Azospirillum brasilense Inoculation in a Maize–Urochloa–Rice Cropping System Promotes Soil Chemical and Biological Changes and Increases Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. History and Initial Characterization of the Experimental Area
2.2. Experimental Design
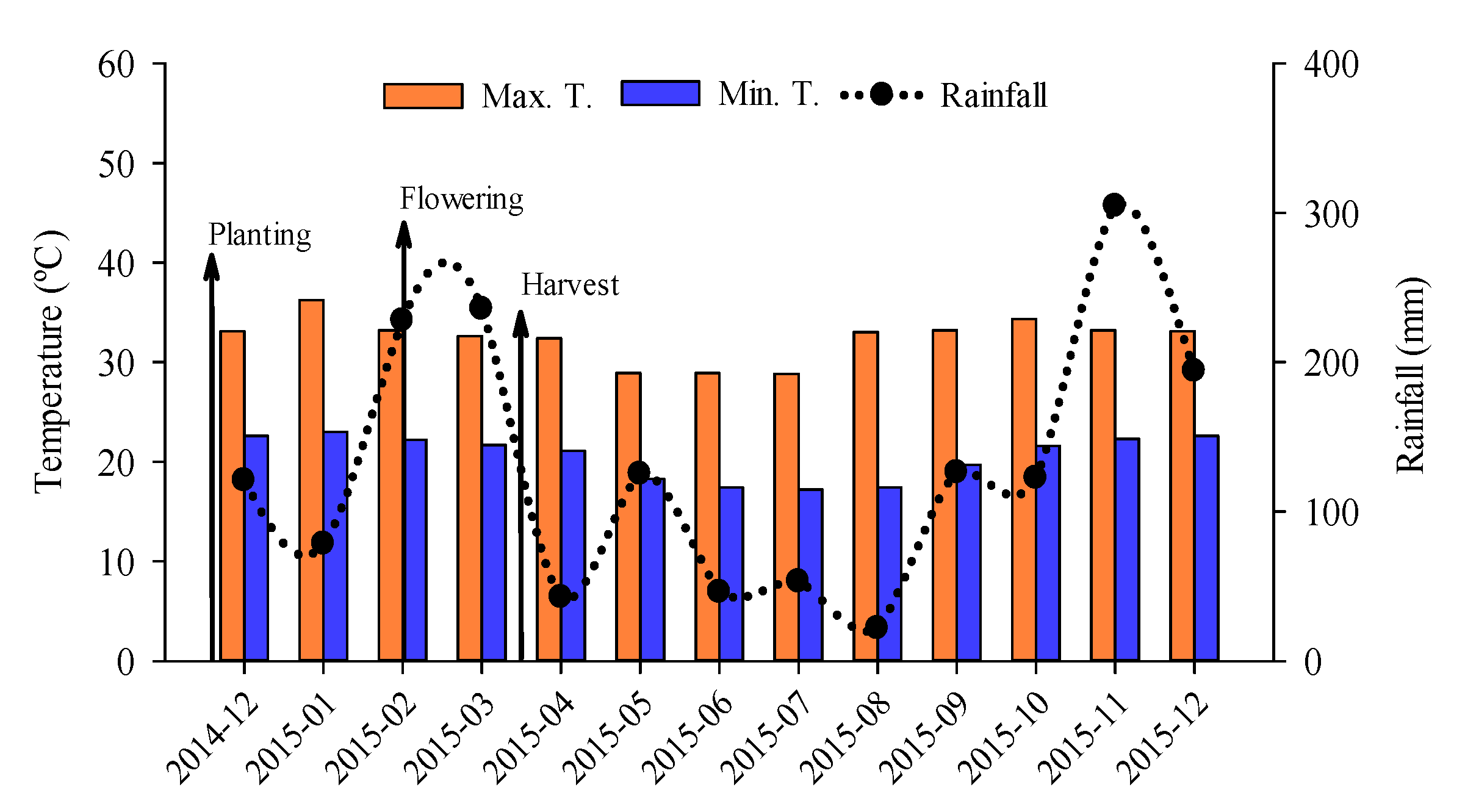
2.3. Implementation and Execution of the Experiment
2.4. Evaluation of Soil Chemical and Biological Attributes
2.5. Rice Development and Productivity
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heinemann, A.B.; Ramirez-Villegas, J.; Rebolledo, M.C.; Neto, G.M.F.C.; Castro, A.P. Upland rice breeding led to increased drought sensitivity in Brazil. Field Crops Res. 2019, 231, 57–67. [Google Scholar] [CrossRef]
- CONAB—Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Grãos; CONAB: Brasília, Brazil, 2023; Volume 10, pp. 1–106.
- Buchi, L.; Wendling, M.; Amossé, C.; Necpalova, M.; Charles, R. Importance of cover crops in alleviating negative effects of reduced soil tillage and promoting fertility in a winter wheat cropping system. Agric. Ecosyst. Environ. 2018, 256, 92–104. [Google Scholar] [CrossRef]
- Ogle, S.M.; Alsaker, C.; Baldock, J.; Bernoux, M.; Breidt, F.J.; McConkey, B.; Regina, K.; Vazquez-Amabile, G.G. Climate and Soil Characteristics Determine Where No-Till management Can Store Carbon in Soils and Mitigate Greenhouse Gas Emissions. Sci. Rep. 2019, 9, 11665. [Google Scholar] [CrossRef]
- Calonego, J.C.; Raphael, J.P.A.; Rigon, J.P.G.; Oliveira Neto, L.; Rosolem, C.A. Soil compaction management and soyben yields with cover crops under no-till and occasional chiseling. Eur. J. Agron. 2017, 85, 31–37. [Google Scholar] [CrossRef]
- Mauricieri, C.; Tolomio, M.; Raimondi, G.; Toffani, A.; Morari, F.; Berti, A.; Borin, M. Organic versus conventional farming: Medium-term evaluation of soil chemical properties. Ital. J. Agron. 2017, 85, 37. [Google Scholar] [CrossRef]
- Koudahe, K.; Allen, S.C.; Djaman, K. Critical review of the impact of cover crops on soil properties. Int. Soil Water Conserv. Res. 2022, 10, 343–354. [Google Scholar] [CrossRef]
- Nugroho, P.A.; Juhos, K.; Prettl, N.; Madarász, B.; Kotroczó, Z. Long-term conservation tillage results in a more balanced soil microbiological activity and higher nutrient supply capacity. Int. Soil Water Conserv. Res. 2023, 11, 528–537. [Google Scholar] [CrossRef]
- Fernandes, M.M.H.; Coelho, A.P.; Silva, M.F.; Fernandes, C. Do fallow in the off-season and crop succession promote differences in soil aggregation in no-tillage systems? Geoderma 2022, 412, 115725. [Google Scholar] [CrossRef]
- Dhiman, V.K.; Rana, N.; Dhiman, V.K.; Pandey, H.; Verma, P.; Singh, D. Effect of rhizobial isolates and nitrogen fertilizers on nursery performace, nodulation behavior and nitrogen activity Dalbergia sissoo Roxb. seedlings. Plant Stress 2022, 4, 100080. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding impact of Azospirillum brasilense strains Ab-V5 and Ab-V6 on the Brazilian agriculture: Lessons that farmers are receptive to adopt new microbial inoculants. Rev. Bras. Ciênc. Solo 2021, 45, e0200128. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Momesso, L.; Bossolani, J.W.; Moretti, L.G.; Oliveira, E.F.; Tubaña, B.S.; Silva, M.A.; Castro, S.G.Q.; Hungria, M. Inoculation with Azospirillum brasilense as a strategy to enhance sugarcane biomass production and bioenergy potential. Eur. J. Agron. 2023, 144, 126749. [Google Scholar] [CrossRef]
- Silva, P.S.T.; Cassiolato, A.M.R.; Galindo, F.S.; Jalal, A.; Nogueira, T.A.R.; Oliveira, C.E.S.; Teixeira Filho, M.C.M. Azospirillum brasilense and zinc rates effect on fungal root colonization and yield of wheat–maize in tropical savannah conditions. Plants 2022, 11, 3154. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Degon, Z.; Ruiz, D.; Pope, J.; Rahmatallah, Y.; Mukherjee, A. The plant growth-promoting bacteria, Azospirillum brasilense, induce a diverse array of genes in rice shoots and promote their growth. Plant Growth Regul. 2022, 97, 143–155. [Google Scholar] [CrossRef]
- Huo, L.; Gao, R.; Hou, X.; Yu, X.; Yang, X. Arbuscular mycorrhizal and dark septate endophyte colonization in Artemisia roots responds differently to environmental gradients in eastern and central China. Sci. Total Environ. 2021, 795, e148808. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Dai, M.D.; Zhu, J.N.; Liu, X.H.; Li, L.; Zhu, X.M.; Wang, J.Y.; Yuan, Z.L.; Lin, F.C. Dark septate endophyte Falciphora oryzae–assisted alleviation of cadmium in rice. J. Hazard. Mater. 2021, 419, e126435. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Shamsy, R.; Liu, A. Arbuscular mycorrhizal fungi–induced tolerance to chromium stress in plants. Environ. Pollut. 2023, 327, 121597. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lubreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA—Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Goncalves, J.L.M.; Gerd, S. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertil de Solos Trop; Instituto Agronômico: Campinas, Brazil, 2001.
- Sabundjian, M.T. Consórcio de Milho e Urochloa ruziziensis e Inoculação com Azospirillum brasilense e Seu Efeito Residual Associado à Adubação Nitrogenada em Feijoeiro de Inverno. Ph.D. Thesis, São Paulo State University, Ilha Solteira, SP, Brazil, 2016; 176p. [Google Scholar]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; Boletim Técnico, 100; Instituto Agronômico: Campinas, Brazil, 1997; 285p.
- Cantarella, H.; Furlani, P.R. Arroz de sequeiro. In Recomendações de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; Raij, B., Van Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 1996; pp. 48–49. [Google Scholar]
- Arf, O.; Rodrigues, R.A.F.; Sá, M.E.; Crusciol, C.A.C. Influência da época de semeadura no comportamento de cultivares de arroz irrigado por aspersão em Selvíria—MS. Pesqui. Agropecuária Bras. 2000, 35, 1967–1976. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 773–777. [Google Scholar] [CrossRef]
- Silva, A.O.; Silva, W.M.; Kurihara, C.H.; Mercante, F.M. Spectrophotometric method for quantification of soil microbial biomass carbon. Afr. J. Biotechnol. 2016, 15, 565–570. [Google Scholar] [CrossRef]
- Anderson, T.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of micorrhizal endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 234–244. [Google Scholar] [CrossRef]
- Jenkins, W.R. A rapid centrifugal–flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Araújo, A.S.F.; Melo, V.M.M.; Pereira, A.P.A.; Pereira, A.P.A.; Lopes, A.C.A.; Rocha, S.M.B.; Araujo, F.F.; Mendes, L.W. Arbuscular mycorrhizal community in soil from different Brazilian Cerrado physiognomies. Rhizosphere 2021, 19, 100375. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; Sena, J.V.S.; Poggere, G.; Reis, A.R.; Corrêa, R.S. Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 2021, 163, 1033913. [Google Scholar] [CrossRef]
- Li, S.; Peng, C.; Wang, C.; Zheng, J.; Hu, Y.; Li, D. Microbial succession and nitrogen cycling in cultured biofilms as affected by the inorganic nitrogen availability. Microb. Ecol. 2016, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Crusciol, C.A.C.; Nascente, A.S.; Borghi, E.; Soratto, R.P.; Martins, P.O. Improving soil fertility and crop yield in a tropical region with palisadegrass cover crops. Agron. J. 2015, 107, 2015. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Roberto, L.A.; Hungria, M.; Corrêa, R.S.; Magri, E.; Correia, T.D. Meta-analysis of maize responses to Azospirillum brasilense inoculation in Brazil: Benefits and lessons to improve inoculation efficiency. Appl. Soil Ecol. 2022, 170, 104276. [Google Scholar] [CrossRef]
- Hungria, M.; Barbosa, J.Z.; Rondina, A.B.L.; Nogueira, M.A. Improving maize Sustainability with partial replacement of N fertilizers by inoculation with Azospirillum brasilense. Agron. J. 2022, 114, 2969–2980. [Google Scholar] [CrossRef]
- Nascente, A.S.; Stone, L.F. Cover crops as affecting soil chemical and physical properties and development of upland rice and soybean cultivated in rotation. Rice Sci. 2018, 25, 340–349. [Google Scholar] [CrossRef]
- Islam, M.R.; Singh, B.; Dijkstra, F.A. Stabilisation of soil organic matter: Interactions between clay and microbes. Biogeochemistry 2022, 160, 145–158. [Google Scholar] [CrossRef]
- Song, G.; Novotny, E.H.; Mao, J.D.; Haynes, M.H. Characterization of transformations of maize residues into soil organic matter. Sci. Total Environ. 2017, 579, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Baptistella, J.L.C.; Llerena, J.P.P.; Domingues-Júnior, A.P.; Fernie, A.R.; Favarin, J.L.; Mazzafera, P. Differential responses of three Urochloa species to low phosphorus availability. Ann. Appl. Biol. 2021, 179, 216–230. [Google Scholar] [CrossRef]
- Rodrigues, G.L.; Matteoli, F.P.; Gazara, R.K.; Rodrigues, P.S.L.; Santos, S.T.; Alves, A.F.; Pedrosa-Silva, F.; Oliveira-Pinheiro, I.; Canedo-Alvarenga, D.; Olivares, F.L.; et al. Characterization of cellular, biochemical and genomic features of the diazotrophic plant growth-promoting bacterium Azospirillum sp. UENF-412522, a novel member of the Azospirillum genus. Microbiol. Res. 2022, 254, 126896. [Google Scholar] [CrossRef] [PubMed]
- Hallama, M.; Pekrun, C.; Pilz, S.; Jarosch, K.A.; Frac, M.; Uksa, M.; Marhan, S.; Kandeler, E. Interactions between cover crops and soil microrganisms increase phosphorus availability in conservation agriculture. Plant Soil 2021, 463, 307–328. [Google Scholar] [CrossRef]
- Diksha, S.K.; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Sithole, N.J.; Magwaza, L.S.; Thibaud, G.R. Long-term impact of no-till conservation agriculture and N-fertilizer on soil aggregate stability, infiltration and distribution of C in different size fractions. Soil Tillage Res. 2019, 190, 147–156. [Google Scholar] [CrossRef]
- Kumari, D.; Kumar, S.; Parveen, H.; Pradhan, A.K.; Kumar, S.; Kumari, R. Long-term impact of conservation agriculture on chemical properties of soil. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2144–2153. [Google Scholar] [CrossRef]
- Uzoh, I.M.; Igwe, C.A.; Okebalama, C.B.; Babalola, O.O. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci. Rep. 2019, 9, 8539. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.T.; Dores, E.F.G.C.; Weber, O.S.W.; Beber, D.C.; Campelo Junior, J.H.; Maia, J.C.S. Soil organic matter doubles the cation exchange capacity of tropical soil under no-till farming in Brazil. J. Sci. Food Agric. 2018, 98, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Stankowski, S.; Jaroszewska, A.; Osinska, B.; Tomaszewicz, T.; Gibczynska, M. Analysis of Long-Term Effect of Tillage System and Pre-Crop on Physicochemical Properties and Chemical Composition of Soil. Agronomy 2022, 12, 2072. [Google Scholar] [CrossRef]
- Garcia, N.F.S.; Arf, O.; Portugal, J.R.; Peres, A.R.; Rodrigues, M.; Penteado, M.S. Doses and application methods of Azospirillum brasilense in irrigated upland rice. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 990–995. [Google Scholar] [CrossRef]
- Sales, L.Z.S.; Garcia, N.F.S.; Martins, J.T.; Buzo, F.S.; Garé, L.M.; Rodrigues, R.A.F.; Arf, O. Inoculação com Azospirillum brasilense e redução da adubação nitrogenada em arroz de terras altas. Res. Soc. Dev. 2021, 10, e9110716345. [Google Scholar] [CrossRef]
- Embrapa—Empresa Brasileira de Pesquisa Agropecuária. Catálogo de Cultivares de Arroz; Embrapa Arroz e Feijão: Santo Antônio de Goiás, Brazil, 2013; p. 11. [Google Scholar]
- Buzo, F.S.; Garé, L.M.; Arf, O.; Portugal, J.R.; Meirelles, F.C.; Garcia, N.F.S. Interaction between thidiazuron and Azospirillum brasilense on yield characteristics and productivity of rice. Rev. Bras. Eng. Agric. Ambient.—Agriambi 2019, 23, 244–249. [Google Scholar] [CrossRef]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- Mei, P.P.; Wang, P.; Yang, H.; Gui, L.G.; Christe, P.; Li, L. Maize/faba bean intercropping with rhizobial inoculation in a reclaimed desert soil enhances productivity and symbiotic N2 fixation and reduces apparent N losses. Soil Tillage Res. 2021, 213, 105154. [Google Scholar] [CrossRef]
- Almeida, K.L.; Ferreira, R.V.; Silva, A.G.; Ferreira, C.J.B.; Braz, G.B.P.; Tavares, R.L.M. Consórcio milho e Brachiaria ruziziensis, época de dessecação e desempenho da soja em sucessão. Res. Soc. Dev. 2020, 9, e13791210867. [Google Scholar] [CrossRef]
- Canisares, L.P.; Rosolem, C.A.; Momesso, L.; Crusciol, C.A.C.; Villega, D.M.; Arango, J.; Ritz, K.; Cantarella, H. Maize-Brachiaria intercropping: A strategy to supply recycled N to maize and reduce soil N2O emissions? Agric. Ecosyst. Environ. 2021, 319, 107491. [Google Scholar] [CrossRef]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.T.; Prates, A.R.; Fernandes, D.M.; Cassiolato, A.M.R.; Maltoni, K.L. Microrganismos e lodo de esgoto compostado no desenvolvimento inicial de mudas de baru em vasos. Eng. Sanitária Ambient. 2022, 27, 1021–1029. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Zhang, W.; Liu, K.; Liu, M.; Shao, X. Cooperation between arbuscular mycorrhizal fungi and plant growth-promoting bacteria and their effects on plant growth and soil quality. PeerJ 2022, 10, e13080. [Google Scholar] [CrossRef] [PubMed]
- Lugo, M.A.; Menoyo, E.; Allione, L.R.; Negritto, M.A.; Henning, J.A.; Anton, A.M. Arbucular mycorrhizas and dark septate endophytes associated with grasses from the Argentine Puna. Mycologia 2018, 110, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Hu, Q.; Liu, J.; He, X. Relationship of root dark septate endophytes and soil factors to plant species and seasonal variation in extremely arid desert in Northwest China. Appl. Soil Ecol. 2022, 175, 104454. [Google Scholar] [CrossRef]
- Chakraborty, K.; Banik, S.; Debnath, A.; Das, A.R.; Saha, A.K. Arbuscular mycorrhiza and dark septate endophyte fungal associations of Oryza sativa L. under field condition: Colonization features and their occurrence. Plant Sci. Today 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Paranavithana, T.M.; Marasinghe, S.; Perera, G.A.D.; Ratnayake, R.R. Effects of crop rotation on enhanced occurrence of arbuscular mycorrhizal fungi and soil carbon stocks of lowland paddy fields in seasonally dry topics. Paddy Water Environ. 2021, 19, 217–226. [Google Scholar] [CrossRef]
- Lima, C.S.; Ceolin, C.; Muller, D.; Lima, J.; Zancan, M.; Chechin, J.; Vey, R.T.; Conceição, G.M.; Pavinato, P.S.; Martin, T.N. Inoculation with Azospirillum brasilense in corn cultivated on cover crops and nitrogen doses. Symbiosis 2022, 87, 237–247. [Google Scholar] [CrossRef]
- Aker, A.M.; Caproni, A.L.; Berbara, R.L.L.; Granha, J.R.D.O.; Silva, C.F.; Pereira, M.G. Arbuscular mycorrhizal fungi in the cerrado biome: Effects of land use system, soil, texture, and seasonality. Rev. Caatinga 2022, 35, 170–180. [Google Scholar] [CrossRef]
- Vieira, L.C.; Silva, D.K.A.; Escobar, I.E.C.; Silva, J.M.; Moura, I.A.; Oleh, F.; Silva, G.A. Changes in an Arbuscular Mycorrhizal Fungi Community Along an Environmental Gradient. Plants 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzade, H.; Ardakani, M.R.; Shadi, A.; Asadi Rahmani, H.; Noormohammadi, M.; Miransari, M. Rice (Oryza sativa L.) nutrient management using mycorrhizal fungi and endophytic Herbaspirillum seropedicae. J. Integr. Agric. 2016, 15, 1385–1394. [Google Scholar] [CrossRef]
- López–Reyes, L.; Carcaño–Montiel, M.G.; Lilia, T.P.; Mendina-de la Rosa, G.; Armando, T.H.R. Antifungal and growth–promoting activity of Azospirillum brasilense in Zea mays L. ssp. mexicana. Arch. Phytopathol. Plant Prot. 2017, 50, 727–743. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Piotrowska–Seget, Z. Plant association with dark septate endophytes: When the going gets tough (and stressful), the tough fungi get going. Chemosphere 2022, 302, 134830. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, A3–A9. [Google Scholar] [CrossRef]
- Xiao, S.S.; Ye, Y.Y.; Xiao, D.; Chen, W.R.; Zhang, W.; Wang, K.L. Effects of tillage on soil N availability, aggregate size, and microbial biomass in a subtropical karst region. Soil Tillage Res. 2019, 192, 187–195. [Google Scholar] [CrossRef]
- Simon, C.A.; Lima, S.F.; Cordeiro, M.S.; Secco, V.A.; Nacata, G.; Silva, A.M.M.; Simon, C.C.; Brasil, M.S. Cover crops as modifying agents of microbiological soil attribute. Aust. J. Crop Sci. 2019, 13, 1578–1585. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Ribeiro, L.R.P.; Marchão, R.L.; Oliveira, A.D.; Pulrolnik, K.; Figueiredo, C.C. Chemical composition of cover crops and soil organic matter pools in no-tillage systems in the Cerrado. Soil Use Manag. 2021, 38, 940–952. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Zhang, J.; Wei, H.; Li, M.; Lan, N.; Luo, H. Intercropping perennial aquatic plants with rice improved paddy field soil microbial biomass, biomass carbon and biomass nitrogen to facilitate soil sustainability. Soil Tillage Res. 2021, 208, 104908. [Google Scholar] [CrossRef]
- Huang, H.; Liu, S.; Du, Y.; Tang, J.; Hu, L.; Chen, X. Carbon Allocation mediated by arbuscular mycorrhizal fungi alters the soil microbial community under various phosphorus levels. Fungal Ecol. 2023, 62, 101227. [Google Scholar] [CrossRef]
- Schäfer, H.; Dannoura, M.; Ataka, M.; Osawa, A. Decomposition rate of extraradical hyphae of arbuscular mycorrhizal fungi decreases rapidly over time and varies by hyphal diameter and season. Soil Biol. Biochem. 2019, 136, 107533. [Google Scholar] [CrossRef]
- Sae-Tun, O.; Bodner, G.; Rosinger, C.; Zechmeister-Boltenstern, S.; Mentler, A.; Keiblinger, K. Fungal biomass and microbial necromass facilitate soil carbon sequestration and aggregate stability under different soil tillage intensities. Appl. Soil Ecol. 2022, 179, 104599. [Google Scholar] [CrossRef]
- Jha, P.; Hati, K.M.; Dalal, R.C.; Dang, Y.P.; Kopittke, P.M.; Menzies, N.W. Soil carbon and nitrogen dynamics in a Vertisol following 50 years of no-tillage, crop stubble retention and nitrogen fertilization. Geoderma 2020, 358, e113996. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.X.; Tian, L.; Zhang, Q. Conservation agriculture practices increase soil microbial biomass carbon and nitrogen in agricultural soils: A global meta–analysis. Soil Biol. Biochem. 2018, 121, 50–58. [Google Scholar] [CrossRef]
- Araújo, T.S.; Gallo, A.S.; Araujo, F.S.; Santos, L.C.; Guimaraes, N.F.; Silva, R.F. Biomassa e atividade microbiana em solo cultivado com milho consorciado com leguminosas de cobertura. Rev. Ciênc. Agrárias 2019, 42, 347–357. [Google Scholar] [CrossRef]

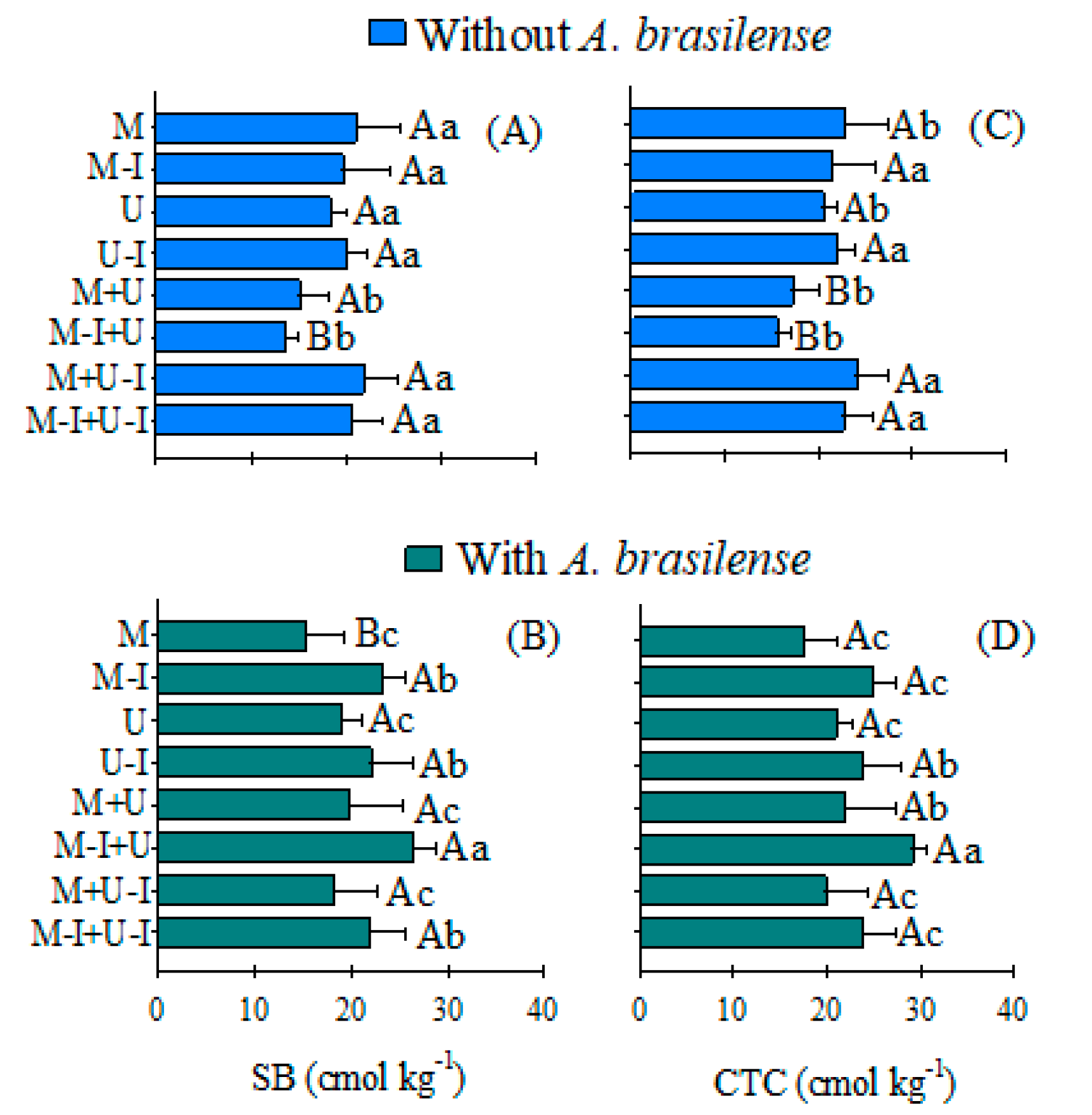
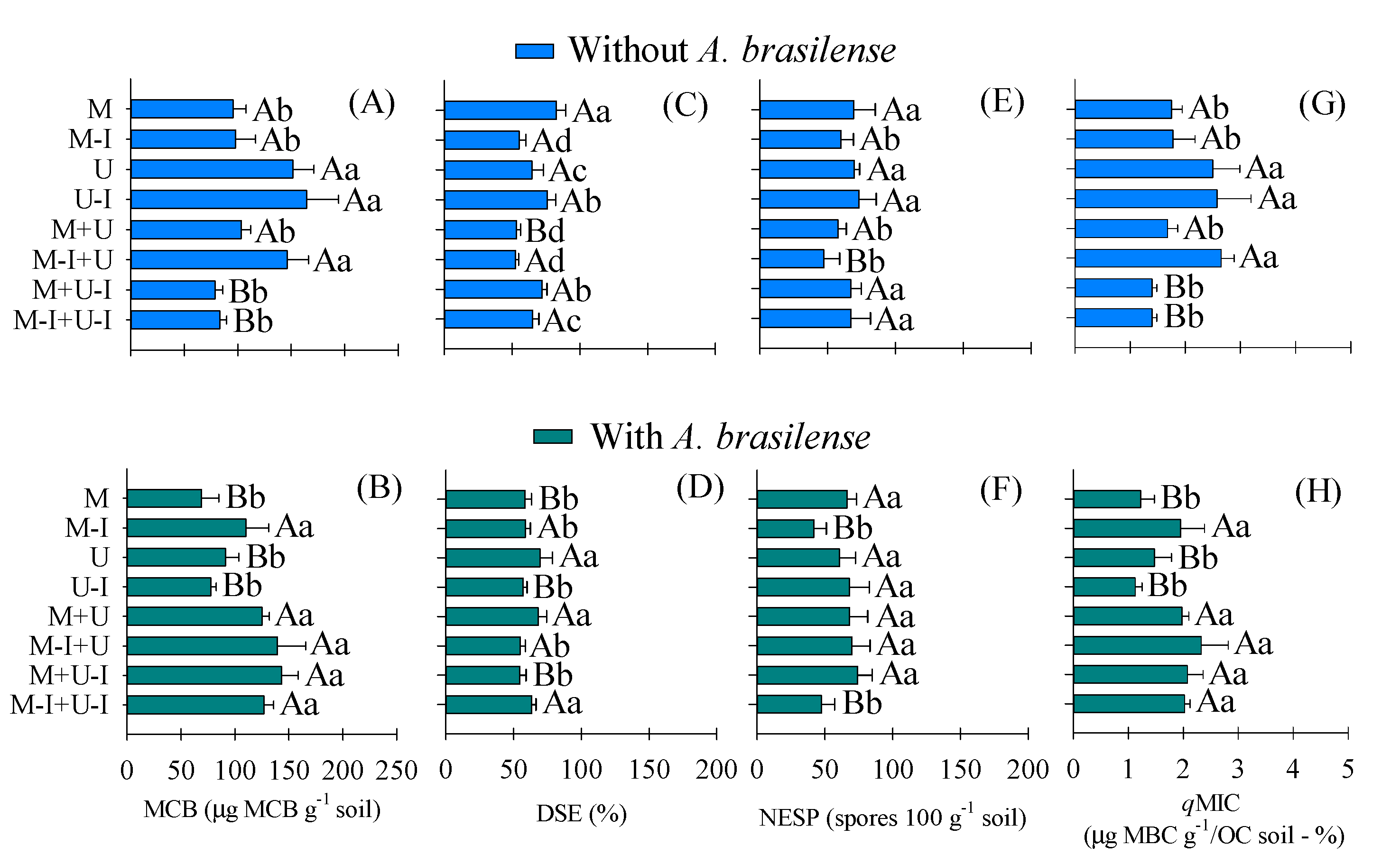
| Treatments | OM | Presine | pH CaCl2 | H + Al | SB | CEC |
|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ––––––––– cmol kg−1 ––––––––– | ||||
| Cover crops (CC) | ||||||
| Maize | 42.6 c | 68.3 a | 6.3 a | 2.0 a | 18.0 b | 20.1 b |
| Inoculated maize (I) | 43.7 c | 66.5 a | 6.6 a | 1.7 a | 21.3 a | 23.0 a |
| Urochloa | 47.4 b | 81.6 a | 6.3 a | 1.9 a | 18.6 b | 20.5 b |
| Inoculated Urochloa (I) | 51.3 a | 75.4 a | 6.4 a | 1.8 a | 20.9 a | 22.7 a |
| Maize + Urochloa | 47.8 b | 82.4 a | 6.3 a | 2.0 a | 17.3 b | 19.4 b |
| Maize–I + Urochloa | 44.4 c | 74.9 a | 6.3 a | 2.4 a | 19.8 a | 22.3 a |
| Maize + Urochloa–I | 48.5 b | 75.1 a | 6.3 a | 1.9 a | 19.8 a | 21.8 a |
| Maize–I + Urochloa–I | 47.2 b | 83.1 a | 6.5 a | 1.8 a | 21.2 a | 23.0 a |
| Plant in succession (PS) | ||||||
| Rice–I | 48.0 a | 78.8 a | 6.5 a | 1.9 a | 20.6 a | 22.6 a |
| Rice | 45.2 b | 73.0 b | 6.3 b | 2.0 a | 18.6 a | 20.6 a |
| General mean | 46.6 | 75.9 | 6.4 | 1.9 | 19.6 | 21.6 |
| F values | ||||||
| CC | 7.54 ** | 2.38 NS | 1.07 NS | 1.76 NS | 2.70 * | 2.28 NS |
| PS | 23.6 * | 31.3 * | 24.2 * | 0.03 NS | 2.23 NS | 2.60 * |
| CC × PS | 1.90 NS | 2.03 NS | 1.66 NS | 0.94 NS | 4.52 ** | 6.42 ** |
| Coefficient of variation (%) | ||||||
| CC | 6.5 | 5.4 | 1.7 | 22.2 | 15.0 | 30.5 |
| PS | 4.9 | 15.1 | 4.6 | 25.8 | 29.1 | 12.7 |
| Treatments | MSPA | AP | NPAN | M100 | MHEC | YIELD |
|---|---|---|---|---|---|---|
| kg ha−1 | m | nº m−2 | g | kg/100 L | kg ha−1 | |
| Cover crops (CC) | ||||||
| Maize | 6485 c | 0.98 a | 290 a | 2.58 a | 51.88 a | 5481 a |
| Inoculated maize (I) | 5868 c | 0.92 b | 305 a | 2.60 a | 52.99 a | 4506 a |
| Urochloa | 7042 b | 0.99 a | 294 a | 2.60 a | 51.20 a | 5757 a |
| Inoculated Urochloa (I) | 6984 b | 0.96 b | 309 a | 2.56 a | 53.23 a | 5449 a |
| Maize + Urochloa | 7385 b | 0.97 b | 301 a | 2.59 a | 51.61 a | 5006 a |
| Maize–I + Urochloa | 8240 b | 0.91 c | 287 a | 2.64 a | 54.08 a | 4214 a |
| Maize + Urochloa–I | 9161 a | 0.96 b | 293 a | 2.59 a | 52.59 a | 5382 a |
| Maize–I + Urochloa–I | 7997 b | 0.93 b | 300 a | 2.55 a | 53.13 a | 4233 a |
| Plant in succession (PS) | ||||||
| Rice–I | 7217 a | 0.96 a | 298 a | 2.48 b | 52.62 a | 5439 a |
| Rice | 7573 a | 0.95 a | 297 a | 2.53 a | 52.55 a | 4568 b |
| General mean | 7395 | 0.95 | 297 | 2.50 | 54.60 | 5004 |
| F values | ||||||
| CC | 3.76 * | 4.18 * | 0.30 NS | 0.92 NS | 1.20 NS | 12.69 NS |
| PS | 2.21 NS | 0.71 NS | 0.01 NS | 1.08 * | 0.02 NS | 1.74 * |
| CC × PS | 0.21 NS | 0.33 NS | 0.67 NS | 0.32 NS | 0.97 NS | 1.94 NS |
| Coefficient of variation (%) | ||||||
| CC | 21.2 | 4.5 | 13.4 | 3.7 | 4.7 | 26.1 |
| PS | 13.2 | 4.2 | 8.5 | 1.2 | 4.1 | 20.0 |
| Treatments | AMF | DSE | NESP | C–CO2 | MBC | OC | qCO2 | qMIC |
|---|---|---|---|---|---|---|---|---|
| –––––– % –––––– | 100 g Soil | μg C–CO2 g−1 Soil | μg MBC g−1 Soil | g kg−1 | μg C–CO2 g−1/μg MBC g−1 Soil | μg MBC g−1/OC Soil–% | ||
| Cover crops (CC) | ||||||||
| Maize | 59.12 d | 70.62 a | 67.75 a | 15.91 a | 82.31 d | 18.57 c | 0.23 a | 1.48 b |
| Inoculated maize (I) | 66.87 b | 57.00 c | 50.75 b | 15.52 a | 103.98 c | 19.07 c | 0.20 b | 1.86 b |
| Urochloa | 62.62 c | 67.12 a | 65.25 a | 15.91 a | 121.32 b | 20.66 c | 0.26 a | 1.98 b |
| Inoculated Urochloa (I) | 74.37 a | 66.50 a | 70.50 a | 16.43 a | 121.03 b | 22.48 a | 0.22 a | 1.85 b |
| Maize + Urochloa | 69.00 b | 60.62 b | 62.87 a | 16.18 a | 114.24 c | 20.87 b | 0.24 a | 1.82 b |
| Maize–I + Urochloa | 58.50 d | 53.62 c | 58.37 b | 16.11 a | 142.81 a | 19.36 c | 0.22 a | 2.48 a |
| Maize + Urochloa–I | 63.37 c | 63.25 a | 70.50 a | 16.76 a | 111.11 c | 21.17 b | 0.20 b | 1.73 b |
| Maize–I + Urochloa–I | 58.00 d | 64.25 a | 57.37 b | 16.60 a | 104.99 c | 20.61 b | 0.20 b | 1.71 b |
| Plant in sucession (PS) | ||||||||
| Rice–I | 66.12 a | 60.56 b | 62.03 a | 16.30 a | 110.28 a | 20.95 a | 0.22 a | 1.78 b |
| Rice | 61.84 b | 65.18 a | 63.81 a | 16.05 a | 115.17 a | 19.75 b | 0.22 a | 1.96 a |
| General mean | ||||||||
| F values | ||||||||
| CC | 42.05 ** | 37.34 ** | 0.55 ** | 1.29 NS | 2.86 ** | 24.23 * | 3.03 * | 23.83 * |
| PS | 18.73 ** | 10.87 ** | 2.68 NS | 1.12 NS | 8.38 NS | 7.43 ** | 0.09 NS | 5.95 ** |
| CC × PS | 2.04 NS | 13.19 ** | 3.67 ** | 1.93 NS | 23.11 ** | 1.91 NS | 1.81 NS | 12.34 ** |
| Coefficient of variation (%) | ||||||||
| CC | 6.2 | 8.3 | 19.5 | 6.8 | 10.2 | 5.0 | 19.2 | 8.3 |
| PS | 4.4 | 5.2 | 15.0 | 6.4 | 15.2 | 6.7 | 16.2 | 17.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.S.T.; Garcia, N.S.; Galindo, F.S.; Arf, O.; Nogueira, T.A.R.; Jani, A.D.; Cassiolato, A.M.R. Azospirillum brasilense Inoculation in a Maize–Urochloa–Rice Cropping System Promotes Soil Chemical and Biological Changes and Increases Productivity. Crops 2024, 4, 211-226. https://doi.org/10.3390/crops4020016
Silva PST, Garcia NS, Galindo FS, Arf O, Nogueira TAR, Jani AD, Cassiolato AMR. Azospirillum brasilense Inoculation in a Maize–Urochloa–Rice Cropping System Promotes Soil Chemical and Biological Changes and Increases Productivity. Crops. 2024; 4(2):211-226. https://doi.org/10.3390/crops4020016
Chicago/Turabian StyleSilva, Philippe Solano Toledo, Nayara Siviero Garcia, Fernando Shintate Galindo, Orivaldo Arf, Thiago Assis Rodrigues Nogueira, Arun Dilipkumar Jani, and Ana Maria Rodrigues Cassiolato. 2024. "Azospirillum brasilense Inoculation in a Maize–Urochloa–Rice Cropping System Promotes Soil Chemical and Biological Changes and Increases Productivity" Crops 4, no. 2: 211-226. https://doi.org/10.3390/crops4020016
APA StyleSilva, P. S. T., Garcia, N. S., Galindo, F. S., Arf, O., Nogueira, T. A. R., Jani, A. D., & Cassiolato, A. M. R. (2024). Azospirillum brasilense Inoculation in a Maize–Urochloa–Rice Cropping System Promotes Soil Chemical and Biological Changes and Increases Productivity. Crops, 4(2), 211-226. https://doi.org/10.3390/crops4020016








