CRISPR-Mediated Analysis of p27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Human Cell Line
2.2. CRISPR-Mediated Generation of p27-T157D, p27-T157A, PAK1-T423E and PAK1-K299R Mutants
2.3. Polymerase Chain Reaction
2.4. Immunoblotting
2.5. Invasion Assay
2.6. Cell Proliferation Assay
2.7. Mouse Studies
2.8. Statistical Analyses
3. Results
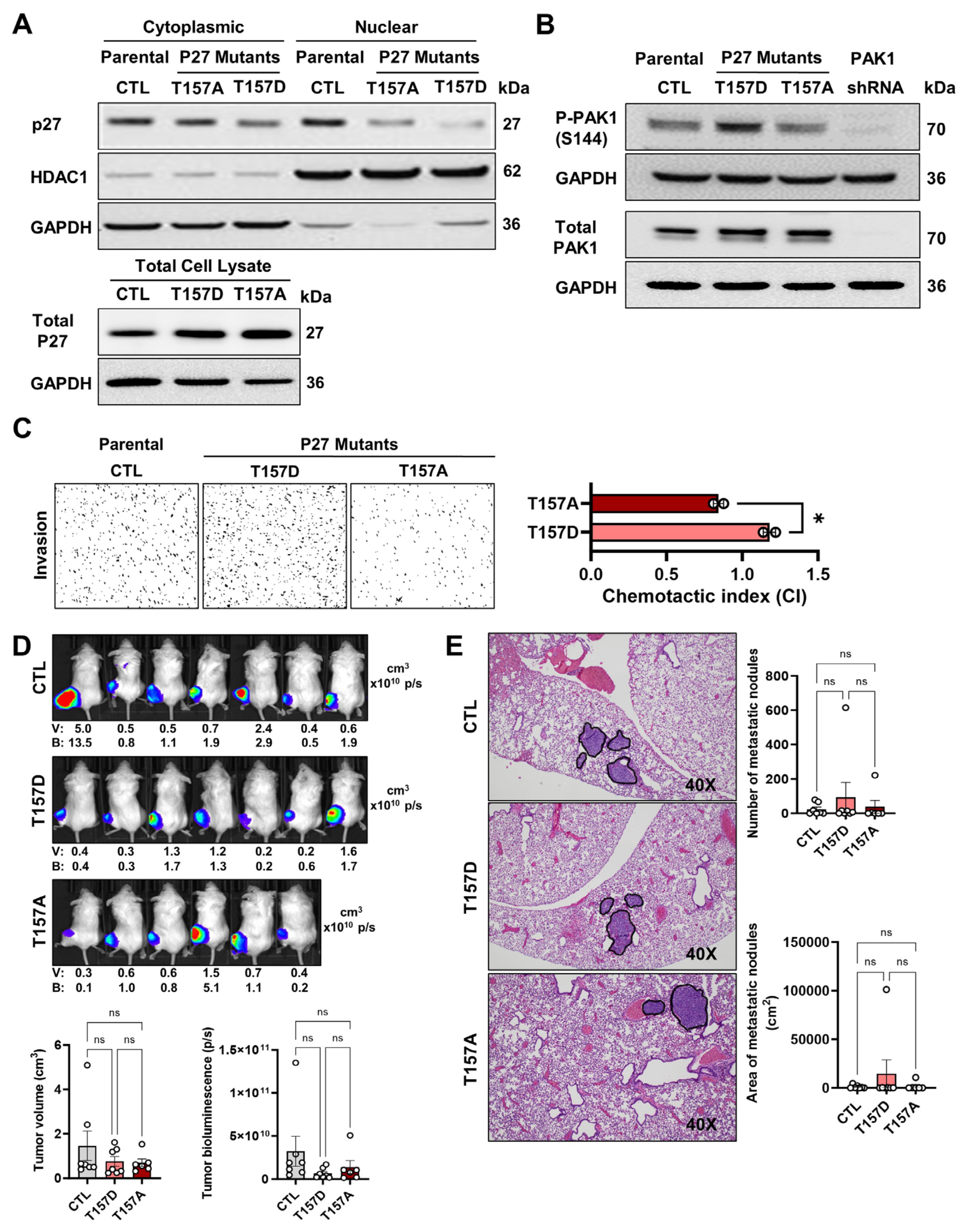
3.1. Effects of p27 T157 Mutations on Cellular Phenotypes and PAK1 Phosphorylation
3.2. Elevated PAK1-S144 Phosphorylation Alone Is Insufficient in Driving OS Pulmonary Metastasis
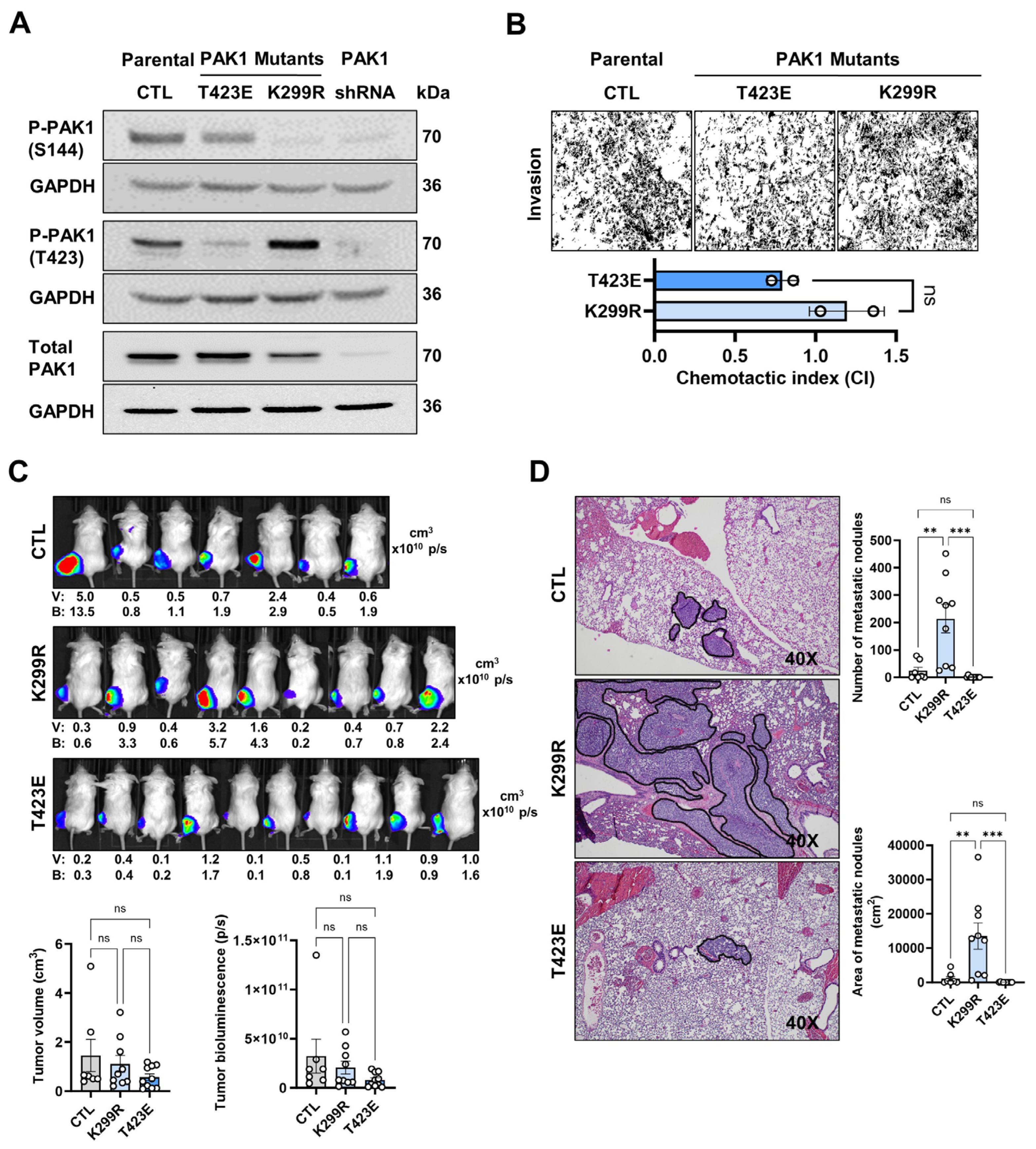
3.3. Increased T423-PAK1 Phosphorylation Promotes OS Metastasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Osteosarcoma |
| P27 | Cyclin-dependent kinase inhibitor 1B |
| PAK1 | P21-activated kinase 1 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
References
- Kager, L.; Zoubek, A.; Pötschger, U.; Kastner, U.; Flege, S.; Kempf-Bielack, B.; Branscheid, D.; Kotz, R.; Salzer-Kuntschik, M.; Winkelmann, W.; et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003, 21, 2011–2018. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Chen, X.; Cates, J.M.M.; Du, Y.C.; Jain, A.; Jung, S.Y.; Li, X.N.; Hicks, J.M.; Man, T.K. Mislocalized cytoplasmic p27 activates PAK1-mediated metastasis and is a prognostic factor in osteosarcoma. Mol. Oncol. 2020, 14, 846–864. [Google Scholar] [CrossRef] [PubMed]
- Razavipour, S.F.; Harikumar, K.B.; Slingerland, J.M. P27 as a transcriptional regulator: New roles in development and cancer. Cancer Res. 2020, 80, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.C.; Jubb, A.M.; Haverty, P.M.; Zhou, W.; Tran, V.; Truong, T.; Turley, H.; O’Brien, T.; Vucic, D.; Harris, A.L.; et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7177–7182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nakka, M.; Kelly, A.J.; Lau, C.C.; Krailo, M.; Barkauskas, D.A.; Hicks, J.M.; Man, T.K. P27 is a candidate prognostic biomarker and metastatic promoter in osteosarcoma. Cancer Res. 2016, 76, 4002–4011. [Google Scholar] [CrossRef]
- Shin, I.; Yakes, F.M.; Rojo, F.; Shin, N.-y.; Bakin, A.V.; Baselga, J.; Arteaga, C.L. PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nat. Med. 2002, 8, 1145–1152. [Google Scholar] [CrossRef]
- Ye, D.Z.; Field, J. PAK signaling in cancer. Cell. Logist. 2012, 2, 105. [Google Scholar] [CrossRef]
- Liang, J.; Zubovitz, J.; Petrocelli, T.; Kotchetkov, R.; Connor, M.K.; Han, K.; Lee, J.-H.; Ciarallo, S.; Catzavelos, C.; Beniston, R.; et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 2002, 8, 1153–1160. [Google Scholar] [CrossRef]
- Zenke, F.T.; King, C.C.; Bohl, B.P.; Bokoch, G.M. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 1999, 274, 32565–32573. [Google Scholar] [CrossRef]
- Chong, C.; Tan, L.; Lim, L.; Manser, E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 2001, 276, 17347–17353. [Google Scholar] [CrossRef]
- Zhou, G.-L.; Zhuo, Y.; King, C.C.; Fryer, B.H.; Bokoch, G.M.; Field, J. Akt Phosphorylation of Serine 21 on Pak1 Modulates Nck Binding and Cell Migration. Mol. Cell. Biol. 2003, 23, 8058–8069. [Google Scholar] [CrossRef]
- Lei, M.; Lu, W.; Meng, W.; Parrini, M.C.; Eck, M.J.; Mayer, B.J.; Harrison, S.C. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 2000, 102, 387–397. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.W.; Wang, Z.X. Structural insights into the autoactivation mechanism of p21-activated protein kinase. Structure 2011, 19, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsen, S.Z.; Gårdsvoll, H.; van Putten, S.; Nørregaard, K.S.; Krigslund, O.; Meilstrup, J.A.; Tran, C.; Jürgensen, H.J.; Melander, M.C.; Nielsen, C.H.; et al. Tumor cell MT1-MMP is dispensable for osteosarcoma tumor growth, bone degradation and lung metastasis. Sci. Rep. 2020, 10, 19138. [Google Scholar] [CrossRef] [PubMed]
- Gyau, B.B.; Wang, J.; Chen, X.; Clement, M.A.; Man, Z.D.; Major, A.M.; Weiser, M.C.; Xu, J.; Hicks, J.; Man, T.K. The metastatic role of the CXCL10-CXCR3 axis and its therapeutic potential in osteosarcoma. J. Bone Oncol. 2025, 52, 100690. [Google Scholar] [CrossRef] [PubMed]
- Kolch, W.; Halasz, M.; Granovskaya, M.; Kholodenko, B.N. The dynamic control of signal transduction networks in cancer cells. Nat. Rev. Cancer 2015, 15, 525–527. [Google Scholar] [CrossRef]
- Kim, J.; Jonasch, E.; Alexander, A.; Short, J.D.; Cai, S.; Wen, S.; Tsavachidou, D.; Tamboli, P.; Czerniak, B.A.; Do, K.A.; et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin. Cancer Res. 2009, 15, 81–90. [Google Scholar] [CrossRef]
- Radu, M.; Semenova, G.; Kosoff, R.; Chernoff, J. PAK signalling during the development and progression of cancer. Nat. Rev. Cancer 2014, 14, 13–25. [Google Scholar] [CrossRef]
- Papakonstanti, E.A.; Stournaras, C. Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol. Biol. Cell 2002, 13, 2946–2962. [Google Scholar] [CrossRef]
- Sells, M.A.; Knaus, U.G.; Bagrodia, S.; Ambrose, D.M.; Bokoch, G.M.; Chernoff, J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997, 7, 202–210. [Google Scholar] [CrossRef]
- Jäkel, H.; Peschel, I.; Kunze, C.; Weinl, C.; Hengst, L. Regulation of p27Kip1 by mitogen-induced tyrosine phosphorylation. Cell Cycle 2012, 11, 1910–1917. [Google Scholar] [CrossRef]
- Lei, M.; Robinson, M.A.; Harrison, S.C. The active conformation of the PAK1 kinase domain. Structure 2005, 13, 769–778. [Google Scholar] [CrossRef]
- Zhao, D.; Besser, A.H.; Wander, S.A.; Sun, J.; Zhou, W.; Wang, B.; Ince, T.; Durante, M.A.; Guo, W.; Mills, G.; et al. Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene 2015, 34, 5447–5459. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Shao, Z.M.; Li, D.Q. Tumor microenvironment: Driving forces and potential therapeutic targets for breast cancer metastasis. Chin. J. Cancer 2017, 36, 36. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Merika, E.E.; Syrigos, K.N.; Saif, M.W. Desmoplasia in pancreatic cancer. Can we fight it? Gastroenterol. Res. Pract. 2012, 2012, 781765. [Google Scholar] [CrossRef]
- Ren, B.; Cui, M.; Yang, G.; Wang, H.; Feng, M.; You, L.; Zhao, Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol. Cancer 2018, 17, 108. [Google Scholar] [CrossRef]
- Bulle, A.; Lim, K.H. Beyond just a tight fortress: Contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 249. [Google Scholar] [CrossRef]
- Yu, J.S.; Chen, W.J.; Ni, M.H.; Chan, W.H.; Yang, S. Der. Identification of the regulatory autophosphorylation site of autophosphorylation-dependent protein kinase (auto-kinase): Evidence that auto-kinase belongs to a member of the p21-activated kinase family. Biochem. J. 1998, 334, 121–131. [Google Scholar] [CrossRef]
- Ng, Y.W.; Raghunathan, D.; Chan, P.M.; Baskaran, Y.; Smith, D.J.; Lee, C.H.; Verma, C.; Manser, E. Why an A-Loop Phospho-Mimetic Fails to Activate PAK1: Understanding an Inaccessible Kinase State by Molecular Dynamics Simulations. Structure 2010, 18, 879–890. [Google Scholar] [CrossRef]
- Zegers, M.M.P.; Forget, M.A.; Chernoff, J.; Mostov, K.E.; Ter Beest, M.B.A.; Hansen, S.H. Pak1 and PIX regulate contact inhibition during epithelial wound healing. EMBO J. 2003, 22, 4155–4165. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Xu, L.; An, H.; Liu, W.; Liu, Y.; Lin, Z.; Xu, J. P21-activated kinase 1 determines stem-like phenotype and sunitinib resistance via NF-κB/IL-6 activation in renal cell carcinoma. Cell Death Dis. 2015, 6, e1637. [Google Scholar] [CrossRef]
- Menard, R.E.; Mattingly, R.R. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cell. Signal. 2003, 15, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Roy, S.; Apolloni, A.; Lane, A.; Hancock, J.F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 1998, 273, 24052–24056. [Google Scholar] [CrossRef] [PubMed]
- Peyssonnaux, C.; Provot, S.; Felder-Schmittbuhl, M.P.; Calothy, G.; Eychène, A. Induction of Postmitotic Neuroretina Cell Proliferation by Distinct Ras Downstream Signaling Pathways. Mol. Cell. Biol. 2000, 20, 7068–7079. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Yarmand, R.; Mandal, M.; Taludker, A.H.; Wang, R.A.; Vadlamudi, R.K.; Kung, H.J.; Kumar, R. Etk/Bmx Tyrosine Kinase Activates Pak1 and Regulates Tumorigenicity of Breast Cancer Cells. J. Biol. Chem. 2001, 276, 29403–29409. [Google Scholar] [CrossRef]
- Rider, L.; Shatrova, A.; Feener, E.P.; Webb, L.; Diakonova, M. JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions. J. Biol. Chem. 2007, 282, 30985–30996. [Google Scholar] [CrossRef]
- King, C.C.; Gardiner, E.M.M.; Zenke, F.T.; Bohl, B.P.; Newton, A.C.; Hemmings, B.A.; Bokoch, G.M. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J. Biol. Chem. 2000, 275, 41201–41209. [Google Scholar] [CrossRef]
- Chang, T.W.; Lin, M.C.; Yu, C.J.; Lee, F.J.S. The phosphorylation of Pak1 by Erk1/2 to drive cell migration requires Arl4D acting as a scaffolding protein. J. Cell Sci. 2025, 138, jcs263812. [Google Scholar] [CrossRef]
- Hullinger, T.G.; Panek, R.L.; Xu, X.; Karathanasis, S.K. p21-activated Kinase-1 (PAK1) Inhibition of the Human Scavenger Receptor Class B, Type I Promoter in Macrophages Is Independent of PAK1 Kinase Activity, but Requires the GTPase-binding Domain. J. Biol. Chem. 2001, 276, 46807–46814. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Reilly, A.M.; Daniels, R.H.; King, C.C.; Olivera, A.; Spiegel, S.; Knaus, U.G. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J. Biol. Chem. 1998, 273, 8137–8144. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, M.; Wang, L.; Liu, J.; Li, Y.; Brakebusch, C.; Mei, Q. P21-activated kinase 1 (PAK1) can promote ERK activation in a kinase-independent manner. J. Biol. Chem. 2013, 288, 20093–20099. [Google Scholar] [CrossRef]
- Higuchi, M.; Onishi, K.; Kikuchi, C.; Gotoh, Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat. Cell Biol. 2008, 10, 1356–1364. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.; B, S.S.S.; Gopinath, P.; Saravanan, R.; Sundaram, S.; Shanmugasundaram, G.; Venkatraman, G.; Rayala, S.K. Pak1 dysregulates pyruvate metabolism in PDAC cells by exerting a phosphorylation-mediated regulatory effect on PDHA1. J. Biol. Chem. 2025, 301, 108409. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Xu, R.; Li, Q.; Huang, X.; Zhang, C.; Yuan, B. PAK2 promotes proliferation, migration, and invasion of lung squamous cell carcinoma through the LIMK1/cofilin signaling pathway. J. Biomed. Res. 2025, 39, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.E.; Box, G.M.; Court, W.; Gale, M.E.; Brown, J.P.; Pinder, S.E.; Eccles, S.A.; Wells, C.M. PAK4 promotes kinase-independent stabilization of RhoU to modulate cell adhesion. J. Cell Biol. 2015, 211, 863–879. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Dong, Z. The Role of p21-Activated Kinases in Cancer and Beyond: Where Are We Heading? Front. Cell Dev. Biol. 2021, 9, 641381. [Google Scholar] [CrossRef]
- He, L.F.; Xu, H.W.; Chen, M.; Xian, Z.R.; Wen, X.F.; Chen, M.N.; Du, C.W.; Huang, W.H.; Wu, J.D.; Zhang, G.J. Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget 2017, 8, 17573–17585. [Google Scholar] [CrossRef]
- Blankenstein, L.J.; Cordes, N.; Kunz-Schughart, L.A.; Vehlow, A. Targeting of p21-Activated Kinase 4 Radiosensitizes Glioblastoma Cells via Impaired DNA Repair. Cells 2022, 11, 2133. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.A.; Mainardi, S.; Dias, M.H.; Bosma, A.; van Dijk, E.; Selig, R.; Albrecht, W.; Laufer, S.A.; Zender, L.; Bernards, R. Small-molecule inhibition of MAP2K4 is synergistic with RAS inhibitors in KRAS-mutant cancers. Proc. Natl. Acad. Sci. USA 2024, 121, e2319492121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, Y.; Wang, Y.; Zhang, M.; Dong, Z.; Liu, Y.; Sun, M. The Potential Treatment Options and Combination Strategies of KRAS-Mutated Lung Cancer. OncoTargets Ther. 2024, 17, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
| Gene | KI Locus | sgRNA | ssODN |
|---|---|---|---|
| PAK1 | K299R | cugaagauucaucugcuuaa | ataatcagctctttcttgggctgctgctgaagattcatctgCCGGatCgccacctgaaatcaagagtatattcaatgtgcaaccatatg |
| PAK1 | T423E | gcagagcaaacggagcacca | ggattctgtgcacagataaccccagagcagagcaaGcgCagcGAAatggtaggaaccccatactggatggcaccagaggttgtgac |
| P27 | T157D | aggaagcgaccugcaaccga | aagcggggccccaaacacattctatggttgggaaagggtcattaccgtcgTCAgcTggtcgcttccttattcctgcgcattgctccgctaaccccgtctgg |
| P27 | T157A | aggaagcgaccugcaaccga | aagcggggccccaaacacattctatggttgggaaagggtcattaccgtcggCAgcTggtcgcttccttattcctgcgcattgctccgctaaccccgtctgg |
| Gene | KI Locus | PCR Primer Fw’ | PCR Primer Rev’ | Restriction Enzyme |
|---|---|---|---|---|
| PAK1 | K299R | gcattcttggcttttgccgtat | ttgactcaggcagatgggttg | HpalI |
| PAK1 | T423E | ccaaaatgggcagcttggac | accacagagaacaccctgga | HhaI |
| P27 | T157D | tgtgtcttttggctccgagg | tgagagggaccgcgatgtat | PvuII |
| P27 | T157A | tgtgtcttttggctccgagg | tgagagggaccgcgatgtat | PvuII |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gyau, B.B.; Xu, J.; Major, A.M.; Hicks, J.; Man, T.-K. CRISPR-Mediated Analysis of p27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis. Onco 2025, 5, 40. https://doi.org/10.3390/onco5030040
Wang J, Gyau BB, Xu J, Major AM, Hicks J, Man T-K. CRISPR-Mediated Analysis of p27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis. Onco. 2025; 5(3):40. https://doi.org/10.3390/onco5030040
Chicago/Turabian StyleWang, Junyan, Benjamin B. Gyau, Jun Xu, Angela M. Major, John Hicks, and Tsz-Kwong Man. 2025. "CRISPR-Mediated Analysis of p27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis" Onco 5, no. 3: 40. https://doi.org/10.3390/onco5030040
APA StyleWang, J., Gyau, B. B., Xu, J., Major, A. M., Hicks, J., & Man, T.-K. (2025). CRISPR-Mediated Analysis of p27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis. Onco, 5(3), 40. https://doi.org/10.3390/onco5030040






