Rebuilding the Marrow In Vitro: Translational Advances in the 3D Modeling of Blood Cancers
Simple Summary
Abstract
1. Introduction
2. Hematological Malignancies and the Microenvironment
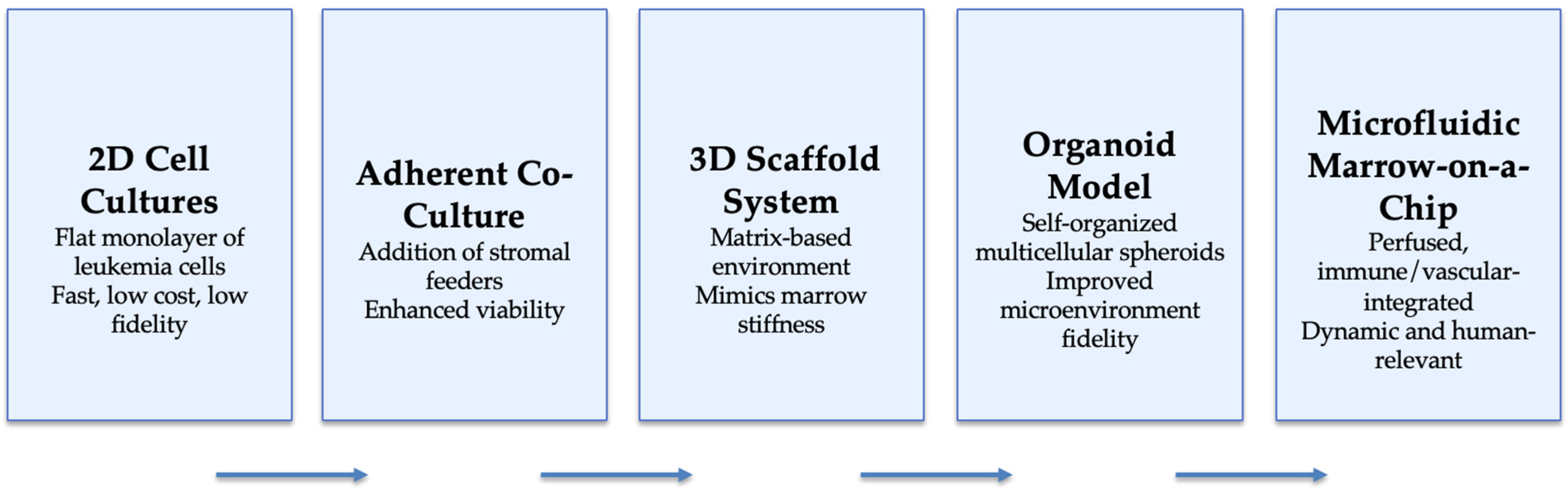
3. Methodologies for 3D Marrow Modeling
3.1. Organoid-like (Marrow-Mimetic) Systems
3.2. Spheroid Models
4. Applications in Hematological Malignancies
4.1. AML and CML Research
4.2. Lymphoma and Myeloma Research
4.3. Cross-Disease Synthesis
5. Personalized Medicine and Drug Screening
6. Challenges and Limitations
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A Report from the International Consensus Classification for Hematolymphoid Neoplasms. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef]
- Doulatov, S.; Notta, F.; Laurenti, E.; Dick, J.E. Hematopoiesis: A Human Perspective. Cell Stem Cell 2012, 10, 120–136. [Google Scholar] [CrossRef]
- Jin, M.Z.; Jin, W.L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and Potential in Organoid Research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap Between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human Organoids in Basic Research and Clinical Applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.A.; Clevers, H. Cancer Modeling Meets Human Organoid Technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The Current State of Animal Models in Research: A Review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Jian, H.; Li, X.; Dong, Q.; Tian, S.; Bai, S. In Vitro Construction of Liver Organoids with Biomimetic Lobule Structure by a Multicellular 3D Bioprinting Strategy. Cell Prolif. 2023, 56, e13465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lyu, X.; Yi, M.; Zhao, W.; Song, Y.; Wu, K. Organoid Technology and Applications in Cancer Research. J. Hematol. Oncol. 2018, 11, 116. [Google Scholar] [CrossRef]
- Andrews, M.G.; Kriegstein, A.R. Challenges of Organoid Research. Annu. Rev. Neurosci. 2022, 45, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, F.; Zhang, H.; Yang, Y.; Lu, J.; Chen, J.; Shen, L.; Pei, G. In vivo Development and Single-Cell Transcriptome Profiling of Human Brain Organoids. Cell Prolif. 2022, 55, e13201. [Google Scholar] [CrossRef] [PubMed]
- Corro, C.; Novellasdemunt, L.; Li, V.S.W. A Brief History of Organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues From ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008, 3, 519–535. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures In Vitro Without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Sato, T.; Van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; Van De Wetering, M.; Clevers, H. Paneth Cells Constitute the Niche for Lgr5 Stem Cells in Intestinal Crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef]
- Patel, S.B.; Kuznetsova, V.; Matkins, V.R.; Franceski, A.M.; Bassal, M.A.; Welner, R.S. Ex Vivo Expansion of Phenotypic and Transcriptomic Chronic Myeloid Leukemia Stem Cells. Exp. Hematol. 2022, 115, 1–13. [Google Scholar] [CrossRef]
- Miller, A.J.; Dye, B.R.; Ferrer-Torres, D.; Hill, D.R.; Overeem, A.W.; Shea, L.D.; Spence, J.R. Generation of Lung Organoids From Human Pluripotent Stem Cells In Vitro. Nat. Protoc. 2019, 14, 518–540. [Google Scholar] [CrossRef]
- Chua, C.W.; Shibata, M.; Lei, M.; Toivanen, R.; Barlow, L.J.; Bergren, S.K.; Badani, K.K.; McKiernan, J.M.; Benson, M.C.; Hibshoosh, H.; et al. Single Luminal Epithelial Progenitors Can Generate Prostate Organoids in Culture. Nat. Cell Biol. 2014, 16, 951–958. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 Alveolar Cells Are Stem Cells in Adult Lung. J. Clin. Invest. 2013, 123, 3025–3036. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina From Human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef]
- Khan, A.O.; Rodriguez-Romera, A.; Reyat, J.S.; Olijnik, A.-A.; Colombo, M.; Wang, G.; Wen, W.X.; Sousos, N.; Murphy, L.C.; Grygielska, B.; et al. Human Bone Marrow Organoids for Disease Modeling, Discovery, and Validation of Therapeutic Targets in Hematologic Malignancies. Cancer Discov. 2023, 13, 364–385. [Google Scholar] [CrossRef]
- Olijnik, A.-A.; Rodriguez-Romera, A.; Wong, Z.C.; Shen, Y.; Reyat, J.S.; Jooss, N.J.; Rayes, J.; Psaila, B.; Khan, A.O. Generating Human Bone Marrow Organoids for Disease Modeling and Drug Discovery. Nat. Protoc. 2024, 19, 2117–2146. [Google Scholar] [CrossRef]
- de Janon, A.; Mantalaris, A.; Panoskaltsis, N. Three-Dimensional Human Bone Marrow Organoids for the Study and Application of Normal and Abnormal Hematoimmunopoiesis. J. Immunol. 2023, 210, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Saleh, F.; Abdin, S.M.; Hashtchin, A.R.; Gensch, I.; Golgath, J.; Oliveira, M.C.; Nguyen, A.H.H.; Gaedcke, S.; Fenske, A.; et al. Standardized Generation of Human iPSC-Derived Hematopoietic Organoids and Macrophages Utilizing a Benchtop Bioreactor Platform Under Fully Defined Conditions. Stem Cell Res. Ther. 2024, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Wu, X.; Tang, X.; Zhang, B.; Fan, T.; He, L.; Pei, X.; Li, Y. Establishment of Human Hematopoietic Organoids for Evaluation of Hematopoietic Injury and Regeneration Effect. Stem Cell Res. Ther. 2024, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Hattangadi, S.M.; Wong, P.; Zhang, L.; Flygare, J.; Lodish, H.F. From Stem Cell to Red Cell: Regulation of Erythropoiesis at Multiple Levels by Multiple Proteins, RNAs and Chromatin Modifications. Blood 2011, 118, 6258–6268. [Google Scholar] [CrossRef]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and Differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, a011601. [Google Scholar] [CrossRef]
- Motazedian, A.; Bruveris, F.F.; Kumar, S.V.; Schiesser, J.V.; Chen, T.; Ng, E.S.; Chidgey, A.P.; Wells, C.A.; Elefanty, A.G.; Stanley, E.G. Multipotent RAG1+ Progenitors Emerge Directly From Haemogenic Endothelium in Human Pluripotent Stem Cell-Derived Haematopoietic Organoids. Nat. Cell Biol. 2020, 22, 60–73. [Google Scholar] [CrossRef]
- Demirci, S.; Haro-Mora, J.J.; Leonard, A.; Drysdale, C.; Malide, D.; Keyvanfar, K.; Essawi, K.; Vizcardo, R.; Tamaoki, N.; Restifo, N.P.; et al. Definitive HSC/Progenitor Cells From Human ESCs Through Serum/Feeder-Free Organoid-Induced Differentiation. Stem Cell Res. Ther. 2020, 11, 493. [Google Scholar] [CrossRef]
- Tamaoki, N.; Siebert, S.; Maeda, T.; Ha, N.-H.; Good, M.L.; Huang, Y.; Vodnala, S.K.; Haro-Mora, J.J.; Uchida, N.; Tisdale, J.F.; et al. Self-Organized Yolk Sac-Like Organoids for the Generation of Multipotent Hematopoietic Progenitor Cells From iPSCs. Cell Rep. Methods 2023, 3, 100460. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, X.; Wang, Q.; Dai, K.; Wang, J.; Liu, C. In vivo Osteo-Organoid Approach for Harvesting Therapeutic HSPCs. J. Vis. Exp. 2024, 204, e66026. [Google Scholar]
- Xu, H.; Jiao, Y.; Qin, S.; Zhao, W.; Chu, Q.; Wu, K. Organoid Technology in Disease Modelling, Drug Development, Personalized Treatment and Regenerative Medicine. Exp. Hematol. Oncol. 2018, 7, 30. [Google Scholar] [CrossRef]
- Tormin, A.; Li, O.; Brune, J.C.; Walsh, S.; Schütz, B.; Ehinger, M.; Ditzel, N.; Kassem, M.; Scheding, S. CD146 Expression on Primary Nonhematopoietic Bone Marrow Stem Cells Is Correlated With In Situ Localization. Blood 2011, 117, 5067–5077. [Google Scholar] [CrossRef] [PubMed]
- Bajénoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal Cell Networks Regulate Lymphocyte Entry, Migration, and Territoriality in Lymph Nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and Its Ligands: Balancing Immunity and Tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Allen, C.D.; Cyster, J.G. Follicular Dendritic Cell Networks of Primary Follicles and Germinal Centers: Phenotype and Function. Semin. Immunol. 2008, 20, 14–25. [Google Scholar] [CrossRef]
- Faria, C.; Gava, F.; Gravelle, P.; Valero, J.G.; Dobaño-López, C.; Van Acker, N.; Quelen, C.; Jalowicki, G.; Morin, R.; Rossi, C.; et al. Patient-Derived Lymphoma Spheroids Integrating Immune Tumor Microenvironment as Preclinical Follicular Lymphoma Models for Personalized Medicine. J. Immunother. Cancer 2023, 11, e007156. [Google Scholar] [CrossRef] [PubMed]
- Kastenschmidt, J.M.; Schroers-Martin, J.G.; Sworder, B.J.; Sureshchandra, S.; Khodadoust, M.S.; Liu, C.L.; Olsen, M.; Kurtz, D.M.; Diehn, M.; Wagar, L.E.; et al. A Human Lymphoma Organoid Model for Evaluating and Targeting the Follicular Lymphoma Tumor Immune Microenvironment. Cell Stem Cell 2024, 31, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Calvo-Vidal, M.N.; Chen, S.; Wu, G.; Revuelta, M.V.; Sun, J.; Zhang, J.; Walsh, M.F.; Nichols, K.E.; Joseph, V.; et al. Germline LSD1/KDM1A Mutations Confer Susceptibility to Multiple Myeloma. Cancer Res. 2018, 78, 2747–2759. [Google Scholar] [CrossRef] [PubMed]
- Britto, L.S.; Balasubramani, D.; Desai, S.; Phillips, P.; Trehan, N.; Cesarman, E.; Koff, J.L.; Singh, A. T Cells Spatially Regulate B Cell Receptor Signaling in Lymphomas Through H3K9me3 Modifications. Adv. Healthc. Mater. 2024, 14, e2401192. [Google Scholar] [CrossRef]
- Rodriguez, C. An Overview of Organoid and Three-Dimensional Models in Multiple Myeloma. Cancer J. 2021, 27, 239–246. [Google Scholar] [CrossRef]
- Verduin, M.; Hoeben, A.; De Ruysscher, D.; Vooijs, M. Patient-Derived Cancer Organoids as Predictors of Treatment Response. Front. Oncol. 2021, 11, 641980. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Li, C.; Huang, J.; Liu, F. Organoids to Study Immune Functions, Immunological Diseases and Immunotherapy. Cancer Lett. 2020, 477, 31–40. [Google Scholar] [CrossRef]
- Shah, S.B.; Carlson, C.R.; Lai, K.; Zhong, Z.; Marsico, G.; Lee, K.M.; Vélez, N.E.F.; Abeles, E.B.; Allam, M.; Hu, T.; et al. Combinatorial Treatment Rescues Tumour-Microenvironment-Mediated Attenuation of MALT1 Inhibitors in B-Cell Lymphomas. Nat. Mater. 2023, 22, 511–523. [Google Scholar] [CrossRef]
- Seet, C.S.; He, C.; Bethune, M.T.; Li, S.; Chick, B.; Gschweng, E.H.; Zhu, Y.; Kim, K.; Kohn, D.B.; Baltimore, D.; et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 2017, 14, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Bosticardo, M.; Pala, F.; Calzoni, E.; Delmonte, O.M.; Dobbs, K.; Gardner, C.L.; Sacchetti, N.; Kawai, T.; Garabedian, E.K.; Draper, D.; et al. Artificial thymic organoids represent a reliable tool to study T-cell differentiation in patients with severe T-cell lymphopenia. Blood Adv. 2020, 4, 2611–2616. [Google Scholar] [CrossRef]
- Lim, S.; van Son, G.J.F.; Yanti, N.L.W.E.; Andersson-Rolf, A.; Willemsen, S.; Korving, J.; Lee, H.-G.; Begthel, H.; Clevers, H. Derivation of Functional Thymic Epithelial Organoid Lines From Adult Murine Thymus. Cell Rep. 2024, 43, 114019. [Google Scholar] [CrossRef]
- Prockop, S.E.; Palencia, S.; Ryan, C.M.; Gordon, K.; Gray, D.; Petrie, H.T. Stromal Cells Provide the Matrix for Migration of Early Lymphoid Progenitors Through the Thymic Cortex. J. Immunol. 2002, 169, 4354–4361. [Google Scholar] [CrossRef]
- Griffith, A.V.; Fallahi, M.; Venables, T.; Petrie, H.T. Persistent Degenerative Changes in Thymic Organ Function Revealed by an Inducible Model of Organ Regrowth. Aging Cell 2012, 11, 169–177. [Google Scholar] [CrossRef]
- Jenkinson, W.E.; Rossi, S.W.; Parnell, S.M.; Jenkinson, E.J.; Anderson, G. PDGFRα-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood 2007, 109, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.F.; Ahn, H.; Schneider, R.S.; Yang, S.N.; Roman-Gonzalez, L.; Melnick, A.M.; Cerchietti, L.; Singh, A. Integrin-Specific Hydrogels as Adaptable Tumor Organoids for Malignant B and T Cells. Biomaterials 2015, 73, 110–119. [Google Scholar] [CrossRef]
- Suematsu, S.; Watanabe, T. Generation of a Synthetic Lymphoid Tissue-Like Organoid in Mice. Nat. Biotechnol. 2004, 22, 1539–1545. [Google Scholar] [CrossRef]
- Giese, C.; Lubitz, A.; Demmler, C.D.; Reuschel, J.; Bergner, K.; Marx, U. Immunological Substance Testing on Human Lymphatic Micro-Organoids In Vitro. J. Biotechnol. 2010, 148, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lenti, E.; Bianchessi, S.; Proulx, S.T.; Palano, M.T.; Genovese, L.; Raccosta, L.; Spinelli, A.; Drago, D.; Andolfo, A.; Alfano, M.; et al. Therapeutic Regeneration of Lymphatic and Immune Cell Functions Upon Lympho-Organoid Transplantation. Stem Cell Rep. 2019, 12, 1260–1268. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated Microfluidic Platform for Dynamic and Combinatorial Drug Screening of Tumor Organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef]
- Licata, J.P.; Schwab, K.H.; Har-El, Y.-E.; Gerstenhaber, J.A.; Lelkes, P.I. Bioreactor Technologies for Enhanced Organoid Culture. Int. J. Mol. Sci. 2023, 24, 11427. [Google Scholar] [CrossRef]
- Cai, H.; Ao, Z.; Wu, Z.; Song, S.; Mackie, K.; Guo, F. Intelligent Acoustofluidics Enabled Mini-Bioreactors for Human Brain Organoids. Lab. Chip 2021, 21, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhao, J.; Li, Y.; Shin, S.R.; Ligresti, G.; Ng, A.H.X.; Bromberg, J.S.; Church, G.; Lemos, D.R.; et al. Rapid Generation of HPSC-Derived High Endothelial Venule Organoids With Functional HEV Structures for In Vitro Immunological Research. Adv. Mater. 2024, 36, e2308760. [Google Scholar] [CrossRef]
- Saleh, J.; Mercier, B.; Xi, W. Bioengineering Methods for Organoid Systems. Biol. Cell 2021, 113, 475–491. [Google Scholar] [CrossRef]
- Lou, Y.R.; Leung, A.W. Next Generation Organoids for Biomedical Research and Applications. Biotechnol. Adv. 2018, 36, 132–149. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, S.H.; Lim, J.; Yoo, J.; Hwang, D.Y. The epidermal growth factor receptor variant type III mutation frequently found in gliomas induces astrogenesis in human cerebral organoids. Cell Prolif. 2021, 54, e12965. [Google Scholar] [CrossRef]
- Di Lullo, E.; Kriegstein, A.R. The Use of Brain Organoids to Investigate Neural Development and Disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, S.; Gu, L.; Li, B.; Xu, F.; Li, C.; Chen, P. High-Throughput Bioengineering of Homogenous and Functional hiPSC-Derived Liver Organoids via Micropatterning Technique. Front. Bioeng. Biotechnol. 2022, 10, 937595. [Google Scholar] [CrossRef]
- Orecchio, F.M.; Tommaso, V.; Santaniello, T.; Castiglioni, S.; Pezzotta, F.; Monti, A.; Butera, F.; Maier, J.A.M.; Milani, P. A Novel Fluidic Platform for Semi-Automated Cell Culture Into Multiwell-Like Bioreactors. Micromachines 2022, 13, 994. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Williams, N.A.; Elrod, A.K.; DesRochers, T.M. Organoids Standardized to a Clinically Validated Drug Response Assay for Truly Predictive In Vitro Drug Response Profiling. Cancer Res. 2022, 82 (Suppl. 12), 3086. [Google Scholar] [CrossRef]
- Al-Kaabneh, A.; El-Zeer, S.; Aljitawi, O. The Potential Role of 3D In Vitro Acute Myeloid Leukemia Culture Models in Understanding Drug Resistance in Leukemia Stem Cells. Cancers 2022, 14, 5252. [Google Scholar] [CrossRef] [PubMed]
- de Janon, A.; Kaza, P.; Deryckere, D.; Graham, D.K.; Takayama, S. High Throughput and Long-Term Human Bone Marrow Perivascular Organoids to Study Tumor Microenvironment and Drug Sensitivity in Acute Myeloid Leukemia. Blood 2024, 144 (Suppl. 1), 4063. [Google Scholar] [CrossRef]
- Sharipol, A.; Lesch, M.L.; Soto, C.A.; Frisch, B.J. Bone Marrow Microenvironment-On-Chip for Culture of Functional Hematopoietic Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 855777. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Körner, Y.; von Bonin, M.; Bornhäuser, M.; Werner, C. A Three-Dimensional Ex Vivo Tri-Culture Model Mimics Cell-Cell Interactions Between Acute Myeloid Leukemia and the Vascular Niche. Haematologica 2017, 102, 1215–1226. [Google Scholar] [CrossRef]
- Ma, C.; Witkowski, M.T.; Harris, J.; Dolgalev, I.; Sreeram, S.; Qian, W.; Tong, J.; Chen, X.; Aifantis, I.; Chen, W. Leukemia-On-A-Chip: Dissecting the Chemoresistance Mechanisms in B Cell Acute Lymphoblastic Leukemia Bone Marrow Niche. Sci. Adv. 2020, 6, eaba5536. [Google Scholar] [CrossRef] [PubMed]
- Aljitawi, O.S.; Li, D.; Xiao, Y.; Zhang, D.; Ramachandran, K.; Stehno-Bitte, L.; Veldhuizen, P.V.; Lin, T.L.; Kambhampati, S.; Garimella, R. A Novel Three-Dimensional Stromal-Based Model for In Vitro Chemotherapy Sensitivity Testing of Leukemia Cells. Exp. Hematol. 2014, 42, 826–830. [Google Scholar]
- Lisi-Vega, L.E.; Pievani, A.; García-Fernández, M.; Forte, D.; Williams, T.L.; Serafini, M.; Méndez-Ferrer, S. Bone Marrow Mesenchymal Stromal Cells Support Translation in Refractory Acute Myeloid Leukemia. Cell Rep. 2025, 44, 115151. [Google Scholar] [CrossRef]
- Kretzschmar, K. Cancer Research Using Organoid Technology. J. Mol. Med. 2021, 99, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Jaafari, N.; Kojabad, A.A.; Shabestari, R.M.; Safa, M. Design and Fabrication of Novel Microfluidic-Based Droplets for Drug Screening on a Chronic Myeloid Leukemia Cell Line. PLoS ONE 2025, 20, e0315803. [Google Scholar] [CrossRef]
- Iefremova, V.; Manikakis, G.; Krefft, O.; Jabali, A.; Weynans, K.; Wilkens, R.; Marsoner, F.; Brändl, B.; Müller, F.-J.; Koch, P.; et al. An Organoid-Based Model of Cortical Development and Disease Identifies Epileptogenesis Mechanisms in Miller-Dieker Syndrome. Cell Rep. 2017, 19, 50–59. [Google Scholar] [CrossRef]
- Boutin, L.; Arnautou, P.; Trignol, A.; Ségot, A.; Farge, T.; Desterke, C.; Soave, S.; Clay, D.; Raffoux, E.; Sarry, J.-E.; et al. Mesenchymal stromal cells confer chemoresistance to myeloid leukemia blasts through Side Population functionality and ABC transporter activation. Haematologica 2020, 105, 987–9998. [Google Scholar] [CrossRef]
- Yang, J.; Friedman, R. Combination strategies to overcome drug resistance in FLT+ acute myeloid leukaemia. Cancer Cell Int. 2023, 23, 161. [Google Scholar] [CrossRef]
- Lee, L.; Hizukuri, Y.; Severson, P.; Powell, B.; Zhang, C.; Ma, Y.; Narahara, M.; Sumi, H.; Hernandez, D.; Rajkhowa, T.; et al. A Novel Combination Regimen of BET and FLT3 Inhibition for FLT3-ITD Acute Myeloid Leukemia. Haematologica 2021, 106, 1022–1033. [Google Scholar] [CrossRef]
- Sharipol, A.; Frisch, B.J. Are We Ready to Integrate 3D Culture Systems in Acute Myeloid Leukemia? Front. Hematol. 2024, 1, 1407698. [Google Scholar]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived From Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Wobus, A.M.; Holzhausen, H.; Jäkel, P.; Schöneich, J. Characterization of a Pluripotent Stem Cell Line Derived From a Mouse Embryo. Exp. Cell Res. 1984, 152, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Zeleniak, A.; Wiegand, C.; Liu, W.; McCormick, C.; K., R.; Alavi, A.; Guan, H.; Bertera, S.; Lakomy, R.; Tajima, A.; et al. De Novo Construction of T Cell Compartment in Humanized Mice Engrafted With iPSC-Derived Thymus Organoids. Nat. Methods 2022, 19, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, F.; Jin, Y.; Ma, Y. Applications of Human Organoids in the Personalized Treatment for Digestive Diseases. Signal Transduct. Target. Ther. 2022, 7, 336. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, F.; Zuo, X.; Li, M. Breakthroughs and Challenges of Organoid Models for Assessing Cancer Immunotherapy: A Cutting-Edge Tool for Advancing Personalised Treatments. Cell Death Discov. 2025, 11, 222. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Magré, L.; A Verstegen, M.M.; Buschow, S.; van der Laan, L.J.W.; Peppelenbosch, M.; Desai, J. Emerging Organoid-Immune Co-Culture Models for Cancer Research: From Oncoimmunology to Personalized Immunotherapies. J. Immunother. Cancer 2023, 11, e006290. [Google Scholar] [CrossRef]
- Sljukic, A.; Green Jenkinson, J.; Niksic, A.; Prior, N.; Huch, M. Advances in liver and pancreas organoids: How far we have come and where we go next. Nat. Rev. Gastroenterol. Hepatol. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Kostine, M.; Pauling, J.D.; Ferreira, A.C.; Stevens, K.; Smith, E.; Eguiluz-Gracia, I.; Studenic, P.; Rodríguez-Carrio, J.; Ramiro, S.; et al. Multidisciplinary collaboration among young specialists: Results of an international survey by the emerging EULAR network and other young organisations. RMD Open 2020, 6, e001398. [Google Scholar] [CrossRef] [PubMed]
| Feature | 2D Culture | 3D Organoids/Spheroids |
|---|---|---|
| Cell architecture | Flat monolayer | Spatially organized multicellular structures |
| Cell–cell interactions | Limited | Extensive, physiologic contacts |
| Oxygen/nutrient gradients | Uniform | Physiologic gradients (hypoxia zones) |
| Stromal and immune integration | Minimal | Incorporates stromal, endothelial, immune cells |
| Genetic stability | Often drifts with passages | Preserves patient-specific clonal diversity |
| Drug response predictivity | Low to moderate | High, correlates with clinical outcomes |
| Throughput | High | Increasing with automation and microfluidics |
| Translational relevance | Limited | Strong correlation with patient response |
| Model Type | Representative Configuration | Key Components | Advantages | Limitations | Translational Utility |
|---|---|---|---|---|---|
| 2D Suspension Cultures | Leukemia cell lines (MOLM-14, MV4-11) in serum/defined media | Leukemic blasts only | Easy, inexpensive, high throughput | Rapid apoptosis of primary AML cells; loss of stemness | Drug screening; signaling assays |
| 2D Adherent Co-Cultures | AML cells on MSC or endothelial feeders | Stromal layer + AML blasts | Improves viability; supports cytokine signaling | Feeder variability; non-physiologic architecture | Testing cytokine or adhesion inhibitors |
| Scaffold-Based 3D Systems | AML + MSC + EC in collagen/Matrigel or PEG hydrogels | ECM scaffold + stromal + hematopoietic | Mimics marrow stiffness, gradient control | Limited vascularization; short-term stability | Mechanistic AML niche modeling |
| Organoid-Like Cultures | Self-assembled aggregates or bioprinted constructs | AML, MSC, EC, immune cells | Preserves phenotype; supports LSC hierarchy | Technically demanding; limited scalability | Personalized drug testing; mechanistic studies |
| Microfluidic ‘Marrow-on-a-Chip’ | Perfused chips with endothelial and stromal chambers | Stromal + hematopoietic + immune | Dynamic flow; oxygen/drug gradients; live imaging | Cost, complexity, throughput limits | Pharmacokinetic modeling; precision-therapy screening |
| Disease | Model Type | Key Components | Applications | Representative References |
|---|---|---|---|---|
| AML | Limited 3D co-culture or bone marrow organoid attempts | Mesenchymal stromal + endothelial + AML blasts | Proof-of-concept modeling of drug resistance and niche protection; early preclinical validation | [24,25,26,27,28,69,70,71,72,73,74,75,79,82] |
| CML | Experimental ex vivo stem cell expansion; microfluidic co-culture | Leukemic stem cells + stromal niche | LSC maintenance and early drug screening; no true organoids yet | [15,16,17,18,19,20,63,73,77,83,84] |
| Lymphoma | Lymphoid organoids or spheroid co-cultures | B/T cells + macrophages + stromal support | Immune checkpoint testing, drug screening, microenvironmental regulation | [40,41,43,47] |
| Multiple Myeloma | Bone marrow or myeloma organoids | Osteoblasts, osteoclasts, plasma cells, MSCs | Modeling bone lesions, IL-6-driven resistance, immune therapy testing | [29,42,44] |
| Anemia and Other Blood Disorders | Hematopoietic and yolk-sac-like 3D organoids | Erythroid progenitors + stromal + endothelial cells | Studying erythropoiesis and anemia pathophysiology | [31,32,33,34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestri, G.; Chatterjee, A. Rebuilding the Marrow In Vitro: Translational Advances in the 3D Modeling of Blood Cancers. Onco 2025, 5, 51. https://doi.org/10.3390/onco5040051
Silvestri G, Chatterjee A. Rebuilding the Marrow In Vitro: Translational Advances in the 3D Modeling of Blood Cancers. Onco. 2025; 5(4):51. https://doi.org/10.3390/onco5040051
Chicago/Turabian StyleSilvestri, Giovannino, and Aditi Chatterjee. 2025. "Rebuilding the Marrow In Vitro: Translational Advances in the 3D Modeling of Blood Cancers" Onco 5, no. 4: 51. https://doi.org/10.3390/onco5040051
APA StyleSilvestri, G., & Chatterjee, A. (2025). Rebuilding the Marrow In Vitro: Translational Advances in the 3D Modeling of Blood Cancers. Onco, 5(4), 51. https://doi.org/10.3390/onco5040051







