Metabolic Reprogramming as a Therapeutic Target in Cancer: A Qualitative Systematic Review (QualSR) of Natural Compounds Modulating Glucose and Glutamine Pathways
Simple Summary
Abstract
1. Introduction
1.1. Cancer Therapeutics: Bridging Genetic and Metabolic Paradigms
1.2. Study Objectives
1.3. Research Question and Study Rationale
- Development of standardized protocols for implementing metabolic approaches in clinical settings.
- Identification of reliable biomarkers for both genetic and metabolic status
- Assessment of aberrant epigenomic modulations in specific therapeutics, such as Demethylase activity in CpG binding to methyl group (CH3).
- Optimization of combination strategies that target both genetic, epigenomic, and metabolic vulnerabilities.
- Investigation of resistance mechanisms in metabolic-targeted therapies
- Development of personalized treatment approaches based on integrated genetic, epigenomic, and metabolic profiles.
2. Materials and Methods
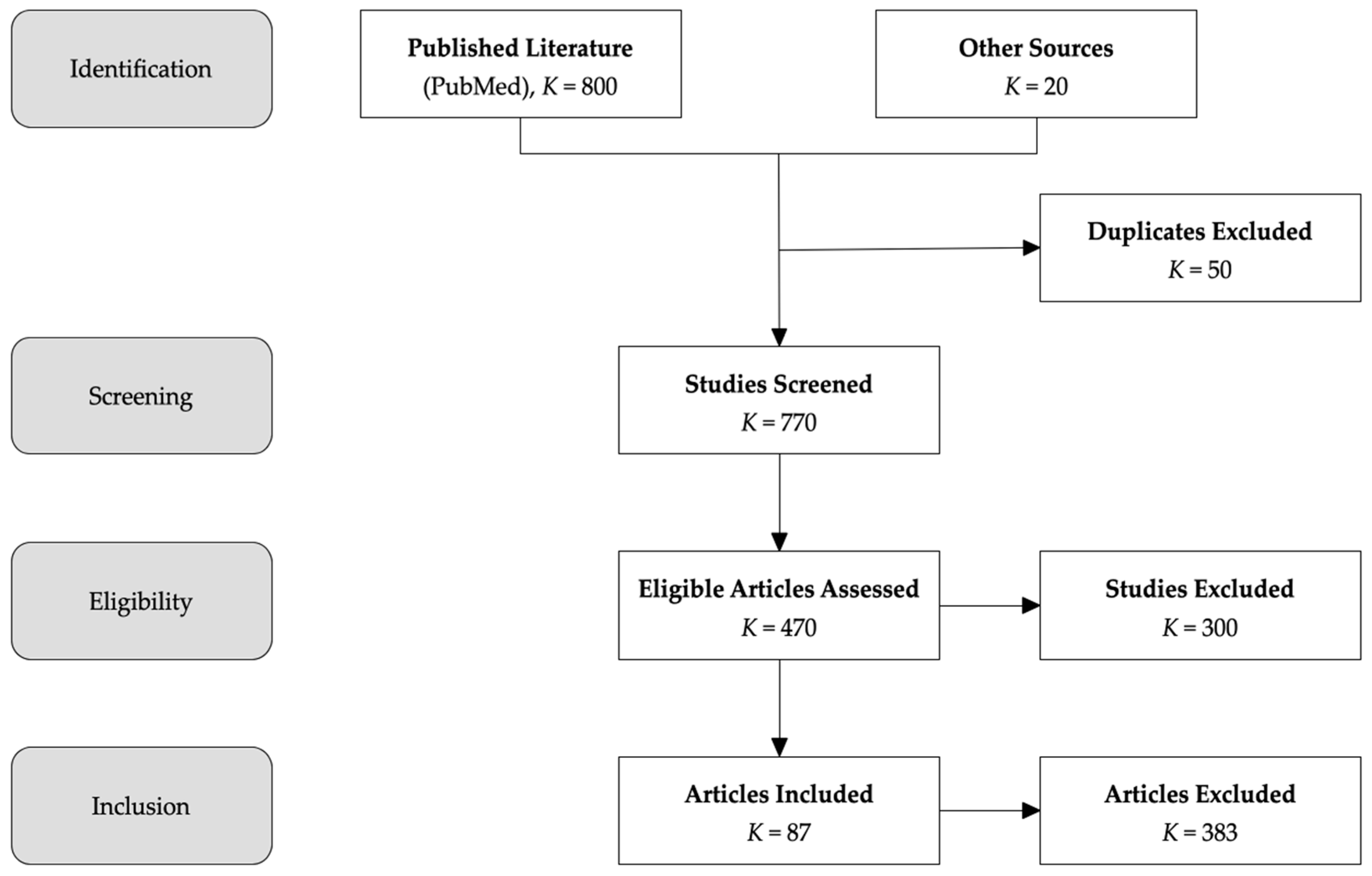
2.1. Literature Search and Study Design
Study Design and PRISMA Compliance
2.2. Search Strategy and Data Sources
- Primary Search Terms: (“cancer metabolism” OR “tumor metabolism” OR “Warburg effect” OR “metabolic reprogramming”) AND (“natural compounds” OR “berberine” OR “curcumin” OR “EGCG” OR “silibinin” OR “resveratrol” OR “quercetin” OR “vitamin D” OR “melatonin”) AND (“glutamine uptake” OR “glutamine metabolism” OR “SLC1A5” OR “ASCT2” OR “metabolic targeting”).
- Secondary Search Terms: (“metabolic therapy” OR “mitochondrial dysfunction” OR “metabolic modulation pathways”) AND (“therapeutic implications” OR “metabolic interventions”).
- Full-Text Review: Articles that passed initial screening underwent full-text assessment based on predefined inclusion and exclusion criteria. Studies failing to meet these criteria were excluded, with reasons for exclusion documented.
- Published in peer-reviewed journals;
- Full-text articles available in English;
- Original research articles or systematic reviews;
- Studies involving human or animal models;
- No restrictions on geographic location or study design;
- Clinical studies with a minimum sample size of 30 participants.
- Non-peer-reviewed literature;
- Case reports and series with n < 30;
- Conference abstracts;
- Opinion pieces;
- Studies without clear methodology;
- Studies without quantifiable outcomes;
- Studies focused on non-cancer-related metabolic disorders.
2.3. Justification for Sample Size Cutoff (≥30 Participants in Clinical Studies)
2.4. Data Extraction and Quality Assessment
- Study Characteristics: Author, year, country, study design, sample size, and cancer type.
- Intervention Details: Type of natural compound, dosage, administration route, and duration.
- Outcome Measures: Metabolic pathway modulation (e.g., inhibition of SLC1A5 transporter, Warburg effect), tumor regression, treatment response rates, and survival outcomes.
- Key Findings: Mechanisms of action, therapeutic efficacy, and clinical implications.
- Quality assessment was conducted using several tools. The Newcastle–Ottawa Scale (NOS) evaluates clinical study quality based on three main domains: selection, comparability, and outcomes. AMSTAR-2 assesses the methodological rigor and reliability of systematic reviews. Finally, ROBINS-I measures the risk of bias in non-randomized trials, examining seven key domains to determine study quality.
- Risk of Bias: The Cochrane Risk of Bias Tool and ROBINS-I tool were used to evaluate bias in randomized and non-randomized studies, respectively.
2.5. Misclassification and Selection Bias
- Documentation of excluded studies;
- Independent verification of inclusion/exclusion decisions;
- Resolution of disagreements by third reviewer.
3. Results and Discussion
3.1. Targeting Oncogenic Signaling and Glutamine Metabolism: EGCG and Berberine
3.2. Modulating Inflammation and Inducing Metabolic Catastrophe: Curcumin and IV Vitamin C
3.3. Direct Metabolic Reprogramming and Epigenetic Influence: Resveratrol, Silibinin, and Vitamin D3
3.4. Restoring Physiological Metabolism and Synergy: Melatonin, Quercetin, and 5-Geranyloxy-7-methoxycoumarin (5GG)
3.5. Remodeling the Tumor Microenvironment: Metabolic Modulation, Immunity, and Metastasis
3.5.1. Metabolic Reprogramming of Immunosuppressive Cells
3.5.2. Targeting Metastasis: Inhibiting the Epithelial-to-Mesenchymal Transition (EMT)
3.5.3. Immunomodulation as a Synergistic Mechanism
4. Translational Solutions and Clinical Evidence
5. Limitations
- Adhere to CONSORT and ARRIVE guidelines, ensuring transparency in effect size reporting, CI inclusion, and appropriate multiple testing correction.
- Incorporate orthogonal validation techniques such as metabolomics and proteomics to substantiate mechanistic claims.
- Conduct biomarker-driven, multi-center clinical trials that integrate metabolic profiling to optimize patient selection.
- Emphasize pharmacokinetic standardization and comparative analysis across formulations (e.g., nanocurcumin vs. native curcumin, oral vs. IV vitamin C).
Safety Data Gaps
6. Conclusions: Clinical Implications and Future Research Directions
6.1. Clinical Implications
- Personalized Metabolic Therapies: Future cancer treatment should incorporate metabolic profiling, allowing oncologists to tailor therapies based on individual metabolic signatures. This could lead to more effective patient stratification and treatment customization. Personalization must extend beyond efficacy biomarkers to include metabolic and genetic risk factors (e.g., G6PD deficiency for ascorbate, CYP polymorphisms for berberine). Standardized monitoring protocols for hepatic, renal, and immune function are critical to mitigate adverse events.
- Combination Strategies with Standard Therapies: Metabolic-targeted treatments can synergize with conventional chemotherapy, immunotherapy, and radiation, reducing tumor resistance and recurrence rates.
- Non-Toxic, Cost-Effective Interventions: Natural metabolic modulators offer a safer and potentially cost-effective alternative to cytotoxic treatments, reducing long-term side effects and improving patient compliance.
- Expanded Treatment Options for Hard-to-Treat Cancers: Aggressive malignancies like glioblastoma, pancreatic cancer, and chronic myeloid leukemia (CML) exhibit high metabolic plasticity. Metabolic inhibition offers a promising avenue for addressing treatment-resistant tumors.
6.2. Ethical and Practical Considerations in Metabolic Therapies
6.3. Conclusion and Final Thoughts
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- LeCompte, M.C.; Brawley, O.W. The Cause of Death in Patients with Cancer. JACC CardioOncology 2023, 5, 67–69. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and Limitations in Traditional Anti-Cancer Therapies: A Comprehensive Review of Surgery, Chemotherapy, Radiation Therapy, and Hormonal Therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a Metabolic Disease: Implications for Novel Therapeutics. Carcinogenesis 2013, 35, 515. [Google Scholar] [CrossRef]
- Gyamfi, J.; Kim, J.; Choi, J. Cancer as a Metabolic Disorder. Int. J. Mol. Sci. 2022, 23, 1155. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y. Cancer Metabolism and Intervention Therapy. Mol. Biomed. 2021, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, F.; Xu, F.; Yang, C. Metabolic Reprogramming and Therapeutic Targeting in Non-Small Cell Lung Cancer: Emerging Insights beyond the Warburg Effect. Front. Oncol. 2025, 15, 1564226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pavlova, N.N.; Thompson, C.B. Cancer Cell Metabolism: The Essential Role of the Nonessential Amino Acid, Glutamine. EMBO J. 2017, 36, 1302–1315. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Park, K.-G. Targeting Glutamine Metabolism for Cancer Treatment. Biomol. Ther. 2018, 26, 19–28. [Google Scholar] [CrossRef]
- Michalak, K.P.; Maćkowska-Kędziora, A.; Sobolewski, B.; Woźniak, P. Key Roles of Glutamine Pathways in Reprogramming the Cancer Metabolism. Oxid. Med. Cell. Longev. 2015, 2015, 964321. [Google Scholar] [CrossRef]
- Li, T.; Copeland, C.; Le, A. Glutamine Metabolism in Cancer. In The Heterogeneity of Cancer Metabolism, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic Reprogramming and Therapeutic Resistance in Primary and Metastatic Breast Cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef]
- Akins, N.S.; Nielson, T.C.; Le, H.V. Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr. Top. Med. Chem. 2018, 18, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mahony, C.B.; Torres, A.; Murillo-Saich, J.; Kemble, S.; Cedeno, M.; John, P.; Bhatti, A.; Croft, A.P.; Guma, M. Dual Inhibition of Glycolysis and Glutaminolysis for Synergistic Therapy of Rheumatoid Arthritis. Arthritis Res. Ther. 2023, 25, 176. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Biological and Therapeutic Impact of Intratumor Heterogeneity in Cancer Evolution. Cancer Cell 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Waarts, M.R.; Stonestrom, A.J.; Park, Y.C.; Levine, R.L. Targeting Mutations in Cancer. J. Clin. Investig. 2022, 132, e154943. [Google Scholar] [CrossRef]
- Aguilar, A.; Mas, L.; Enríquez, D.; Vallejos, C.; Gutarra, R.; Flores, C.J. Impact of Targeted Therapy on the Survival of Patients with Advanced-Stage Non-Small Cell Lung Cancer in Oncosalud—AUNA. Cancer Control 2022, 29, 10732748211068637. [Google Scholar] [CrossRef]
- Pagliarini, R.; Shao, W.; Sellers, W.R. Oncogene Addiction: Pathways of Therapeutic Response, Resistance, and Road Maps toward a Cure. EMBO Rep. 2015, 16, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Hoque, N.; Hossain, M.; Rahaman, M.; Chowdhury, R.; Alam, M.; Hossain, M.; Tama, R. Molecular Mechanism of Cell Signaling Pathways of Cancer Cells in Forward and Reverse Mutation: A Perspective Review. J. Cancer Tumor Int. 2024, 14, 46–57. [Google Scholar] [CrossRef]
- Bahrami, H. On the Origin of the Warburg Effect in Cancer Cells: Controlling Cancer as a Metabolic Disease. Asian Pac. J. Cancer Biol. 2024, 9, 75–80. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N. Fundamentals of Bacterial Physiology and Metabolism; Springer Nature: Singapore, 2021; ISBN 978-981-16-0722-6. [Google Scholar]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Masson, N.; Ratcliffe, P.J. Hypoxia Signaling Pathways in Cancer Metabolism: The Importance of Co-Selecting Interconnected Physiological Pathways. Cancer Metab. 2014, 2, 3. [Google Scholar] [CrossRef]
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef] [PubMed]
- Bertram, R.; Gram Pedersen, M.; Luciani, D.S.; Sherman, A. A Simplified Model for Mitochondrial ATP Production. J. Theor. Biol. 2006, 243, 575–586. [Google Scholar] [CrossRef]
- Cooper, G.M. The Mechanism of Oxidative Phosphorylation. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Broeks, M.H.; Meijer, N.W.F.; Westland, D.; Bosma, M.; Gerrits, J.; German, H.M.; Ciapaite, J.; Van Karnebeek, C.D.M.; Wanders, R.J.A.; Zwartkruis, F.J.T.; et al. The Malate-Aspartate Shuttle Is Important for de Novo Serine Biosynthesis. Cell Rep. 2023, 42, 113043. [Google Scholar] [CrossRef]
- Bose, S.; Zhang, C.; Le, A. Glucose Metabolism in Cancer: The Warburg Effect and Beyond. In The Heterogeneity of Cancer Metabolism; Le, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1311, pp. 3–15. ISBN 978-3-030-65767-3. [Google Scholar]
- Seyfried, T.N.; Lee, D.C.; Duraj, T.; Ta, N.L.; Mukherjee, P.; Kiebish, M.; Arismendi-Morillo, G.; Chinopoulos, C. The Warburg Hypothesis and the Emergence of the Mitochondrial Metabolic Theory of Cancer. J. Bioenerg. Biomembr. 2025, 57, 57–83. [Google Scholar] [CrossRef]
- Halama, A.; Suhre, K. Advancing Cancer Treatment by Targeting Glutamine Metabolism—A Roadmap. Cancers 2022, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Moro, L. Mitochondrial Dysfunction in Aging and Cancer. J. Clin. Med. 2019, 8, 1983. [Google Scholar] [CrossRef]
- Wang, S.-F.; Tseng, L.-M.; Lee, H.-C. Role of Mitochondrial Alterations in Human Cancer Progression and Cancer Immunity. J. Biomed. Sci. 2023, 30, 61. [Google Scholar] [CrossRef]
- Dong, L.-F.; Kovarova, J.; Bajzikova, M.; Bezawork-Geleta, A.; Svec, D.; Endaya, B.; Sachaphibulkij, K.; Coelho, A.R.; Sebkova, N.; Ruzickova, A.; et al. Horizontal Transfer of Whole Mitochondria Restores Tumorigenic Potential in Mitochondrial DNA-Deficient Cancer Cells. eLife 2017, 6, e22187. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and Cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Li, Y.; Wei, S.; He, G.; Liu, J.; Li, X.; Yang, S.; Li, D.; Lin, W.; et al. Glutamine Addiction in Tumor Cell: Oncogene Regulation and Clinical Treatment. Cell Commun. Signal. 2024, 22, 12. [Google Scholar] [CrossRef]
- Alfarsi, L.H.; Ansari, R.E.; Erkan, B.; Fakroun, A.; Craze, M.L.; Aleskandarany, M.A.; Cheng, K.W.; Ellis, I.O.; Rakha, E.A.; Green, A.R. SLC1A5 Is a Key Regulator of Glutamine Metabolism and a Prognostic Marker for Aggressive Luminal Breast Cancer. Sci. Rep. 2025, 15, 2805. [Google Scholar] [CrossRef]
- Cunha, A.; Silva, P.M.A.; Sarmento, B.; Queirós, O. Targeting Glucose Metabolism in Cancer Cells as an Approach to Overcoming Drug Resistance. Pharmaceutics 2023, 15, 2610. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, Z.C.; Song, M.G.; Di Magliano, M.P.; Lyssiotis, C.A.; Kim, S.E. Nutrient Transporters: Connecting Cancer Metabolism to Therapeutic Opportunities. Oncogene 2023, 42, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, R.; Maeda, K.; Aki, S.; Osawa, T. Metabolic Adaptations of Cancer in Extreme Tumor Microenvironments. Cancer Sci. 2023, 114, 1200–1207. [Google Scholar] [CrossRef]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.M.R.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A.; et al. Designing a Broad-Spectrum Integrative Approach for Cancer Prevention and Treatment. Semin. Cancer Biol. 2015, 35, S276–S304. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sarma, D.K.; Verma, V.; Nagpal, R.; Kumar, M. Unveiling the Future of Metabolic Medicine: Omics Technologies Driving Personalized Solutions for Precision Treatment of Metabolic Disorders. Biochem. Biophys. Res. Commun. 2023, 682, 1–20. [Google Scholar] [CrossRef]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.-C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting Cancer Vulnerabilities with High-Dose Vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Delgir, S.; Bastami, M.; Ilkhani, K.; Safi, A.; Seif, F.; Alivand, M.R. The Pathways Related to Glutamine Metabolism, Glutamine Inhibitors and Their Implication for Improving the Efficiency of Chemotherapy in Triple-Negative Breast Cancer. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108366. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, A.; Pecoraro, G.; Giurato, G.; Nassa, G.; Rizzo, F.; Saggese, P.; Martinez, C.A.; Scafoglio, C.; Tarallo, R. Regulation of Metabolic Reprogramming by Long Non-Coding RNAs in Cancer. Cancers 2021, 13, 3485. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Acosta, L.; Byham-Gray, L.; Kurzer, M.; Samavat, H. Hepatotoxicity with High-Dose Green Tea Extract: Effect of Catechol-O-Methyltransferase and Uridine 5’-Diphospho-Glucuronosyltransferase 1A4 Genotypes. J. Diet. Suppl. 2023, 20, 850–869. [Google Scholar] [CrossRef]
- Cao, J.; Han, J.; Xiao, H.; Qiao, J.; Han, M. Effect of Tea Polyphenol Compounds on Anticancer Drugs in Terms of Anti-Tumor Activity, Toxicology, and Pharmacokinetics. Nutrients 2016, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tan, M.; Cai, Q. The Warburg Effect in Tumor Progression: Mitochondrial Oxidative Metabolism as an Anti-Metastasis Mechanism. Cancer Lett. 2015, 356, 156–164. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Qureshi, M.Z.; Khalid, S.; Attar, R.; Martinelli, C.; Sabitaliyevich, U.Y.; Nurmurzayevich, S.B.; Taverna, S.; Poltronieri, P.; Xu, B. Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers 2019, 11, 478. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A Natural Isoquinoline Alkaloid with Antitumor Activity: Studies of the Biological Activities of Berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-Dose Intravenous Vitamin C, a Promising Multi-Targeting Agent in the Treatment of Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, B.; Wang, H.; Yi, F. Glutamine Metabolic Reprogramming in Hepatocellular Carcinoma. Front. Mol. Biosci. 2023, 10, 1242059. [Google Scholar] [CrossRef]
- Liu, C.-S.; Zheng, Y.-R.; Zhang, Y.-F.; Long, X.-Y. Research Progress on Berberine with a Special Focus on Its Oral Bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Kamble, S.; Deshkar, S.; Kothapalli, L.; Chitlange, S. Bioavailability of Berberine: Challenges and Solutions. İstanbul J. Pharm. 2021, 51, 141–153. [Google Scholar] [CrossRef]
- Shi, L.; Wang, W.; Jing, C.; Hu, J.; Liao, X. Berberine and Health Outcomes: An Overview of Systematic Reviews. BMC Complement. Med. Ther. 2025, 25, 147. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Saha, P.; Chatterjee, B.; Chaudhary, A.A.; Lall, R.; Srivastava, A.K. Complexity of Tumor Microenvironment: Therapeutic Role of Curcumin and Its Metabolites. Nutr. Cancer 2023, 75, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The Roles of Curcumin in Regulating the Tumor Immunosuppressive Microenvironment (Review). Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef]
- Vishvakarma, N.K. Novel Antitumor Mechanisms of Curcumin: Implication of Altered Tumor Metabolism, Reconstituted Tumor Microenvironment and Augmented Myelopoiesis. Phytochem. Rev. 2014, 13, 717–724. [Google Scholar] [CrossRef]
- Cao, X.; Yi, Y.; Ji, M.; Liu, Y.; Wang, D.; Zhu, H. The Dual Role of Vitamin C in Cancer: From Antioxidant Prevention to Prooxidant Therapeutic Applications. Front. Med. 2025, 12, 1633447. [Google Scholar] [CrossRef]
- Li, W.-N.; Zhang, S.-J.; Feng, J.-Q.; Jin, W.-L. Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment. Cancers 2022, 14, 2608. [Google Scholar] [CrossRef]
- Dandoti, S. Mechanisms Adopted by Cancer Cells to Escape Apoptosis–A Review. Biocell 2021, 45, 863–884. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in Cancer: From Molecular Mechanisms to Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Peng, C.; Peng, F. Potential Roles and Mechanisms of Curcumin and Its Derivatives in the Regulation of Ferroptosis. Int. J. Biol. Sci. 2024, 20, 4838–4852. [Google Scholar] [CrossRef]
- Dytrych, P.; Kejík, Z.; Hajduch, J.; Kaplánek, R.; Veselá, K.; Kučnirová, K.; Skaličková, M.; Venhauerová, A.; Hoskovec, D.; Martásek, P.; et al. Therapeutic Potential and Limitations of Curcumin as Antimetastatic Agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef]
- Foroutan, Z.; Butler, A.E.; Zengin, G.; Sahebkar, A. Curcumin and Ferroptosis: A Promising Target for Disease Prevention and Treatment. Cell Biochem. Biophys. 2024, 82, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Thongpon, P.; Intuyod, K.; Chomwong, S.; Pongking, T.; Klungsaeng, S.; Muisuk, K.; Charoenram, N.; Sitthirach, C.; Thanan, R.; Pinlaor, P.; et al. Curcumin Synergistically Enhances the Efficacy of Gemcitabine against Gemcitabine-Resistant Cholangiocarcinoma via the Targeting LAT2/Glutamine Pathway. Sci. Rep. 2024, 14, 16059. [Google Scholar] [CrossRef]
- Wilson, M.K.; Baguley, B.C.; Wall, C.; Jameson, M.B.; Findlay, M.P. Review of High-dose Intravenous Vitamin C as an Anticancer Agent. Asia Pac. J. Clin. Oncol. 2014, 10, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Rago, C.; Cheong, I.; Pagliarini, R.; Angenendt, P.; Rajagopalan, H.; Schmidt, K.; Willson, J.K.V.; Markowitz, S.; Zhou, S.; et al. Glucose Deprivation Contributes to the Development of KRAS Pathway Mutations in Tumor Cells. Science 2009, 325, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Hawk, M.A.; Schafer, Z.T. Mechanisms of Redox Metabolism and Cancer Cell Survival during Extracellular Matrix Detachment. J. Biol. Chem. 2018, 293, 7531–7537. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of Poly (ADP-Ribose) Polymerase (PARP) Mechanisms of Action and Rationale for Targeting in Cancer and Other Diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ Consumption by PARP1 in Response to DNA Damage Triggers Metabolic Shift Critical for Damaged Cell Survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef]
- Podyacheva, E.; Toropova, Y. The Role of NAD+, SIRTs Interactions in Stimulating and Counteracting Carcinogenesis. Int. J. Mol. Sci. 2023, 24, 7925. [Google Scholar] [CrossRef]
- Zam, W.; Ahmed, I.; Yousef, H. The Warburg Effect on Cancer Cells Survival: The Role of Sugar Starvation in Cancer Therapy. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 30–38. [Google Scholar] [CrossRef] [PubMed]
- PDQ Cancer Genetics Editorial Board. Genetics of Breast and Gynecologic Cancers (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Jung, K.-H.; Lee, J.H.; Thien Quach, C.H.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol Suppresses Cancer Cell Glucose Uptake by Targeting Reactive Oxygen Species–Mediated Hypoxia-Inducible Factor-1α Activation. J. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Pirouzpanah, M.B.; Sabzichi, M.; Pirouzpanah, S.; Chavoshi, H.; Samadi, N. Silibilin-Induces Apoptosis in Breast Cancer Cells by Modulating P53, P21, Bak and Bcl-Xl Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lee, S.-Y.; Lin, C.-L.; Tu, T.-H.; Chen, L.H.; Chen, Y.J.; Huang, H.-C. Co-Treatment with Quercetin and 1,2,3,4,6-Penta- O -Galloyl-β- D -Glucose Causes Cell Cycle Arrest and Apoptosis in Human Breast Cancer MDA-MB-231 and AU565 Cells. J. Agric. Food Chem. 2013, 61, 6430–6445. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Lee, Y.-H.; Sharma, A.R.; Park, J.-B.; Jagga, S.; Sharma, G.; Lee, S.-S.; Nam, J.-S. Quercetin Induces Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells through Modulation of Foxo3a Activity. Korean J. Physiol. Pharmacol. 2017, 21, 205. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Qiao, J.; Cao, B. Silibinin Suppresses Glioblastoma Cell Growth, Invasion, Stemness, and Glutamine Metabolism by YY1/SLC1A5 Pathway. Transl. Neurosci. 2024, 15, 20220333. [Google Scholar] [CrossRef]
- Bai, Z.-L.; Tay, V.; Guo, S.-Z.; Ren, J.; Shu, M.-G. Silibinin Induced Human Glioblastoma Cell Apoptosis Concomitant with Autophagy through Simultaneous Inhibition of mTOR and YAP. BioMed Res. Int. 2018, 2018, 6165192. [Google Scholar] [CrossRef]
- Binienda, A.; Ziolkowska, S.; Pluciennik, E. The Anticancer Properties of Silibinin: Its Molecular Mechanism and Therapeutic Effect in Breast Cancer. Anticancer Agents Med. Chem. 2020, 20, 1787–1796. [Google Scholar] [CrossRef]
- Navasardyan, I.; Zaravinos, A.; Bonavida, B. Therapeutic Implications of Targeting YY1 in Glioblastoma. Cancers 2024, 16, 2074. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Matthews, D.; LaPorta, E.; Simmons, K.M.; Beaudin, S.; Welsh, J. The Impact of Vitamin D in Breast Cancer: Genomics, Pathways, Metabolism. Front. Physiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Cucielo, M.S.; Cesário, R.C.; Silveira, H.S.; Gaiotte, L.B.; Dos Santos, S.A.A.; De Campos Zuccari, D.A.P.; Seiva, F.R.F.; Reiter, R.J.; De Almeida Chuffa, L.G. Melatonin Reverses the Warburg-Type Metabolism and Reduces Mitochondrial Membrane Potential of Ovarian Cancer Cells Independent of MT1 Receptor Activation. Molecules 2022, 27, 4350. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rorsales-Corral, S.; De Almeida Chuffa, L.G. Melatonin Inhibits Warburg-Dependent Cancer by Redirecting Glucose Oxidation to the Mitochondria: A Mechanistic Hypothesis. Cell. Mol. Life Sci. 2020, 77, 2527–2542. [Google Scholar] [CrossRef]
- Bastani, S.; Akbarzadeh, M.; Rastgar Rezaei, Y.; Farzane, A.; Nouri, M.; Mollapour Sisakht, M.; Fattahi, A.; Akbarzadeh, M.; Reiter, R.J. Melatonin as a Therapeutic Agent for the Inhibition of Hypoxia-Induced Tumor Progression: A Description of Possible Mechanisms Involved. Int. J. Mol. Sci. 2021, 22, 10874. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Zhai, K.; Varghese, E.; Samuel, S.M.; Šudomová, M.; Lucansky, V.; Kassayova, M.; Pec, M.; et al. Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers 2021, 13, 3018. [Google Scholar] [CrossRef]
- Tavartkiladze, A.; Simonia, G.; Lou, R.; Revazishvili, P.; Kasradze, D.; Maisuradze, M.; Khutsishvili, R.; Andronikashvili, I.; Nozadze, P. Targeting Glycolysis in Tumor Cells: Therapeutic Inhibition of GLUT1 and LDH-A Using Phloretin and Melatonin for Metabolic Reprogramming and Tumor Regression in Triple Negative Breast Cancer. Immunogenet. Open Access 2024, 9, 1–19. Available online: https://www.longdom.org/open-access/targeting-glycolysis-in-tumor-cells-therapeutic-inhibition-of-glut1-and-ldha-using-phloretin-and-melatonin-for-metabolic-reprogram-110826.html#ai (accessed on 4 September 2025).
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting Immune System against Cancer by Melatonin: A Mechanistic Viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef]
- Sakai, C.; Nishikawa, H. Immunosuppressive Environment in Tumors. Gan Kagaku Ryoho Cancer Chemother. 2018, 45, 222–226. [Google Scholar]
- Sugiura, A.; Rathmell, J.C. Metabolic Barriers to T Cell Function in Tumors. J. Immunol. 2018, 200, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- Johnson, M.O.; Siska, P.J.; Contreras, D.C.; Rathmell, J.C. Nutrients and the Microenvironment to Feed a T Cell Army. Semin. Immunol. 2016, 28, 505–513. [Google Scholar] [CrossRef]
- Zalpoor, H.; Aziziyan, F.; Liaghat, M.; Bakhtiyari, M.; Akbari, A.; Nabi-Afjadi, M.; Forghaniesfidvajani, R.; Rezaei, N. The Roles of Metabolic Profiles and Intracellular Signaling Pathways of Tumor Microenvironment Cells in Angiogenesis of Solid Tumors. Cell Commun. Signal. 2022, 20, 186. [Google Scholar] [CrossRef]
- Buruiană, A.; Gheban, B.-A.; Gheban-Roșca, I.-A.; Georgiu, C.; Crișan, D.; Crișan, M. The Tumor Stroma of Squamous Cell Carcinoma: A Complex Environment That Fuels Cancer Progression. Cancers 2024, 16, 1727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wei, F.; Sun, G.; Wen, Y.; Xiang, J.; Su, F.; Zhan, L.; Nian, Q.; Chen, Y.; Zeng, J. Natural Compounds Targeting Glycolysis as Promising Therapeutics for Gastric Cancer: A Review. Front. Pharmacol. 2022, 13, 1004383. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Zeng, A.; Song, L. Revisiting of Cancer Immunotherapy: Insight from the Dialogue between Glycolysis and PD-1/PD-L1 Axis in the Tumor Microenvironment. Int. J. Biol. Sci. 2025, 21, 1202–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Ren, Y.; Zhang, Q.; Yi, P.; Cheng, C. Metabolic Modulation of Immune Checkpoints and Novel Therapeutic Strategies in Cancer. Semin. Cancer Biol. 2022, 86, 542–565. [Google Scholar] [CrossRef]
- Venkatraman, S.; Balasubramanian, B.; Thuwajit, C.; Meller, J.; Tohtong, R.; Chutipongtanate, S. Targeting MYC at the Intersection between Cancer Metabolism and Oncoimmunology. Front. Immunol. 2024, 15, 1324045. [Google Scholar] [CrossRef] [PubMed]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers 2021, 13, 210. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Qin, W.; Yang, Y.; Li, X.; Liu, R. Metabolic Regulation of Myeloid-Derived Suppressor Cells in Tumor Immune Microenvironment: Targets and Therapeutic Strategies. Theranostics 2025, 15, 2159–2184. [Google Scholar] [CrossRef]
- Lim, S.A. Metabolic Reprogramming of the Tumor Microenvironment to Enhance Immunotherapy. BMB Rep. 2024, 57, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Mojsilovic, S.S.; Mojsilovic, S.; Villar, V.H.; Santibanez, J.F. The Metabolic Features of Tumor-Associated Macrophages: Opportunities for Immunotherapy? Anal. Cell. Pathol. 2021, 2021, 5523055. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Peng, W.-B.; Zhang, P.; Yang, X.-P.; Zhou, Q. Lactate in the Tumour Microenvironment: From Immune Modulation to Therapy. EBioMedicine 2021, 73, 103627. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Li, P.; Zhang, Z.; Zeng, M.; Liang, Z.; Li, D.; Wang, L.; Chen, Y.; Liang, Y.; et al. Reprogramming of Glutamine Metabolism and Its Impact on Immune Response in the Tumor Microenvironment. Cell Commun. Signal. 2022, 20, 114. [Google Scholar] [CrossRef]
- Schwager, S.C.; Mosier, J.A.; Padmanabhan, R.S.; White, A.; Xing, Q.; Hapach, L.A.; Taufalele, P.V.; Ortiz, I.; Reinhart-King, C.A. Link between Glucose Metabolism and Epithelial-to-Mesenchymal Transition Drives Triple-Negative Breast Cancer Migratory Heterogeneity. iScience 2022, 25, 105190. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Arner, E.N.; Rathmell, J.C. Metabolic Programming and Immune Suppression in the Tumor Microenvironment. Cancer Cell 2023, 41, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Biggi, A.F.B.; Simioni, P.U. Inhibition of PD-1 Protein by the CRISPR-Cas9 Method as Antitumor Therapy of Non-Small Cell Lung Cancers. Rev. Fac. Ciênc. Médicas Sorocaba 2019, 21, 2–7. [Google Scholar] [CrossRef]
- Duraj, T.; Kalamian, M.; Zuccoli, G.; Maroon, J.C.; D’Agostino, D.P.; Scheck, A.C.; Poff, A.; Winter, S.F.; Hu, J.; Klement, R.J.; et al. Clinical Research Framework Proposal for Ketogenic Metabolic Therapy in Glioblastoma. BMC Med. 2024, 22, 578. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Magalhães, M.; Pereira-Silva, M.; Caldas, M.; Ferreira, L.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Targeting Cancer Via Resveratrol-Loaded Nanoparticles Administration: Focusing on In Vivo Evidence. AAPS J. 2019, 21, 57. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.-M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Landis-Piwowar, K.; Chen, D.; Foldes, R.; Chan, T.-H.; Dou, Q.P. Novel Epigallocatechin Gallate Analogs as Potential Anticancer Agents: A Patent Review (2009–Present). Expert Opin. Ther. Pat. 2013, 23, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.E.; Chinnaiyan, A.M.; Lerner, S.P.; Swanton, C.; Rubin, M.A. The Emergence of Precision Urologic Oncology: A Collaborative Review on Biomarker-Driven Therapeutics. Eur. Urol. 2017, 71, 237–246. [Google Scholar] [CrossRef]
- Dai, J.; Zhou, F.X.; Xu, H.; Jiang, C.Q.; Wang, W.B.; Jiang, H.G.; Wang, Q.Y.; Wang, Y.; Xia, L.; Wu, H.; et al. Efficacy and Safety of High-Dose Vitamin C Combined with Total Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer (HCCSC R02 Study). Int. J. Radiat. Oncol. 2023, 117, e291–e292. [Google Scholar] [CrossRef]
- Guo, P.; Cai, C.; Wu, X.; Fan, X.; Huang, W.; Zhou, J.; Wu, Q.; Huang, Y.; Zhao, W.; Zhang, F.; et al. An Insight into the Molecular Mechanism of Berberine Towards Multiple Cancer Types Through Systems Pharmacology. Front. Pharmacol. 2019, 10, 857. [Google Scholar] [CrossRef] [PubMed]
| Aspect | Genetic-Centric Approach | Metabolic-Centric Approach | Integrated Approach |
|---|---|---|---|
| Primary Target | Specific mutations/pathways | Cellular metabolism (e.g., Warburg effect, glutamine addiction) | Multiple cellular systems (genetic and metabolic) |
| Response Rate | 20–35% (this can vary significantly depending on the specific cancer type and the targeted mutation) | Under investigation | Potentially higher |
| Resistance Development | Common (6–12 months) (combination therapies and targeted drug combinations are being explored to overcome this limitation) | Less understood | May be reduced |
| Patient Selection | Based on genetic profiling | Based on metabolic markers | Multi-parameter selection (genetic and metabolic) |
| Cost | Often very high | Generally lower | Moderate to high |
| Implementation Complexity | High | Moderate | High |
| Strengths | Effective for specific subtypes with known mutations | Broad applicability, complements genetic therapies | Comprehensive targeting may improve efficacy |
| Limitations | High rates of resistance, limited durability | Limited standardized protocols, variable efficacy | Complex implementation, requires tailored patient selection and protocols |
| Key Therapeutic Agents | Small-molecule inhibitors, monoclonal antibodies | Natural compounds (e.g., Vitamin C, berberine, curcumin); metabolic inhibitors | Combination therapies targeting both genetic and metabolic vulnerabilities |
| Future Potential | Improved patient stratification, potential for combination with metabolic interventions | Personalized, metabolic-targeted protocols | Enhanced durability and personalized regimens by combining genetic and metabolic therapies |
| Criterion | Inclusion | Exclusion |
|---|---|---|
| Study Type | RCTs, systematic reviews, mechanistic studies | Case reports (n < 30), conference abstracts, opinion pieces |
| Cancer Focus | Metabolic-targeted cancer therapies (glucose/glutamine metabolism) | Non-cancer metabolic disorders |
| Outcome Measures | Tumor regression, metabolic modulation, treatment response rates | Studies without quantifiable outcomes |
| Compound | Primary Mechanism(s) | Reported Effects | Side Effects/Limitations | Clinical/Preclinical Notes |
|---|---|---|---|---|
| Curcumin | Inhibits NF-κB, TNF-α, IL-6; induces ferroptosis; inhibits SLC1A5/LAT1 | ↓ Inflammation; ↑ antioxidant capacity; ferroptosis induction | Poor oral bioavailability; rapid metabolism | Enhanced gemcitabine efficacy in resistant cholangiocarcinoma; ↓ TNF-α, IL-6 in clinical studies |
| Berberine (BBR) | Inhibits Akt/mTOR; suppresses SLC1A5; miRNA-mediated regulation | ↓ Glutamine uptake; G1 arrest; ↓ tumor proliferation | CYP3A4 inhibition; gastrointestinal upset | Strong preclinical evidence in HCC, LUAD, BLCA with q < 10−10 |
| EGCG | Inhibits glutaminase; ↓ ERα, IGFBP-2; ↑ p53/p21 | ↓ Proliferation; ↑ apoptosis | Short half-life (~3.4 h); hepatotoxicity at high doses | Synergistic with tamoxifen; clinical promise in ERα-negative tumors |
| Resveratrol | ↓ HIF-1α, GLUT1; ROS modulation | ↓ Glycolytic flux; ↑ mitochondrial respiration | Low bioavailability; variable plasma stability | ↓ 18F-FDG uptake in vivo; anti-glycolytic effects in TNBC and colon carcinoma |
| Silibinin | YY1/SLC1A5 axis inhibition | ↓ Glutamine metabolism; ↑ apoptosis in breast and glioblastoma | Limited human clinical validation | Promising preclinical evidence against glioblastoma |
| Vitamin C (IV) | GLUT1-mediated uptake; pro-oxidant at pharmacological doses | ROS induction; NAD+ depletion; sensitization to therapy | Hemolysis risk in G6PD deficiency | 44.4% pathologic complete response in rectal cancer with CRT |
| Vitamin D3 | ↑ G6PD, TXNIP modulation; regulates glycolysis and OXPHOS | ↓ Proliferation; metabolic reprogramming | Hypercalcemia at supraphysiological dosing | Linked to prognosis in breast cancer via G6PD expression |
| Melatonin | Inhibits PDK; ↓ HIF-1α stabilization; restores OXPHOS | ↓ Glycolysis; ↓ tumor proliferation; ↑ apoptosis | Limited pharmacokinetic data; underpowered trials | Potential synergy with chemo/radiotherapy; metabolic reprogramming evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enwere, M.; Irobi, E.; Chime, V.; Ezeogu, A.; Onu, A.; El Hussein, M.T.; Ogungbade, G.; Davies, E.; Omoniwa, O.; Omale, C.; et al. Metabolic Reprogramming as a Therapeutic Target in Cancer: A Qualitative Systematic Review (QualSR) of Natural Compounds Modulating Glucose and Glutamine Pathways. Onco 2025, 5, 43. https://doi.org/10.3390/onco5030043
Enwere M, Irobi E, Chime V, Ezeogu A, Onu A, El Hussein MT, Ogungbade G, Davies E, Omoniwa O, Omale C, et al. Metabolic Reprogramming as a Therapeutic Target in Cancer: A Qualitative Systematic Review (QualSR) of Natural Compounds Modulating Glucose and Glutamine Pathways. Onco. 2025; 5(3):43. https://doi.org/10.3390/onco5030043
Chicago/Turabian StyleEnwere, Michael, Edward Irobi, Victoria Chime, Ada Ezeogu, Adamu Onu, Mohamed Toufic El Hussein, Gbadebo Ogungbade, Emmanuel Davies, Omowunmi Omoniwa, Charles Omale, and et al. 2025. "Metabolic Reprogramming as a Therapeutic Target in Cancer: A Qualitative Systematic Review (QualSR) of Natural Compounds Modulating Glucose and Glutamine Pathways" Onco 5, no. 3: 43. https://doi.org/10.3390/onco5030043
APA StyleEnwere, M., Irobi, E., Chime, V., Ezeogu, A., Onu, A., El Hussein, M. T., Ogungbade, G., Davies, E., Omoniwa, O., Omale, C., Neufeld, M., Akagwu, O., Atim, T., & Holmes, L., Jr. (2025). Metabolic Reprogramming as a Therapeutic Target in Cancer: A Qualitative Systematic Review (QualSR) of Natural Compounds Modulating Glucose and Glutamine Pathways. Onco, 5(3), 43. https://doi.org/10.3390/onco5030043






