Genotypic Variability in Response to Heat Stress and Post-Stress Compensatory Growth in Mungbean Plants (Vigna radiata [L.] Wilczek)
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Glasshouse Experiment
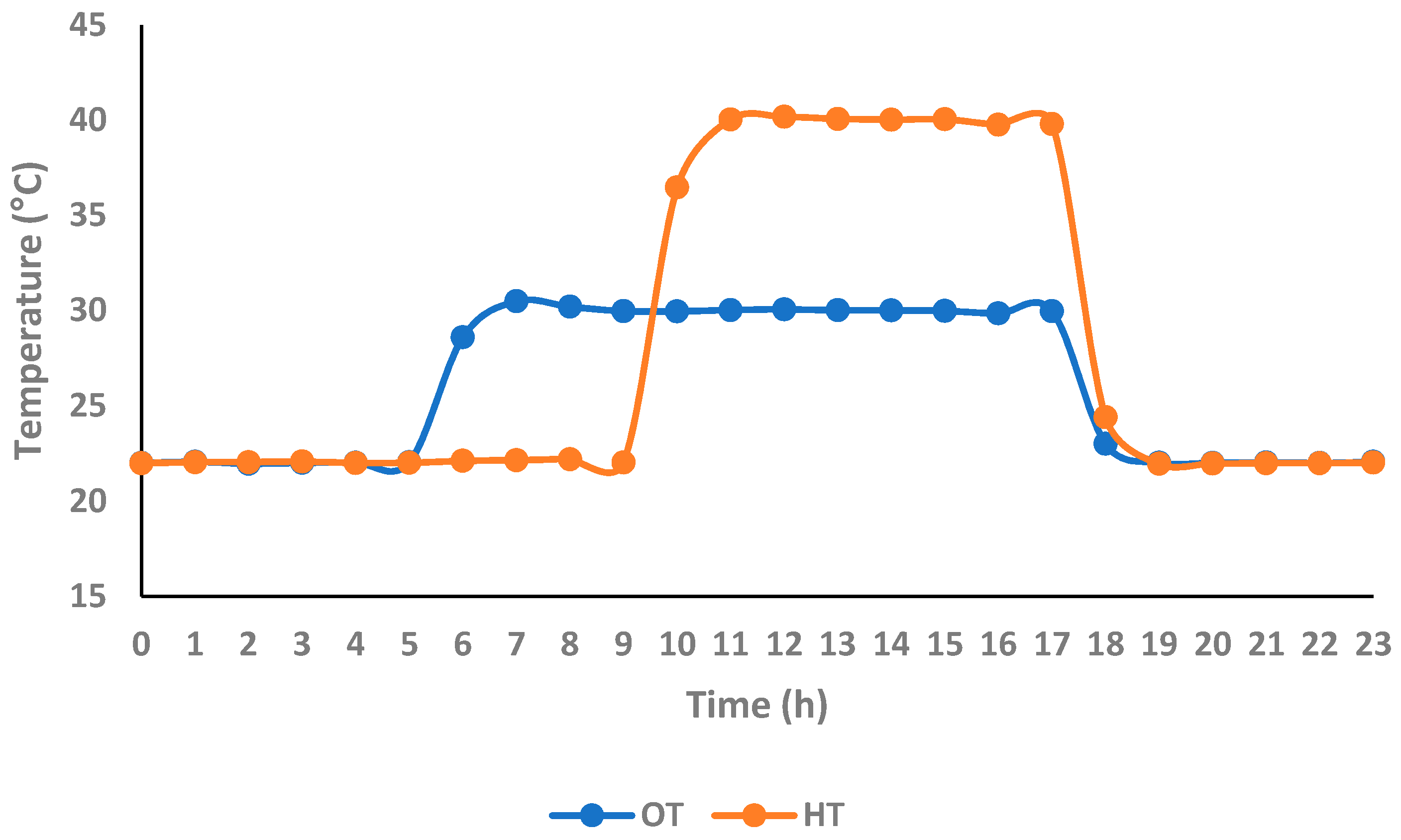
2.2.1. Experimental Details
2.2.2. Measurements
2.3. Field Experiment
2.4. Statistical Analyses
3. Results
3.1. Glasshouse Experiment
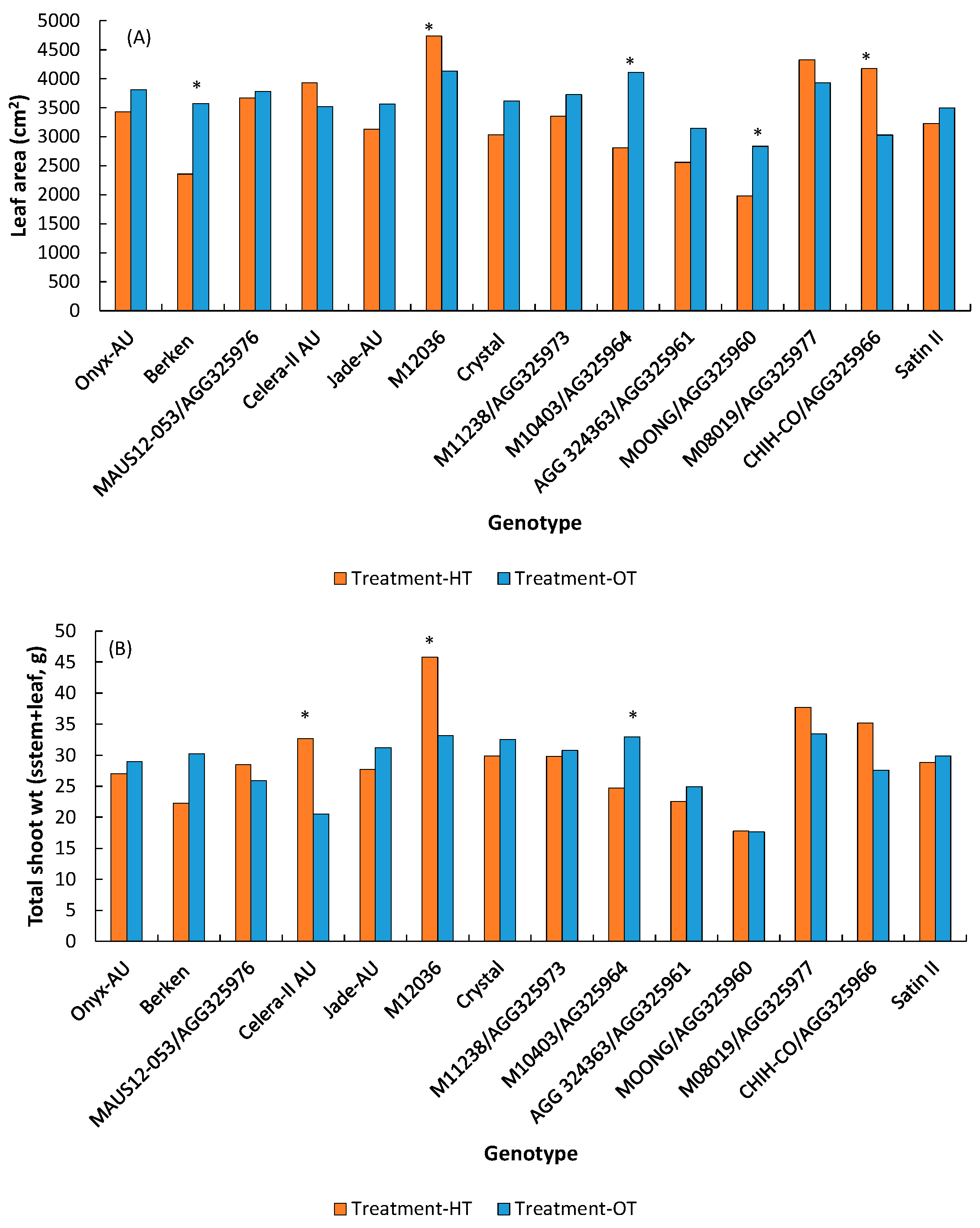
3.1.1. Shoot Morphology
3.1.2. Phenology/Flowering
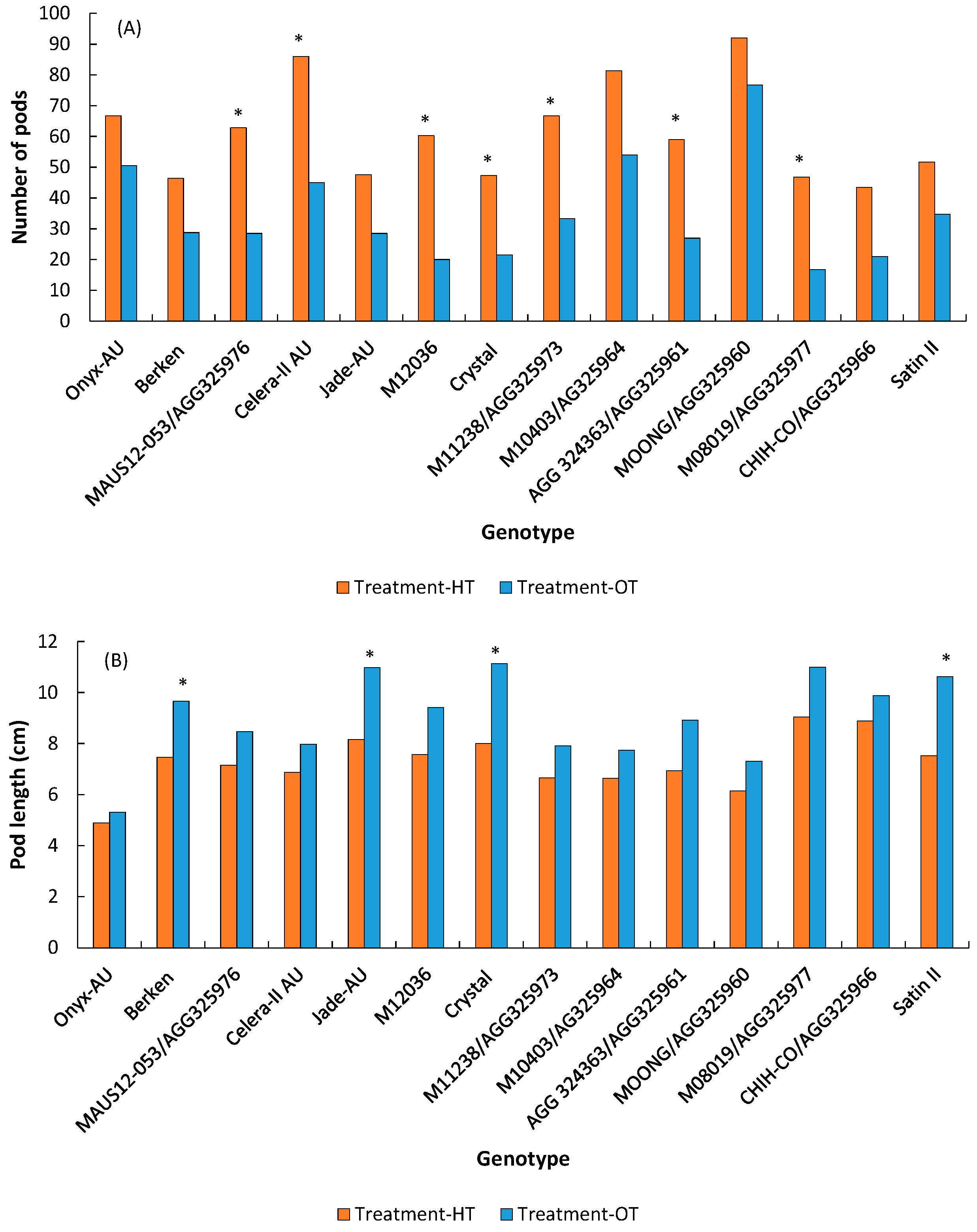
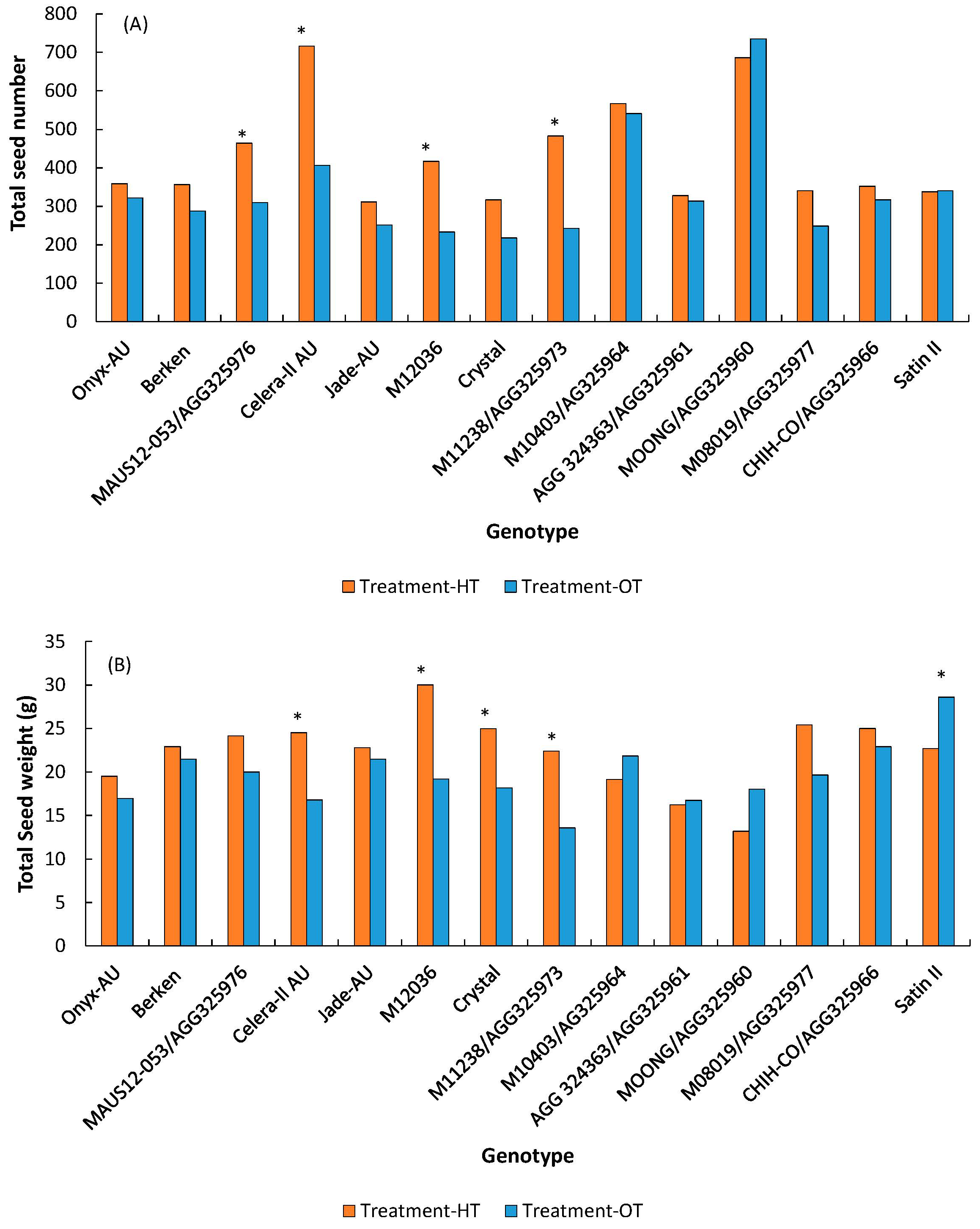
3.1.3. Pods/Seeds
3.2. Field Experiment
4. Discussion
4.1. Temperature Threshold for Pollen Viability and Grain Yield in Mungbean Plants
4.2. Impaired Pollen Viability Had No Impact on Grain Yield in This Study
4.3. Post-Heat-Stress Compensatory Growth Response in Mungbean Plants
4.4. Evidence for Post-Heat-Stress Compensatory Growth in Other Studies
4.5. Performance of Genotypes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, S.S.; Beebe, S.; Crespi, M.; Delbreli, B.; Gonzaliz, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Foyer, C.H.; Nguyen, H.T.; Lam, H.M.; Siddique, K.H.M. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.M.; Schreinemachers, P. Global status and economic importance of mungbean. In The Mungbean Genome; Nair, R.M., Schafleitner, R., Lee, S.-H., Eds.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Chauhan, Y.; Williams, R. Physiological and agronomic strategies to increase mungbean yield in climatically variable environments of Northern Australia. Agronomy 2018, 8, 83. [Google Scholar] [CrossRef]
- Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, I.; Mosquera, G.; Bueno, G.; Blair, M.W. Genetic improvement of common beans and the challenges of climate change. In Crop Adaptation of Climate Change, 1st ed.; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; Wiley: Oxford, UK, 2011; pp. 356–369. [Google Scholar] [CrossRef]
- Basu, P.S.; Singh, U.; Kumar, A.; Praharaj, C.S.; Shivran, R.K. Climate change and its mitigation strategies in pulse production. Indian J. Agron. 2016, 61, S71–S82. [Google Scholar]
- Bhardwaj, R.; Lone, J.K.; Pandey, R.; Mondal, N.; Dhandapani, R.; Meena, S.K.; Khan, S.G. Insights into morphological and physio-biochemical adaptive responses in mungbean (Vigna radiata L.) under heat stress. Front Genet. 2023, 14, 1206451. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; Ahuja, L.H., Saseendran, S.A., Eds.; Advances in Agricultural Systems Modelling Series 1; ASA-CSSA: Madison, WI, USA, 2008; pp. 301–355. [Google Scholar]
- Petkova, V.; Nikolova, V.; Kalapchieva, S.H.; Stoeva, V.; Topalova, E.; Angelova, S. Physiological response and pollen viability of Pisum sativum genotypes under high temperature influence. Acta Hortic. 2009, 830, 665–671. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, R.; Kaur, N.; Bhandhari, K.; Kaushal, N.; Gupta, K.; Bains, T.S.; Nayyar, H. Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol. Plant. 2011, 33, 2091–2101. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Tan, D.K.Y.; Gaur, P.M.; Raju, T.N.; Trethowan, R.M. High temperature tolerance in chickpea and its implications for plant improvement. Crop Past. Sci. 2012, 63, 419–428. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Tokachichu, R.N.; Trethowan, R.M.; Tan, D.K.Y.; Gaur, P.M.; Mallikarjuna, N. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Singh, V.; van Oosterom, E.J.; Chapman, S.; Jordan, D.R.; Hammer, G.L. Genotypic variability in growth and development of sorghum in response to high temperature. Funct. Plant Biol. 2013, 40, 439–448. [Google Scholar] [CrossRef]
- Kaur, R.; Bains, T.S.; Bindumadhava, H.; Nayyar, H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015, 197, 527–541. [Google Scholar] [CrossRef]
- Singh, V.; Nguyen, C.T.; van Oosterom, E.J.; Chapman, S.; Jordan, D.R.; Hammer, G.L. Genotypic differences in pollen viability and seed set to short episodes of high temperature stress during reproductive development in sorghum. Crop Sci. 2016, 56, 1–12. [Google Scholar] [CrossRef]
- Patriyawaty, N.R.; Rachaputi, C.N.R.; George, D.; Douglas, C. Genotypic variability for tolerance to high temperature stress during reproductive phase in mungbean [Vigna radiata (L.) Wilczek]. Sci. Hortic. 2018, 227, 132–141. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhu, Y.; Jones, A.; Rose, R.J.; Song, Y. Heat stress in legume seed setting: Effects, causes, and future prospects. Front. Plant Sci. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Sharma, L.; Singh, I.; Bains, T.S.; Siddique, H.M.; Bindumadhava, H.; Nair, R.M.; Nayyar, H. Securing reproductive function in mungbean grown under high temperature environment with exogenous application of proline. Plant Physiol. Biochem. 2019, 140, 136–150. [Google Scholar] [CrossRef] [PubMed]
- IPCC. IPCC-SR15, Global Warming of 1.5 °C. Available online: http://www.ipcc.ch/report/sr15/ (accessed on 17 June 2019).
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Berger, J.D.; Warkentin, T.; Asseng, S.; Ratnakumar, P.; Rao, K.P.C.; Gaur, P.M.; Munier-Jolain, N.; Larmure, A.; Voisin, A.-S.; et al. Adaptation of grain legumes to climate change: A review. Agron. Sust. Develop. 2012, 32, 31–44. [Google Scholar] [CrossRef]
- Luria, G.; Rutley, N.; Lazsar, I.; Harper, J.F.; Miller, G. Direct analysis of pollen fitness by flow cytometry: Implications for pollen responses to stress. The Plant J. 2019, 98, 942–952. [Google Scholar] [CrossRef]
- Monterroso, V.A.; Wien, H.C. Flower and pod abscission due to heat stress in beans. J. Am. Soc. Horti. Sci. 1990, 115, 631–634. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2012, 59, 823–843. [Google Scholar] [CrossRef]
- Siebers, M.H.; Yendrek, C.R.; Drag, D.; Locke, A.M.; Acosta, L.R.; Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Ort, D.R. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Glob. Chang. Biol. 2015, 21, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Twell, D.; Firon, N. Pollen development at high temperature: From acclimation to collapse. Plant Physiol. 2017, 173, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Young, T.E.; Ling, J.; Geisler-Lee, C.J.; Tanguay, R.L.; Caldwell, C.; Gallie, D.R. Developmental and thermal regulation of the maize heat shock protein HSP101. Plant Physiol. 2001, 127, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Hiyama, T. Heat-Shock Proteins and Temperature Stress, Handbook of Plant and Crop Stress; Marcel Dekker: New York, NY, USA, 1999; pp. 399–416. [Google Scholar]
- Bindumadhava, H.; Sharma, L.; Nair, R.M.; Nayyar, H.; Riley, J.J.; Easdown, W. High-temperature-tolerant mungbean (Vigna radiata L.) lines produce better yields when exposed to higher CO2 levels. J. Crop Improv. 2018, 32, 418–430. [Google Scholar] [CrossRef]
- HanumanthaRao, B.; Nair, R.M.; Nayyar, H. Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front. Plant Sci. 2016, 7, 957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Priya, M.; Bindumadhava, H.; Nair, R.M.; Nayyar, H. Influence of high temperature stress on growth, phenology and yield performance of mungbean [Vigna radiata (L.) Wilczek] under managed growth conditions. Sci. Hortic. 2016, 213, 379–391. [Google Scholar] [CrossRef]
- Tickoo, J.L.; Gajraj, R.; Matho Manji, C. Plant type in Mungbean (Vigna radiata L. Wilczek). In Proceedings Recent Advances in mungbean Research, Indian Society of Pulses Research and Development; Asthana, A.N., Kim, D.H., Eds.; Indian Institute of Pulses Research: Kanpur, India, 1996; pp. 197–213. [Google Scholar]
- Clarke, H.J.; Siddique, K.H.M. Response of chickpea genotypes to low temperature stress during reproductive development. Field Crops Res. 2004, 90, 323–334. [Google Scholar] [CrossRef]
- Wang, J.; Gan, Y.T.; Clarke, F.; McDonald, C.L. Response of chickpea yield to high temperature stress during reproductive development. Crop Sci. 2006, 46, 2171–2178. [Google Scholar] [CrossRef]
- Heidmann, I.; Berardino, M.C. Impedance flow cytometry as a tool to analyze microspore and pollen quality. Meth. Molec. Biol. 2017, 1669, 339–354. [Google Scholar] [CrossRef]
- Fisher, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, C.G.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Tainan, Taiwan, 13–18 August 1992; pp. 257–270. [Google Scholar]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. New York, USA. 2021. Available online: https://www.xlstat.com (accessed on 15 July 2021).
- Siddique, K.H.M.; Loss, S.P.; Regan, K.L.; Jettner, R.L. Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Aust. J. Agric. Res. 1999, 50, 375–387. [Google Scholar] [CrossRef]
- Farooq, M.; Nadeem, F.; Gogoi, N.; Ullah, A.; Alghamdi, S.S.; Nayyar, H.; Siddique, K.H.M. Heat stress in grain legumes during reproductive and grain-filling phases. Crop Past. Sci. 2017, 68, 985–1005. [Google Scholar] [CrossRef]
- Nair, R.M.; Pandey, A.K.; War, A.R.; HanumanthaRao, B.; Shwe, T.; Alam, A.; Pratap, A.; Malik, S.R.; Karimi, R.; Mbeyagala, E.K. Biotic and abiotic constraints in mungbean production-progress in genetic improvement. Front. Plant Sci. 2019, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Cober, E.; Tanner, J. Performance of related indeterminate and tall determinate soybean in short-season areas. Crop Sci. 1995, 35, 361–364. [Google Scholar] [CrossRef]
- Kwak, M.; Toro, O.; Debouck, D.G.; Gepts, P. Multiple origins of determinate growth habit in domesticated common bean (Phaseolus vulgaris). Ann. Bot. 2012, 110, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Patriyawaty, N.R.; Rachaputi, C.N.R.; George, D. Physiological mechanisms underpinning tolerance to high temperature stress during reproductive phase in mungbean [Vigna radiata (L.) Wilczek]. Environ. Exp. Bot. 2018, 150, 188–197. [Google Scholar] [CrossRef]
- Iqbal, J.; Shabbir, G.; Shah, K.N.; Hassan, F.U.; Qayyum. Deciphering of genotype x environment interaction to identify stable heat-tolerant mungbean genotypes by GGE biplot analysis. J. Soil Sci. Plant Nutr. 2021, 21, 2551–2561. [Google Scholar] [CrossRef]
- Simões-Araújo, J.L.; Rumjanek, N.G.; Margis-Pinheiro, M. Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. Braz. J. Plant Physiol. 2003, 15, 33–41. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Lawn, R.J. Agronomic studies on Vigna spp. In South-eastern Queensland. II. Vegetative and reproductive response of cultivars to sowing date. Aust. J. Agric. Res. 1979, 30, 871–882. [Google Scholar] [CrossRef]
- Lawn, R.J.; Ahn, C.S. Mungbean (Vigna radiata.L, Wilczek/Vigna mungo. L, Hepper). In Grain Legume Crops; Summerfield, R., Roberts, E.H., Eds.; Collins: London, UK, 1985; pp. 584–623. [Google Scholar]
- Chikukura, L.; Bandyopadhyay, S.; Naresh Kumar, S.; Pathak, S. Effect of elevated temperature stress on growth, yield and yield attributes of mungbean (Vigna radiata L.) in semi-arid north-west India. Curr. Adv. Agric. Sci. 2017, 9, 18–22. [Google Scholar] [CrossRef]
- Ha, S. High Temperature Stress Response of Agronomic Traits in Soybean and Mungbean. Master’s Thesis, Department of Plant Science, The Graduate School of Seoul National University, Seoul, Republic of Korea, 2016. Available online: https://s-space.snu.ac.kr/bitstream/10371/125588/1/000000132835.pdf (accessed on 15 July 2021).
- Basu, P.S.; Pratap, A.; Gupta, S.; Sharma, K.; Tomar, R.; Singh, N.P. Physiological traits for shortening crop duration and improving productivity of greengram (Vigna Radiata L. Wilczek) under high temperature. Front. Plant Sci. 2019, 10, 1508. [Google Scholar] [CrossRef] [PubMed]



| Genotype | Days to Flowering | Days to Maturity | Seed Size | Weight of 50 Seeds (g) |
|---|---|---|---|---|
| Jade-AU | 42 | 70 | Large | 3.5 |
| Crystal | 38 | 72 | Large | 3.7 |
| Celera-II AU | 44 | 74 | Small–Medium | 2.8 |
| Berken | 40 | 69 | Medium–Large | 3.2 |
| Satin II | 42 | 71 | Medium | 3.0 |
| AGG 324363/AGG325961 | 44 | 74 | Medium | 2.8 |
| MOONG/AGG325960 | 41 | 72 | Small | 1.2 |
| M08019/AGG325977 | 41 | 78 | Large | 4.0 |
| CHIH-CO/AGG325966 | 42 | 70 | Large | 3.5 |
| M10403/AG325964 | 40 | 73 | Small | 1.7 |
| MAUS12-053/AGG325976 | 42 | 70 | Medium | 2.9 |
| M11238/AGG325973 | 38 | 70 | Medium | 2.5 |
| M12036 | 42 | 72 | Medium–Large | 3.2 |
| Onyx-Au (black gram) | 38 | 75 | Small | 2.5 |
| Plant Growth Parameter | Plant Traits | HT | OT | Pr. (Temp.) | Pr. (Genotype) | Pr. (G × T) |
|---|---|---|---|---|---|---|
| Shoot morphology | Plant height (cm) | 73.5 | 67.3 | ** | *** | *** |
| Number of nodes | 15.1 | 9.6 | *** | ns | ns | |
| Secondary branching | 42.6 | 29.0 | *** | *** | * | |
| Total leaf number | 80.8 | 75.6 | * | *** | * | |
| Leaf area (cm2) | 3335.7 | 3590.6 | * | *** | *** | |
| Stem dry wt. (g) | 16.1 | 14.1 | *** | *** | *** | |
| Leaf dry wt. (g) | 13.8 | 14.6 | ns | *** | ns | |
| Total shoot wt. (g) | 29.3 | 28.6 | ns | *** | ** | |
| Days to first flower | 37.3 | 34.9 | *** | *** | ns | |
| Phenology | Days to 50% flower | 41.0 | 39.1 | ** | ns | ns |
| Pollen viability (%) | 29.6 | 67.2 | *** | ns | ns | |
| Pods | Total number of pods | 61.3 | 34.7 | *** | *** | 0.069 |
| Pod size/length (cm) | 7.3 | 9.0 | *** | *** | *** | |
| Unfertilized pods | 5.8 | 0.1 | *** | *** | *** | |
| Seeds | Total seed number/plant | 431.1 | 340.5 | *** | *** | *** |
| Unviable seeds/plant | 28.4 | 1.0 | *** | ns | ns | |
| Number of seeds per pod | 7.1 | 10.5 | *** | *** | ** | |
| Viable seed wt. (g/seed) | 0.061 | 0.065 | ** | *** | ns | |
| Total seed wt. (g/plant) | 22.4 | 19.7 | *** | *** | ** |
| Genotype | OT | HT | STI | SSI/CGI |
|---|---|---|---|---|
| Satin II | 28.6 | 22.7 * | 1.69 | −1.50 |
| CHIH-CO/AGG325966 | 22.9 | 25.0 | 1.49 | 0.65 |
| M10403/AG325964 | 21.9 | 19.1 | 1.09 | −0.90 |
| Berken | 21.5 | 22.9 | 1.28 | 0.49 |
| Jade-AU | 21.5 | 22.8 | 1.27 | 0.45 |
| MAUS12-053/AGG325976 | 20.0 | 24.2 | 1.26 | 1.52 |
| M08019/AGG325977 | 19.7 | 25.4 | 1.30 | 2.12 |
| M12036 | 19.2 | 30.0 * | 1.50 | 4.09 |
| Crystal | 18.2 | 25.0 * | 1.18 | 2.73 |
| MOONG/AGG325960 | 18.0 | 13.2 | 0.62 | −1.94 |
| Onyx-AU | 17.0 | 19.5 | 0.86 | 1.08 |
| Celera-II AU | 16.8 | 24.5 * | 1.07 | 3.34 |
| AGG 324363/AGG325961 | 16.8 | 16.2 | 0.71 | −0.23 |
| M11238/AGG325973 | 13.6 | 22.4 * | 0.79 | 4.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Collins, M. Genotypic Variability in Response to Heat Stress and Post-Stress Compensatory Growth in Mungbean Plants (Vigna radiata [L.] Wilczek). Crops 2024, 4, 270-287. https://doi.org/10.3390/crops4030020
Singh V, Collins M. Genotypic Variability in Response to Heat Stress and Post-Stress Compensatory Growth in Mungbean Plants (Vigna radiata [L.] Wilczek). Crops. 2024; 4(3):270-287. https://doi.org/10.3390/crops4030020
Chicago/Turabian StyleSingh, Vijaya, and Marisa Collins. 2024. "Genotypic Variability in Response to Heat Stress and Post-Stress Compensatory Growth in Mungbean Plants (Vigna radiata [L.] Wilczek)" Crops 4, no. 3: 270-287. https://doi.org/10.3390/crops4030020
APA StyleSingh, V., & Collins, M. (2024). Genotypic Variability in Response to Heat Stress and Post-Stress Compensatory Growth in Mungbean Plants (Vigna radiata [L.] Wilczek). Crops, 4(3), 270-287. https://doi.org/10.3390/crops4030020






