Selenium Biofortification of Allium Species
Abstract
1. Introduction
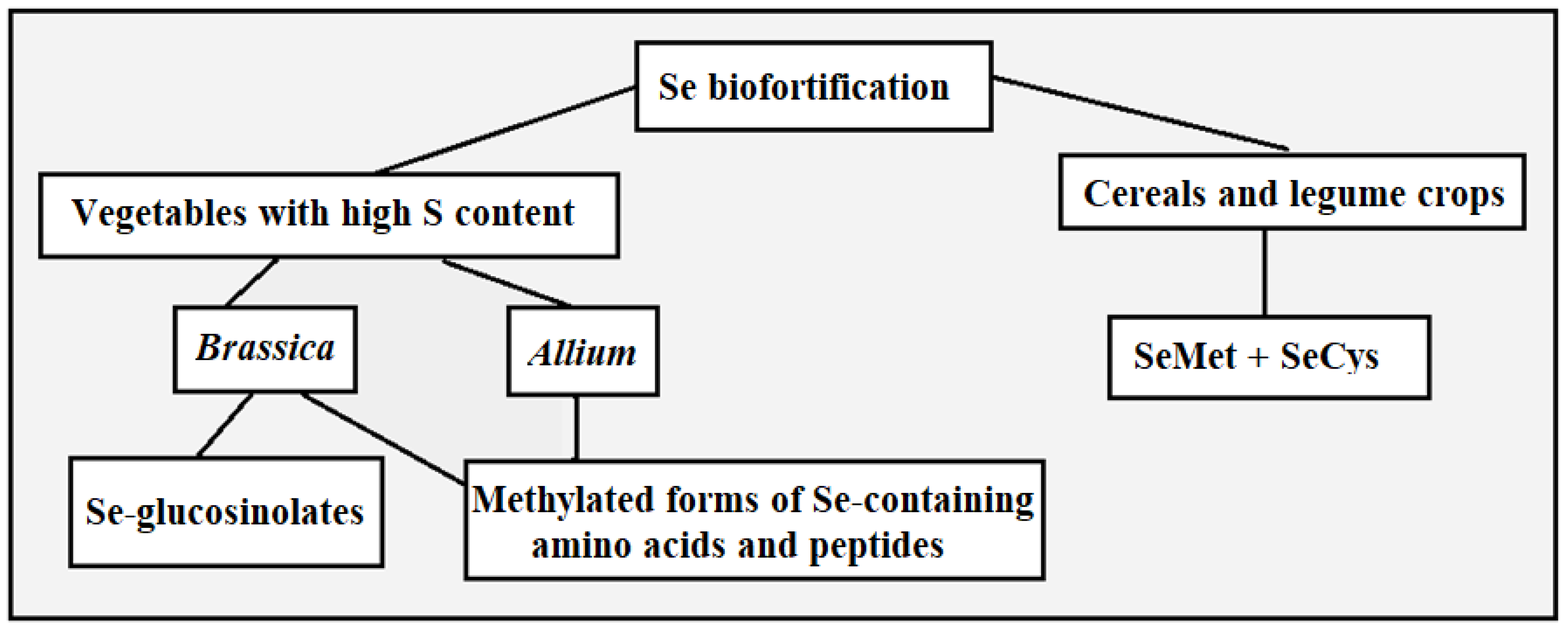
2. Vegetables as Targets of Se Biofortification
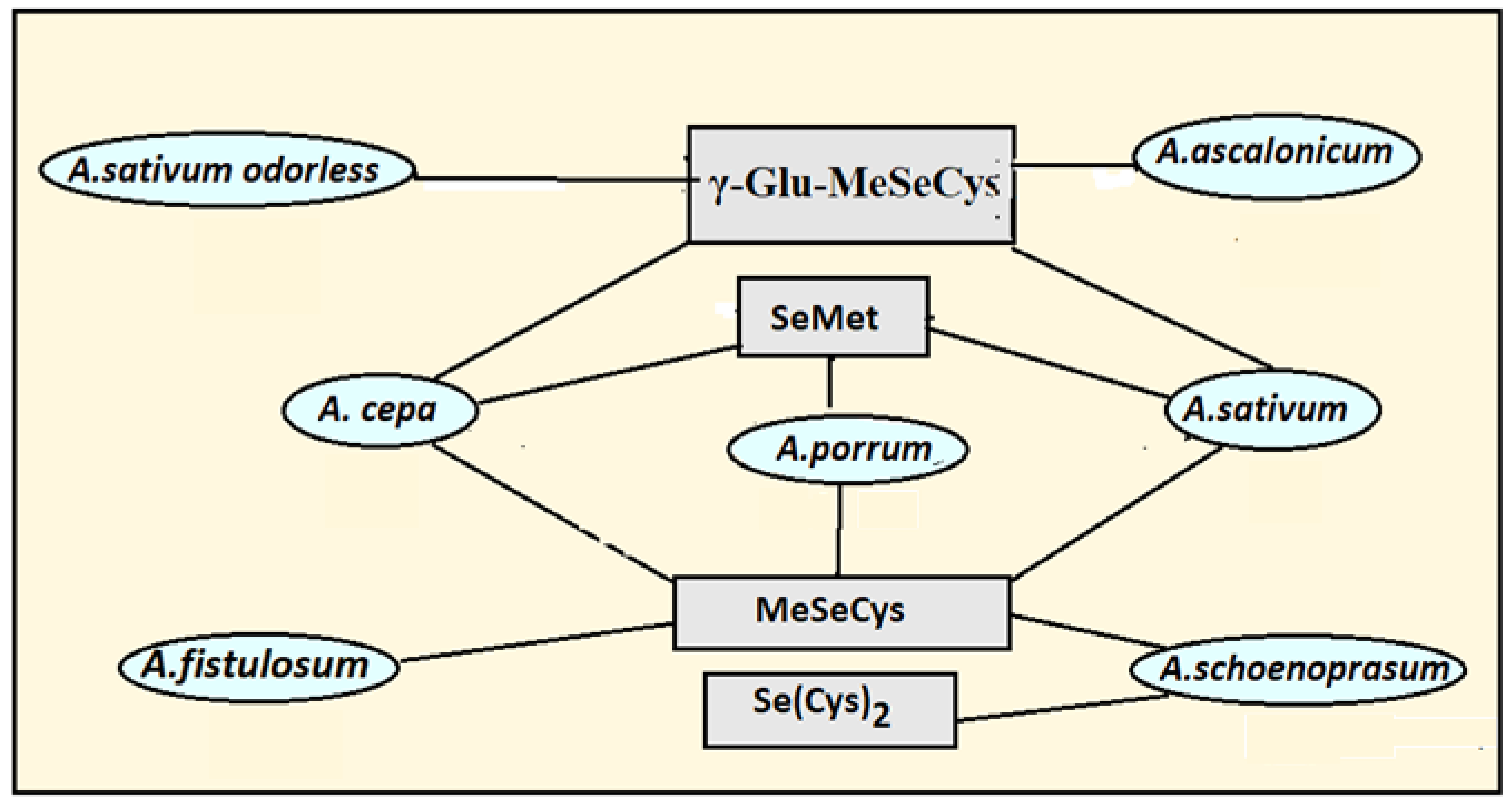
3. Peculiarities of Allium Species Biofortification with Se
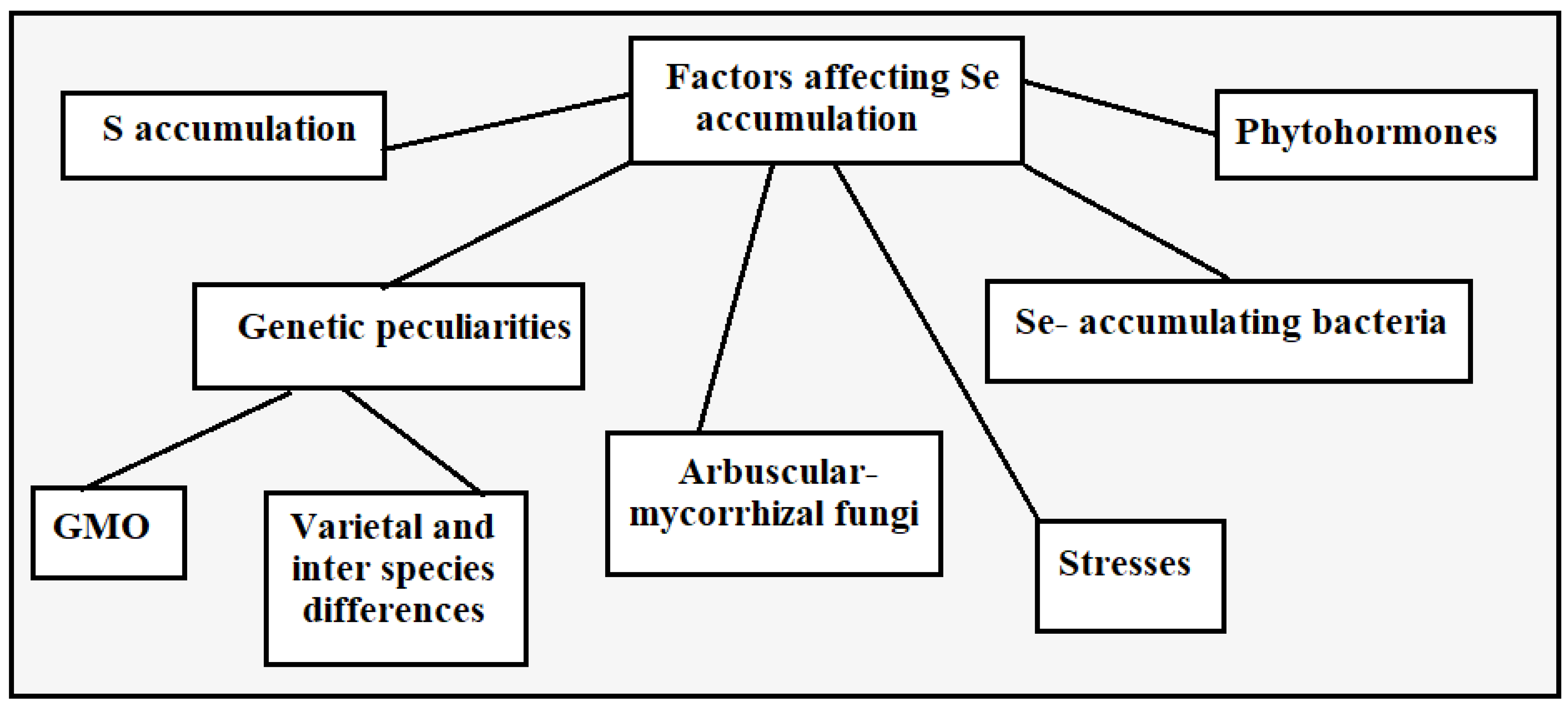
4. Arbuscular Mycorrhizal Fungi Utilization
5. Biochar Application
6. Selenium–Sulphur Interaction
7. Hormonal Regulation
8. Methods of Se Supply
9. Effect of Se Biofortification on Product Quality
10. Changes in Mineral Composition
11. Functional Food Products
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khurana, A.; Allawadhi, P.; Singh, V.; Khurana, I.; Yadav, P.; Sathua, K.B.; Allwadhi, S.; Banothu, A.K.; Navik, U.; Bharani, K.K. Antimicrobial and anti-viral effects of selenium nanoparticles and selenoprotein based strategies: COVID-19 and beyond. J. Drug Deliv. Sci. Technol. 2023, 86, 104663. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Gao, X. Editorial: Selenium and human health. Front. Nutr. 2023, 10, 1269204. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, H.; Wang, S.; Zhang, Y. Association of habitually low intake of dietary selenium with new-onset stroke: A retrospective cohort study (2004–2015 China Health and Nutrition Survey). Front. Public Health 2023, 10, 1115908. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poet, D.; McGrath, S.P. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
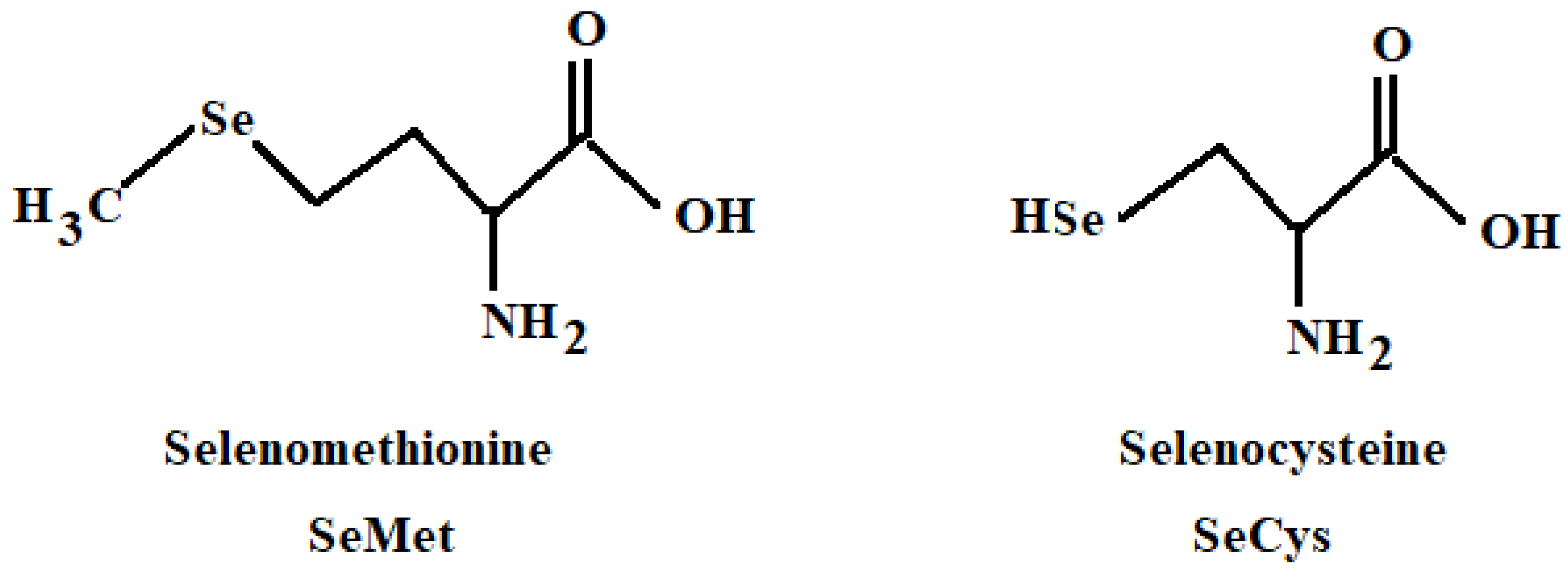
- Hu, J.; Wang, Z.; Zhang, L.; Peng, J.; Huang, T.; Yang, X.; Jeong, B.R.; Yang, Q. Seleno-Amino Acids in Vegetables: A Review of Their Forms and Metabolism. Front. Plant Sci. 2022, 13, 804368. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Waterland, N.; Moon, Y. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Kaur, M.P.; Devi, U.; Mukta, A.; Kaur, A.; Dhillon, G.S.; Padhy, A.K.; Sharma, H.; Sharma, A.; Kaur, S. Chapter 9—Wheat biofortification: A molecular breeding outlook. In QTL Mapping in Crop Improvement; Wani, S.H., Wang, D., Pratap Singh, G., Eds.; Academic Press: Boston, MA, USA, 2023; pp. 163–201. [Google Scholar] [CrossRef]
- Ferrari, L.; Cattaneo, D.M.I.R.; Abbate, R.; Manoni, M.; Ottoboni, M.; Luciano, A.; von Holst, C.; Pinotti, L. Advances in selenium supplementation: From selenium-enriched yeast to potential selenium-enriched insects, and selenium nanoparticles. Anim. Nutr. 2023, 14, 193–203. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef] [PubMed]
- Radawiec, A.; Szulc, W.; Rutkowska, B. Selenium Biofortification of Wheat as a Strategy to Improve Human Nutrition. Agriculture 2021, 11, 144. [Google Scholar] [CrossRef]
- Yan, G.; Wu, L.; Hou, M.; Jia, S.; Jiang, L.; Zhang, D. Effects of selenium application on wheat yield and grain selenium content: A global meta-analysis. Field Crops Res. 2024, 307, 109266. [Google Scholar] [CrossRef]
- Broadle, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.-J.; Breward, N. Biofortification of UK food crops with Selenium. Proc. Nutr. Soc. 2006, 65, 169–181. [Google Scholar] [CrossRef]
- Poluboyarinov, P.A.; Moiseeva, I.Y.; Mikulyak, N.I.; Golubkina, N.A.; Kaplun, A.P. New synthesis of cysteine and selenocystine enantiomers and their derivatives News of Higher Educational Technologies. Ser. Chem. Chem. Technol. 2022, 65, 19–29. (In Russian) [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminene, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Kovalsky, J.G.; Golubkina, N.A.; Papazyan, T.T.; Senkevich, O.A. The human selenium status in Khabarovsk land, 2018. Trace Elem. Med. 2019, 20, 45–53. [Google Scholar] [CrossRef]
- Kippa, A.P.; Strohmb, D.; Brigelius-Flohéa, R.; Schomburgc, L.; Bechtholdb, A.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society (DGE) Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An overview of Selenium intake, metabolism and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.S.; Freeman, J.; Arroyo, I. Accumulation and speciation of selenium in biofortified vegetables grown under high boron and saline field conditions. Food Chem. 2020, 5, 100073. [Google Scholar] [CrossRef]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Juárez-Maldonado, A.; Rivas-Martínez, E.N.; Benavides-Mendoza, A. Selenium and Sulfur to Produce Allium Functional Crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef]
- Lavu, R.V.; Du Laing, G.; Van De Wiele, T.; Pratti, V.L.; Willekens, K.; Vandecasteele, B.; Tack, F. Fertilizing soil with Selenium fertilizers: Impact on concentration, speciation, and bio accessibility of Selenium in leek (Allium ampeloprasum). J. Agric. Food Chem. 2012, 60, 10930–10935. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Kalderis, D.; Massas, I. Selenium Biofortification of lettuce plants (Lactuca sativa L.) as affected by Se species, Se rate, and a biochar co-application in a calcareous Soil. Agronomy 2022, 12, 131. [Google Scholar] [CrossRef]
- El-Nayoumy, K.; Sinha, R.; Pinto, J.T.; Rivlin, R.S. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J. Nutr. 2006, 136, 864S–869S. [Google Scholar] [CrossRef] [PubMed]
- Ogra, Y.; Ishiwata, K.; Iwashita, Y.; Suzuki, K.T. Simultaneous speciation of selenium and sulfur species in selenized odorless garlic (Allium sativum L. Shiro) and shallot (Allium ascalonicum) by HPLC–inductively coupled plasma-(octopole reaction system)-mass spectrometry and electrospray ionization-tandem mass spectrometry. J. Chrom. A 2005, 1093, 118–125. [Google Scholar] [CrossRef]
- Arı, B.; Öz, E.; Can, S.Z.; Bakırdere, S. Bioaccessibility and bioavailability of selenium species in Se-enriched leeks (Allium porrum) cultivated by hydroponically. Food Chem. 2022, 372, 131314. [Google Scholar] [CrossRef]
- Kapolna, E.; Shah, M.; Caruso, J.A.; Fodor, P. Selenium speciation studies in Se-enriched chives (Allium schoenoprasum) by HPLC-ICP–MS. Food Chem. 2007, 101, 1398–1406. [Google Scholar] [CrossRef]
- Kápolna, E.; Fodor, P. Speciation analysis of Selenium enriched green onions (Allium fistulosum) by HPLC-ICP-MS. Microchem. J. 2006, 84, 56–62. [Google Scholar] [CrossRef]
- Shah, M.; Kannamkumarath, S.S.; Wuilloud, J.C.A.; Wuilloud, R.G.; Caruso, J.A. Identification and characterization of Selenium species in enriched green onion (Allium fistulosum) by HPLC-ICP-MS and ESI-ITMS. J. Anal. At. Spectrom. 2004, 19, 381–386. [Google Scholar] [CrossRef]
- Larsen, E.H.; Lobinski, R.; Burger-Meÿer, K.; Hansen, M.; Ruzik, R.; Mazurowska, L.; Rasmussen, P.H.; Sloth, J.J.; Scholten, O.; Kik, C. Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal. Bioanal. Chem. 2006, 385, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Kápolna, E.; Laursen, K.H.; Husted, S.; Larsen, E.H. Bio-fortification and isotopic labelling of Se metabolites in onions and carrots following foliar application of Se and 77 Se. Food Chem. 2012, 133, 650–657. [Google Scholar] [CrossRef]
- Francioso, A.; Conrado, A.B.; Mosca, L.; Fontana, M. Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology. Oxid. Med. Cell. Longev. 2020, 2020, 8294158. [Google Scholar] [CrossRef]
- Bertolino, F.A.; Stege, P.W.; Salinas, E.; Raba, M.G.A.J. Electrochemical Study of the Antioxidant Activity and the Synergic Effect of Selenium with Natural and Synthetic Antioxidants. Anal. Lett. 2010, 43, 2078–2090. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Ip, C.; Lisk, D.J. Efficacy of cancer prevention by high-selenium garlic is primary dependent on the action of selenium. Carcinogenesis 1995, 16, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Birringer, M.; Block, E.; Kotrebai, M.; Tyson, J.F.; Uden, P.C.; Lisk, D.J. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J. Agric. Food Chem. 2000, 48, 2062–2070. Available online: http://scholarworks.umass.edu/chem_faculty_pubs/1046 (accessed on 19 September 2024). [CrossRef]
- de Vahl, E.; Svanberg, I. Traditional uses and practices of edible cultivated Allium species (fam. Amaryllidaceae) in Sweden. J. Ethnobiol. Ethnomed. 2022, 18, 14. [Google Scholar] [CrossRef]
- Lundegårdh, B.; Botek, P.; Schulzov, V.; Hajšlov, J.; Strömberg, A.; Andersson, H.C. Impact of different green manures on the content of S-alk(en)yl-L-cysteine sulfoxides and L-ascorbic acid in leek (Allium porrum). J. Agric. Food Chem. 2008, 56, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pan, Y.; Ali, A.; Zhang, S.; Li, X.; Qi, X.; Liu, H.; Meng, H.; Cheng, Z. Agronomic Biofortification of Garlic through Selenium and Arbuscular Mycorrhizal Fungi Application. Horticulturae 2021, 7, 230. [Google Scholar] [CrossRef]
- Slekovac, M.; Goessler, W. Accumulation of Se in natural plants and selenium supplemented vegetable and selenium speciation by HPLC-ICPMS. Chem. Spec. Bioavail. 2005, 17, 63–73. [Google Scholar] [CrossRef]
- Gandea, A.; Zagrean-Tuza, C.; Covaci, E.; Frentiu, T.; Marincas, O.; Gal, E.; Mot, A.C. Unravelling the bridge of polyphenol composition and mineralomics in Se biofortified edible Allium species. J. Food Comp. Anal. 2024, 135, 106648. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of Selenium Biofortification and Beneficial Microorganism Inoculation on Yield, Quality and Antioxidant Properties of Shallot Bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Seredin, T.; Kriachko, T.; Caruso, G. Nutritional features of leek cultivars and effect of selenium-enriched leaves from Goliath variety on bread physical quality and antioxidant attributes. Ital. J. Food Sci. 2019, 31, 288–300. [Google Scholar] [CrossRef]
- Golubkina, N.; Folmanis, G.; Tananaev, I. Comparative evaluation of selenium accumulation by Allium species after foliar application of selenium nano particles, sodium selenite and sodium selenate. Dokl. Biol. Sci. 2012, 444, 230–2333. (In Russian) [Google Scholar] [CrossRef]
- Zhang, Y.; Frankenberger, W.T. Formation of dimethylselenonium compounds in soil. Environ. Sci. Technol. 2000, 34, 776–783. [Google Scholar] [CrossRef]
- Golubkina, N.; Krivenkov, L.; Sekara, A.; Vasileva, V.; Tallarita, A.; Caruso, G. Prospects of Arbuscular Mycorrhizal Fungi Utilization in Production of Allium Plants. Plants 2020, 9, 279. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Kumar, P.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Sugihara, S.; Kondo, M.; Chihara, Y.; Yuji, M.; Hattori, H.; Yoshida, M. Preparation of selenium-enriched sprouts and identification of their selenium species by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Biosci. Biotechnol. Biochem. 2004, 68, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Golubkina, N.; Tallarita, A.; Abdelhamid, M.T.; Sekara, A. Biodiversity, Ecology, and Secondary Metabolites Production of Endophytic Fungi Associated with Amaryllidaceae Crops. Agriculture 2020, 10, 533. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid.-Based Complement. Altern. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef]
- FAOSTAT 2015. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 8 August 2015).
- Brewster, J.L. Onions and Other Vegetable Alliums; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Serra, A.D.B. Agronomy of onions. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 187–232. [Google Scholar]
- Patharajan, S.; Raaman, N. Influence of arbuscular mycorrhizal fungi on growth and selenium uptake by garlic plants. Arch. Phytopathol. Plant. Prot. 2012, 45, 138–151. [Google Scholar] [CrossRef]
- Moreno Jiménez, E.; Ferrol, N.; Corradi, N.; Peñalosa, J.M.; Rillig, M.C. The potential of arbuscular mycorrhizal fungi to enhance metallic micronutrient uptake and mitigate food contamination in agriculture: Prospects and challenges. New Phytol. 2024, 242, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Madawala, H.M.S.P. Arbuscular mycorrhizal fungi as biofertilizers: Current trends, challenges, and future prospects, Chapter 7. Biofertilizers 2021, 1, 83–93. [Google Scholar] [CrossRef]
- Yadav, S.P.S.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Paudel, P.; Shrestha, J.; et al. Biochar application: A sustainable approach to improve soil health. J. Agric. Food Res. 2023, 11, 100498. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Alkhasha, A.; Ibrahim, H.M. Effect of biochar particle size on water retention and availability in a sandy loam soil. J. Saudi Chem. Soc. 2020, 24, 1042–1050. [Google Scholar] [CrossRef]
- Aneseyee, A.B.; Wolde, T. Effect of biochar and inorganic fertilizer on the soil properties and growth and yield of onion (Allium cepa) in tropical Ethiopia. Sci. World J. 2021, 1, e5582697. [Google Scholar] [CrossRef]
- Abdelrasheed, K.G.; Mazrou, Y.; Omara, A.E.-D.; Osman, H.S.; Nehela, Y.; Hafez, E.M.; Rady, A.M.S.; El-Moneim, D.A.; Alowaiesh, B.F.; Gowayed, S.M. Soil Amendment Using Biochar and Application of K-Humate Enhance the Growth, Productivity, and Nutritional Value of Onion (Allium cepa L.) under Deficit Irrigation Conditions. Plants 2021, 10, 2598. [Google Scholar] [CrossRef]
- Praus, L.; Száková, J.; Steiner, O.; Goessler, W. Rapeseed (Brassica napus L.) biofortification with selenium: How do sulphate and phosphate influence the efficiency of selenate application into soil? Arch. Agron. Soil. Sci. 2019, 65, 2059–2072. [Google Scholar] [CrossRef]
- Mobini, M.; Khoshgoftarmanesh, A.H.; Ghasemi, S. Biofortification of onion bulb with selenium at different levels of sulfate. J. Plant Nutr. 2019, 42, 269–277. [Google Scholar] [CrossRef]
- Cheng, B.; Lian, H.F.; Liu, Y.Y.; Yu, X.H.; Sun, Y.L.; Sun, X.D.; Shi, Q.H.; Liu, S.Q. Effects of Selenium and Sulfur on antioxidants and physiological parameters of garlic plants during senescence. J. Integr. Agric. 2016, 15, 566–572. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Randle, W.M. Selenate concentration affects selenium and sulfur uptake and accumulation by ‘Granex 23’ onions. J. Amer. Soc. Hort. Sci. 1997, 122, 721–726. [Google Scholar] [CrossRef]
- Rebecca, A.J. Effect of Selenium, Sulphur and their Interaction on Yield, Contents and Uptake by Onion (Allium cepa L.). Int. J. Pure App. Biosci. 2017, 5, 1403–1410. [Google Scholar] [CrossRef]
- Golubkina, N. Selenium biorhythms and hormonal regulation. In Selenium: Sources, Functions and Health Effects; Nova Science Publishers: New York, NY, USA, 2012; pp. 33–75. [Google Scholar]
- Bandehagh, A.; Dehghanian, Z.; Gougerdchi, V.; Hossain, M.A. Selenium: A Game Changer in Plant Development, Growth, and Stress Tolerance, via the Modulation in Gene Expression and Secondary Metabolite Biosynthesis, Phyton-International. J. Exp. Bot. 2023, 92, 2301–2324. [Google Scholar] [CrossRef]
- Golubkina, N.; Dobrutskaya, H.; Novoselov, J. Hormonal regulation of selenium accumulation by plants. Veg. Crops Russ. 2015, 3, 104–107. [Google Scholar] [CrossRef]
- Khrikina, J.; Golubkina, N.; Nikulshin, V.; Grigoryants, I.; Bogachev, V. Selenium accumulation by garlic Allium sativum L. Bull. Russ. Acad. Sci. 2007, 5, 32–33. (In Russian) [Google Scholar]
- Galeas, M.L.; Zhang, L.H.; Freeman, J.L.; Wegner, M.; Pilon-Smits, E.A. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyper-accumulators and related non-accumulators. New Phytol. 2007, 173, 517–525. [Google Scholar] [CrossRef]
- Freeman, J.L.; Zhang, L.H.; Marcus, M.A.; Fakra, S.; Pilon-Smits, R.A.H. Spatial imaging, speciation and quantification of selenium in the hyperaccumulator plant Stragalus bisulcatus and Stanleya pinnata. Plant Physiol. 2006, 142, 124–134. [Google Scholar] [CrossRef]
- Machado, B.Q.V.; Pereira, B.J.; Rezende, G.F.; Filho, A.B.C. Onion quality and yield after agronomic biofortification with selenium. Chilian J. Agric. Res. 2024, 84, 372–379. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium- and Small-Scale Operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Stylianidis, G.P.; Manta, V.; Karousis, E.N.; Tzanaki, A.; Dimitriadi, D.; Bouzas, E.A.; Siyiannis, V.F.; Constantinou-Kokotou, V.; Chorianopoulou, S.N. Floret Biofortification of Broccoli Using Amino Acids Coupled with Selenium under Different Surfactants: A Case Study of Cultivating Functional Foods. Plants 2023, 12, 1272. [Google Scholar] [CrossRef]
- Dobrzyńska, M.; Drzymała-Czyż, S.; Woźniak, D.; Drzymała, S.; Przysławski, J. Natural Sources of Selenium as Functional Food Products for Chemoprevention. Foods. 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Amerian, M.; Vafa, M.K.; Palangi, A.; Gohari, G.; Ntatsi, G. Potential Assessment of Selenium for Improving Yield and Nitrogen Use Efficiency in Garlic (Allium sativum L.). Agrotech. Ind. Crops 2024, 4, 106–112. [Google Scholar]
- Sohrabi, M.; Mehrjerdi, M.Z.; Karimi, S.; Tavallali, V. Using gypsum and selenium foliar application for mineral biofortification and improving the bioactive compounds of garlic ecotypes. Ind. Crops and Prod. 2020, 154, 112742. [Google Scholar] [CrossRef]
- Keshari, P.; Sharma, S.; Yadav, V.; Majumdar, R.S.; Teotia, S. Effect of selenium treatment on the physico-chemical and phytochemical properties of Allium sativum L. Int. J. Plant Res. Vegetos 2023, 37, 1534–1542. [Google Scholar] [CrossRef]
- Põldma, P.; Tõnutare, T.; Viitak, A.; Luik, A.; Moor, U. Effect of Selenium treatment on mineral nutrition, bulb size, and antioxidant properties of garlic (Allim sativum L.). J. Agric. Food Chem. 2011, 59, 5498–5503. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Antoshkina, M.; Agafonov, A.; Nadezhkin, S. Intervarietal differences in accumulation of biologically active compounds by Allium cepa L. Messenger Russ. Agric. Sci. 2016, 2, 51–55. [Google Scholar]
- Põldma, P.; Moor, U.; Tõnutare, T.; Herodes, K.; Rebane, R. Selenium treatment under field conditions affects mineral nutrition, yield and antioxidant properties of bulb onion (Allium cepa L.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 167–181. [Google Scholar]
- de Paiva, L.G.; Grangeiro, L.C.; do Nascimento, C.W.A.; Costa, R.M.C.; Pereira, N.A.E.; de Lima, R.B.; de P. Souza, B.; de A. Carmo, L.H.; Oliveira, R.R.T.; Morais, É.G. Selenium as an inorganic biostimulant in onion grown in a semi-arid climate Brazilian. J. Agric. Environ. Eng. 2024, 28, e279061. [Google Scholar]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef]
- Vuković, S.; Moravčević, D.; Gvozdanović-Varga, J.; Dojčinović, B.; Vujošević, A.; Pećinar, I.; Kilibarda, S.; Kostić, A.Ž. Elemental Profile, General Phytochemical Composition and Bioaccumulation Abilities of Selected Allium Species Biofortified with Selenium under Open Field Conditions. Plants 2023, 12, 349. [Google Scholar] [CrossRef]
- Amagova, Z.; Matsadze, V.; Golubkina, N.; Seredin, T.; Caruso, G. Selenium biofortification of wild garlic. Veg. Crops Russ. 2018, 4, 76–80. [Google Scholar] [CrossRef]
- Pérez, M.B.; Lipinski, V.M.; Filippini, M.F.; Chacón-Madrid, K.; Arruda, M.A.Z.; Wuilloud, R.G. Selenium biofortification on garlic growth and other nutrients accumulation. Hortic. Bras. 2019, 37, 294–301. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, A.; Prakash, R.; Prakash, N.T. Selenium accumulation and Se-induced anti-oxidant activity in Allium cepa. Environ. Inform. Arch. 2007, 5, 328–336. [Google Scholar]
- Tsuneyoshi, T.; Yoshida, J.; Sasaoka, T. Hydroponic cultivation offers a practical means of producing Selenium enriched garlic. J. Nutr. 2006, 136, 870–872. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Y.; Li, Y.-F.; Hu, Y.; Peng, X.; Dong, Y.; Li, B.; Chen, C.; Chai, Z. Selenium inhibits the phytotoxicity of mercury in garlic (Allium sativum). Environ. Res. 2013, 125, 75–81. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. S. Afr. J. Bot. 2019, 121, 447–455. [Google Scholar] [CrossRef]
- Selenium-Enriched Onions and Preparation Method Thereof. CN Patent CN103004567B, 28 December 2012.
- Kopsell, D.A.; Randle, W.M. Selenium affects the S-alk(en)yl cysteine sulfoxides among short-day onion cultivars. J. Am. Soc. Hortic. Sci. 1999, 124, 307–311. [Google Scholar] [CrossRef]
- Golubkina, N.; Seredin, T.; Baranova, H.; Startseva, L.; Agafonov, A.; Ushakova, O. Prospects of selenium biofortified Allium seedling production. Veg. Crops Russ. 2018, 6, 50–54. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2022, 12, 44. [Google Scholar] [CrossRef]
- Asgher, M.; Rehaman, A.; Islam, S.N.U.; Arshad, M.; Khan, N.A. Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants. Agriculture 2023, 13, 1083. [Google Scholar] [CrossRef]
- Golubkina, N.; Seredin, T.; Koshevarov, A.; Shilo, L.; Baranova, H.; Pavlov, L. Garlic powder biofortified with selenium. Microelem. Med. 2018, 19, 43–50. [Google Scholar] [CrossRef]
- Vollmannová, A.; Bojnanská, T.; Musilová, J.; Lidiková, J.; Cifrová, M. Quercetin as one of the most abundant represented biological valuable plant components with remarkable chemoprotective effects—A review. Heliyon 2024, 10, E33342. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Dey, S.; Raychaudhuri, S.S. Selenium biofortification improves bioactive composition and antioxidant status in Plantago ovata Forsk., a medicinal plant. Genes Environ. 2023, 45, 38. [Google Scholar] [CrossRef]
- Bystrická, J.; Kavalcová, P.; Musilová, J.; Tomáš, J.; Tóth, T.; Orsák, M. Selenium and Its influence on the content of polyphenol compounds in onion (Allium cepa L.). J. Microbiol. Biotechnol. Food Sci. 2015, 4, 23–26. [Google Scholar] [CrossRef]
- Skrypnik, L.; Feduraev, P.; Golovin, A.; Maslennikov, P.; Styran, T.; Antipina, M.; Riabova, A.; Katserov, D. The Integral Boosting Effect of Selenium on the Secondary Metabolism of Higher Plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.; Valverde, J.; Finn, L.; Gaffney, M.; Rai, D.K.; Brunton, N. A note on the effectiveness of selenium supplementation of Irish-grown Allium crops. Irish J. Agric. Food Res. 2014, 53, 91–99. [Google Scholar]
- Adhikari, P. Biofortification of Selenium in Broccoli (Brassica oleracea L. var.italica) and Onion (Allium cepa L.). Master’s Thesis, Norwegian University of Life Sciences, Ås Municipality, Norway, 2012. [Google Scholar]
- Gazhemi, K.; Bolandnazar, S.; Tabatbaei, S.J.; Pirdazhti, H.; Arzanlou, M.; Ebrahimzadeh, M.A. Antioxidant properties of garlic as affected by selenium and humic acid treatmenrts. NZJ Crop Hortic. Sci. 2015, 43, 173–181. [Google Scholar]
- Shokri, M.; Zare Mehrjerdi, M.; Karimi, S.; Tavallali, V. Foliar Application of Selenium Improved Drought Tolerance and Quality of Garlic. Available online: https://ssrn.com/abstract=4663102 (accessed on 13 December 2023).
- Golubkina, N.A. Prospects of onion (Allium cepa L.) fertilization by selenium. Mini-review. Trace Elem. Med. 2016, 17, 4–9. [Google Scholar] [CrossRef]
- Hu, W.; Su, Y.; Yang, R.; Xie, Z.; Gong, H. Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field. Horticulturae 2023, 9, 1126. [Google Scholar] [CrossRef]
- Hu, W.; Su, Y.; Zhou, J.; Zhu, H.; Guo, J.; Huo, H.; Gong, H. Foliar application of silicon and selenium improves the growth, yield and quality characteristics of cucumber in field conditions. Sci. Hort. 2022, 294, 110776. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef]
- Cunha, M.L.O.; de Mello Prado, R. Synergy of Selenium and Silicon to Mitigate Abiotic Stresses: A Review. Gesunde Pflanz. 2023, 75, 1461–1474. [Google Scholar] [CrossRef]
- Al-Salami, M.O.G.; Alhasnawi, N.J.R. Effect of Water Stress and Selenium Spraying on Vegetative and Yield Indicators of Garlic Allium sativum L. IOP Conf. Ser. Earth Environ. Sci. 2023, 1262, 052042. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effect of selenium application on phenylalanine ammonia-lyase (PAL) activity, phenol leakage and total phenolic content in garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- Afton, S.E.; Caruso, J.A. The effect of Se antagonism on the metabolic fate of Hg in Allium fistulosum. J. Anal. At. Spectrom. 2009, 24, 759–766. [Google Scholar] [CrossRef]
- Bybordi, A.; Saadat, S.; Zargaripour, P. The effect of zeolite, selenium and silicon on qualitative and quantitative traits of onion grown under salinity conditions. Arch. Agron. Soil Sci. 2018, 64, 520–530. [Google Scholar] [CrossRef]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Secur. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Odunlade, T.V.; Famuwagun, A.A.; Taiwo, K.A.; Gbadamosi, S.O.; Oyedele, D.J.; Adebooye, O.C. Chemical Composition and Quality Characteristics of Wheat Bread Supplemented with Leafy Vegetable Powders. J. Food Qual. 2017, 2017, 9536716. [Google Scholar] [CrossRef]
- Radovanović, B.; Mladenović, J.; Radovanović, A.; Pavlović, R.; Nikolić, V. Phenolic Composition, Antioxidant, Antimicrobial and Cytotoxic Activites of Allium porrum L. (Serbia) Extracts. J. Food Nutr. Res. 2015, 3, 564–569. [Google Scholar] [CrossRef]
- Garvin, D.F.; Hareland, G.; Gregoire, B.R.; Finley, J.W. Impact of Wheat Grain Selenium Content Variation on Milling and Bread Baking. Cereal Chem. 2011, 88, 195–200. [Google Scholar] [CrossRef]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The Chemical Compositions of the Volatile Oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef]
- Rahman, M.M.; Fazlic, V.; Saad, N.W. Antioxidant properties of raw garlic (Allium sativum) extract. Int. Food Res. J. 2012, 19, 589–591. [Google Scholar]
- Ryu, J.H.; Kang, D. Physicochemical properties, biological activity, health benefits, and general limitations of aged black garlic: A Review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and Antioxidant Properties of Black Garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- Method for Processing Selenium-Rich Black Garlic Product Pat. CN 103798702A, 9 October 2013.
- Golubkina, N.A.; Seredin, T.M.; Kosheleva, O.V.; Soldatenko, A.V. Effect of selenium on biochemical characteristics of «Black garlic». Trace Elem. Med. 2010, 18, 3–7. [Google Scholar]
- Choe, U.; Yu, L.; Wang, T.T.Y. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef]
- Pannico, A.A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef]
- Tan, L.; Nuffer, H.; Feng, J.; Kwan, S.H.; Chen, H.; Tong, X.; Kong, L. Antioxidant Properties and Sensory Evaluation of Microgreens from Commercial and Local Farms. Food Sci. Hum. Wellness 2020, 9, 45–51. [Google Scholar] [CrossRef]


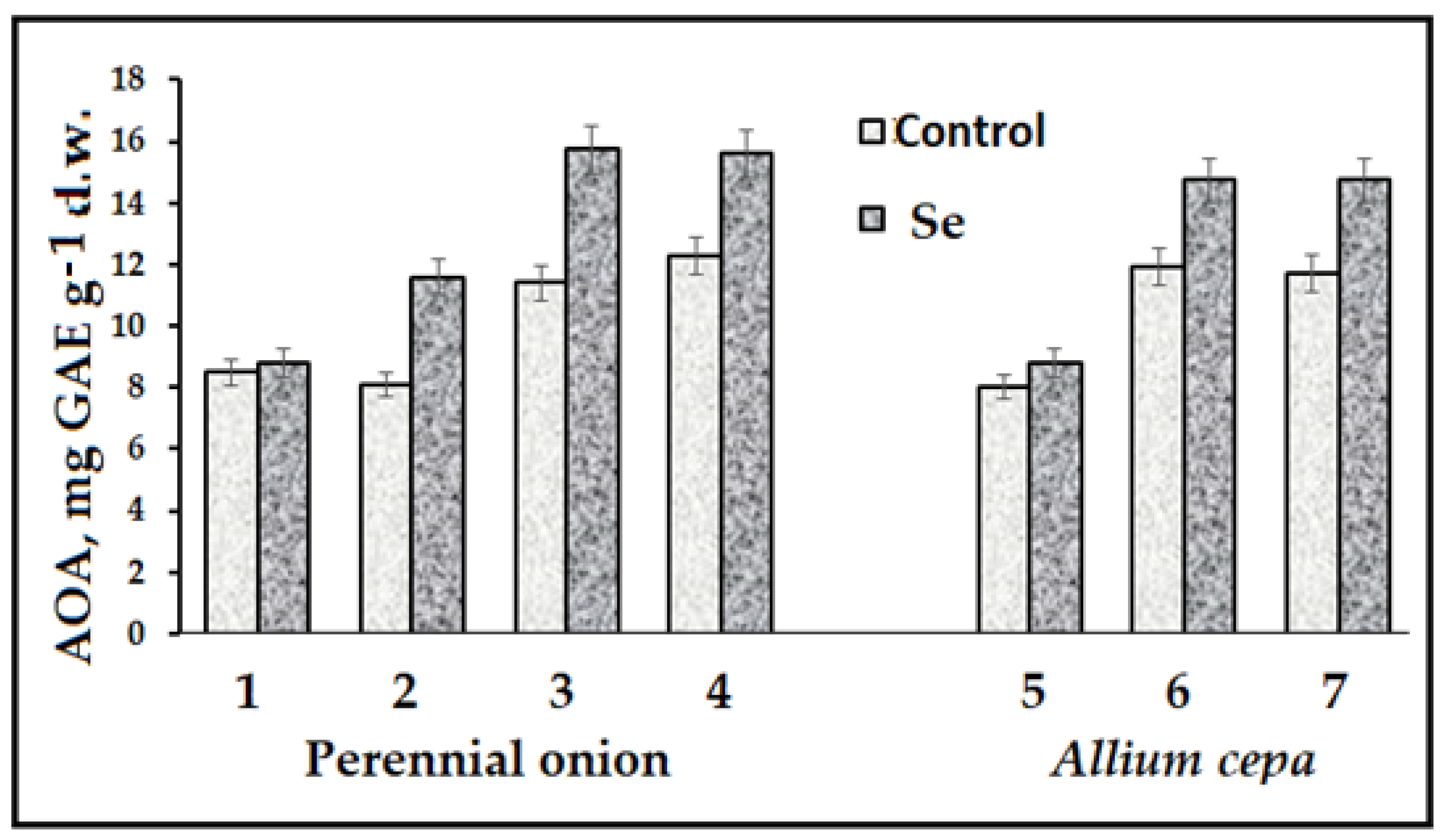
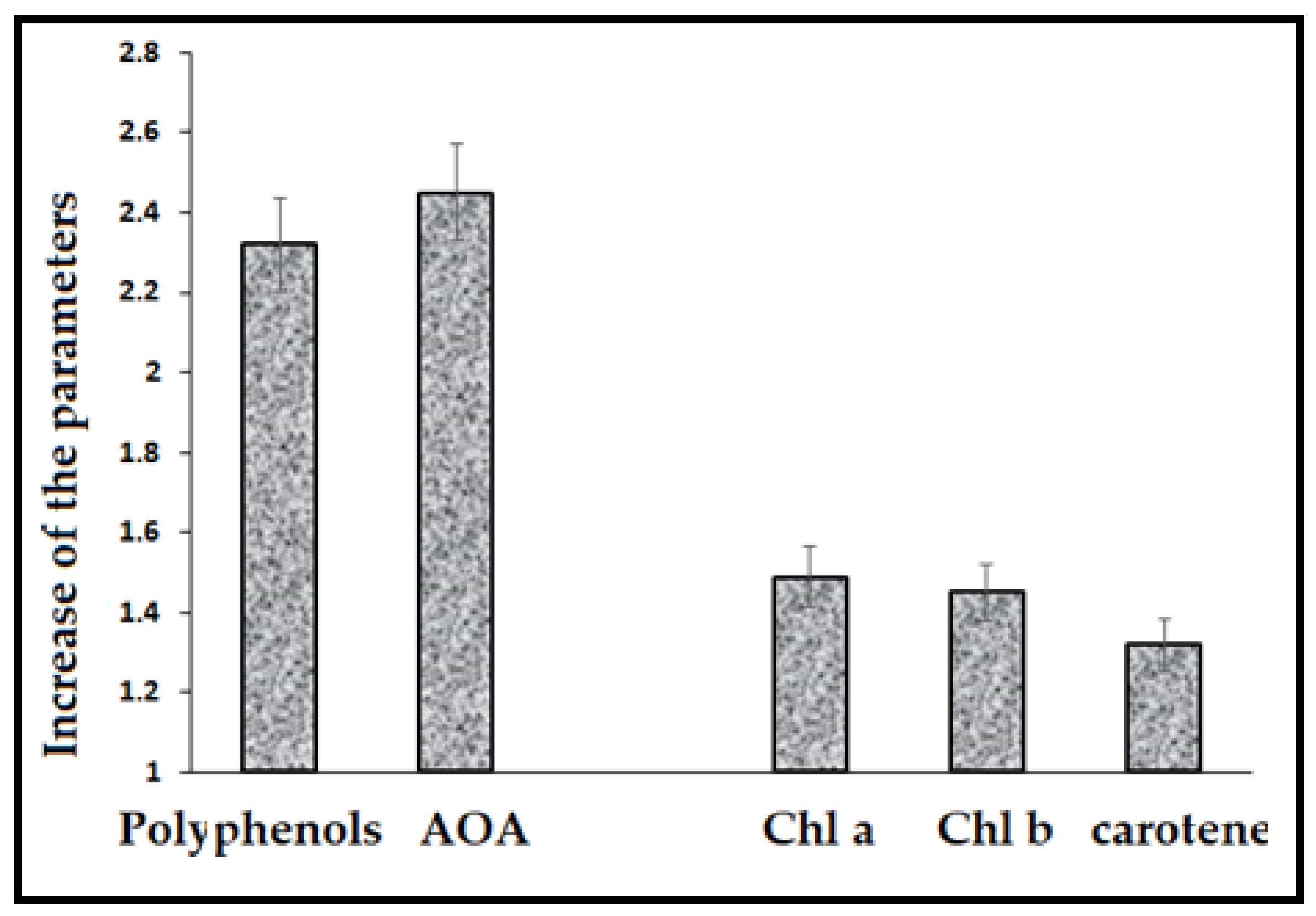

| Phytohormone | Biochemical Effect | Ref. |
|---|---|---|
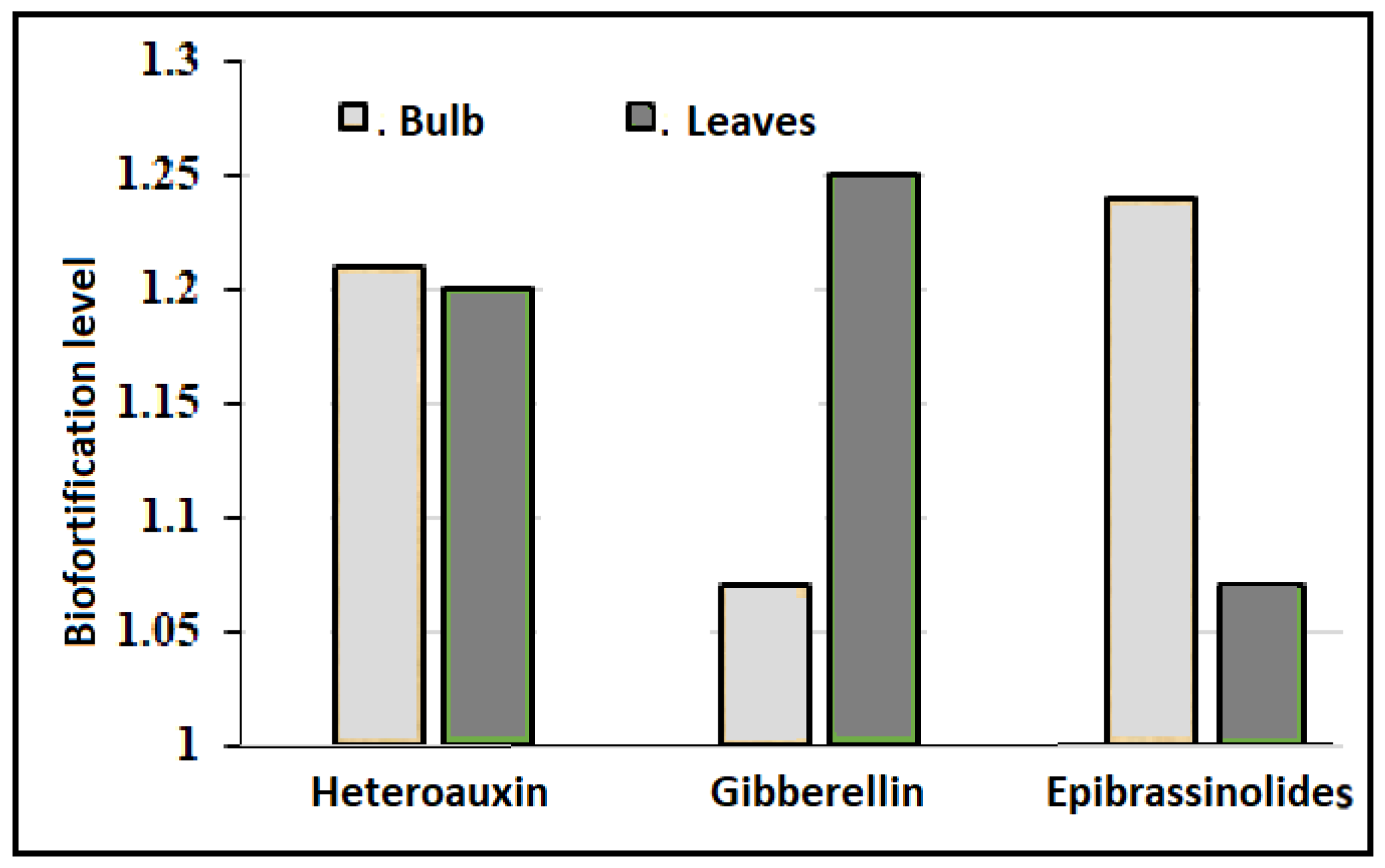
| Epibrassinolides | Increase in Se accumulation by garlic leaves by 25% | [72,73] |
| Heteroauxin | High Se levels in young garlic leaves and reproductive organs, Se content increase in leaves and bulbs by 15–16% via heteroauxin supply (125 mg L−1) | [73,74] |
| Gibberellin | Se accumulation increase in garlic cloves by 26% under single plant spray using gibberellin solution (125 mg L−1) | [73] |
| Methyl jasmonic acid, ethylene, salicylic acid | Hyperaccumulators with extremely high Se content possess higher levels of these hormones compared to non-accumulators | [75] |
| Species | Se Form | Effect of Foliar Se Supply | Ref. |
|---|---|---|---|
| A. sativum | Se (VI) | Maximum efficiency of N utilization and yield at 150 kg ha−1 N and 10 mg selenate L−1 | [81] |
| Gypsum (0–20–40 ppm S) + Se (VI) (1–15–30 ppm Se) | Increased AOC, TP, sugar, and allicin under joint application of high S and low Se, which provides better results with foliar Se supply than soil Se treatment at the optimal concentration of 10 mg Se L−1 | [82] | |
| Se (VI) 0.5–10 mg selenate L−1 | Pro-oxidant effect of 8–10 mg L−1 selenate solution increases proline and SOD and decreases total protein, sugar, phenolics, and flavonoids | [83] | |
| 10–100 mg L−1 Se (VI) | 10–50 mg Se L−1 increased yield and AOA | [84] | |
| A. cepa | Se (VI) 50 mg L−1 | Varietal differences in sugar, TP, and AOA levels | [85] |
| 0–200 g Se (VI) ha−1 | The highest yield at 100 g ha−1 had no effect on pungency and mineral composition, with foliar supply more beneficial than soil supplementation | [76] | |
| 10–100 µg Se mL−1 | The highest yield, AOC, and TP were at 50 µg Se mL−1 | [86] | |
| 15–60 g Se ha−1 | Out of 2 cultivars, only one showed significant increases in yield and dry weight | [87] | |
| A. cepa, A. sativum | AMF + Se (VI) 0.26 mM solution | The increases in yield, monosaccharides, Fl, and mineral content under AMF and Se supply were highly specific, differing between garlic and onion grown in similar environmental conditions | [88] |
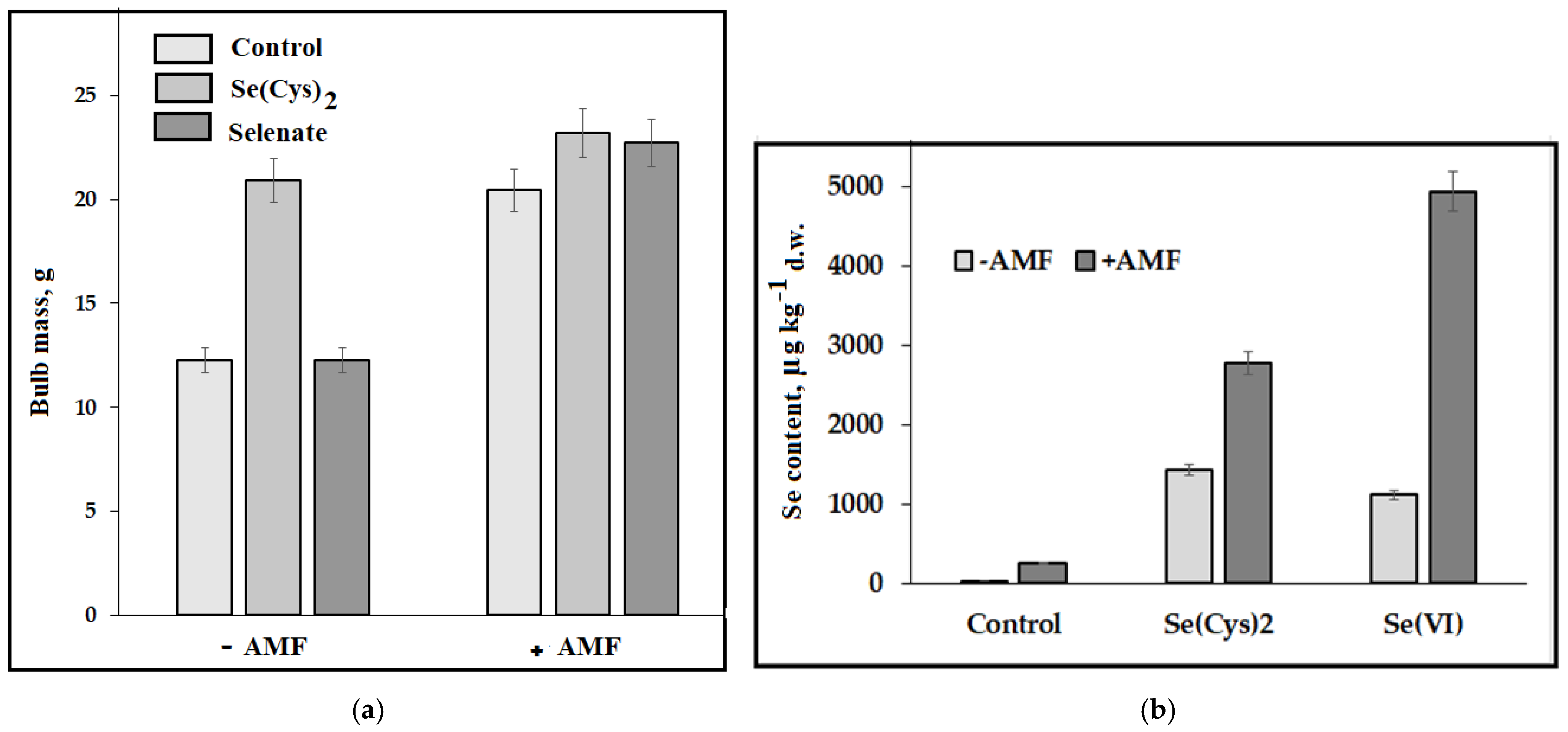
| A. cepa L. Aggregatum group | AMF + Se (VI)/Se(Cys)2 0.26 mM solution | AMF increased Se accumulation; Se(Cys) increased yield more efficiently without AMF supply, and the opposite phenomenon was recorded for Se (VI) | [46] |
| A. schoenoprasum, A. odorum | 10–30 g Se (VI) ha−1 | Increase in TP and Fl in A. schoenoprasum in both seasons, and in A. odorum only in drought conditions; with 10 g ha−1 was the highest mineral content without drought, and under drought, it was 20 g ha−1 for A. schoenoprasum and 30 g ha−1 for A. odorum. | [89] |
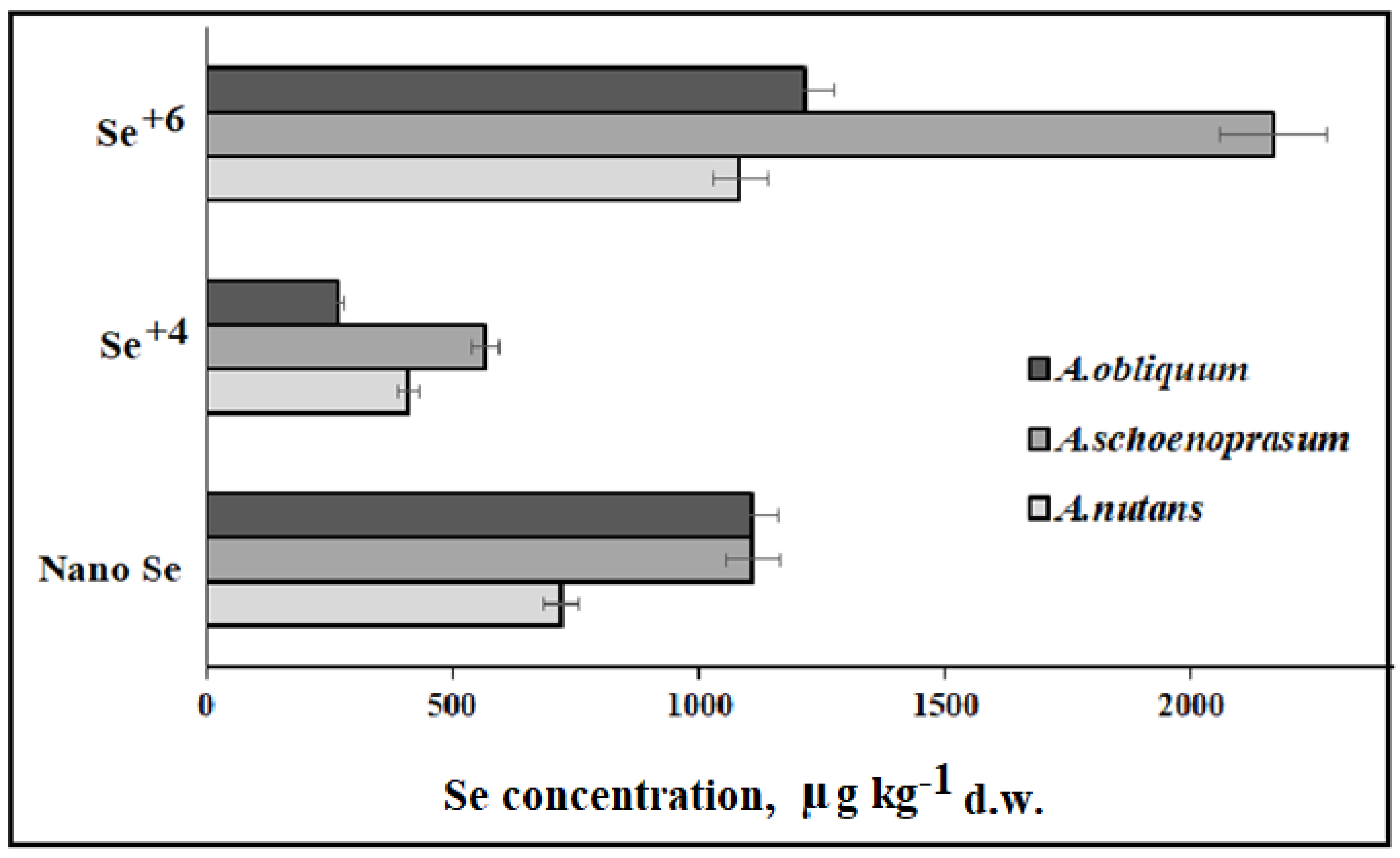
| A. nutans, A. obliquum, A. schoenoprasum | Se (VI) | Leaf Se levels were affected by genetic factors and the chemical form of Se, with the highest values in A. schoenoprasum and Se (VI) | [47] |
| A. ursinum | Se (VI) 50 mg L−1 | Increases in chlorophyll, carotene, TP, and AOC; Cr, Fe, V increased and Si decreased | [90] |
| A. porrum | 75 mg Se (VI) m−2 | Increases in TP and monosaccharides in pseudo-stems and TP in leaves | [47] |
| Species | Se Form | Effect of Soil Se Supply | Ref. |
|---|---|---|---|
| A. sativum | 0–15 kg ha−1 Se (VI) and Se (IV) | Decreased accumulation of Mg, Mn, Cu, Fe, P, and S, but increased Zn content, with no effect on garlic growth; Se content was up to 49.5 mg kg−1 d.w. Se supplementation led to significant modifications of the accumulation and distribution of Zn, Mg, Mn, Fe, Cu, P, and S between leaves, bulbs, and roots | [91] |
| A. cepa | Se (VI)/Se (IV) | Bulbing was delayed under Se supply. Se (VI) induced higher AOA than Se (IV); Se distribution was as follows: bulbs ≥ leaves > roots | [92] |
| A. fistulosum, A. cepa, A. ampelo-prasum, A. schoe-noprasum, A. sene-scens, subsp. montanum, A. obliquum | 3, 5, 20 mg kg−1 Se (VI) | Se biofortification led to evident changes in 57 polyphenol profiles, with species-specific variations. The optimal concentration was 5 mg Se L−1 | [45] |
| A. fistulosum | 1, 3, 5, 15 mg Se (IV) L−1 | No growth inhibition; the maximum Se level was 30 mg kg−1 d.w. | [34] |
| Se (IV)/SeMet 10–100 mg L−1 | The amounts of SeCys and MeSeCys depend on the chemical form and dose of Se supplied; Se accumulation levels in cases of SeMet are significantly higher than under Se (IV) supply | [32] |
| Species | Se Form | Effect of Se Supply in Hydroponic System | Ref. |
|---|---|---|---|
| A. sativum | 0–3–5 µM L−1 | Lower levels of lipid peroxidation SOD activity, higher levels of GPx and catalase in garlic shoots | [67] |
| Se (VI)/Se (IV) 50 µM L−1 | Garlic seedlings produced more methylated forms of Se amino acids under Se (VI) than Se (IV); Se–S antagonism was observed | [93] | |
| > 1 mg L−1 Se(VI)/Se (IV) | Se (VI) and Se (IV) at high concentrations (>1 mg L−1) inhibited Hg accumulation to the same extent | [94] | |
| 4–16 mg L−1 Se(VI) | Se alleviated salt stress and improved TP in plants | [95] | |
| A. cepa | 0.5–2 mg L−1 | At low concentrations, Se stimulated S accumulation; Se levels decreased according to the following: leaves > roots > bulbs | [68] |
| Nano-Se: 10–30 early stage; 20–60 middle stage; 10–20 mg L−1 late stage | Optimal conditions to obtain onion seedlings with the highest content of organic Se | [96] | |
| 2 mg L−1 Se (VI) | Se decreased S content in bulbs | [97] | |
| A. schoeno-prasum | 10–100 mg L−1 Se(IV) or SeMet | Se (IV) elicited more inorganic Se in the resulting product than SeMet. The Se concentration reached 200 mg kg−1 d.w. | [31] |
| A. porrum | Se (VI)/Se (IV) | A. porrum may accumulate more than 1000 mg Se kg−1 d.w. without growth inhibition; despite the higher bioavailability of Se (VI), Se (IV) led to significantly higher organic Se (MeSeCys and SeMet) | [30] |
| A. fistulosum, A. nutants, A. obliquum, A. altaicum, 3 cvs of A. cepa | 10 mg Se (VI) L−1 | Species and varietal differences in Se accumulation levels, AOC, and TP. Se concentration decreased according to the following: A. fistulosum > A. nutans > A. obliquum > A. altaicum | [98] |
| Conditions | Effect | References |
|---|---|---|
| Drought, vegetative experiment, foliar selenate supply | Allicin, Se, and S increased in bulbs, and water-soluble proteins and protein in leaves | [111] |
| Foliar selenate supply, field conditions | Yield increased by 1.32 times | [117] |
| Salinity, 60 MM NaCl. Hydroponics, 16 mg L−1 selenate supply | A low dose of Se enhanced plant tolerance to salt stress and decreased oxidative injury by boosting the activities of antioxidants, decreasing garlic MDA and increasing photosynthetic pigments, yield, and enzymes of antioxidant activity | [95,118] |
| Selenate, selenite 1 mg L−1 + mercury salts | Inhibition of Hg accumulation and yield increased | [94] |
| Selenite, perlite | Inhibition of Hg accumulation in A. fistulosum | [119] |
| Selenate, semi-arid conditions | Increase of A. cepa yield | [87] |
| Salinity, selenate, silt loam soil with 8 dS m−1 salinity, 0.5 and 1 kg Se ha−1 | A. cepa yield increase and decrease in Na+ accumulation. Maximum yield occurred with enhancements in the physiological and qualitative indices of Allium cepa | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Nemtinov, V.; Amagova, Z.; Skrypnik, L.; Nadezhkin, S.; Murariu, O.C.; Tallarita, A.V.; Caruso, G. Selenium Biofortification of Allium Species. Crops 2024, 4, 602-622. https://doi.org/10.3390/crops4040042
Golubkina N, Nemtinov V, Amagova Z, Skrypnik L, Nadezhkin S, Murariu OC, Tallarita AV, Caruso G. Selenium Biofortification of Allium Species. Crops. 2024; 4(4):602-622. https://doi.org/10.3390/crops4040042
Chicago/Turabian StyleGolubkina, Nadezhda, Victor Nemtinov, Zarema Amagova, Liubov Skrypnik, Sergey Nadezhkin, Otilia Cristina Murariu, Alessio Vincenzo Tallarita, and Gianluca Caruso. 2024. "Selenium Biofortification of Allium Species" Crops 4, no. 4: 602-622. https://doi.org/10.3390/crops4040042
APA StyleGolubkina, N., Nemtinov, V., Amagova, Z., Skrypnik, L., Nadezhkin, S., Murariu, O. C., Tallarita, A. V., & Caruso, G. (2024). Selenium Biofortification of Allium Species. Crops, 4(4), 602-622. https://doi.org/10.3390/crops4040042










