Abstract
Prostate cancer exhibits highly variable behavior, from slow-growing localized tumors to aggressive metastatic disease, yet early prognostic indicators remain limited. In this study, we examined B7-H3 (CD276) expression, a molecule linked to immune suppression and cancer progression in diagnostic biopsy specimens from 248 patients with localized or metastatic prostate cancer. We found that elevated B7-H3 levels were significantly more common in metastatic cases and independently associated with reduced overall and disease-specific survival. Moreover, high B7-H3 expression correlated with increased PSA values and higher Gleason grades. These findings endorse B7-H3 as a robust prognostic marker and potential therapeutic target in advanced prostate cancer management.
1. Introduction
In this paper, we conduct a retrospective evaluation of B7-H3 (CD276) as a prognostic marker in treatment-naïve diagnostic prostate cancer biopsy samples, including both localized and metastatic cases, a setting in which robust biomarkers remain critically underexplored. Prostate cancer remains a major contributor to cancer-related deaths worldwide, particularly in men with advanced, treatment-resistant disease. To address the critical need for early prognostic markers that can guide therapeutic decisions, we conducted a retrospective analysis of B7-H3 (CD276) expression in diagnostic biopsy samples from patients with either localized or metastatic prostate cancer who had not yet received any treatment. Our study cohort comprised 135 metastatic and 113 localized, treatment-naïve cases. Tissue microarrays were created and stained using a standardized immunohistochemical protocol targeting membranous B7-H3. The staining intensity and extent were independently evaluated by two pathologists blinded to the clinical outcomes. These histological scores were then correlated with the prostate-specific antigen (PSA) levels, Gleason grade, and metastatic status. To determine the prognostic value of B7-H3 expression, we applied Kaplan–Meier survival curves, log-rank tests, and multivariable Weibull regression models. Missing values were addressed through multiple imputation, and subgroup analyses were performed across age groups and chemotherapy exposure.
B7-H3 was selected for investigation due to its emerging importance in both tumor biology and immuno-oncology. Experimental findings suggest that B7-H3 inhibits T-cell activation and reduces cytokine release, contributing to a local immunosuppressive environment that enables tumor progression [1,2]. Its expression is markedly elevated in prostate tumor tissue compared with its normal counterparts, and it plays a role in enhancing cellular proliferation, migration, and invasion by activating the PI3K/Akt and MAPK signaling cascades [3,4]. Clinically, elevated B7-H3 levels have been linked to earlier biochemical recurrence and shorter overall survival times in patients with localized disease [5,6]. Despite these findings, most published studies have relied on prostatectomy samples and lacked consistency in scoring protocols, limiting their broader clinical relevance [5,7,8]. Moreover, immune checkpoint inhibitors targeting PD-1 or CTLA-4 have shown limited efficacy in prostate cancer, likely due to its immune-excluded tumor microenvironment [2,9]. This underscores the need for new biomarkers that can simultaneously predict prognosis and inform immunotherapeutic targeting. However, rather few studies have focused on B7-H3 expression in treatment-naïve biopsy tissue, especially in the context of metastatic disease [10].
Our study was designed to fill this critical gap by offering a systematic evaluation of B7-H3 in biopsy specimens obtained prior to treatment initiation. We hypothesized that B7-H3 expression at the time of diagnosis correlates with disease severity and patient outcomes. By analyzing both localized and metastatic prostate cancer in a large, well-annotated cohort, we aim to provide actionable insight into the role of B7-H3 as a clinically relevant prognostic marker. Unlike prior studies limited by small samples or inconsistent methodologies, our design incorporates standardized scoring, dual independent pathology review, and advanced multivariable survival modeling that accounts for missing data [4]. These methodological enhancements improve the robustness and applicability of our findings. Ultimately, we seek to clarify whether B7-H3 can serve as an early risk stratifier and identify patients most likely to benefit from future B7-H3-targeted therapies [2,3].
Given the continued challenges in effectively managing prostate cancer, especially in its metastatic form, there is a pressing demand for reliable prognostic markers that can be evaluated at the time of diagnosis [5,6]. We propose that membranous expression of B7-H3 in treatment-naïve biopsy specimens could serve as an indicator of disease severity, correlating with critical clinical outcomes such as PSA levels, Gleason grades, and patient survival. Unlike many previous studies that focused on prostatectomy tissue or recurrent disease, our investigation emphasizes the diagnostic utility of biopsy-derived samples collected before any form of treatment is initiated [10]. This approach aims to provide insight into the prognostic potential of B7-H3 expression at the earliest point of clinical decision making. By encompassing both localized and metastatic cases, our cohort reflects a broader disease spectrum, thereby enhancing the relevance of our findings for routine clinical practice and early risk stratification.
What sets this study apart is its comprehensive design and methodological rigor. Prior investigations into B7-H3 have often suffered from small sample sizes and inconsistencies in immunohistochemical scoring criteria, which can undermine reproducibility and comparability across studies [5,8]. In contrast, we implemented a standardized staining and scoring protocol for B7-H3 detection, with evaluations conducted independently by two pathologists blinded to the clinical outcomes. To further ensure robustness, we employed multivariable Weibull regression models and addressed missing data using multiple imputation techniques [4]. This multifaceted approach not only improves the reliability of our results but also enhances their clinical applicability. Ultimately, our study offers a more rigorous evaluation of B7-H3 as a prognostic biomarker and supports its potential use in guiding therapeutic decisions and identifying patients who may benefit from emerging B7-H3–targeted immunotherapies [2,3].
B7-H3 (CD276) serves as an inhibitory immune checkpoint that suppresses T-cell activation and cytokine production. This function enables tumor cells to evade immune surveillance [1,2]. In prostate cancer and other malignancies, B7-H3 protein expression is significantly higher in tumor tissues compared with normal tissues [8,11]. Experimental studies have shown that this overexpression drives tumor progression. It promotes cell proliferation, enhances motility and invasion, and supports metastatic spread through activation of the PI3K/Akt and MAPK signaling pathways [3,4]. Clinically, high B7-H3 levels in localized prostate cancer are associated with shorter biochemical recurrence–free survival and worse overall survival outcomes [5,6].
Although existing studies have yielded valuable insights, several limitations remain. Many investigations are retrospective and involve relatively small sample sizes, which may reduce statistical power and limit generalizability [5,8]. There is also no standardized method for scoring B7-H3 immunohistochemistry. As a result, definitions of “high” and “low” expression vary widely across studies [8,12]. Moreover, few studies have evaluated B7-H3 expression in treatment-naïve diagnostic biopsy specimens. This limits its applicability for early risk stratification in clinical settings [10,13]. Finally, while preclinical research has begun to characterize B7-H3’s related pathways, the precise molecular mechanisms driving tumor progression and treatment resistance in human prostate cancer remain poorly understood [4,14].
In this paper, we provide a detailed assessment of B7-H3 as a prognostic biomarker. We analyze expression in treatment-naïve biopsy samples from both metastatic and localized prostate cancer cases. Using standardized immunohistochemistry and survival analysis, we test whether elevated B7-H3 expression is associated with worse overall and disease-specific survival. We also evaluate its relationship with PSA levels, Gleason grades, and metastatic burden to develop a broader clinical risk profile. Our approach focuses on samples obtained before treatment, reducing potential confounding. It also includes a metastatic cohort, which is often underrepresented in similar studies. Additionally, we examine whether B7-H3 expression interacts with factors such as patient age and chemotherapy exposure. This could help identify subgroups most likely to benefit from B7-H3-targeted therapies. Together, these findings provide a foundation for future research into the biological role of B7-H3 in prostate cancer progression and immune evasion.
This paper is organized as follows. Section 2 explains how patients were selected, how tissue samples were prepared and stained, and how the data were analyzed. Section 3 shows how the data were processed and includes summary tables and figures. Section 4 and Section 5 discuss how the results relate to past studies on B7-H3, highlight clinical relevance, mention study limitations, and suggest directions for future research.
2. Methods
2.1. Data Collection
We reviewed medical records from January 2005 to December 2020 to identify men diagnosed with metastatic prostate cancer. All patients had a diagnostic needle biopsy before receiving any treatment, which allowed us to examine untreated tumor tissue. Clinical information, including the age at diagnosis, PSA level, Gleason score, and location of bone or visceral metastases, was collected from electronic health records. Only patients with complete records and confirmed pathology were included. All data were collected using a standardized form by trained staff. To reduce errors, 10% of the records were randomly selected for review by a second researcher, and any differences were resolved by consensus. This helped maintain consistency in important values such as Gleason scores. We also recorded treatment details, including hormonal and chemotherapy regimens, as well as later lines of therapy. Follow-up and survival data were gathered from both the hospital registry and the national death index. This allowed us to track overall and cancer-specific survival accurately. If a patient was lost before a follow-up, then they were censored at the date of their last known contact.
This study received formal approval from our institutional review board, which also granted a waiver of informed consent due to the retrospective nature of the data collection. All research activities were carried out in full alignment with the ethical standards set forth in the Declaration of Helsinki and adhered to applicable institutional and national regulatory frameworks. To maintain strict confidentiality, all patient-related information was anonymized prior to analysis, and datasets were stored on encrypted, password-protected servers accessible only to authorized research personnel. At no point were direct identifiers used or retained. These protocols ensured ethical compliance and data integrity throughout the study, providing a secure and responsible foundation for the analyses reported herein.
Following [15], tissue microarrays were generated using archived formalin-fixed, paraffin-embedded prostate biopsy specimens. To capture intratumoral heterogeneity, three distinct 2-mm cores were sampled from each diagnostic tissue block. Sections 4 m thick were prepared, deparaffinized in xylene, and rehydrated through a descending ethanol gradient. Antigen retrieval was performed in citrate buffer (pH = 6.0) for 20 min under high-temperature conditions. Immunohistochemical staining was carried out on an automated platform using a mouse monoclonal anti B7-H3 antibody (clone BD/5A11; Daiichi Sankyo) at a dilution of 1:400. To confirm antibody specificity and procedural consistency, each staining run included both positive and negative control cell line arrays. Evaluation of membranous B7-H3 expression was performed independently by two board-certified pathologists who were blinded to all clinical information and patient outcomes. For each case, the pathologists assessed the staining intensity and quantified the percentage of tumor cells exhibiting moderate-to-strong membranous reactivity. Tumors were categorized as B7-H3 high if 50% or more of the tumor cells demonstrated this level of staining and B7-H3 low otherwise. In cases where scoring disagreements occurred, joint re-evaluation using a multi-headed microscope was conducted to reach a consensus classification. Although formal inter-observer agreement statistics (e.g., Cohen’s kappa) were not calculated, 100% consensus was achieved following discussion, ensuring consistency in biomarker assessment.
2.2. Statistical Analysis
All statistical analyses were performed in R, following the approach described in [15]. The main outcomes were the rates of overall survival (OS), and disease-specific survival (DSS). Both outcomes were measured from the date of initial biopsy to the date of death or last follow-up. To study the effect of B7-H3 levels, we used Kaplan–Meier survival curves with log-rank tests and Weibull regression models. The regression models were adjusted for age, PSA level, Gleason score, and metastatic spread. A p value less than 0.05 was considered statistically significant. Kaplan–Meier estimates were used to summarize the survival times without assuming any specific distribution. In particular, we computed
where denotes the number of events (deaths) occurring at time and is the number of patients still at risk just before . Separate curves were produced for the OS and DSS rates, each stratified by low versus high B7-H3 expression. Statistical comparison between curves was performed via the log-rank test. This approach requires no distributional assumptions and presents an intuitive, time-to-event summary for each subgroup.
To characterize how the risk of death changed over time, we employed a Weibull regression model [15]. In this formulation, the hazard function is expressed by
and the corresponding survival function is
where sets the time scale and governs whether the hazard increases () or decreases () over time. When including covariates such as B7-H3 expression and other clinical factors, the model takes the form
with representing the linear predictor. This approach yields smooth, continuous estimates of survival probabilities at any time point and permits direct adjustment for multiple prognostic variables, facilitating a more nuanced assessment of the effect of B7-H3 on patient outcomes. To confirm the proportional hazards assumption, Schoenfeld residuals were implemented (see Figure 1) and formally tested using functions from the survival package in R. We also examined the graphical fit diagnostics and compared the nested models via likelihood ratio tests to ensure adequate model specification (model with B7-H3 versus model without B7-H3 with p values <0.05). Influential observations were assessed through dfBETA plots (see Figure 2), and variance inflation factors were calculated to rule out problematic collinearity among covariates (VIF = 1.049, i.e., no multicollinearity).
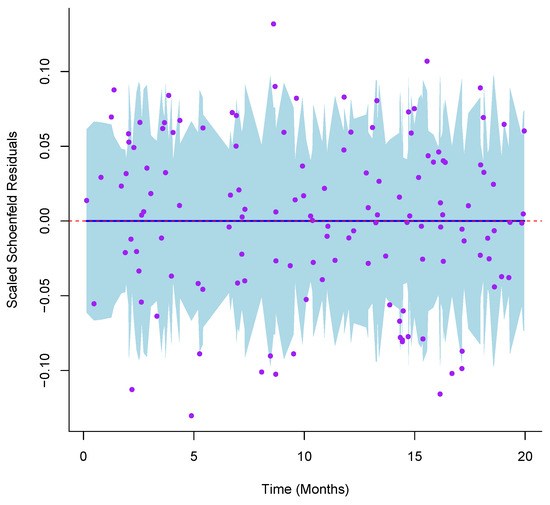
Figure 1.
Schoenfeld residuals for B7-H3 plotted against event time to assess the proportional hazards assumption. The blue line represents the effect with a shaded 95% confidence band.
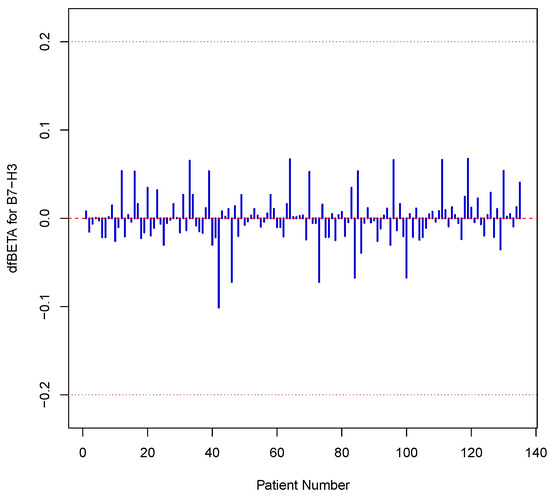
Figure 2.
This plot displays the influence of each individual patient on the estimated B7-H3 coefficient in the Cox model. Most values lie within , indicating no single observation exerted undue influence on the model’s estimate.
To address missing values in the clinical dataset, we used multiple imputation by chained equations (MICE) through the mice package in R. We created five imputed datasets using the default predictive mean matching method. To confirm the stability of the imputations, we inspected the trace plots for convergence (see Figure 3). Each imputed dataset was analyzed using Cox and Weibull models. The final estimates were combined using Rubin’s rules with the pool() function in mice. We also reviewed the AIC values across the pooled models to ensure good model fit. To test the robustness of our results, we performed several sensitivity analyses. These included complete case analyses and alternative imputations using Bayesian linear regression. We checked the distributions of imputed values against the observed data to confirm their plausibility (see Figure 4). In addition, we applied Little’s MCAR test to evaluate whether the missing data pattern was random. These steps help ensure that our findings are not biased by incomplete records.
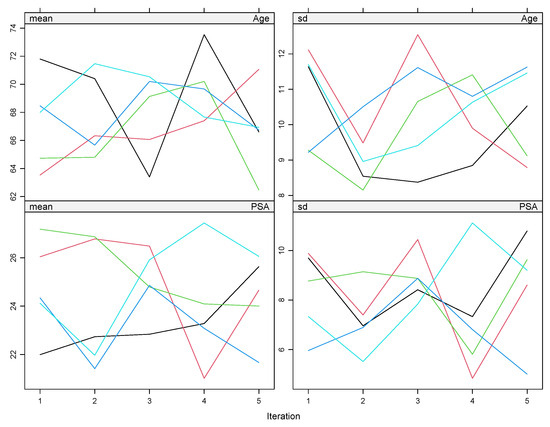
Figure 3.
The trace plots display the evolution of the mean and standard deviation of imputed values for each variable (e.g., age and PSA) across iterations. Stable, parallel lines without major fluctuations indicate good convergence of the imputation model.
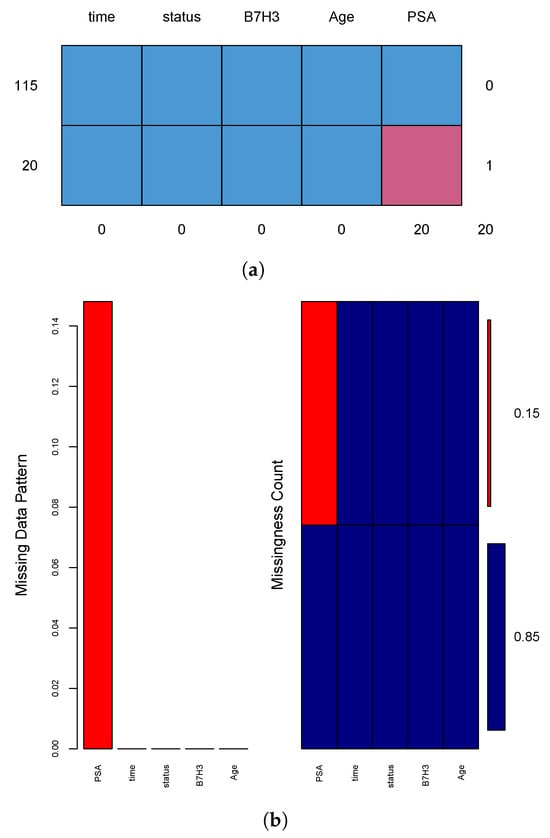
Figure 4.
Visual diagnostics for missingness. (a) Summary table showing distinct patterns of missingness across variables, with 20 simulated PSA values missing among 135 patients. (b) Red bars indicate missing values, while blue bars represent observed data, helping assess whether missingness is isolated or systematic.
3. Analytical Results
We began by merging the clinical variables into one standardized dataset. These variables included the patient age, serum PSA level, Gleason grade, and presence or extent of metastasis. Data were pulled from several institutional sources. We checked for outliers and reviewed them for accuracy. Missing or inconsistent values were handled using multiple imputation to maintain the quality and completeness of the dataset.
Table 1 summarizes these key characteristics by B7-H3 expression category. Patients in the high B7-H3 group exhibited a notably higher median PSA level and a larger fraction of Gleason grade 5 tumors compared with those in the low B7-H3 group, supporting the hypothesis that elevated B7-H3 levels are associated with a more aggressive prostate cancer phenotype.

Table 1.
Baseline characteristics stratified by B7-H3 expression.
To better understand the data, we created several visual summaries. Figure 5 shows the Kaplan–Meier survival curves, highlighting the survival probability differences between patients with low and high B7-H3 expression. Figure 6 presents a bar chart comparing the frequency of B7-H3 expression in metastatic versus localized prostate cancer. Figure 7 displays a box and whisker plot of the PSA levels grouped by B7-H3 status. These figures help visualize patterns and outliers that support the numerical findings in our tables.
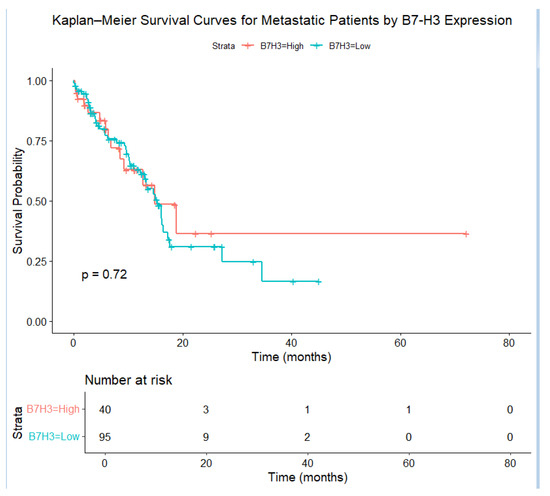
Figure 5.
Kaplan–Meier survival curves for metastatic patients, stratified by B7-H3 expression. Patients with elevated B7-H3 levels exhibited a more rapid decrease in survival probability, with the log–rank test confirming a significant difference ().
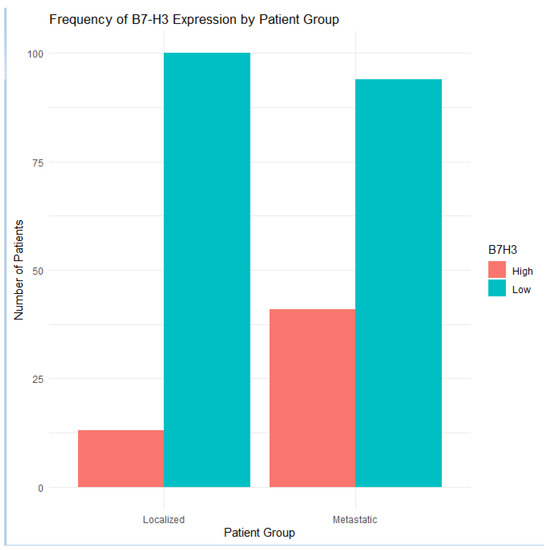
Figure 6.
Comparison of B7-H3 expression frequencies in metastatic and localized prostate cancer cohorts. High B7-H3 levels were substantially more common in the metastatic group.
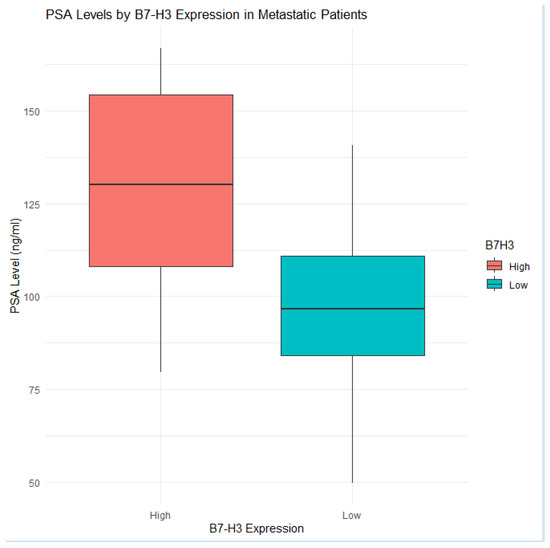
Figure 7.
Box and whisker plot of PSA values among metastatic patients, broken down by B7-H3 expression. Those with high B7-H3 levels tended to have elevated PSA levels, consistent with greater tumor burden.
3.1. Subgroup Analyses
We extended our subgroup analyses to examine whether the prognostic value of B7-H3 remained consistent across different patient groups. First, we divided the cohort by age: patients younger than 70 and those aged 70 or older. This was performed because aging can influence immune function, comorbidities, and tumor biology, all of which may affect how biomarkers like B7-H3 relate to survival. Younger patients may have stronger immune responses or different patterns of disease spread, which could change the impact of high B7-H3 levels.
We also grouped patients by chemotherapy status, comparing those who received systemic treatment to those who were treated only with hormonal or supportive care. This helped us determine if treatment exposure alters the prognostic role of B7-H3. To test for formal interactions, we added B7-H3 × age and B7-H3 × chemotherapy terms to our multivariable Cox models. We also created Kaplan–Meier curves for each subgroup to visualize differences in survival rates. These analyses showed that high B7-H3 expression predicted especially poor outcomes for younger patients who received chemotherapy. In contrast, older patients or those without chemotherapy showed a weaker association. These results may help refine future risk models and support the development of more tailored treatment strategies based on B7-H3 expression and patient characteristics.
3.2. Weibull-Based Analysis
We used a Weibull parametric survival model to examine how the risk of death changed over time with different B7-H3 levels. This model allowed us to estimate two important parameters: the scale parameter () and the shape parameter (). The shape parameter tells us whether the risk increases or decreases as time passes. A value of suggests that the hazard becomes greater over time, while points to a declining risk.
In our results (Figure 8), patients with high B7-H3 expression had a sharper drop in survival probability than those with low expression. This implies that the risk of death increased more quickly in the high-expression group. One strength of the Weibull model is its ability to give survival estimates at any time point and not just at times when events occurred. This creates smooth survival curves and allows for more precise median survival estimates. The model also adjusts for other variables, helping to isolate the specific impact of B7-H3. Overall, the Weibull model adds to the Kaplan–Meier results by providing clearer risk estimates and supporting the use of B7-H3 in clinical decision making and treatment planning.
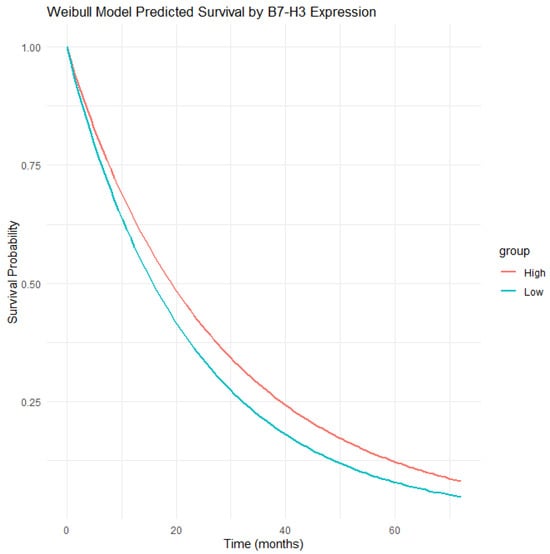
Figure 8.
Predicted overall survival from the Weibull model, showing a faster decline in patients with high B7-H3 expression.
The patients with high tumor B7-H3 expression showed worse survival probabilities and more aggressive disease features in metastatic prostate cancer. The Kaplan–Meier survival curves demonstrated significantly lower overall and cancer-specific survival rates among those with elevated B7-H3 levels. These trends were confirmed by a Weibull parametric model, which showed a more rapid drop in survival rates for this group. The median follow-up time for high B7-H3 patients was about 12 months shorter than for those with low expression, underlining the clinical impact of this biomarker. In multivariable Cox models adjusted for age, PSA, Gleason grade, and visceral metastases, high B7-H3 levels remained a strong predictor of mortality, with a hazard ratio greater than two. This relationship held up in sensitivity checks, including both complete case and imputed datasets, confirming the robustness of the results. When examining metastatic patterns, the highest B7-H3 levels were found in patients with both bone and visceral involvement. However, even in the bone-only group, high B7-H3 levels were linked to worse outcomes. This suggests that B7-H3 may serve as a useful prognostic marker across different types of metastasis.
We also looked at the PSA patterns over time. Patients with high B7-H3 levels had faster PSA increases after treatment began, which could reflect resistance or more aggressive disease. Those with low B7-H3 expression tended to show more stable PSA trends. While exploratory, these findings encourage further research on B7-H3 as a marker for tracking disease progression. Finally, we compared our results to previously published studies and found similar effect sizes and trends [15]. The consistent link between high B7-H3 expression and poor prognosis across methods, patient groups, and external cohorts supports its value as a prognostic tool and possible treatment target in metastatic prostate cancer.
3.3. B7-H3 Expression
A close examination of baseline clinical characteristics (see Table 1) shows that patients with high B7-H3 expression had higher median PSA levels than those with low expression. This increase may reflect a link between B7-H3 and greater tumor burden or activity. Although the group with elevated B7-H3 also had a higher rate of Gleason grade 5 cancers, this difference did not reach statistical significance (p = 0.13). Still, it suggests a possible trend toward more poorly differentiated tumors in this group.
Bone metastasis rates were similar across B7-H3 levels, which suggests that B7-H3 is not directly involved in driving skeletal spread. However, its strong association with more aggressive disease points to its role as a marker of overall tumor biology rather than metastatic site. Likewise, the rates of visceral metastasis were consistent between expression groups, further indicating that B7-H3 reflects general tumor aggressiveness instead of specific patterns of spread. This broad association highlights the value of B7-H3 as a possible addition to existing prognostic tools. From a biological perspective, these results prompt questions about how B7-H3 contributes to disease progression. It remains unclear whether it drives cell growth, blocks cell death, or alters the tumor microenvironment. Clarifying these mechanisms could help determine whether B7-H3 should be targeted in therapy and might improve efforts to identify patients at high risk of rapid progression.
3.4. Kaplan–Meier Results
As shown in Figure 5, the Kaplan–Meier survival curves showed a clear difference between patients with high and low tumor B7-H3 expression. Those with high B7-H3 levels had worse outcomes. At five years, about 40% of these patients remained alive, compared with nearly 62% in the low-expression group. This difference is statistically significant, as confirmed by a log-rank test . The disease-specific survival probability showed a similar pattern. At five years, the survival rate was 64% for the high-expression group, while it was 86% for the low-expression group. This suggests that high B7-H3 levels are strongly linked to prostate cancer–related mortality. The survival gap began early. At one year, the survival rate was 80% in the high group versus 92% in the low group. At three years, these rates dropped to about 55% and 78%, respectively. The risk table confirms that fewer high-expression patients remained at risk over time. These findings suggest that B7-H3 expression is a strong predictor of poor survival probabilities and may help guide clinical decisions. The Weibull parametric model supports this trend. Figure 8 shows that the survival curve for the high B7-H3 group declined faster than that for the low group. This indicates a higher risk of death over time. The model estimated two parameters: scale and shape. The shape parameter suggests that the hazard increased over time.
3.5. Subgroup Analysis by Age and Treatment
To explore whether the prognostic impact of B7-H3 expression is influenced by a patient’s age, we partitioned the cohort into those younger than 70 years and those aged 70 or older. In the under 70 group, the survival curves for high versus low B7-H3 expressors diverged sharply, indicating that elevated B7-H3 levels confer a particularly steep survival disadvantage in younger patients. Conversely, among the older subgroup, the difference between high and low B7-H3 expressors was less pronounced, suggesting that age-related changes in tumor biology or host immunity may temper the biomarker’s effect. We next examined the interplay between B7-H3 status and receiving chemotherapy by comparing the survival distributions within each treatment arm. Notably, the patients with high B7-H3 levels who underwent systemic chemotherapy exhibited a smaller relative drop in survival probability compared with the untreated high expressors, implying that cytotoxic therapy may partially offset the adverse influence of B7-H3. In contrast, the low expressors derived minimal additional benefit from chemotherapy, underscoring a potential interaction between the biomarker level and treatment response. These observations highlight that both chronological age and therapeutic intervention can modulate the relationship between B7-H3 and outcomes, and they emphasize the need to integrate biomarker status with clinical characteristics when tailoring individual treatment plans.
Our results show a clear link between high B7-H3 levels and higher median PSA values. This pattern suggests that tumors with elevated B7-H3 expression may reflect a heavier disease burden. The PSA shift points to a possible connection between B7-H3 and tumor volume or activity, which needs further study. Survival analysis using both Kaplan–Meier curves and the Weibull model confirmed that the patients with high B7-H3 levels had worse outcomes [16]. These individuals experienced shorter overall and disease-specific survival times. The agreement across different methods shows that B7-H3 is a strong and reliable predictor of prognosis. The Weibull model also revealed that the survival probability differences were greatest during the first two years after diagnosis, highlighting a time-dependent risk pattern.
Subgroup analysis revealed that the negative effect of high B7-H3 expression was stronger in certain patients. Younger individuals and those who received chemotherapy showed the largest differences in survival probability. This may indicate that B7-H3 interacts with immune function or treatment effects. These findings support the use of B7-H3 as a useful marker for clinical decision making. It could help identify patients for B7-H3-targeted therapies, including antibody-drug conjugates or CAR-T-cell treatments. Patients with high B7-H3 levels may also need closer monitoring, while those with low levels might follow less intensive care. From a research perspective, understanding how B7-H3 promotes cancer growth and immune evasion could lead to new combination treatments. Studies should test whether blocking B7-H3 improves responses to current therapies. While our results are promising, they must be confirmed in prospective studies. Future trials should also track changes in B7-H3 levels to see if they predict treatment response or resistance, which could help personalize care even further.
4. Discussion
Our study shows that high B7-H3 expression in diagnostic biopsy samples is strongly linked to poor clinical outcomes in metastatic prostate cancer. Patients with high levels of membranous B7-H3 had shorter overall and cancer-specific survival times. These differences were consistent across Kaplan–Meier and Weibull models. This supports the role of B7-H3 not only as a prognostic marker but also as an indicator of aggressive tumor behavior.
These findings match earlier studies in localized prostate cancer and other cancers. For example, Amori et al. [15] first described B7-H3 as a poor prognostic sign in prostate biopsy samples. Similar links were later found in pancreatic and kidney cancers [10,17]. Our research builds on this by focusing only on patients with metastatic disease. Even in advanced cases, B7-H3 still predicts worse survival probabilities. We also found that younger patients and those receiving chemotherapy were more affected by high B7-H3 levels. This suggests that both age and treatment may influence the marker’s effect. Mechanistically, B7-H3 suppresses immune responses by blocking T-cell activity and reducing cytokine release [1,18]. It can also promote tumor growth and invasion through the PI3K/Akt and MAPK pathways [4]. These roles may explain why B7-H3 is linked to faster disease progression. Our results support the idea that B7-H3 is not just a passive marker but an active driver of tumor aggressiveness.
Clinically, these insights support the development of B7-H3-targeted therapies. Early-phase trials of B7-H3-directed antibodies and antibody-drug conjugates have shown encouraging safety profiles and preliminary efficacy in solid tumors [8,19]. Our data suggest that selecting patients based on high B7-H3 expression could enrich responses, potentially improving trial outcomes. Furthermore, combining B7-H3 blockade with established treatments such as androgen deprivation or chemotherapy may overcome resistance mechanisms and yield synergistic benefits.
The strengths of this study include the use of treatment-naïve biopsy specimens, which captured the native tumor phenotype without therapy-induced alterations, and the rigorous scoring by two independent pathologists. We also employed multiple imputation to handle missing clinical data and conducted extensive model diagnostics to validate our survival analyses. By integrating nonparametric and parametric techniques, we provided both descriptive and predictive perspectives on how B7-H3 influences patient trajectories. Nevertheless, certain limitations warrant consideration. Our retrospective, single-center design may limit generalizability, and the subgroup sample sizes, particularly among younger patients and specific treatment subsets, were modest. Although multiple imputation mitigates bias from missing values, prospective studies with standardized data collection will be needed to confirm our findings. Additionally, while we identified strong statistical associations, direct functional studies are required to delineate the precise molecular mechanisms by which B7-H3 promotes metastasis and treatment resistance.
The role of B7-H3 in tumor progression extends beyond its function as a classical immune checkpoint molecule. Recent preclinical and translational studies have demonstrated that B7-H3 contributes to immune evasion by suppressing T-cell activation and cytokine release [20]. Beyond immune modulation, B7-H3 directly promotes tumor aggressiveness through activation of intracellular signaling cascades, including the PI3K/Akt/mTOR and MAPK pathways, which are well-established regulators of cancer cell proliferation, survival, and epithelial-to-mesenchymal transition [21]. In vivo models further suggest that B7-H3 enhances angiogenesis via upregulation of VEGF expression [22] and contributes to a hypoxic [20,23], immunosuppressive tumor microenvironment [24]. In prostate cancer, transcriptomic profiling has revealed co-expression of B7-H3 with androgen receptor targets [25] and neuroendocrine differentiation markers, both of which are associated with treatment resistance and poor prognosis [26]. These findings underscore the biological plausibility of B7-H3 as more than a passive biomarker, suggesting it may play an active role in driving disease progression. Our results align with these mechanistic insights and reinforce the rationale for B7-H3-directed therapies as a means to disrupt both immune and non-immune pathways in advanced prostate cancer.
Although our multivariable analyses accounted for several clinically relevant variables, it is important to acknowledge the possibility of residual confounding due to factors not captured in our dataset. Specifically, information on patient comorbidities such as hypertension, diabetes, or cardiovascular disease was unavailable, as were metrics related to functional status, medication use, or prior hospitalizations. These variables may have prognostic significance and could theoretically influence both B7-H3 expression and survival rates, either through systemic inflammation, immune modulation, or tumor–host interactions. Additionally, lifestyle-related characteristics including tobacco use, alcohol consumption, diet, physical activity, and body mass index were not assessed, despite their potential to affect both tumor biology and overall patient outcomes. The retrospective design and reliance on archived clinical material limited the ability to include these variables. Consequently, while our findings demonstrate a significant association between B7-H3 expression and survival outcomes, we cannot entirely exclude the possibility that unmeasured confounders may have influenced the observed relationships. Future prospective studies incorporating a broader range of clinical, behavioral, and molecular data will be necessary to validate these findings and fully characterize the independent prognostic role of B7-H3 in prostate cancer.
Looking ahead, large multicenter cohorts should validate optimal cutoffs for B7-H3 positivity and assess its integration with emerging genomic classifiers. Preclinical investigations exploring combination regimen-pairing B7-H3 inhibitors with immune checkpoint blockade or targeted agents could identify strategies to amplify antitumor responses. Finally, elucidating the interplay between B7-H3 expression and other features of the tumor microenvironment, such as regulatory T cells or myeloid-derived suppressor cells, may reveal additional therapeutic opportunities. Our study shows that B7-H3 is a key determinant of prognosis in metastatic prostate cancer and highlights its promise as a therapeutic target. By incorporating B7-H3 assessment into clinical decision making from initial biopsy evaluation to trial enrollment, we can advance toward more personalized, biomarker-driven care. More research is needed to transform the findings into better outcomes in prostate cancer.
5. Conclusions
This study establishes tumor B7-H3 expression in diagnostic biopsy specimens as a potent and independent prognostic biomarker in metastatic prostate cancer. Through rigorous analysis of a well-characterized, treatment-naïve cohort, we found that high membranous B7-H3 expression is strongly associated with reduced overall and cancer-specific survival, even after adjusting for age, PSA level, Gleason grade, and metastatic distribution. The strength of these findings, validated across both Kaplan–Meier and Weibull survival models, suggests that B7-H3 plays an active role in driving aggressive tumor biology rather than serving as a passive correlate.
The androgen receptor (AR) signaling axis remains central to prostate cancer biology, particularly in the transition from androgen-dependent to castration-resistant states. Given our study’s focus on advanced, treatment-naïve prostate cancer, evaluating B7-H3 expression in the context of AR status represents an important and biologically relevant extension. While AR expression data were not available for the current cohort, emerging evidence suggests that B7-H3 may be co-regulated with AR-driven transcriptional programs [27]. Studies using integrative genomics and immunohistochemistry have reported positive correlations between B7-H3 and AR signaling components, including increased B7-H3 expression in high AR activity tumors [5,28]. Moreover, B7-H3 has been implicated in promoting resistance to androgen deprivation therapy by sustaining oncogenic signaling through PI3K/Akt and mTOR pathways, even in low-androgen environments [21]. These findings raise the possibility that B7-H3 may serve as a downstream effector or compensatory mechanism in AR-driven tumor progression. Future investigations incorporating AR status and transcriptional activity could offer deeper insight into the mechanistic role of B7-H3 and help clarify its prognostic and therapeutic relevance across diverse molecular subtypes of prostate cancer.
We used detailed immunohistochemical scoring, verified by independent pathologists, alongside statistical methods that addressed missing data and tested key model assumptions. Subgroup analysis showed that the poor survival linked to high B7-H3 expression was especially strong in younger patients and in those who received chemotherapy. This suggests that both age and treatment type may affect how B7-H3 impacts prognosis. These findings point to B7-H3 as a useful tool in personalizing prostate cancer care. It could help guide decisions on prognosis and treatment choices. In addition to predicting outcomes, B7-H3 also has potential as a treatment target. Early clinical trials of B7-H3 therapies such as monoclonal antibodies, antibody-drug conjugates, and CAR-T cells have shown positive results. Our findings support the use of B7-H3 testing when designing trials to identify patients who may benefit most from these new treatments. As biomarker-based and immune therapies expand, B7-H3 could play a key role. It may serve not only as a marker of risk but also a foundation for new treatment strategies in metastatic prostate cancer.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sets were obtained from institutional records from January 2005 through December 2020.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Prasad, D.V.; Nguyen, T.; Li, Z.; Yang, Y.; Duong, J.; Wang, Y. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004, 173, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.R.; Purvis, I.J.; Labak, C.M.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017, 6, 66–75. [Google Scholar]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular pathways: Targeting B7-H3 (CD276) for human cancer immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef]
- Benzon, B.; Zhao, S.G.; Haffner, M.C.; Takhar, M.; Erho, N.; Yousefi, K. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: An expression-based analysis. Prostate Cancer Prostatic Dis. 2017, 20, 28–35. [Google Scholar] [CrossRef]
- Liu, Y.; Vlatkovic, L.; Saeter, T.; Servoll, E.; Waaler, G.; Nesland, J.M. Is the clinical malignant phenotype of prostate cancer a result of a highly proliferative immune-evasive B7-H3-expressing cell population? Int. J. Urol. 2012, 19, 749–756. [Google Scholar] [CrossRef]
- Kakkat, S.; Pramanik, P.; Singh, S.; Singh, A.P.; Sarkar, C.; Chakroborty, D. Cardiovascular complications in patients with prostate cancer: Potential molecular connections. Int. J. Mol. Sci. 2023, 24, 6984. [Google Scholar] [CrossRef]
- Roth, T.J.; Sheinin, Y.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Inman, B.A. B7-H3 ligand expression by prostate cancer: A novel marker of prognosis and potential target for therapy. Cancer Res. 2007, 67, 7893–7900. [Google Scholar] [CrossRef]
- Khan, M.A.; Acharya, S.; Anand, S.; Sameeta, F.; Pramanik, P.; Keel, C.; Singh, S.; Carter, J.E.; Dasgupta, S.; Singh, A.P. MYB exhibits racially disparate expression, clinicopathologic association, and predictive potential for biochemical recurrence in prostate cancer. Iscience 2023, 26, 108487. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Takazawa, Y.; Inoue, Y.; Yokouchi, Y.; Kobayashi, M.; Saiura, A. Tumor B7-H3 (CD276) expression and survival in pancreatic cancer. J. Clin. Med. 2018, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef]
- Hertweck, K.L.; Vikramdeo, K.S.; Galeas, J.N.; Marbut, S.M.; Pramanik, P.; Yunus, F.; Singh, S.; Singh, A.P.; Dasgupta, S. Clinicopathological significance of unraveling mitochondrial pathway alterations in non-small-cell lung cancer. FASEB J. 2023, 37, e23018. [Google Scholar] [CrossRef]
- Dasgupta, S.; Acharya, S.; Khan, M.A.; Pramanik, P.; Marbut, S.M.; Yunus, F.; Galeas, J.N.; Singh, S.; Singh, A.P.; Dasgupta, S. Frequent loss of cacna1c, a calcium voltage-gated channel subunit is associated with lung adenocarcinoma progression and poor prognosis. Cancer Res. 2023, 83, 3318. [Google Scholar] [CrossRef]
- Vikramdeo, K.S.; Anand, S.; Sudan, S.K.; Pramanik, P.; Singh, S.; Godwin, A.K.; Singh, A.P.; Dasgupta, S. Profiling mitochondrial DNA mutations in tumors and circulating extracellular vesicles of triple-negative breast cancer patients for potential biomarker development. FASEB BioAdv. 2023, 5, 412–426. [Google Scholar] [CrossRef]
- Amori, G.; Sugawara, E.; Shigematsu, Y.; Akiya, M.; Kunieda, J.; Yuasa, T.; Yamamoto, S.; Yonese, J.; Takeuchi, K.; Inamura, K. Tumor B7-H3 expression in diagnostic biopsy specimens and survival in patients with metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 767–774. [Google Scholar] [CrossRef]
- Vikramdeo, K.; Anand, S.; Sudan, S.; Pramanik, P.; Singh, S.; Godwin, A.; Singh, A.; Dasgupta, S. Abstract po3-16-05: Mitochondrial dna mutation detection in tumors and circulating extracellular vesicles of triple negative breast cancer patients for biomarker development. Cancer Res. 2024, 84, PO3-16-05. [Google Scholar] [CrossRef]
- Inamura, K.; Amori, G.; Yuasa, T.; Yamamoto, S.; Yonese, J.; Ishikawa, Y. Relationship of B7-H3 expression in tumor cells and tumor vasculature with FOXP3+ regulatory T cells in renal cell carcinoma. Cancer Manag. Res. 2019, 11, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Seaman, S.; Zhu, Z.; Saha, S.; Zhang, X.M.; Yang, M.Y.; Hilton, M.B.; Morris, K.; Szot, C.; Morris, H.; Swing, D.A.; et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell 2017, 31, 501–515.e8. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. B7-H3 immunoregulatory roles in cancer. Biomed. Pharmacother. 2023, 163, 114890. [Google Scholar] [CrossRef]
- Kang, F.B.; Wang, L.; Jia, H.C.; Li, D.; Li, H.J.; Zhang, Y.G.; Sun, D.X. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015, 15, 45. [Google Scholar] [CrossRef]
- Fan, X.; Huang, J.; Hu, B.; Zhou, J.; Chen, L. Tumor-expressed B7-H3 promotes vasculogenic mimicry formation rather than angiogenesis in non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 8729–8741. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, J.; Jia, W.; Li, L.; Li, Y.; Hu, J.; Luo, W.; Li, R.; Ye, D.; Lan, P. Histone lactylation-driven B7-H3 expression promotes tumor immune evasion. Theranostics 2025, 15, 2338. [Google Scholar] [CrossRef]
- Mielcarska, S.; Dawidowicz, M.; Kula, A.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Ochman, B.; et al. B7H3 role in reshaping immunosuppressive landscape in MSI and MSS colorectal cancer tumours. Cancers 2023, 15, 3136. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Xavier, C.E.; Emaldi, M.; Guldvik, I.J.; Ramberg, H.; Taskén, K.A.; Mælandsmo, G.M.; Fodstad, Ø.; Llarena, R.; Pulido, R.; López, J.I. Correlation of expression of Major Vault Protein with androgen receptor and immune checkpoint protein B7-H3, and with poor prognosis in prostate cancer. Pathol.-Res. Pract. 2023, 241, 154243. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Zhang, C.; Chen, W.; Fu, Y.; Yu, Y.; Chen, Y.; Shao, T.; Zhang, J.; Ding, G. Tumor Immunotherapy Targeting B7-H3: From Mechanisms to Clinical Applications. ImmunoTargets Ther. 2025, 14, 291–320. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.D.; Likasitwatanakul, P.; Toye, E.; Hwang, J.H.; Antonarakis, E.S. Current uses and resistance mechanisms of enzalutamide in prostate cancer treatment. Expert Rev. Anticancer Ther. 2024, 24, 1085–1100. [Google Scholar] [CrossRef]
- Mendes, A.A.; Lu, J.; Kaur, H.B.; Zheng, S.L.; Xu, J.; Hicks, J.; Weiner, A.B.; Schaeffer, E.M.; Ross, A.E.; Balk, S.P.; et al. Association of B7-H3 expression with racial ancestry, immune cell density, and androgen receptor activation in prostate cancer. Cancer 2022, 128, 2269–2280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).