Simple Summary
Cancer affects people of all ages, sexes, and ethnicities. Surgery, chemotherapy, radiotherapy, and immunotherapy are commonly used to treat cancers, but due to the evolving nature of cancers, there is a constant need to find novel and supplemental strategies for treatment. Numerous in vitro and in vivo studies have demonstrated that different biotics such as prebiotics, probiotics, synbiotics, and postbiotics can prevent certain cancers and slow the progression of others through a variety of mechanisms. These include modulating the immune system, regulating proliferative and apoptotic pathways, and generating epigenetic changes in cancer cells. In this review, we summarize the therapeutic potential of prebiotics, probiotics, synbiotics, and postbiotics and conclude by addressing the need for more robust clinical trials to maximize the therapeutic potential of these agents.
Abstract
Cancer is a public health concern due to the incidence, prevalence, morbidity, and mortality associated with it. While chemotherapy, radiotherapy, surgery, and immunotherapy are common treatments, there is still ongoing research to find targeted and innovative therapies that are more efficacious. The effect of probiotics on cancer progression and treatment has been actively investigated using different in vitro and in vivo models. Similarly, the role of prebiotics alone or in combination with probiotics, referred to as synbiotics, has also been evaluated in the context of cancers. Recently, the therapeutical potential of postbiotics is also being determined. Many studies have demonstrated that these agents can have onco-suppressive effects and can also prevent cancer in some instances. In this review, we summarize the different studies that have utilized these therapeutics in the prevention and treatment of a variety of cancers. We also discuss the different molecular mechanisms that enable these agents to be effective against cancers. Finally, we address safety and the need for more robust clinical trials that will aid in designing strategies involving these biotics in the prevention and treatment of cancer.
1. Introduction
According to the Cancer Facts and Figures 2025 publication by the American Cancer Society, there were 20 million new cancer cases globally in 2022. Additionally, 9.7 million people died due to cancer in the same year [1]. Cancer is caused by a variety of factors that include genetic predisposition, exposure to carcinogens in the environment, infectious agents, and disruption of the microbiome [2,3]. People with mutations in DNA repair genes such as BRCA1, BRCA2, and MLH1; in tumor suppressor genes like p53; or with chromosomal translocations, as observed in BCR-ABL, are at a higher risk of developing different types of cancers [4]. Exposure to high-intensity radiation, chemicals, alcohol, tobacco, and other carcinogens in the environment also promotes tumorigenesis [5]. Different infectious agents, such as human papillomavirus, hepatitis B and C viruses, Helicobacter pylori, and Chlamydia trachomatis, have been shown to promote tumorigenesis [6]. The human microbiome is comprised of a diverse and dynamic group of microorganisms that are found in different parts of the body [7]. The microbes in the microbiome are mostly commensal organisms that regulate many different physiological processes in our body to promote homeostasis [8]. Changes in the microbiome, especially the gastrointestinal or gut microbiome, can promote tumorigenesis.
The costs associated with the treatment of cancer are enormous, and it has been predicted that by 2030, USD 246 billion will be spent on the management and treatment of cancer [9]. This widespread nature and the costs associated with the treatment of cancer make it a global public health concern. Chemotherapy, radiotherapy, and surgical strategies are commonly employed in the treatment of cancers. In more recent times, immunotherapy, gene therapy, and novel therapeutics have been designed for treatment [10]. Due to the heterogeneity of different cancers and the evolution of cancers to existing therapies, there is ongoing research to find supplemental and more efficacious therapeutic strategies for the prevention and treatment of cancers. One approach that scientists and clinicians are actively exploring, is to support or restore a healthy microbiome in patients. Among the most promising tools in this approach are prebiotics, probiotics, synbiotics, and postbiotics, all of which are gaining traction in both laboratory research and clinical trials for their potential to prevent, slow, or treat cancer.
In this review, we will address how dysbiosis of the intestinal microbiome contributes to cancer and the role of different biotics in combating this dysbiosis. We discuss the effects of these agents against different cancers and molecular mechanisms that aid in the prevention and treatment of different cancers. We conclude by highlighting safety concerns and emphasizing the need for more robust and comprehensive clinical trials to design effective therapeutic strategies involving different biotics in the treatment and prevention of cancer.
2. Gut Microbiome Homeostasis and Dysbiosis
The human gut is home to an astonishingly diverse array of microbes. Most of the microbes are bacteria, but archaea, fungi, and viruses are also present [11,12]. This microbial ecosystem forms the gut microbiome that influences many different aspects of human health and well-being. In this section, we will focus on the bacterial communities that are found in the gut microbiome.
2.1. Composition of the Gut Microbiome and Gut Homeostasis
The gut microbiome harbors more than 1013 bacteria that belong to ~4000 species [13]. Most bacteria in the gut microbiome belong to the phyla Firmicutes (current name Bacillota), Bacteroidetes (current name Bacteroidota), Proteobacteria (current name Pseudomonadota), Verrucomicrobia (current name Verrucomicrobiota), Actinobacteria (current name Actinomycetota), and Fusobacteria (current name Fusobacteriota) [14]. Of these phyla, ~90% of the bacteria belong to the phyla Firmicutes and Bacteroidetes [15]. As shown in Figure 1, the gut microbiome aids with digestion, regulates metabolism, supports immune function, influences brain signaling, and contributes to the overall health of an individual [16]. Thus, the gut microbiome supports homeostasis by promoting resilience and helps in resisting adverse outcomes. The gut microbiome regulates many physiological processes through the production of different compounds that come under the group of postbiotics [17]. Postbiotics include short chain fatty acids (SCFAs), vitamins, peptides, and even non-viable microbes that provide health benefits to the host [18].
Changes in the gut microbiome occur over a person’s lifetime and are influenced by diet, cultural habits, exercise, obesity, therapeutics, infections, and many other factors [14]. The changes to the microbiome caused by these factors do not always result in dysbiosis. Hence, homeostasis can still persist when there are subtle changes to the diversity of the gut microbiome. However, in many instances, the changes in the gut microbiome communities no longer support health and well-being, resulting in dysbiosis [19].
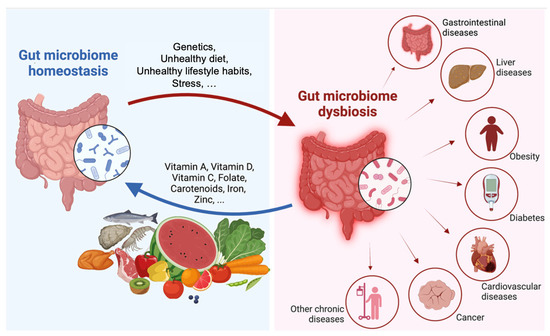
Figure 1.
Factors contributing to gut homeostasis and dysbiosis. Diet, genetics, and lifestyle can determine if the gut microbiome promotes homeostasis or dysbiosis in the host. Alteration of the gut microbiome that results in dysbiosis can cause different ailments. Reproduced with permission from Lin, D; 2023 [20].
2.2. Factors Affecting Gut Dysbiosis
Dysbiosis results in changes in metabolites produced by the microbes that can disrupt different physiological processes and induce inflammation [19,21]. The dysbiotic state is usually associated with lower levels of beneficial metabolites that include SCFAs and vitamins. Additionally, some microbial communities in the dysbiotic state synthesize metabolites that are harmful, cause DNA damage, weaken the intestinal barrier, induce inflammation, and affect immune responses [22]. Dysbiosis contributes to different disorders like obesity, inflammatory bowel disease, and even cancer [23,24]. Some of the main drivers of dysbiosis are infections, intake of antimicrobial agents to combat infections, and diet [20].
Antimicrobial agents like antibiotics can disrupt the microbial ecosystems in the gut and change the diversity of microorganisms in the gut microbiome, leading to dysbiosis. Antibiotic-induced dysbiosis is known to drive infections caused by Clostridioides difficile and Helicobacter pylori [25]. Pathogens that infect the gut can also cause changes in the gut microbiome that result in dysbiosis [26]. Infections associated with pathogens such as Salmonella spp., Escherichia coli, and Toxoplasma gondii are known to trigger dysbiosis as part of their pathogenesis profile by competing with members of the gut microbiome and inducing inflammation [26,27,28,29].
Diet acts as a double-edged sword, playing a vital role in maintaining homeostasis on one hand and promoting dysbiosis on the other. A well-balanced diet is known to maintain homeostasis, while undernourishment and diets rich in fats are associated with dysbiosis. A large population in low and medium socio-economic countries suffers dysbiosis and its effects due to undernourishment and malnutrition. Transplantation of gut microbiota from undernourished children into germ-free mice resulted in stunted growth in the mice, while normal growth was observed in germ-free mice transplanted with gut microbiota from healthy children [30]. Reconstitution of gut microbiota using probiotics in malnourished children can provide positive outcomes like reduced incidence of diarrhea [31]. Diets rich in fats have been shown to promote dysbiosis. One study demonstrated that a high-fat diet decreased protective bacterial species such as Parabacteroides distasonis, which resulted in weakened gut barrier function and increased inflammation. The decrease in protective species correlated with an increase in other unfavorable bacterial genera such as Alistipes. Furthermore, when the dysbiotic microbiota from these mice was transferred into germ-free mice, tumor development occurred, suggesting that the microbiome itself played a key role in promoting cancer [32]. Another study that compared the components of Western diets with Mediterranean diets found that the components of Western diets led to a decrease in gut microbial diversity with an increase in the population of unfavorable Firmicutes [33]. Several common food additives in processed foods, such as emulsifiers and artificial sweeteners, cause changes in the gut microbiota, which result in adverse functional effects such as reduced immunity, loss of gut integrity, and inflammation [34].
2.3. Dysbiosis and Cancer
Diet-induced dysbiosis of the gut microbiome has received attention among the various causes leading to colorectal cancer (CRC) [35]. CRC patients exhibit decreased bacterial diversity, lower abundance of commensal bacteria such as Akkermansia muciniphila, Lactobacillus rhamnosus, and Bifidobacterium breve, and higher levels of pro-carcinogenic bacteria such as Fusobacterium spp., Escherichia coli, Bacteroides spp., and Streptococcus spp., thus indicating that dysbiosis of intestinal microbiota is a potential risk and indicator of the onset of CRC [36]. Dysbiosis promotes tumorigenesis by affecting host immunity, metabolic signaling, and microbial metabolite production. Immune-impaired mice developed CRC due to gut microbial shifts that activated IL-6 (a pro-inflammatory cytokine) and STAT3 (a transcription factor involved in oncogenic signaling and immune regulation), reinforcing how inflammation and dysbiosis are connected [37]. Dysbiosis can also negatively impact treatment outcomes. For example, melanoma patients with lower microbiome diversity responded poorly to immunotherapy, suggesting that microbial composition may influence systemic immune responses and treatment outcomes [38].
Restoration of the gut microbiome to tackle the dysbiosis, and thereby the carcinogenesis associated with the dysbiosis, is a strategy that has gained more traction in recent years. One method to achieve this is by fecal microbiota transplantation (FMT) that involves introducing normal fecal microflora in the form of either feces or mixtures of bacterial strains found in healthy individuals into individuals with dysbiosis through oral capsules, colonoscopy, or enemas [39]. Two clinical trials involving FMT as a therapeutic in patients with immunotherapy-resistant cancers demonstrated positive outcomes in 30–40% of patients [40,41]. Some challenges associated with FMT include determining optimal doses and safety concerns. It is estimated that adverse events have been associated with ~40% patients even though most adverse events are minor and transient [42]. Another popular strategy for addressing dysbiosis is the employment of prebiotics, probiotics, synbiotics, or postbiotics. In this review, we will delve into different studies that have utilized these biotics as therapeutics.
3. Prebiotics, Probiotics, Synbiotics, and Postbiotics
The ‘biotics’, as shown in Figure 2, include prebiotics, probiotics, synbiotics, and postbiotics. These agents are becoming more popular as therapeutics for a wide variety of diseases, including cancer. In this section, we describe different features of these biotics.
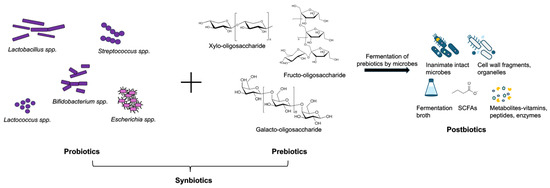
Figure 2.
Different types of prebiotics, probiotics, synbiotics, and postbiotics. Synbiotics refer to the combination of probiotics, that include different bacterial species, and prebiotics, that include non-digestible dietary fibers. Postbiotics include a wide variety of substances such as bacterial components, short chain fatty acids (SCFAs), metabolites, and fermentation broths.
3.1. Prebiotics
Prebiotics are non-digestible compounds that nourish beneficial bacteria already living in the gut [43]. As shown in Figure 2, some common prebiotics include fibers such as fructo-oligosaccharides (FOS), xylo-oligosaccharides, galacto-oligosaccharides (GOS), and human milk oligosaccharides (HMOs) [44,45]. Since these fibers are not broken down in the stomach or small intestine, they reach the colon in an intact form, where they are fermented by microbes into postbiotics like SCFAs. Some commonly formed SCFAs are acetate, propionate, and butyrate. These SCFAs support gut lining integrity, regulate inflammation, and influence metabolism [46]. For example, inulin, a type of FOS, can stimulate the growth of Bifidobacterium adolescentis and Faecalibacterium prausnitzii, which are major butyrate producers [47]. Butyrate serves as a key energy source for colonocytes, helps maintain the integrity of the gut lining, and suppresses inflammation [48]. Thus, prebiotics promote homeostasis by promoting the growth of bacteria that provide health benefits to the host.
3.2. Probiotics
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are live microorganisms that confer a health benefit to the host when administered in adequate amounts [49]. Probiotics do not have to colonize in the gut to provide benefits to the host; they can provide benefits while passing through the digestive system. Lactobacillus and Bifidobacterium species are commonly used probiotics (Figure 2). Other probiotics that have been used belong to the genera Lactococcus, Streptococcus, Bacillus, Escherichia and the yeast Saccharomyces [50]. These beneficial microbes help strengthen the intestinal barrier, balance immune responses, and regulate the growth of harmful bacteria [51]. The growth of probiotics is promoted by prebiotics, which can be fermented by probiotics and other microbes to form postbiotic compounds that benefit the host [52].
3.3. Synbiotics
Synbiotics harness the benefits of both probiotics and prebiotics, supplying live beneficial microbes along with the nutrients they require to thrive (Figure 2). This pairing can improve the survival and activity of probiotics in the gut and amplify their effects [53]. Synbiotics function in a synergistic manner by providing greater benefits to the host than the prebiotic or probiotic alone. Synbiotics have been used as therapeutics for the treatment of cancer, inflammatory diseases, and infectious diseases [54,55]. In this review, we will focus on the therapeutic potential of synbiotics in the context of cancer.
3.4. Postbiotics
Postbiotics encompass a wide variety of substances obtained from microorganisms. According to ISAPP, postbiotics include preparations of inanimate or non-viable microorganisms and/or their components that confer a health benefit on the host [18]. Many postbiotics are obtained from probiotics and synbiotics, and hence they are promising therapeutics, especially in scenarios where probiotics and synbiotics cannot be used, such as immunocompromised patients, very young individuals, and patients with weak intestinal barriers [56,57]. Common postbiotics include heat-inactivated probiotics, SCFAs, and fermentation broths, as well as substances isolated from them [58,59,60].
3.5. Therapeutic Potential of the Biotics
Prebiotics, probiotics, and synbiotics have been used as therapeutics for the prevention and/or treatment of infectious diseases, inflammatory diseases, and cancer [61]. Prophylactic supplementation of vaginally administered probiotics significantly decreased the incidence of recurrent urinary tract infections in the presence or absence of oral probiotics [62], while synbiotic administration of Lactobacillus rhamnosus GG, Lactobacillus rhamnosus LC705, Propionibacterium freudenreichii subsp. shermanii, Bifidobacterium breve Bb99, and galacto-oligosaccharide in infants for 6 months resulted in fewer respiratory tract infections than the placebo group [63]. Prebiotics, probiotics, and synbiotics have aided in the treatment of infections caused by Clostridioides difficile, Helicobacter pylori, and viruses that cause gastrointestinal infections [64,65,66].
Probiotics and synbiotics have also been used as therapeutics for the treatment of inflammatory bowel disease (IBD). Administration of Bifidobacterium longum 536 in patients with mild to moderate ulcerative colitis resulted in a significant decrease in the ulcerative colitis disease activity index and Rachmilewitz endoscopic index [67]. Similarly, patients with acute ulcerative colitis that were provided with a probiotic preparation of four Lactobacillus species, three Bifidobacterium species, and Streptococcus thermophilus, achieved significant remission compared to the placebo group (42.7% compared to 15.7% of the placebo group) [68].
There are many studies that have investigated the ability of pre-, pro-, syn-, and postbiotics in the prevention and treatment of different types of cancers [69,70,71,72,73]. They have also been studied for use in adjuvant therapies [74]. In the next section we will review the mechanisms employed by the biotics to mediate their anti-tumor effects.
4. Mechanisms of Anti-Cancer Effects Displayed by Different Biotics
Prebiotic, probiotic, synbiotic, and postbiotic strategies have garnered significant attention for their ability to influence cancer development and progression by modulating inflammation, immunity, apoptosis, and oncogenic signaling, as depicted in Figure 3 [75]. These approaches are rooted in the understanding that the microbiome acts not only as a passive inhabitant of the gut but also as an active regulator of host physiology. This section outlines the mechanisms employed by these agents, highlighting relevant experimental and clinical evidence that supports the development of microbiome-based therapeutic strategies.
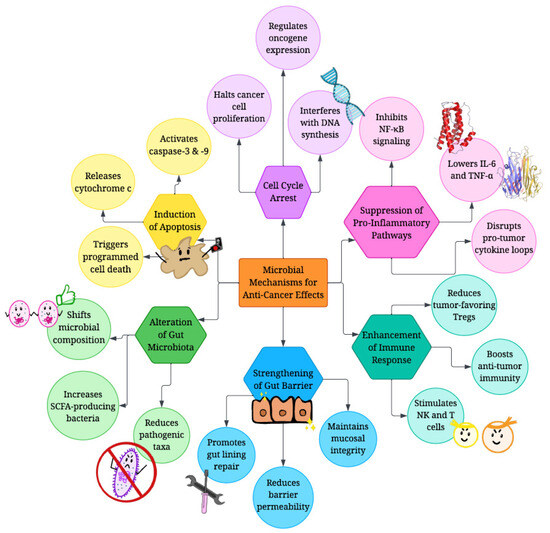
Figure 3.
Molecular mechanisms employed by biotics to combat cancer. Biotics mediate anti-cancer effects mainly by altering gut microbiota composition, promoting cell cycle arrest and apoptosis in cancer cells, preventing inflammation, activating immune cells, and strengthening the gut barrier.
4.1. Modulation of Oncogenic Pathways
Tumorigenesis is often marked by overactivation of growth and survival pathways such as PI3K/Akt, NF-kB, and MAPK [76,77,78]. These cascades govern essential cellular functions, and alterations in these pathways are common in multiple cancer types, making them attractive targets for intervention. Certain microbes may help turn off these oncogenic pathways and thereby inhibit survival and growth of cancer cells. As shown in Figure 4, probiotics can block oncogenic signaling pathways in multiple ways. Lactobacillus plantarum inhibits oral cancer cell proliferation by downregulating phosphorylated ERK1/2 and upregulating PTEN, thereby impairing MAPK signaling and reactivating tumor suppressor checkpoints [79]. Bifidobacterium infantis was able to suppress PI3K-Akt-mTOR signaling via PD-L1 activation in colon epithelial cells, resulting in increased Foxp3 expression and regulatory T-cell development [80]. Postbiotics like cell-free extracts and heat-killed bacteria can also affect these pathways [81]. These results underscore the ability of beneficial microbes and microbial products to interfere with key intracellular pathways and reestablish immune equilibrium in the tumor microenvironment.
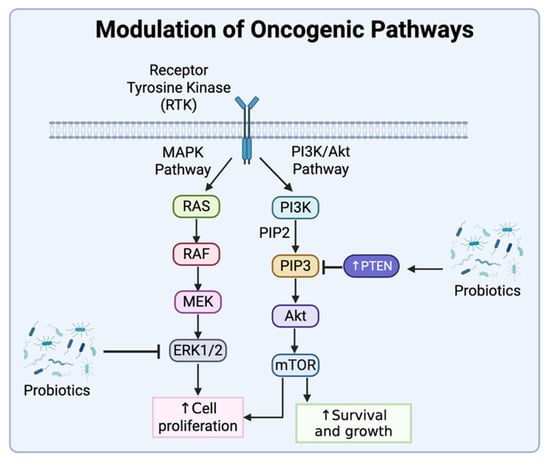
Figure 4.
Modulation of oncogenic pathways by probiotics. Probiotics can suppress oncogenic pathways that promote cell survival, growth, and proliferation of cancer cells. For example, some probiotics can inhibit the mitogen-activated protein kinase (MAPK) signaling pathway by suppressing the activation of extracellular signal-regulated kinase (ERK) 1/2, resulting in inhibition of cell proliferation. Other probiotics can increase the expression and activity of the phosphatase PTEN that targets the phosphoinositide 3-kinase (PI3K) signaling pathway by dephosphorylating phosphatidylinositol (3,4,5)-trisphosphate (PIP3). This results in the subsequent inhibition of the mechanistic target of rapamycin (mTOR), which is a kinase that plays important roles in cell survival, growth, and proliferation. Created in BioRender. Tafla, T. (2025) https://BioRender.com/6wys1r0 (accessed on 10 August 2025).
4.2. Induction of Apoptosis
Programmed cell death is a tightly regulated process essential for removing damaged or malignant cells. Tumors often suppress this process to ensure survival and resistance to treatment. While apoptosis is the most common and well-studied form of programmed cell death, other forms include pyroptosis, necroptosis, and ferroptosis. There are many studies that show that probiotics can induce apoptosis in cancer cells through the intrinsic mitochondrial pathway. As shown in Figure 5, this pathway involves key regulators such as the Bcl-2 family of proteins and downstream caspases, both of which are commonly disrupted in cancers [82]. Lactobacillus plantarum 5BL isolated from vaginal microbiota increased the Bax/Bcl-2 ratio and activated caspase-9 in HeLa cells, culminating in DNA fragmentation and sub-G1 arrest [83]. In a chemically induced colon cancer rat model, Lactobacillus paracasei X12 administration significantly reduced tumor incidence and progression due to increased apoptosis via caspase-3 activation and reduced expression of anti-apoptotic Bcl-2 proteins, reinforcing the potential of probiotic interventions in cancer therapy [84]. In another study, heat-killed Lactobacillus reuteri MG5346 and Lactobacillus casei MG4584 suppressed tumor growth in a xenograft mouse model of human colorectal carcinoma by activating the caspase-9-dependent apoptosis pathway. This resulted in substantial tumor size reduction in mice [85]. We could not find reports on probiotics inducing other forms of programmed cell death in tumors but did find reports on postbiotics that induced pyroptosis and ferroptosis in human cells. Reuterin, a metabolite produced by Lactobacillus reuteri, was able to induce pyroptosis in a preclinical model of human hepatocellular cancer cells, while γ-linoleic acid, a postbiotic molecule released into the supernatant of L. plantarum MM89 culture, induced ferroptosis by mitochondrial damage in colon cancer cells [86,87].
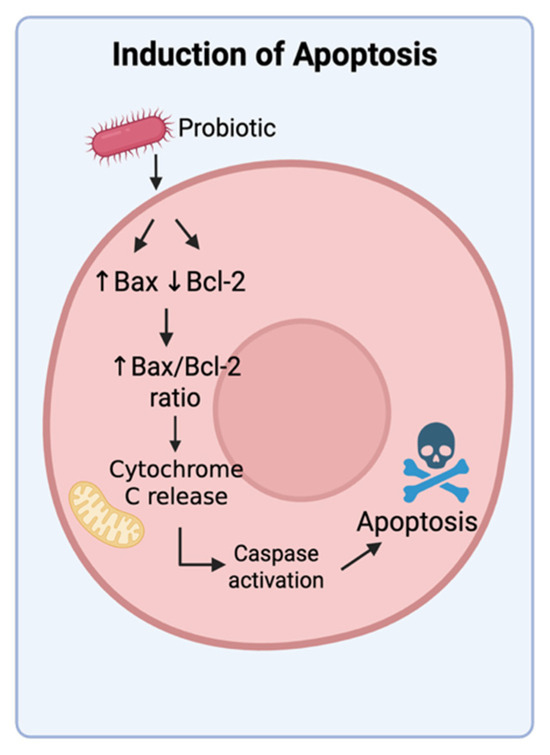
Figure 5.
Induction of apoptosis by probiotics. Probiotics can promote apoptosis in cancer cells by increasing the expression of pro-apoptotic Bax and anti-apoptotic Bcl-2. This increase in the Bax/Bcl-2 ratio activates the intrinsic pathway of apoptosis by releasing cytochrome c from the mitochondria, resulting in activation of caspases that induce apoptosis. Created in BioRender. Tafla, T. (2025) https://BioRender.com/723nhcu (accessed on 10 August 2025).
4.3. Modulating Inflammatory Cytokine Production
Chronic inflammation contributes to carcinogenesis by promoting DNA damage, cellular proliferation, and resistance to apoptosis. As shown in Figure 6, the signaling pathways involved in inflammatory responses activate NF-κB and MAPK, which induce the production of pro-inflammatory molecules, including pro-inflammatory cytokines [88]. These cytokines can promote tumorigenesis when expressed for long periods of time, as observed during chronic inflammation. Early intervention targeting these cascades can have profound impacts on cancer prevention [88]. Probiotics can reduce the expression of pro-inflammatory molecules while increasing the levels of anti-inflammatory cytokines like IL-10, thereby preventing chronic inflammation [89]. In an LPS-induced colitis mouse model, oral administration of Bifidobacterium bifidum E3 and Bifidobacterium longum subsp. infantis E4 significantly reduced expression of IL-6 and TNF-α by attenuating TLR4-mediated activation of NF-κB and MAPK signaling, thereby improving epithelial barrier function [90]. In another study, gut microbiota-derived indole-3-acetic acid (IAA) improved precancerous intestinal inflammation by enhancing IL-35 secretion, a potent immunosuppressive cytokine. This shift to an anti-inflammatory environment contributed to reduced tumorigenesis and shows how bacterial metabolites can reshape immune responses to favor cancer prevention [91]. There are studies that also evaluated the effect of probiotics on NLRP3 activation and inflammasome formation, which aids in the production of IL-1β, a proinflammatory cytokine. Different probiotics were able to suppress NLRP3 activation in various mammalian models, such as rodents, canines, and hamsters. Mixed results were obtained from porcine models. Depending on the probiotic used, there was either suppression or no change in NLRP3 activation [92]. These findings indicate that targeted microbial interventions can actively modulate inflammatory pathways involved in tumorigenesis. Microbiota-based approaches that reduce pro-inflammatory cytokines or block receptor-mediated activation of these pathways may serve as adjunctive or even primary strategies in cancers with strong inflammatory etiologies.
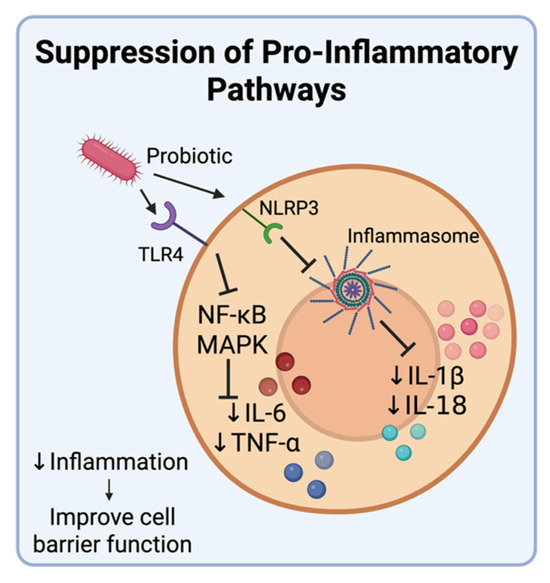
Figure 6.
Suppression of pro-inflammatory pathways by probiotics. Probiotics inhibit the production of pro-inflammatory cytokines like IL-6, TNFα, IL-18, and IL-1β by inhibiting the MAPK signaling pathway and suppressing activation of the pattern recognition receptor NLRP3 as well as transcription factor nuclear factor κB (NF-κB). Created in BioRender. Tafla, T. (2025) https://BioRender.com/4eq1l9s (accessed on 10 August 2025).
4.4. Immune Modulation
Immune escape is a hallmark of cancer, where tumors evade immune detection or suppress immune responses [93]. Probiotics can counteract this by activating natural killer (NK) cells, inducing regulatory T-cells (Tregs), promoting B-cells to produce IgA, and modulating the Th1/Th2 response (Figure 7) [94]. These effects help restore immune surveillance and create a tumor-hostile microenvironment. Aerosolized Lactobacillus strains administered in a murine lung metastasis model enhanced the cytotoxic effects of NK cells and IFN-γ production, resulting in reduced tumor burden [95]. Oral administration of Bifidobacterium bifidum FL228.1 and Bifidobacterium bifidum FL276.1 in a mouse model resulted in significantly higher levels of IgA in the intestine [96]. Similarly, higher levels of IgA and IgG were observed in the serum of patients with CRC who were provided with a mixture of Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis before a radical colorectal resection. Additionally, probiotic administration resulted in lower incidences of postoperative complications [97]. Probiotic supplementation with Lactobacillus casei Shirota and Bifidobacterium breve in mice with urothelial cancer undergoing gemcitabine and cisplatin chemotherapy enhanced anti-tumor immune responses and reduced recurrence by activating cytotoxic T-cells and dendritic cells in the tumor microenvironment [98]. Some probiotics, including lactobacilli, have also been shown to promote Th1 responses and downregulate Th2 responses, thereby promoting an anti-tumor response [99]. Thus, probiotics can modulate immune responses, helping the body fight tumors more effectively. In addition to probiotics, prebiotics and postbiotics have also been shown to modulate immune responses [100,101]. These immune-based effects reinforce the view that biotics can both reverse immune suppression and prime host defense systems.
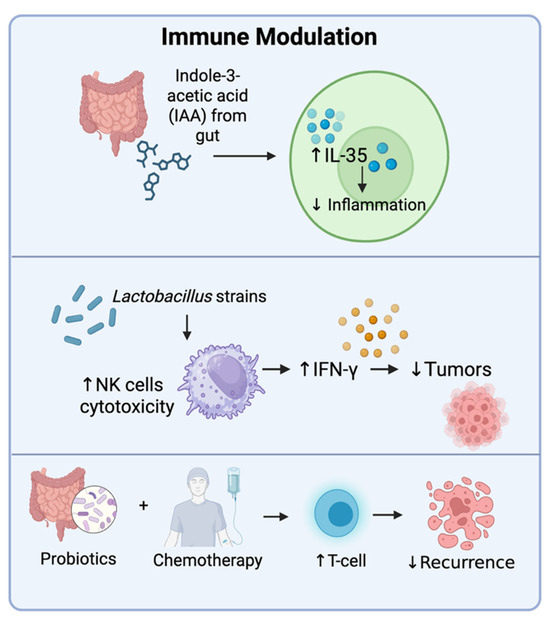
Figure 7.
Modulation of immune responses by different biotics. Probiotics can modulate immune cell function by creating metabolites like IAA that upregulate the expression of IL-35, resulting in reduced inflammation. Some probiotics can increase the cytotoxic properties of natural killer (NK) cells and induce the production of interferon γ (IFN-γ) that activates immune cells and reduces tumor growth as well as the recurrence of tumors. Created in BioRender. Tafla, T. (2025) https://BioRender.com/jefc17f (accessed on 10 August 2025).
4.5. Epigenetic Modulation via Microbial Metabolites
Microbial fermentation of prebiotics generates metabolites that can directly influence gene regulation, potentially bypassing conventional genetic resistance pathways. SCFAs like butyrate, which are fermentation products derived from dietary fibers, can serve as epigenetic modulators in addition to being metabolic byproducts, thus highlighting the close connection between diet, the microbiota, and host gene expression (Figure 8). Butyrate functions as a histone deacetylase (HDAC) inhibitor, promoting chromatin relaxation and reactivating silenced tumor suppressor genes [102]. In addition to butyrate, propionate, which is another SCFA, can also mediate epigenetic changes. A study in a transgenic mouse model of mammary cancer showed that inulin supplementation resulted in higher sodium propionate levels in blood. Sodium propionate was able to inhibit the activity of histone deacetylases and DNA methyltransferases, which resulted in an increase in p53 expression [103]. These epigenetic changes contributed to a significant reduction in mammary tumor incidence and delayed tumor development in the mice. Thus, biotics are able to regulate gene expression by modulating epigenetic changes.
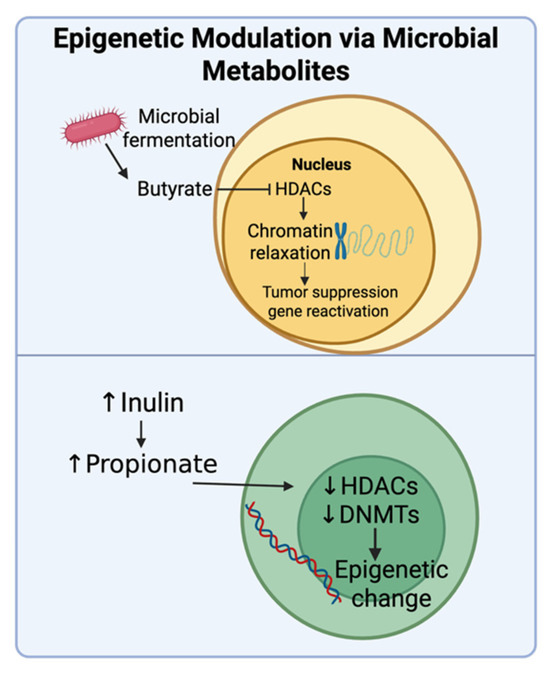
Figure 8.
Postbiotics like butyrate and propionate that are generated through microbial fermentation of different substances, including inulin, inhibit histone deacetylases (HDACs) and DNA methyltransferases (DNMTs). This results in epigenetic changes such as the expression of tumor suppressor genes that promote anti-cancer effects. Created in BioRender. Tafla, T. (2025) https://BioRender.com/6yqzqem (accessed on 10 August 2025).
5. Role of Prebiotics, Probiotics, Synbiotics, and Postbiotics in the Prevention and Treatment of Cancer
Recently, the anti-neoplastic activity exhibited by probiotic strains, prebiotics, and postbiotic molecules and combinations thereof has been gaining immense attention. In vitro and in vivo studies have proven the protective effects of the intake of salutary bacteria like probiotics against cancer [72]. Several trials in animal models and cancer patients suggest improvement in health upon consumption of prebiotic molecules such as FOS, inulin, β-glucans, and resistant starch, individually as well as in the form of synbiotics [69,104]. Some of the studies supporting the effects of biotic treatments against some common forms of cancer have been summarized in this section.
5.1. Colorectal Cancer
CRC is responsible for approximately half a million cancer-related deaths worldwide. Since the colon is home to probiotic bacteria, the ingestion of different biotics is now becoming a relevant therapeutic option for the prevention or treatment of CRC. The biotics exert anti-tumorigenic effects by improving immunity, reducing inflammation, strengthening epithelial barriers, and regulating signaling pathways that control proliferation and apoptosis [105].
5.1.1. Prevention and Treatment of CRC Using Different Biotics
Different studies have demonstrated that treatment or administration of prebiotics can prevent and reduce the growth and progression of CRC in in vitro and in vivo models [104,106]. Treatment of HT-29 cells with exopolysaccharides purified from different species of Lactobacillus induced apoptosis [107]. The incidence of tumors in the colon was dramatically reduced in mouse and rat dimethylhydrazine-induced colon tumor models when the animals were provided with resistant starch as part of their diet [108,109]. Administration of mice with triterpenoid saponins obtained from Gynostemma pentaphyllum promoted the growth of Bifidobacterium animalis in the mouse gut and reduced the number of polyps by 40.68% [110,111]. Similarly, chemically induced colorectal cancer in rats showed a 57.5% decrease in polyps when they were provided with GOS derived from lactulose [112]. Clinical trials associated with determining the effects of prebiotics on CRC show inconclusive or contradictory information, especially related to dietary fibers. One study showed that there was no correlation between intake of dietary fiber and risk of developing colorectal adenomas, while in another study, intake of dietary fiber decreased the risk of colorectal adenomas [113,114,115]. To address this contradiction, meta-analyses have been performed. A meta-analysis of 20 studies encompassing 11,000 candidates with adenomas concluded that high intake of dietary fiber was associated with a lower risk of colorectal adenomas [116]. Benefits are observed when prebiotics are provided in combination with probiotics, as shown in Table 1.
There are many studies that have investigated the effects of probiotics on the prevention and treatment of colorectal cancer [69,74]. Lactobacillus plantarum YYC-3 treatment could prevent early-stage colorectal cancer by downregulating the NF-KB and Wnt pathways, resulting in anti-inflammatory and tumor inhibitory effects. Furthermore, it was more effective in preventing cancer than its corresponding cell-free supernatant [117]. A novel probiotic strain of Pediococcus pentosus reduced DNA fragmentation and HDAC activity in the colon cancer cell line HCT-116 and effectively mitigated azoxymethane-induced colon cancer in mice [118]. Oral administration of Butyrivibrio fibrisolvens MDT-1, a probiotic known to produce high amounts of butyrate, significantly reduced the number of aberrant cryptic foci in a 1,2-dimethyhydrazine mouse model [119].
Several studies have revealed that synbiotics, a combination of pre- and probiotics, lead to improved results compared to either biotic alone. A combination of inulin and Bifidobacterium longum was more successful at decreasing aberrant cryptic foci than their individual treatments in a rodent model [120]. A synbiotic composed of Lactobacillus casei strain Shirota and Bifidobacterium breve strain Yakult suppressed tumor development by downregulating the expression of IL-6, TNF-α, COX-2, and STAT-3 in a mouse model [121]. A combination of resistant dextrin, isomalto-oligosaccharides, FOS, and stachyose along with the probiotic strains of Bifidobacterium longum, Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus plantarum reduced the migration, proliferation, and invasion of the CT26 colorectal cancer cell line. Oral administration of this mixture in a CT26-induced colon carcinoma BALB/c mouse model resulted in significantly smaller tumors [122]. More studies related to using synbiotics as therapeutics are listed in Table 1.
Metabolites secreted by probiotic species, their fermentation broth, and other postbiotic excipients can overcome the adverse effects of colon cancer [123]. The cell-free fraction of kefir, a fermented milk product rich in Lactobacilli, polysaccharides, and proteins, exerts an anti-proliferative effect on Caco-2 and HT-29 cells [124]. Ethyl acetate extracts of Lactobacillus plantarum and Lactobacillus rhamnosus could induce intrinsic apoptosis in CaCo-2 cells but not in the normal HUVEC cells [125]. Similarly, peptidoglycan obtained from Lactobacillus spp. could reduce the growth of tumors generated through the injection of CT26 colon cancer cells in a BALB/c mouse model [126]. Oat beta-glucans altered the gut microbiota and cytokine profile and improved the integrity of the intestinal barrier in azoxymethane-induced colon cancer models [127].
Clinical trials associated with probiotic administration and the outcome of cancer show conflicting information, mainly due to differences in agents, doses, duration, and confounding variables that were not accounted for. A synbiotic combination of oligofructose-enriched inulin, Lactobacillus rhamnosus, and Bifidobacterium lactis modified the immune profile in patients with polypectomies and reduced colorectal proliferation [128]. On the other hand, a similar result was not found when a combination of wheat bran and Lactobacillus casei was employed in another study [129]. However, in the case of yogurt, there is agreement in the literature that yogurt consumption reduces the risk of CRC compared to no consumption [130,131]. Thus, there is a need for controlled and robust clinical trials to determine the strains, dose, and duration of biotic administration for the prevention and treatment of CRC.
5.1.2. Effect of Biotics on Side Effects of CRC Treatment
The effect of biotics on side effects associated with treatments like surgery or chemotherapy has been evaluated in different clinical trials. A clinical trial that provided a mixture of six viable strains comprised of Lactobacillus acidophilus BCMC® 12,130, Lactobacillus lactis BCMC® 12,451, Lactobacillus casei subsp. BCMC® 12,313, Bifidobacterium longum BCMC® 02120, Bifidobacterium bifidum BCMC® 02290, and Bifidobacterium infantis BCMC® 02129 to colorectal patients post-surgery found that while administration of the probiotic mixture did not reduce diarrhea, there was a significant reduction in the levels of different circulating cytokines in serum [132]. The researchers did not determine the effect of probiotics on the severity of diarrhea, as was performed by another group that studied the effect of probiotic or synbiotic supplementation in patients undergoing chemotherapy [133]. When Lactobacillus rhamnosus GG with or without guar gum was administered orally to patients undergoing adjuvant chemotherapy after surgery, there was no significant difference in the overall incidence of diarrhea, but there was a significant decrease in the frequency of grade 3 or 4 diarrhea in the group that received the probiotics [22%] compared to the group that did not [37%]. There was no added benefit when guar gum was provided with Lactobacillus rhamnosus GG. A similar result was obtained where patients who consumed tablets containing live Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus while undergoing chemotherapy showed significantly less diarrhea [16% vs. 40%], constipation [8% vs. 28%], and abdominal pain [3% vs. 24%] [134]. Meta-analyses that determined the efficacy and impact of prebiotics, probiotics, and synbiotics in CRC-related clinical trials also found that probiotics may reduce the adverse side effects associated with CRC treatment [36,135,136]. Thus, probiotics and synbiotics can prove to be useful therapeutics in reducing undesirable side effects associated with treatment of CRC.
5.2. Cervical Cancer
Infections caused by human papillomavirus [HPV] are the driving force behind most cervical cancers, which is one of the most prevalent cancers in women. Lactobacillus is one of the predominant species in the vaginal tract that supports the maintenance of pH and improves cytokine expression, thus facilitating HPV clearance and inducing apoptosis in cancer cells, ultimately playing a key role in combating cervical cancer [137].
5.2.1. Prevention and Treatment of Cervical Cancer Using Biotics
Cervical cancer can be prevented through effective clearance of HPV from the host [138]. A study involving 14,151 women demonstrated that intake of dietary fiber was negatively associated with HPV infection [139]. In another study, intravaginal transplantation of capsules containing the probiotic Lactobacillus crispatus chen-01 and sweet potato flour in women with high-risk HPV infection resulted in significantly lower HPV viral load and reduced vaginal inflammation [140].
In addition to preventing cervical cancer, pre-, pro-, and postbiotics can also inhibit the progression of cervical cancer. Chito-oligosaccharides, prebiotics obtained from chitosan, inhibited the proliferation of many HPV-positive and negative cervical cancer cell lines and induced anti-tumorigenic effects, apoptosis, and autophagy in HPV-negative C33A cells [141]. Administration of live Lactobacillus debrueckii subsp. lactis in a xenograft U14-induced tumor model involving BALB/c mice resulted in significantly smaller tumors and increased expression of E-cadherin, a key molecule inhibiting the Wnt/β-catenin pathway, thereby preventing the development and metastasis of cancer [142]. Postbiotic molecules produced by different Lactobacillus species have been shown to inhibit the proliferation of different HPV-positive cervical cancer cell lines. Supernatants from Lactobacillus crispatus, Lactobacillus jensenii, and Lactobacillus gasseri inhibited the proliferation of Caski cells [143], while postbiotic molecules secreted by Lactobacillus fermentum species lead to enhanced apoptosis of HeLa cells [144].
5.2.2. Effect of Biotics on Traditional Cervical Cancer Treatments
There have been a few clinical trials demonstrating that probiotics can increase the efficacy of traditional cancer treatments. A mouse model of cervical cancer demonstrated that when pessaries loaded with cisplatin and Lactobacillus rhamnosus were administered, the tumor volume was lower in the condition that involved cisplatin and Lactobacillus rhamnosus than either therapeutic used alone [145]. Clinical trials performed in the late 1900s demonstrated that supplementing probiotics with radiation therapy resulted in better outcomes. A study involving more than 200 patients with stage IIIB cervical cancer found that supplementing radiotherapy with Lactobacillus casei YIT9018 resulted in lesser leukopenia, prolonged survival, and longer relapse-free intervals [146]. In patients undergoing pelvic radiotherapy for cervical cancer, VSL#3 (containing four Lactobacillus species, three Bifidobacterium species, and Streptococcus thermophilus) reduced post-radiotherapy diarrhea compared to the placebo group (31.6% vs. 51.8%) [147]. Similar to CRC, administration of probiotics in patients undergoing radiation therapy for the treatment of cervical cancer significantly reduces both diarrhea and abdominal pain [148].
While the literature suggests the use of probiotics as a therapeutic option for the treatment of cervical cancer, there is a need for further in vivo and clinical trials to confirm the potential of pre-, syn-, and postbiotics in cervical cancer treatment.
5.3. Breast Cancer
Breast cancer is one of the most common cancers in women and a leading cause of death. In 2022, there were 2.3 million new cases of female breast cancer and 670,000 deaths worldwide [149]. Since the late 1900s, studies on the effect of prebiotics and probiotics on breast cancer have been performed. In this section we provide an overview of the effects of different biotics on prevention, treatment, and traditional therapies related to breast cancer.
5.3.1. Prevention and Treatment of Breast Cancer Using Biotics
A study in the Netherlands determined that consumption of fermented milk products decreases the risk of breast cancer, while milk consumption did not [150]. In 1999, the anti-carcinogenic and mammary tumor growth inhibiting potential of prebiotics such as inulin, pectin, and oligofructose was reported in a methylnitrosourea-induced mammary carcinogenesis model involving Sprague Dawley female rats [151]. Since then, numerous studies have demonstrated the beneficial effects of different biotics in the treatment of breast cancer.
Prebiotics like inulin, starch, and FOS can inhibit the proliferation and induce apoptosis in different breast cancer cell lines and mouse models. Pectic oligosaccharides, a class of prebiotics derived from apple and citrus, were seen to promote apoptosis in the MDA-MB-231 breast cancer cell line [152]. Inulin supplementation was able to delay tumor latency and inhibit tumor growth in a transgenic mouse model of estrogen-negative mammary cancer [103]. Different non-digestible carbohydrates such as FOS, resistant maltodextrin, and high amylose cornstarch were able to limit the proliferation of MCF-7 tumor growth in an athymic xenograft mouse model [153]. Smaller tumors, improved cytokine profiles, and lower VEGF levels were observed upon administration of prebiotic-oligofructose in fecal microbiome-transplanted mice with breast cancer that were treated with paclitaxel [154].
Probiotics also have therapeutic effects against breast cancer. Oral administration of Lactobacillus acidophilus ATCC4356 in BALB/c mice transplanted with adenocarcinoma breast tumors resulted in significantly smaller tumors [155]. When milk fermented with Lactobacillus casei CRL 431 was provided to BALB/c mice harboring 4T1-induced mammary cancer, the tumors formed were smaller, less invasive, and unable to metastasize as effectively as observed in the control group and a group provided with normal milk [156]. The mice provided with fermented milk had better survival, with 50% survival observed compared with 28.5% survival in the control group and 25% survival in the milk group. A similar finding was observed when kefir, a traditional fermented milk product with probiotics, was provided to BALB/c mice with a 4T1-induced mammary cancer model [157].
A population-based case-control study involving 306 breast cancer patients and 662 controls found that consumption of beverages containing Lactobacillus casei Shirota and isoflavones reduced the incidence of breast cancer [158]. Probiotic microbes and local breast microbiota secrete metabolites such as folates and SCFAs that participate in epigenetic processes and exert anti-mutagenic and anti-genotoxic effects, thus serving as protective agents against breast cancer [159]. Extracts of kefir inhibited the proliferation of MCF-7 breast cancer cells but not normal mammary epithelial cells, implying that its bioactive components specifically acted on the tumor cells [160].
5.3.2. Effect of Biotics on Breast Cancer Treatments
The effects of administration of synbiotics in breast cancer patients are summarized in Table 1. Probiotics can help alleviate undesirable side effects associated with the treatment of breast cancer. Breast cancer is commonly treated by chemotherapy and estrogen deprivation therapy, which results in symptomatic vaginal atrophy due to genitourinary syndrome of menopause [161]. Providing postmenopausal breast cancer patients with a probiotic capsule containing Lactobacillus crispatus LbV 88, Lactobacillus rhamnosus LbV 96, Lactobacillus jensenii LbV 116, and Lactobacillus gasseri LbV 150N resulted in an improvement of vaginal microbiota in 63% of patients compared to 36% in the control group [162]. Chemotherapy is also associated with cognitive impairment in patients, and a study involving intake of probiotic capsules containing Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis in breast cancer patients undergoing adjuvant chemotherapy demonstrated that probiotics protected the cognitive functions of patients [163].
5.4. Other Cancers
Prebiotics, probiotics, synbiotics, and postbiotics have been used in other cancers as well. Lactobacilli exert protective effects against both the axillary lymph node and lung metastases. Lactobacillus casei YIT9018 has been shown to suppress pulmonary and regional lymph node metastases in mice and guinea pigs [164]. Yazdi et al., showed that oral administration of selenium nanoparticle-enriched Lactobacillus brevis increased IFN-γ and IL-17 levels, enhanced NK cell-mediated cytotoxicity, and decreased metastasis of tumors to the liver. Additionally, there was improved survivability of affected animals [165]. Inflammation caused by Helicobacter pylori is the leading cause of gastric cancer. Probiotic strains Lactobacillus acidophilus and Lactobacillus bulgaricus can inhibit Helicobacter pylori adhesion to GES-1 cells in vitro. A mixture of Lactobacillus fermentum, Lactobacillus casei, and Lactobacillus rhamnosus reduced the production of the pro-inflammatory cytokines IFN-γ and IL-1β, thereby attenuating inflammation in infected mouse models [166]. Probiotic bacteria such as Bifidobacteria spp. and Streptomyces salivarius have been used to alleviate the symptoms of oral mucositis in patients undergoing chemotherapy for head, neck, and thyroid cancers [167]. Fu et al., explored the effects of using the postbiotic antimicrobial peptide nisin, produced by Lactococcus lactis, in treating oropharyngeal cancer in conjunction with radiation therapy. Nisin leads to enhanced shrinkage of tumors and improved apoptotic activity in the affected area [168]. Skin commensals such as Staphylococus epidermis are known to exert protection against UV-B-induced cutaneous papillomas and reduce the occurrence of non-melanoma skin cancer by producing molecules such as 6-N-hydroxyaminopurine that have anti-proliferative effects on neoplastic cells [169]. Melanoma patients with lower microbiome diversity responded poorly to immunotherapy, suggesting that microbial composition may influence systemic immune responses and treatment outcomes [38].
5.5. Engineered Probiotics and Cancer
In recent years, there has been an increase in engineering microorganisms for a variety of therapeutic applications, and this concept is being applied to probiotics as well [170,171]. Some studies have engineered the intestinal probiotic Escherichia coli (E. coli) Nissle strain for therapeutic purposes. A study engineered E. coli Nissle 1917 to express p53, Tum-5, or both p53 and Tum-5 and administered these engineered probiotics to BALB/c mice bearing SMMC-7721 tumors. The engineered probiotics inhibited the expression of Ki67 while increasing the expression of caspase 3, resulting in significant inhibition of tumor growth in the animals [172]. E. coli Nissle 1917 has also been engineered to synthesize and release nanobodies in tumors that can bind to PD-L1 and CTLA4, proteins expressed by cancers to evade the immune system [173,174]. Mice treated with the engineered E. coli Nissle strain showed slower tumor growth and more presence of activated T-cells [174]. In another approach, E. coli Nissle has been engineered to synthesize and release cyclic dinucleotides in hypoxic conditions that can activate the STING pathway in immune cells, resulting in interferon production [175]. A phase I trial using this engineered E. coli strain, called SYNB1891, demonstrated that the microbe was safe to be injected alone or in combination with the chemotherapeutic atezolizumab [176]. In addition to the E. coli Nissle strain, Limosilactobacillus reuteri has been engineered to release the cytokine IL-22 in tumors. Mice with ovarian cancer that were treated with this engineered probiotic showed better survival and were able to undergo radiotherapy with better outcomes than the control group [177]. As time progresses, we expect to see more engineered probiotics for the treatment of cancer.
While several studies claim the protective effects of different biotics as therapeutics, there are inconsistencies due to the complexity of carcinogenesis, dose-dependent and strain-dependent effects of probiotics, and safety concerns [178,179]. This calls for an increase in well-designed in vivo studies and clinical trials to confirm the effects of the biotics as preventive and curative therapeutics against cancer.

Table 1.
Summary of randomized clinical trials carried out to study the effect of synbiotic interventions in cancer patients.
Table 1.
Summary of randomized clinical trials carried out to study the effect of synbiotic interventions in cancer patients.
| Reference | Synbiotic Combination | Type of Cancer | Type of Trial | Effect |
|---|---|---|---|---|
| Rafter, et al. (2007) [128]. | Oligofructose-enriched inulin (SYN1) + Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 | CRC | Double-blind, placebo-controlled trial of a synbiotic food composed of the synbiotic conducted in 37 colon cancer patients and 43 polypectomized patients. | Change in fecal flora, reduced IL-2 production, increased necrosis, reduced genotoxicity. |
| Flesch, et al. (2017) [180]. | Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifi dobacterium, and fructo-oligosaccharide (FOS) | CRC | Double-blind randomized trial of 91 patients with 42 patients in the placebo group and 49 patients in the synbiotic treated group. | Decreased postoperative wound infection, inhibition of pathogens, and shortened hospital time |
| Krebs, (2016) [181]. | Synbiotic 2000 FORTE (Medipharm). It consists of Pediacoccus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp. Paracasei, and Lactobacillus plantarum 2362 + 2.5 g of each of betaglucan, inulin, pectin, and resistant starch. | CRC | Randomized, controlled, double-blind trial of 54 patients who underwent surgery for colorectal cancer | Enriched colon microbiota, reduction in hospitalization time. Reduction in immune markers was not significantly different from the control group. |
| Farshi Radvar, et al. (2020) [182]. | Synbiotic capsules (Protexin) contained Lactobacillus casei PXN 37, Lactobacillus rhamnosus PXN 54, Streptococcus thermophilus 81 PXN 66, Bifidobacterium breve PXN 25, Lactobacillus acidophilus PXN 35, Bifidobacterium longum PXN 30, 82 Lactobacillus bulgaricus PXN 39, and Fructo-oligosaccharide | Rectal Cancer | Double-blind randomized parallel trial of 38 patients with 19 patients in the placebo group and 19 in the synbiotic treated group. | Increased intake of carbohydrates and proteins, reduction in matrix metalloproteins in patients undergoing chemoradiotherapy |
| Nascimento, et al. (2020) [183]. | Lactobacillus reuteri and soluble fiber (Nestlé) | Prostate cancer | Randomized, double-blind, placebo-controlled pilot trial of 20 patients with 10 patients in the placebo group and 10 in the synbiotic treated group. | Synbiotics prevented rectal inflammation/proctitis caused by radiation therapy for cancer |
| Sugawara, et al. (2006) [184]. | Lactobacillus casei strain Shirota; Bifiel (Yakult Honsha) containing Bifidobacterium breve strain Yakult; and galactooligosaccharide (Oligomate 55, Yakult Honsha) | Biliary cancer | Randomized controlled trial of 81 patients | Decrease in IL-6, CRP, and WBCs. Increase in beneficial bacteria population, decrease in pathogenic bacteria. |
| Tanaka, et al. (2012) [185]. | Yakult BL Seichoyaku (Yakult Honsha, Tokyo) Bifidobacterium breve strain Yakult (B. breve strain Yakult), Lactobacillus casei strain Shirota, and galacto-oligosaccharides (Oligomate S-HP; Yakult Honsha). | Esophageal cancer | Randomized controlled trial of 64 patients undergoing surgery | Reduced inflammation, increase in beneficial bacteria and SCFA production, 20% reduced infection occurrence in comparison to control |
| Sugimoto, et al. (2023) [186]. | Lacticaseibacillus paracasei strain Shirota, Bifidobacterium breve strain Yakult, and galacto-oligosaccharides | Esophageal cancer | Ancillary study to a randomized controlled trial in 73 cancer patients | Reduced febrile neutropenia and diarrhea in patients receiving neoadjuvant chemotherapy, correlated to an increase in beneficial bacteria and SCFA production. |
| Motoori, et al. (2017) [187]. | Yakult BL Seichoyaku containing Bifidobacterium breve strain Yakult and Lactobacillus casei strain and galacto-oligosaccharides | Esophageal cancer | Open-labeled randomized prospective clinical trial of 67 advanced-stage patients | Increased SCFA production reduced lymphopenia and diarrhea and reduced side effects of chemotherapy. |
| Manifar, et al. (2023) [188]. | FamiLact-Bifidobacterium breve, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus rhamnosus, streptococcus salivarius +FOS | Oral Cancer | Double-blind randomized clinical trial on 64 oral cancer patients undergoing radiotherapy | Reduced oral mucositis after radiotherapy, changes in microbiome. |
| Koopaie, et al. (2025) [189]. | Synbiotic mouthwash containing Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus salivarius, Lactobacillus reuteri, Bifidobacterium lactis, Bifidobacterium longum, Bifidobacterium bifidum and FOS | Head and neck cancer | Triple-blind, placebo-controlled, randomized clinical trial of 44 patients with squamous carcinoma. 22 patients were in the placebo group and 22 in the synbiotic treated group. | Non-significant reduction in salivary TLR 2, significant delay in onset of mucositis and intensity in synbiotic group compared to placebo group. |
| Sommacal, et al. (2015) [190]. | Lactobacillus acidophilus 10, Lactobacillus rhamnosus HS 111, Lactobacillus casei 10, Bifidobacterium bifidum, and fructooligosaccharides (FOS) | Periampullary neoplasm | Randomized double-blind clinical trial of 46 patients with 23 patients in the placebo group and 23 in the synbiotic treated group. | Reduced rate of infection and hospitalization period |
| Monshikarimi, et al. (2020) [191]. | L. rhamnosus Heriz I and soluble1–3,1–6,D-beta glucan | Breast cancer | Randomized double-blind placebo-controlled clinical trial in 30 patients | Significantly improved functional scale scores and reduced symptoms in patients receiving chemotherapy. |
| Tirgar, et al. (2024) [192]. | Lactobacillus casei, L. acidophilus, L. rhamnosus, L. salivarius, L. reuteri, Bifidobacterium lactis, B. longum, and B. bifidum + FOS + Vitamin D | Breast cancer | Double-blind randomized trial of 76 patients | Increased anti-inflammatory index ratio in symbiotic group |
| Khazaei, et al. (2023) [193]. | Lactocare® (Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Lactobacillus helveticus, Lactobacillus lactis, Lactobacillus paraplantarum, Bifidobacterium bifidum, Streptococcus thermophilus and Lactobacillus gasseri + Fructo-oligosaccharides) | Breast cancer | Double-blind randomized clinical trial on 67 patients with 33 patients in the placebo group and 34 in the synbiotic treated group. | Reduced side effects of chemotherapy, nausea, fatigue, and anorexia. |
6. Safety Considerations Associated with Biotic Therapies
Despite the easy availability and plethora of beneficial effects obtained through the administration of different biotics in the prevention and treatment of cancer, several drawbacks of these remedies limit their full-fledged utilization in cancer therapy. Concerns about possible adverse outcomes, choosing appropriate strains of probiotics for patients considering the uniqueness of individual microbiomes, determining the appropriate dose due to heterogeneity in commercial preparations, risks of metabolic disorders, and other factors contribute to hesitancy in their use among oncologists [194,195]. Inconsistencies in effects of these agents due to storage, viability, delivery to target site, and functionality further affect their effective use in therapy [72]. Additionally, there are many in vivo animal studies that support the anti-tumor effects of biotics, but tumors induced by chemicals differ from tumors that naturally arise in the human body. Hence, the anti-tumor effects of probiotics observed in animal models cannot be assumed to be similar in humans.
Probiotics, prebiotics, and synbiotics are quite safe, with few adverse outcomes reported. A prospective study comprising 499 cancer patients undergoing anticancer therapy found that 28.5% (~143 patients) took probiotic supplements, and 12 (~8.5%) patients described negative side effects, the most common being diarrhea and obstipation [196]. A systematic retrospective study of 1530 cancer patients across seventeen studies reported probiotic-related bacteremia/fungemia in five cases out of 756 that consumed probiotics [197]. Thus, even though there are some probiotic Lactobacillus spp., such as Lactobacillus rhamnosus CBT LR5 and Lactobacillus plantarum DR7®, that have received the ‘Generally Recognized as Safe’ status from the United States Food and Drug Administration for consumption purposes, it is still imperative that adverse outcomes and appropriate doses for all probiotic strains be rigorously tested so that clinicians can choose the appropriate strains for therapeutic purposes.
Furthermore, it is imperative that probiotics are screened for any antimicrobial resistance genes before being used as a therapeutic so that appropriate antibiotics can be prescribed in the event of infections that arise due to the probiotics. Additionally, probiotics harboring antimicrobial resistance genes may pass the genes to other commensals in the body [198]. Many oncology patients are administered high doses of antibiotics, so a simultaneous probiotic treatment may pose the threat of horizontal transfer of antibiotic resistance genes, which is undesirable [199].
The use of pre- and postbiotics, which are inanimate in nature, helps overcome some of the safety concerns of probiotics while providing similar advantages to probiotics. Further understanding of the mechanism of action of biotics on tumors can aid in the development of high-efficiency therapeutics for the treatment of cancer.
7. Conclusions
Altogether, prebiotics, probiotics, synbiotics, and postbiotics offer promising, microbiome-focused strategies for promoting health and potentially lowering cancer risk. Moving forward, understanding exactly how these agents work—whether by shifting microbial populations, producing bioactive compounds, or changing how the host responds—will be key to developing effective, personalized cancer prevention and treatment approaches. It will also aid in the design of precision biotics that can deliver specific pro-apoptotic factors, tumor suppressor genes, and immune modulators for personalized therapy for cancer patients in the future.
Author Contributions
Conceptualization, J.K.I.; writing—original draft preparation, T.T., A.B., and J.K.I.; writing—review and editing, T.T., A.B., and J.K.I.; visualization, T.T., A.B., and J.K.I.; supervision, J.K.I.; project administration, J.K.I. All authors contributed equally to writing and figure creation, with each author responsible for drafting individual sections. J.K.I. compiled and finalized the full manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BRCA | Breast cancer gene |
| BCR-Abl | Breakpoint cluster region-Abelson |
| CRC | Colorectal cancer |
| DNMT | DNA methyltransferase |
| DSB | Double-strand break |
| FMT | Fecal microbiota transplantation |
| FOS | Fructo-oligosaccharides |
| GOS | Galacto-oligosaccharides |
| HDAC | Histone deacetylase |
| HMOs | Human milk oligosaccharides |
| HPV | Human papillomavirus |
| IAA | Indole acetic acid |
| IgA | Immunoglobulin A |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| MAPK | Mitogen-activated protein kinase |
| MLH1 | mutL homolog 1 |
| NK cells | Natural killer cells |
| mTOR | Mechanistic target of rapamycin |
| PI3K | Phosphoinositide 3-kinase |
| PIP3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| SCFAs | Short chain fatty acids |
| Tregs | Regulatory T-cells |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: A diagnostic and therapeutic perspective. Cancer Biol. Ther. 2023, 24, 2240084. [Google Scholar] [CrossRef]
- Wang, Q. Cancer predisposition genes: Molecular mechanisms and clinical impact on personalized cancer care: Examples of Lynch and HBOC syndromes. Acta Pharmacol. Sin. 2016, 37, 143–149. [Google Scholar] [CrossRef]
- Madia, F.; Worth, A.; Whelan, M.; Corvi, R. Carcinogenicity assessment: Addressing the challenges of cancer and chemicals in the environment. Environ. Int. 2019, 128, 417–429. [Google Scholar] [CrossRef]
- Ofoezie, E.F.; Ogbonna, C.A.; Olisakwe, S.C.; Anunobi, C.J.; George, E.T.; Babarinde, S.; Chuweumeka, C.G.; Ogbonna, U.E.; Amafili, C.C.; Alisigwe, C.V.; et al. Role of infectious agents in cancer pathogenesis and therapy. Microbe 2025, 6, 100284. [Google Scholar] [CrossRef]
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Enewold, L.; Zhao, J.; Zeruto, C.A.; Yabroff, K.R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1304–1312. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Leonard, J.M.; Toro, D.D. Defining the Microbiome Components (Bacteria, Viruses, Fungi) and Microbiome Geodiversity. Surg. Infect. 2023, 24, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Rosenberg, E. Diversity of bacteria within the human gut and its contribution to the functional unity of holobionts. NPJ Biofilms Microbiomes 2024, 10, 134. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8836–8847. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the power of postbiotics: A revolutionary approach to nutrition for humans and animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Lin, D.; Medeiros, D.M. The microbiome as a major function of the gastrointestinal tract and its implication in micronutrient metabolism and chronic diseases. Nutr. Res. 2023, 112, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Vimal, J.; Himal, I.; Kannan, S. Role of microbial dysbiosis in carcinogenesis & cancer therapies. Indian. J. Med. Res. 2020, 152, 553–561. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, J.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Kesavelu, D.; Jog, P. Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis. 2023, 10, 20499361231154443. [Google Scholar] [CrossRef]
- Winter, S.E.; Bäumler, A.J. Gut dysbiosis: Ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2316579120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Fahmawi, A.; Christian, D.A.; Fang, Q.; Radaelli, E.; Chen, L.; Sullivan, M.C.; Misic, A.M.; Ellringer, J.A.; Zhu, X.Q.; et al. Infection-induced intestinal dysbiosis is mediated by macrophage activation and nitrate production. mBio 2019, 10, e00935-19. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, N.H.; Sanad, Y.M.; Han, J.; Foley, S.L. Current knowledge and perspectives of potential impacts of Salmonella enterica on the profile of the gut microbiota. BMC Microbiol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensan, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; O’Ferrall, S.; Krych, Ł.; O’Mahony, E.; Namusoke, H.; Lanyero, B.; Kot, W.; Nabukeera Barungi, N.; Michaelsen, K.F.; Molgaard, C.; et al. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes 2020, 11, 855–867. [Google Scholar] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.; Sung, J.J.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijjaro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The detrimental impact of ultra-processed foods on the human gut microbiome and gut barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef]
- Fan, X.; Jin, Y.; Chen, G.; Ma, X.; Zhang, L. Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion 2021, 102, 508–515. [Google Scholar] [CrossRef]
- Moreira, M.M.; Carriço, M.; Capelas, M.L.; Pimenta, N.; Santos, T.; Ganhão-Arranhado, S.; Mäkitie, A.; Ravasco, P. The impact of pre-, pro- and synbiotics supplementation in colorectal cancer treatment: A systematic review. Front. Oncol. 2024, 14, 1395966. [Google Scholar] [CrossRef]
- Garrett, W.S.; Punit, S.; Gallini, C.A.; Michaud, M.; Zhang, D.; Sigrist, K.S.; Lord, G.M.; Glickman, J.N.; Glimcher, L.H. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 2009, 16, 208–219. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Fecal transplants as a microbiome-based therapeutic. Curr. Opin. Microbiol. 2020, 56, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal microbiota transplantation: Current challenges and future landscapes. Clin. Microbiol. Rev. 2024, 37, e0006022. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Barile, D.; Rastall, R.A. Human milk and related oligosaccharides as prebiotics. Curr. Opin. Biotechnol. 2013, 24, 214–219. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Gulati, M.; Wadhwa, S.; Vishwas, S.; Sharma, D.S.; Corrie, L.; Alam, A.; Alnasser, S.M.; Alkhayl, F.F.A.; Parveen, Z.; et al. Multifaceted role of synbiotics as nutraceuticals, therapeutics and carrier for drug delivery. Chem. Biol. Interact. 2022, 368, 110223. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu, Y.; Abreu, A.T.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The clinical evidence for postbiotics as microbial therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Caetano, M.A.F.; Castelucci, P. Role of short chain fatty acids in gut health and possible therapeutic approaches in inflammatory bowel diseases. World J. Clin. Cases 2022, 10, 9985–10003. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, T.R. Production, formulation, and application of postbiotics in the treatment of skin conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Iyer, J.K. Insights into bacteriotherapy: Harnessing bacteria for the greater good. Biomed. J. Sci. Tech. Res. 2025, 62, 54253–54261. [Google Scholar] [CrossRef]
- Gupta, V.; Mastromarino, P.; Garg, R. Effectiveness of prophylactic oral and/or vaginal probiotic supplementation in the prevention of recurrent urinary tract infections: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2024, 78, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: Randomized, double-blind, placebo-controlled trial. Pediatrics 2008, 122, 8–12. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Han, Z.; He, K.; Zhang, Y.; Wu, D.; Chen, H. The effects of probiotics supplementation on Helicobacter pylori standard treatment: An umbrella review of systematic reviews with meta-analyses. Sci. Rep. 2024, 14, 10069. [Google Scholar] [CrossRef] [PubMed]
- Al Sharaby, A.; Abugoukh, T.M.; Ahmed, W.; Ahmed, S.; Elshaikh, A.O. Do probiotics prevent Clostridium difficile-associated diarrhea? Cureus 2022, 14, e27624. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Lamas, A.; Del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic effects against virus infections: New weapons for an old war. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar]
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131. [Google Scholar] [CrossRef]
- Peitsidou, K.; Karantanos, T.; Theodoropoulos, G.E. Probiotics, prebiotics, synbiotics: Is there enough evidence to support their use in colorectal cancer surgery? Dig. Surg. 2012, 29, 426–438. [Google Scholar] [CrossRef]
- Gutiérrez Salmeán, G.; Delgadillo González, M.; Rueda Escalona, A.A.; Leyva Islas, J.A.; Castro-Eguiluz, D. Effects of prebiotics, probiotics, and synbiotics on the prevention and treatment of cervical cancer: Mexican consensus and recommendations. Front. Oncol. 2024, 14, 1383258. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The role of probiotics in cancer prevention. Cancers 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.; Shang, X.; Effat, K.; Kanwal, F.; He, X.; Li, Y.; Xu, C.; Niu, W.; War, A.R.; Zhang, Y. The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer. J. Food Biochem. 2022, 46, e14302. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Morgos, D.T.; Stefani, C.; Miricescu, D.; Greabu, M.; Stanciu, S.; Nica, S.; Stanescu-Spinu, I.I.; Balan, D.G.; Balcangiu-Stroescu, A.E.; Coculescu, E.C.; et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int. J. Mol. Sci. 2024, 25, 1848. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Asoudeh-Fard, A.; Barzegari, A.; Dehnad, A.; Bastani, S.; Golchin, A.; Omidi, Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signaling pathways. BioImpacts 2017, 7, 193–198. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, Y.; Li, Y. Bifidobacterium infantis promotes Foxp3 expression in colon cells via PD-L1-mediated inhibition of the PI3K-Akt-mTOR signaling pathway. Front. Immunol. 2022, 13, 871705. [Google Scholar] [CrossRef]
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and cellular mechanisms influenced by postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin Hassan, M.I.; Habib, S.; Islam, S. Apoptosis: A comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef]
- Jam, S.A.M.; Morshedi, M.; Khosroushahi, A.Y.; Eftekharsadat, A.T.; Alipour, M.; Alipour, B. Preventive and tumor-suppressive effects of Lactobacillus paracasei x12 in rat model of colorectal cancer. Iran. J. Pharm. Res. 2020, 19, 330–342. [Google Scholar]
- Kim, S.J.; Kang, C.H.; Kim, G.H.; Cho, H. Anti-tumor effects of heat-killed L. reuteri MG5346 and L. casei MG4584 against human colorectal carcinoma through caspase-9-dependent apoptosis in xenograft model. Microorganisms 2022, 10, 533. [Google Scholar] [CrossRef]
- Cui, L.; Xu, X.; Fan, H.; Wan, X.; Chen, Q.; Zhang, J.; Tao, C.; Du, Z.; Wang, Y.; Zhang, J.; et al. Reuterin promotes pyroptosis in hepatocellular cancer cells through mtDNA-mediated STING activation and caspase 8 expression. Cancer Lett. 2024, 601, 217183. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Dai, M.; Qiu, C.; Sun, Q.; Fan, T.; Guo, Y.; Zhao, L.; Jiang, Y. γ-Linolenic acid derived from Lactobacillus plantarum MM89 induces ferroptosis in colorectal cancer. Food Funct. 2025, 16, 1760–1771. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, Y.; Xie, Q.; Lv, X.; Zhou, L.; Smith, E.E.; Cao, T.; Zhang, Y.; Li, B.; Huo, G.; et al. Bifidobacterium bifidum E3 combined with Bifidobacterium longum subsp. infantis E4 improves LPS-induced intestinal injury by inhibiting the TLR4/NF-κB and MAPK signaling pathways in vivo. J. Agric. Food Chem. 2023, 71, 8915–8930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, Y.; Yang, Y.; Zhang, Y.; Xu, C.; Yang, R. Gut microbiota derived indole-3-acetic acid ameliorates precancerous inflammatory intestinal milieu to inhibit tumorigenesis through IL-35. J. Immunother. Cancer 2025, 13, e011155. [Google Scholar] [CrossRef]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics regulating inflammation via NLRP3 inflammasome modulation: A potential therapeutic approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: A strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018, 24, 3528–3538. [Google Scholar] [CrossRef]
- Zhao, M.; Liang, X.; Meng, Y.; Lu, H.; Lin, K.; Gong, P.; Liu, T.; Yi, H.; Pan, J.; Zhang, Y.; et al. Probiotics induce intestinal IgA secretion in weanling mice potentially through promoting intestinal APRIL expression and modulating the gut microbiota composition. Food Funct. 2024, 15, 4862–4873. [Google Scholar] [CrossRef]
- Zhang, J.W.; Du, P.; Yang, B.R.; Gao, J.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Oda, Y.; Owari, T.; Iida, K.; Ohnishi, S.; Fujii, T.; Nishimura, N.; Miyamoto, T.; Shimizu, T.; Ohnishi, K.; et al. Probiotics enhances anti-tumor immune response induced by gemcitabine plus cisplatin chemotherapy for urothelial cancer. Cancer Sci. 2023, 114, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Rodrigues e-Lacerda, R.; Barra, N.G.; Kukje Zada, D.; Robin, N.; Mehra, A.; Schertzer, J.D. Postbiotic Impact on Host Metabolism and immunity provides therapeutic potential in metabolic disease. Endocr. Rev. 2025, 46, 60–79. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef]
- Wu, H.; Van Der Pol, W.J.; Dubois, L.G.; Morrow, C.D.; Tollefsbol, T.O. Dietary supplementation of inulin contributes to the prevention of estrogen receptor-negative mammary cancer by alteration of gut microbial communities and epigenetic regulations. Int. J. Mol. Sci. 2023, 24, 9015. [Google Scholar] [CrossRef]
- Mishra, P.; Badiyani, V.M.; Jain, S.; Subramanian, S.; Maharaj, S.V.; Kumar, A.; Singh, B.N. Prebiotics: Ignored player in the fight against cancer. Cancer Rep. 2023, 6, e1870. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther. 2006, 5, 1265–1269. [Google Scholar] [CrossRef]
- Mahdavi, M.; Laforest-Lapointe, I.; Massé, E. Preventing colorectal cancer through prebiotics. Microorganisms 2021, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Marinovic, M. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis 2006, 27, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, P.; Xiao, Z. Resistant starch prevents tumorigenesis of dimethylhydrazine-induced colon tumors via regulation of an ER stress-mediated mitochondrial apoptosis pathway. Int. J. Mol. Med. 2018, 41, 1887–1898. [Google Scholar] [CrossRef]
- Chen, L.; Brar, M.S.; Leung, F.C.C.; Hsiao, W.L.W. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 2016, 7, 31226–31242. [Google Scholar] [CrossRef]
- Liao, W.; Khan, I.; Huang, G.; Chen, S.; Liu, L.; Leong, W.K.; Li, X.A.; Wu, J.; Wendy Hsiao, W.L. Bifidobacterium animalis: The missing link for the cancer-preventive effect of Gynostemma pentaphyllum. Gut Microbes 2021, 13, 1847629. [Google Scholar] [CrossRef]
- Fernández, J.; Moreno, F.J.; Olano, A.; Clemente, A.; Villar, C.J.; Lombó, F. A galacto-oligosaccharides preparation derived from lactulose protects against colorectal cancer development in an animal model. Front. Microbiol. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Lanza, E.; Corle, D.; Lance, P.; Iber, F.; Caan, B.; Shike, M.; Weissfeld, J.; Burt, R.; Cooper, M.R.; et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. N. Engl. J. Med. 2000, 342, 1149–1155. [Google Scholar] [CrossRef]
- Peters, U.; Sinha, R.; Chatterjee, N.; Subar, A.F.; Ziegler, R.G.; Kulldorff, M.; Bresalier, R.; Weissfeld, J.L.; Flood, A.; Schatzkin, A.; et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet 2003, 361, 1491–1495. [Google Scholar] [CrossRef]
- Tantamango, Y.M.; Knutsen, S.F.; Beeson, L.; Fraser, G.; Sabate, J. Association between dietary fiber and incident cases of colon polyps: The adventist health study. Gastrointest. Cancer Res. 2011, 4, 161–167. [Google Scholar]
- Ben, Q.; Sun, Y.; Chai, R.; Qian, A.; Xu, B.; Yuan, Y. Dietary, fiber intake reduces risk for colorectal adenoma: A meta-analysis. Gastroenterology 2014, 146, 689–699.e6. [Google Scholar] [CrossRef]
- Yue, Y.; Ye, K.; Lu, J.; Wang, X.; Zhang, S.; Liu, L.; Yang, B.; Nassar, K.; Xu, X.; Pang, X.; et al. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed. Pharmacother. 2020, 127, 110159. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Ohkawara, S.; Furuya, H.; Nagashima, K.; Asanuma, N.; Hino, T. Oral administration of Butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J. Nutr. 2005, 135, 2878–2883. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Rumney, C.J.; Coutts, J.T.; Lievense, L.C. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis 1998, 19, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hinoi, T.; Adachi, T.; Miguchi, M.; Niitsu, H.; Kochi, M.; Sada, H.; Sotomaru, Y.; Sakamoto, N.; Sentani, K.; et al. Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model. PLoS ONE 2019, 14, e0216393. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224. [Google Scholar] [CrossRef]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Abbasi, A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 1787–1803. [Google Scholar] [CrossRef]
- Khoury, N.; El-Hayek, S.; Tarras, O.; El-Sabban, M.; El-Sibai, M.; Rizk, S. Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Int. J. Oncol. 2014, 45, 2117–2127. [Google Scholar] [CrossRef]
- Amin, M.; Navidifar, T.; Saeb, S.; Barzegari, E.; Jamalan, M. Tumor-targeted induction of intrinsic apoptosis in colon cancer cells by Lactobacillus plantarum and Lactobacillus rhamnosus strains. Mol. Biol. Rep. 2023, 50, 5345–5354. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Y.H.; Le, G.W.; Ma, X.Y. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J. Gastroenterol. 2005, 11, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Guzowska, M.; Dziendzikowska, K.; Kopiasz, Ł.; Gajewska, M.; Wilczak, J.; Harasym, J.; Czerwińska, M.; Gromadzka-Ostrowska, J. Oat beta-glucans modulate the gut microbiome, barrier function, and immune responses in an in vivo model of early-stage colorectal cancer. Int. J. Mol. Sci. 2024, 25, 13586. [Google Scholar] [CrossRef]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int. J. Cancer 2005, 116, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Yoon, L.S.; Michels, K.B.; Tranfield, W.; Jacobs, J.P.; May, F.P. The impact of prebiotic, probiotic, and synbiotic supplements and yogurt consumption on the risk of colorectal neoplasia among adults: A systematic review. Nutrients 2022, 14, 4937. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, W.; Cai, S.; Feng, X.; Chen, Y.; Cheng, X.; Ma, J.; Ma, W.; Tian, Z.; Yang, W. Evaluation of the efficacy of probiotics in the chemoradiotherapy of colorectal cancer: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2025, 25, 312. [Google Scholar] [CrossRef] [PubMed]
- Amitay, E.L.; Carr, P.R.; Gies, A.; Laetsch, D.C.; Brenner, H. Probiotic/synbiotic treatment and postoperative complications in colorectal cancer patients: Systematic review and meta-analysis of randomized controlled trials. Clin. Transl. Gastroenterol. 2020, 11, e00268. [Google Scholar] [CrossRef]
- Kandati, K.; Belagal, P.; Nannepaga, J.S.; Viswanath, B. Role of probiotics in the management of cervical cancer: An update. Clin. Nutr. ESPEN 2022, 48, 5–16. [Google Scholar] [CrossRef]
- Xu, P.; Mageswaran, U.M.; Nisaa, A.A.; Balasubramaniam, S.D.; Rajendran, D.; Ismail, E.H.B.E.; Kadir, M.N.; Oon, C.E.; Tan, C.S.; Sany, S.B.; et al. Roles of probiotics against HPV through the gut-vaginal axis. Int. J. Gynecol. Obstet. 2025, 169, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, F.; Chen, C.; Chen, S.; Huang, X.; Wang, Y.; Qiu, P.; Deng, G.; Gao, J. Dietary fiber and human papillomavirus infection among US women: The national health and nutrition examination survey, 2003–2016. Nutr. Cancer 2021, 73, 2515–2522. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gu, L.; Li, Y.; Chen, S.; You, J.; Fan, L.; Wang, Y.; Zhao, L. Chitooligosaccharides display anti-tumor effects against human cervical cancer cells via the apoptotic and autophagic pathways. Carbohydr. Polym. 2019, 224, 115171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Du, X.; Yu, W.; Jiang, J.; Geng, Y.; Guo, X.; Fan, X.; Ma, C. Lactobacilli inhibit cervical cancer cell migration in vitro and reduce tumor burden in vivo through upregulation of E-cadherin. Oncol. Rep. 2017, 38, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory effect of vaginal Lactobacillus supernatants on cervical cancer cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef]
- Asoudeh-Fard, A.; Beygi, M.Y.; Parsaei, A.; Mohkam, M.; Asoudeh-Fard, M.; Gholami, A. Postbiotic metabolites derived from Lactobacillus fermentum as potent antiproliferative bioresources on HeLa cells with promising biocompatibility. BMC Complement. Med. Ther. 2024, 24, 420. [Google Scholar] [CrossRef]
- Negi, D.; Singh, A.; Joshi, N.; Mishra, N. Cisplatin and probiotic biomass loaded pessaries for the management of cervical cancer. Anticancer. Agents Med. Chem. 2019, 20, 589–598. [Google Scholar] [CrossRef]
- Okawa, T.; Niibe, H.; Arai, T.; Sekiba, K.; Noda, K.; Takeuchi, S.; Hashimoto, S.; Ogawa, N. Effect of LC9018 combined with radiation therapy on carcinoma of the uterine cervix. A phase III, multicenter, randomized, controlled study. Cancer 1993, 72, 1949–1954. [Google Scholar] [CrossRef]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912. [Google Scholar] [CrossRef]
- Linn, Y.H.; Thu, K.K.; Win, N.H.H. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: A randomized double-blind placebo-controlled study. Probiotics Antimicrob. Proteins 2019, 11, 638–647. [Google Scholar] [CrossRef]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- van’t Veer, P.; Dekker, J.M.; Lamers, J.W.; Kok, F.J.; Schouten, E.G.; Brants, H.A.; Sturmans, F.; Hermus, R.J. Consumption of fermented milk products and breast cancer: A case-control study in The Netherlands. Cancer Res. 1989, 49, 4020–4023. [Google Scholar] [PubMed]
- Taper, H.S.; Roberfroid, M. Influence of inulin and oligofructose on breast cancer and tumor growth. J. Nutr. 1999, 129, 1488S–1491S. [Google Scholar] [CrossRef]
- Delphi, L.; Sepehri, H.; Khorramizadeh, M.R.; Mansoori, F. Pectic-oligosaccharides from apples induce apoptosis and cell cycle arrest in MDA-MB-231 cells, a model of human breast cancer. Asia Pac. J. Cancer Prev. 2015, 16, 5265–5271. [Google Scholar] [CrossRef]
- Kondegowda, N.G.; Meaney, M.P.; Baker, C.; Ju, Y.H. Effects of non-digestible carbohydrates on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Nutr. Cancer 2011, 63, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sampsell, K.; Wang, W.; Ohland, C.; Mager, L.F.; Pett, N.; Lowry, D.E.; Sales, K.M.; McNeely, M.L.; McCoy, K.D.; Culos-Reed, S.N.; et al. Exercise and prebiotic fiber provide gut microbiota-driven benefit in a survivor to germ-free mouse translational model of breast cancer. Cancers 2022, 14, 2722. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Soltan Dallal, M.M.; Hassan, Z.M.; Holakuyee, M.; Agha Amiri, S.; Abolhassani, M.; Mahdavi, M. Oral administration of Lactobacillus acidophilus induces IL-12 production in spleen cell culture of BALB/c mice bearing transplanted breast tumour. Br. J. Nutr. 2010, 104, 227–232. [Google Scholar] [CrossRef]
- Aragón, F.; Carino, S.; Perdigón, G.; De Moreno De LeBlanc, A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J. Immunother. 2015, 38, 185–196. [Google Scholar] [CrossRef]
- Zamberi, N.R.; Abu, N.; Mohamed, N.E.; Nordin, N.; Keong, Y.S.; Beh, B.K.; Zakaria, Z.A.B.; Nik Abdul Rahman, N.M.A.; Alitheen, N.B. The antimetastatic and antiangiogenesis effects of Kefir water on murine breast cancer cells. Integr. Cancer Ther. 2016, 15, NP53-66. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: A case-control study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Actis, S.; Cazzaniga, M.; Bounous, V.E.; D’Alonzo, M.; Rosso, R.; Accomasso, F.; Minella, C.; Biglia, N. Emerging evidence on the role of breast microbiota on the development of breast cancer in high-risk patients. Carcinogenesis 2023, 44, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Dianatpour, A.; Ghafouri-Fard, S. The role of probiotics in cancer treatment: Emphasis on their in vivo and in vitro anti-metastatic effects. Int. J. Mol. Cell Med. 2017, 6, 66. [Google Scholar]
- López, D.M.L. Management of genitourinary syndrome of menopause in breast cancer survivors: An update. World J. Clin. Oncol. 2022, 13, 71–100. [Google Scholar] [CrossRef]
- Marschalek, J.; Farr, A.; Marschalek, M.L.; Domig, K.J.; Kneifel, W.; Singer, C.F.; Kiss, H.; Petricevic, L. Influence of orally administered probiotic Lactobacillus strains on vaginal microbiota in women with breast cancer during chemotherapy: A randomized placebo-controlled double-blinded pilot study. Breast Care 2017, 12, 335–339. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomized, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Yokokura, T.; Azuma, I. Anti-tumour activity of Lactobacillus casei on Lewis lung carcinoma and line-10 hepatoma in syngeneic mice and guinea pigs. Cancer Immunol. Immunother. 1985, 20, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J. Pharm. Sci. 2013, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Fallahi, F.; Tamtaji, O.R.; Tajiknia, V.; Banikazemi, Z.; Fathizadeh, H.; Abbasi-Kolli, M.; Aschner, M.; Ghandali, M.; Sahebkar, A.; et al. An update on the effects of probiotics on gastrointestinal cancers. Front. Pharmacol. 2021, 12, 680400. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Boutell, S.; Taylor, M.W.; Douglas, R.G.; Biswas, K. Randomized, double-blind, placebo-controlled trial of oral probiotic Streptococcus salivarius M18 on head and neck cancer patients post-radiotherapy: A pilot study. Sci. Rep. 2020, 10, 13201. [Google Scholar] [CrossRef]
- Fu, W.; Kapila, L.Y. Nisin, a probiotic bacteriocin, combined with limited chemoradiation therapy in head and neck cancer. Arch. Clin. Med. Case Rep. 2021, 05, 531–536. [Google Scholar] [CrossRef]
- Torabi, S.; Nahidi, Y.; Ghasemi, S.Z.; Reihani, A.; Samadi, A.; Ramezanghorbani, N.; Nazari, E.; Davoudi, S. Evaluation of skin cancer prevention properties of probiotics. Genes. Nutr. 2025, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lyu, Y.; Liu, X.; Jia, X.; Cui, F.; Wu, X.; Deng, S.; Yue, C. Engineered probiotics. Microb. Cell Fact. 2022, 21, 72. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Wan, L.; Lu, J.; Wei, H.; Deng, K.Y.; Wei, J.; Xin, H.B. Attenuated Salmonella engineered with an apoptosis-inducing factor (AIF) eukaryotic expressing system enhances its anti-tumor effect in melanoma in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 3517–3528. [Google Scholar] [CrossRef]
- He, L.; Yang, H.; Tang, J.; Liu, Z.; Chen, Y.; Lu, B.; He, H.; Tang, S.; Sun, Y.; Liu, F. Intestinal probiotics E. coli Nissle 1917 as a targeted vehicle for delivery of p53 and Tum-5 to solid tumors for cancer therapy. J. Biol. Eng. 2019, 13, 58. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Lia, I.; Vincent, R.; Coker, C.; Castro, S.; Treuting, P.M.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 2020, 12, eaax0876. [Google Scholar] [CrossRef]
- Leventhal, D.S.; Sokolovska, A.; Li, N.; Plescia, C.; Kolodziej, S.A.; Gallant, C.W.; Christmas, R.; Gao, J.R.; James, M.J.; Abin-Fuentes, A.; et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 2020, 11, 2739. [Google Scholar] [CrossRef]
- Luke, J.J.; Piha-Paul, S.A.; Medina, T.; Verschraegen, C.F.; Varterasian, M.; Brennan, A.M.; Riese, R.J.; Sokolovska, A.; Strauss, J.; Hava, D.L.; et al. Phase I study of SYNB1891, an engineered E. coli Nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies. Clin. Cancer Res. 2023, 29, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Hamade, D.F.; Epperly, M.W.; Fisher, R.; Hou, W.; Shields, D.; van Pijkeren, J.P.; Leibowitz, B.J.; Coffman, L.G.; Wang, H.; Huq, M.S.; et al. Genetically engineered probiotic Limosilactobacillus reuteri releasing IL-22 (LR-IL-22) modifies the tumor microenvironment, enabling irradiation in ovarian cancer. Cancers 2024, 16, 474. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Bedani, R.; Saad, S.M.I. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: An update for current perspectives and future challenges. Br. J. Nutr. 2015, 114, 1993–2015. [Google Scholar] [CrossRef] [PubMed]
- Flesch, A.T.; Tonial, S.T.; Contu, P.d.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef]
- Krebs, B. Prebiotic and synbiotic treatment before colorectal surgery-randomised double blind trial. Coll. Antropol. 2016, 40, 35–40. [Google Scholar]
- Farshi Radvar, F.; Mohammad-Zadeh, M.; Mahdavi, R.; Andersen, V.; Nasirimotlagh, B.; Faramarzi, E.; Lotfi Yagin, N. Effect of synbiotic supplementation on matrix metalloproteinase enzymes, quality of life and dietary intake and weight changes in rectal cancer patients undergoing neoadjuvant chemoradiotherapy. Med. J. Nutrition Metab. 2020, 13, 225–235. [Google Scholar] [CrossRef]
- Nascimento, M.; Caporossi, C.; Eduardo Aguilar-Nascimento, J.; Michelon Castro-Barcellos, H.; Teixeira Motta, R.; Reis Lima, S. Efficacy of synbiotics to reduce symptoms and rectal inflammatory response in acute radiation proctitis: A randomized, double-blind, placebo-controlled pilot trial. Nutr. Cancer 2020, 72, 602–609. [Google Scholar] [CrossRef]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: A randomized controlled trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yano, M.; Motoori, M.; Kishi, K.; Miyashiro, I.; Ohue, M.; Ohigashi, H.; Asahara, T.; Nomoto, K.; Ishikawa, O. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: A prospective randomized controlled trial. Surgery 2012, 152, 832–842. [Google Scholar] [CrossRef]
- Sugimoto, T.; Atobe, S.; Kado, Y.; Takahashi, A.; Motoori, M.; Sugimura, K.; Miyata, H.; Yano, M.; Tanaka, K.; Doki, Y.; et al. Gut microbiota associated with the mitigation effect of synbiotics on adverse events of neoadjuvant chemotherapy in patients with esophageal cancer: A retrospective exploratory study. J. Med. Microbiol. 2023, 72, 001723. [Google Scholar] [CrossRef]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Manifar, S.; Koopaie, M.; Jahromi, Z.M.; Kolahdooz, S. Effect of synbiotic mouthwash on oral mucositis induced by radiotherapy in oral cancer patients: A double-blind randomized clinical trial. Support. Care Cancer 2023, 31, 31. [Google Scholar] [CrossRef]
- Koopaie, M.; Vaziri, S.; Manifar, S.; Younespour, S.; Kolahdooz, S. Efficacy of synbiotic mouthwash on salivary TLR2 levels and oral mucositis in head and neck cancer patients undergoing radiotherapy: A randomized clinical trial. Support. Care Cancer 2025, 33, 481. [Google Scholar] [CrossRef]
- Sommacal, H.M.; Bersch, V.P.; Vitola, S.P.; Osvaldt, A.B. Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: A randomized, double-blind clinical trial. Nutr. Cancer 2015, 67, 457–462. [Google Scholar] [CrossRef]
- Monshikarimi, A.; Ostadrahimi, A.; Asghari Jafarabadi, M.; EivaziZiaei, J.; Barzeghari, A.; Esfahani, A.; Payahoo, L.; Aamazadeh, F.; Farrin, N. Does combination of Lactobacillus rhamnosus Heriz I and beta glucan improve quality of life in women with breast cancer receiving chemotherapy? A randomized double-blind placebo-controlled clinical trial. Nutr. Food Sci. 2020, 50, 569–578. [Google Scholar] [CrossRef]
- Tirgar, A.; Rezaei, M.; Ehsani, M.; Salmani, Z.; Rastegari, A.; Jafari, E.; Khandani, B.K.; Nakhaee, N.; Khaksari, M.; Moazed, V. Exploring the synergistic effects of vitamin D and synbiotics on cytokines profile, and treatment response in breast cancer: A pilot randomized clinical trial. Sci. Rep. 2024, 14, 21372. [Google Scholar] [CrossRef]
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: A randomized placebo-controlled double-blind clinical trial. BMC Complement. Med. Ther. 2023, 23, 339. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Wong, A. Accounting for the health risk of probiotics. Heliyon 2024, 10, e27908. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Mego, M.; Semanova, M.; Wachsmannova, L.; Adamcikova, Z.; Stevurkova, V.; Drgona, L.; Zajac, V. Probiotic survey in cancer patients treated in the outpatient department in a comprehensive cancer center. Integr. Cancer Ther. 2017, 16, 188–195. [Google Scholar] [CrossRef]
- Redman, M.G.; Ward, E.J.; Phillips, R.S. The efficacy and safety of probiotics in people with cancer: A systematic review. Ann. Oncol. 2014, 25, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Ye, H.; Zhou, X.; Wang, J.; Zhang, L.; Sun, W.; Duan, C.; Fan, M.; Zhou, W.; Bi, C.; et al. Evaluating the health risk of probiotic supplements from the perspective of antimicrobial resistance. Microbiol. Spectr. 2025, 13, e0001924. [Google Scholar] [CrossRef] [PubMed]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic resistance propagation through probiotics. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).