |||
|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Textural Class (USDA) |
| 60.10 | 39.10 | 0.80 | Sandy loam |
| Media | pH | EC (mS/m) | Specific Gravity (g/cm3) | Loss on Ignition (%) | Total Carbon (%) | Total Nitrogen (%) |
|---|---|---|---|---|---|---|
| Soil | 5.05 | 8.20 | 2.41 | 22.10 | 6.18 | 0.36 |
| Soil + biochar | 5.12 | 14.60 | 2.39 | 23.30 | 6.48 | 0.34 |
| Biochar (RHB) | 10.47 | - | - | - | 49.27 | 0.53 |
| Media | Height (cm) | NL | RCD (mm) | SDW (g) | LFW (g) | LDW (g) | TLA (cm2) | FAGB (g) | DAGB (g) |
|---|---|---|---|---|---|---|---|---|---|
| Rice husk biochar | 31.70 a | 12.60 a | 5.24 a | 1.56 a | 8.86 a | 1.78 a | 253.1 a | 13.8 a | 3.36 a |
| STDEV | ±3.68 | ±2.61 | ±1.10 | ±0.73 | ±3.37 | ±0.59 | ±61.71 | ±5.63 | ±1.24 |
| Soil | 27.21 b | 12.13 a | 4.68 b | 1.37 a | 7.89 a | 1.66 a | 224.14 b | 12.18 a | 3.03 a |
| STDEV | ±2.19 | ±2.86 | ±0.95 | ±0.60 | ±2.25 | ±0.44 | ±41.23 | ±4.01 | ±0.92 |
| p | 0.00 | 0.43 | 0.02 | 0.23 | 0.15 | 0.33 | 0.02 | 0.15 | 0.23 |
| F (df) | 10.45 (1.74) | 0.63 (1.74) | 5.69 (1.74) | 1.46 (1.74) | 2.17 (1.74) | 0.96 (1.74) | 5.80 (1.74) | 2.07 (1.74) | 1.47 (1.74) |
| ω2 | 0.111 | 0.00 | 0.06 | 0.01 | 0.02 | 0.00 | 0.06 | 0.01 | 0.01 |
| Height (cm) | NL | RCD (mm) | SFW (g) | SDW (g) | LFW (g) | LDW (g) | TLA (g) | FAGB (g) | DAGB (g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Height | 1.00 | 0.72 | 0.82 | 0.88 | 0.86 | 0.74 | 0.52 | 0.541 | 0.82 | 0.78 |
| NL | 0.72 | 1.00 | 0.86 | 0.89 | 0.90 | 0.81 | 0.63 | 0.55 | 0.88 | 0.86 |
| RCD | 0.82 | 0.86 | 1.00 | 0.93 | 0.93 | 0.79 | 0.63 | 0.50 | 0.87 | 0.87 |
| SFW | 0.88 | 0.89 | 0.93 | 1.00 | 0.99 | 0.89 | 0.71 | 0.62 | 0.96 | 0.95 |
| SDW | 0.86 | 0.90 | 0.93 | 0.99 | 1.00 | 0.86 | 0.67 | 0.58 | 0.95 | 0.94 |
| LFW | 0.74 | 0.81 | 0.79 | 0.89 | 0.86 | 1.00 | 0.91 | 0.87 | 0.98 | 0.97 |
| LDW | 0.52 | 0.63 | 0.63 | 0.71 | 0.67 | 0.91 | 1.00 | 0.84 | 0.85 | 0.89 |
| TLA | 0.54 | 0.55 | 0.50 | 0.62 | 0.58 | 0.87 | 0.84 | 1.00 | 0.78 | 0.76 |
| FAGB | 0.82 | 0.87 | 0.87 | 0.96 | 0.95 | 0.98 | 0.85 | 0.78 | 1.00 | 0.99 |
| DAGB | 0.78 | 0.86 | 0.87 | 0.95 | 0.94 | 0.97 | 0.89 | 0.76 | 0.99 | 1.00 |
| Variable | Water Status | Growing Media | Water Status * Growing Media | |||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| Plant height | 5.08 | 0.05 | 23.46 | <0.00 | 6.11 | 0.02 |
| Root collar diameter (RCD) | 50.27 | <0.00 | 25.47 | <0.00 | 2.08 | 0.16 |
| Number of leaves (NL) | 5.41 | 0.05 | 1.49 | 0.23 | 0.31 | 0.58 |
| Chlorophyll | 13.75 | <0.00 | 7.07 | 0.02 | 0.87 | 0.35 |
| Leaf loss | 10.00 | 0.00 | 6.40 | 0.02 | 0.40 | 0.53 |
| Total leaf area (TLA) | 3.35 | 0.08 | 0.89 | 0.35 | 0.01 | 0.75 |
| Average leaf area | 4.46 | 0.07 | 9.06 | 0.01 | 0.39 | 0.54 |
| Specific leaf area | 100.21 | <0.00 | 5.31 | 0.05 | 7.58 | 0.01 |
| Mean leaf relative water content | 197.05 | <0.00 | 0.79 | 0.38 | 0.33 | 0.57 |
| Leaf fresh weight (LFW) | 35.81 | <0.00 | 0.23 | 0.63 | 0.27 | 0.61 |
| Leaf dry weight (LDW) | 38.32 | <0.00 | 0.00 | 0.96 | 0.57 | 0.46 |
| Stem fresh weight (SFW) | 24.90 | <0.00 | 5.43 | 0.05 | 0.00 | 0.95 |
| Stem dry weight (SDW) | 13.95 | <0.00 | 4.62 | 0.05 | 0.00 | 0.99 |
| Fresh above-ground biomass (FAGB) | 22.59 | <0.00 | 0.99 | 0.33 | 0.14 | 0.72 |
| Dry above-ground biomass (DAGB) | 31.54 | <0.00 | 0.37 | 0.55 | 0.30 | 0.59 |
| Rice husk biochar stressed leaves | ||||||||
| Leaf 3 | Leaf 4 | Leaf 5 | Leaf 6 | Leaf 7 | Leaf 8 | Leaf 9 | Leaf10 | |
| Leaf 3 | 1.000 | 0.494 | 0.310 | 0.000 | <0.0001 | <0.0001 | <0.0001 | - |
| Leaf 4 | 0.494 | 1.000 | 0.097 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | - |
| Leaf 5 | 0.310 | 0.097 | 1.000 | 0.006 | 0.000 | <0.0001 | <0.0001 | - |
| Leaf 6 | 0.000 | <0.0001 | 0.006 | 1.000 | 0.018 | 0.003 | 0.000 | - |
| Leaf 7 | <0.0001 | <0.0001 | 0.000 | 0.018 | 1.000 | 0.601 | 0.175 | - |
| Leaf 8 | <0.0001 | <0.0001 | <0.0001 | 0.003 | 0.601 | 1.000 | 0.353 | - |
| Leaf 9 | <0.0001 | <0.0001 | <0.0001 | 0.000 | 0.175 | 0.353 | 1.000 | - |
| Soil-Stressed leaves | ||||||||
| Leaf 3 | Leaf 4 | Leaf 5 | Leaf 6 | Leaf 7 | Leaf 8 | Leaf 9 | Leaf10 | |
| Leaf 3 | 1.000 | 0.017 | 0.003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | - |
| Leaf 4 | 0.017 | 1.000 | 0.307 | 0.000 | <0.0001 | <0.0001 | <0.0001 | - |
| Leaf 5 | 0.003 | 0.307 | 1.000 | 0.002 | <0.0001 | <0.0001 | <0.0001 | - |
| Leaf 6 | <0.0001 | 0.000 | 0.002 | 1.000 | 0.020 | 0.000 | <0.0001 | - |
| Leaf 7 | <0.0001 | <0.0001 | <0.0001 | 0.020 | 1.000 | 0.001 | <0.0001 | - |
| Leaf 8 | <0.0001 | <0.0001 | <0.0001 | 0.000 | 0.001 | 1.000 | 0.357 | - |
| Leaf 9 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.357 | 1.000 | - |
| Rice husk biochar non-stressed leaves | ||||||||
| Leaf 3 | Leaf 4 | Leaf 5 | Leaf 6 | Leaf 7 | Leaf 8 | Leaf 9 | leaf 10 | |
| Leaf 3 | 1.000 | 0.721 | 0.277 | 0.004 | 0.002 | 0.000 | 0.000 | 0.029 |
| Leaf 4 | 0.721 | 1.000 | 0.147 | 0.001 | 0.001 | 0.000 | 0.000 | 0.018 |
| Leaf 5 | 0.277 | 0.147 | 1.000 | 0.021 | 0.010 | 0.002 | 0.002 | 0.064 |
| Leaf 6 | 0.004 | 0.001 | 0.021 | 1.000 | 0.832 | 0.238 | 0.297 | 0.897 |
| Leaf 7 | 0.002 | 0.001 | 0.010 | 0.832 | 1.000 | 0.285 | 0.359 | 0.968 |
| Leaf 8 | 0.000 | 0.000 | 0.002 | 0.238 | 0.285 | 1.000 | 0.815 | 0.392 |
| Leaf 9 | 0.000 | 0.000 | 0.002 | 0.297 | 0.359 | 0.815 | 1.000 | 0.441 |
| leaf 10 | 0.029 | 0.018 | 0.064 | 0.897 | 0.968 | 0.392 | 0.441 | 1.000 |
| Soil non-stressed leaves | ||||||||
| Leaf 3 | Leaf 4 | Leaf 5 | Leaf 6 | Leaf 7 | Leaf 8 | Leaf 9 | leaf 10 | |
| Leaf 3 | 1.000 | 0.859 | 0.761 | 0.161 | 0.108 | 0.011 | 0.032 | 0.056 |
| Leaf 4 | 0.859 | 1.000 | 0.611 | 0.094 | 0.054 | 0.004 | 0.014 | 0.025 |
| Leaf 5 | 0.761 | 0.611 | 1.000 | 0.237 | 0.163 | 0.014 | 0.045 | 0.078 |
| Leaf 6 | 0.161 | 0.094 | 0.237 | 1.000 | 0.936 | 0.151 | 0.354 | 0.553 |
| Leaf 7 | 0.108 | 0.054 | 0.163 | 0.936 | 1.000 | 0.081 | 0.302 | 0.504 |
| Leaf 8 | 0.011 | 0.004 | 0.014 | 0.151 | 0.081 | 1.000 | 0.633 | 0.253 |
| Leaf 9 | 0.032 | 0.014 | 0.045 | 0.354 | 0.302 | 0.633 | 1.000 | 0.642 |
| leaf 10 | 0.056 | 0.025 | 0.078 | 0.553 | 0.504 | 0.253 | 0.642 | 1.000 |
| Variable | Water Status (Non-Stressed vs. Post-Stress | Growing Media | Water Status * Growing Media | |||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| Plant height | 1.00 | 0.32 | 13.10 | <0.00 | 0.05 | 0.82 |
| Root collar diameter (RCD) | 20.93 | <0.00 | 22.28 | <0.00 | 0.08 | 0.77 |
| Total leaf area (TLA) | 1.82 | 0.19 | 5.78 | 0.02 | 0.26 | 0.61 |
| Average leaf area | 10.96 | 0.00 | 2.31 | 0.14 | 1.86 | 0.18 |
| Leaf fresh weight (LFW) | 0.03 | 0.86 | 4.33 | 0.05 | 0.51 | 0.48 |
| Stem fresh weight (SFW) | 5.92 | 0.02 | 4.92 | 0.03 | 0.27 | 0.61 |
| Stem dry weight (SDW) | 8.99 | 0.01 | 5.41 | 0.03 | 0.67 | 0.42 |
| Fresh above-ground biomass (FAGB) | 0.52 | 0.48 | 4.79 | 0.04 | 0.45 | 0.51 |
| Dry above-ground biomass (DAGB) | 1.63 | 0.21 | 4.27 | 0.05 | 1.46 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazibuko, D.M.; Maskey, S.; Kurashina, K.; Okazawa, H.; Oshima, H.; Kato, T.; Kikuno, H. Effects of Biochar on Growth, Response to Water Stress, and Post-Stress Recovery in Underutilized Vegetable Hibiscus sabdariffa from Malawi. Crops 2025, 5, 13. https://doi.org/10.3390/crops5020013
Mazibuko DM, Maskey S, Kurashina K, Okazawa H, Oshima H, Kato T, Kikuno H. Effects of Biochar on Growth, Response to Water Stress, and Post-Stress Recovery in Underutilized Vegetable Hibiscus sabdariffa from Malawi. Crops. 2025; 5(2):13. https://doi.org/10.3390/crops5020013
Chicago/Turabian StyleMazibuko, Dickson Mgangathweni, Sarvesh Maskey, Kiseki Kurashina, Hiromu Okazawa, Hiroyuki Oshima, Taku Kato, and Hidehiko Kikuno. 2025. "Effects of Biochar on Growth, Response to Water Stress, and Post-Stress Recovery in Underutilized Vegetable Hibiscus sabdariffa from Malawi" Crops 5, no. 2: 13. https://doi.org/10.3390/crops5020013
APA StyleMazibuko, D. M., Maskey, S., Kurashina, K., Okazawa, H., Oshima, H., Kato, T., & Kikuno, H. (2025). Effects of Biochar on Growth, Response to Water Stress, and Post-Stress Recovery in Underutilized Vegetable Hibiscus sabdariffa from Malawi. Crops, 5(2), 13. https://doi.org/10.3390/crops5020013






