Prostate Cancer and Dietary Sugar Intake: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. The Protocol and Registration
2.2. The Search Strategy
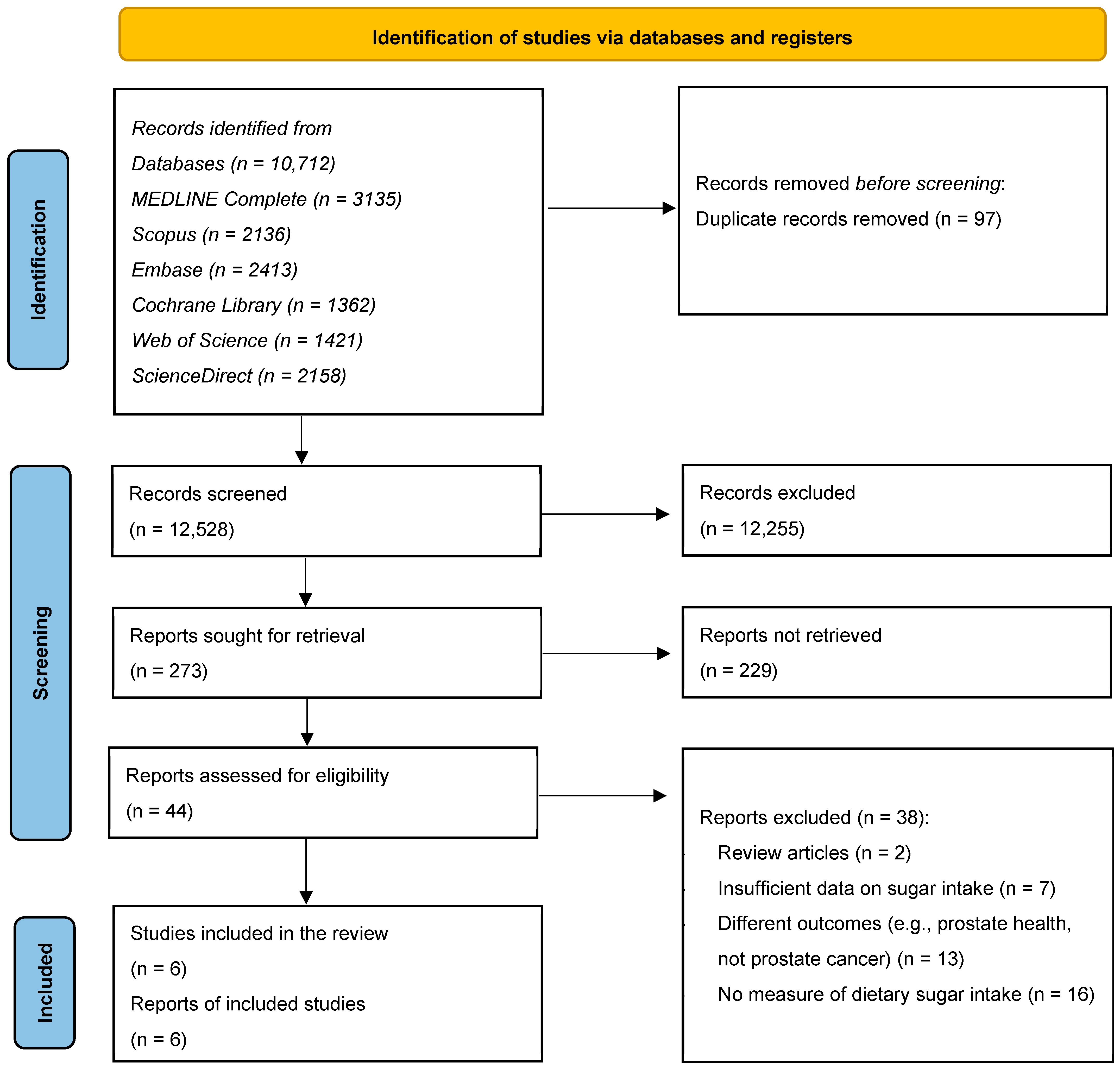
2.3. Selection of the Studies
2.4. The Inclusion and Exclusion Criteria
- (i)
- Included adult men (≥18 years);
- (ii)
- Measured sugar intake quantitatively (e.g., total sugars, added sugars, sugar-sweetened beverages);
- (iii)
- Reported prostate cancer risk, incidence, or mortality as a primary or secondary outcome;
- (iv)
- Reported sugar intake as an independent exposure or measured it in combination with other nutrients without disaggregation;
- (v)
- Were observational studies (cohort, case–control, or cross-sectional);
- (vi)
- Were published in English in peer-reviewed journals.
- (i)
- Did not report the effect estimates or necessary data (e.g., odds ratios, hazard ratios, relative risks);
- (ii)
- Focused on animal or in vitro models;
- (iii)
- Were editorials, reviews, conference abstracts, or theses;
- (iv)
- Included participants with diagnosed cancer at the baseline (except in mortality outcome studies).
2.5. The Data Extraction
2.6. The Study Exposure
2.7. The Study Outcomes
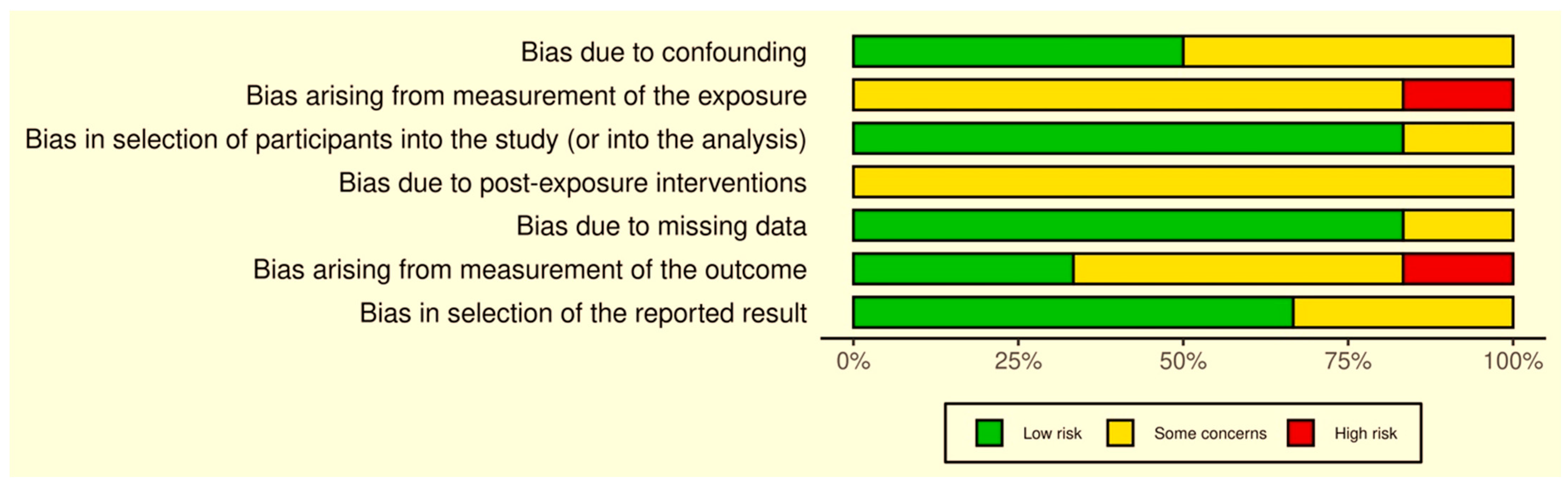
2.8. Quality Evaluation
3. Results
3.1. The Characteristics of the Included Studies
3.2. The Studies’ Exposure and Outcome Assessment Methods
3.3. Confounding Factors
3.4. The Study Findings
3.5. Quality Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Kensler, K.H.; Rebbeck, T.R. Cancer Progress and Priorities: Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer Cases Expected to Double Worldwide Between 2020 and 2040, New Analysis Suggests. Available online: https://www.mrcctu.ucl.ac.uk/news/news-stories/2024/april/prostate-cancer-cases-expected-to-double-worldwide-between-2020-and-2040-new-analysis-suggests/ (accessed on 13 May 2025).
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Khaled, K. The Role of Healthy Dietary Patterns in Managing Chronic Low-Grade Inflammation—A Literature Review. Am. J. Biomed. Res. 2024, 24, 636–641. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, Y.; Wu, L.; Li, C.; Ni, S.; Liu, Q.; Ni, X.; Sun, Q. Mg-HA-C/c Composites Promote Osteogenic Differentiation and Repair Bone Defects through Inhibiting MiR-16. Front. Bioeng. Biotechnol. 2022, 10, 838842. [Google Scholar] [CrossRef]
- Bowers, L.W.; Rossi, E.L.; O’Flanagan, C.H.; deGraffenried, L.A.; Hursting, S.D. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front. Endocrinol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Della Corte, K.; Perrar, I.; Penczynski, K.; Schwingshackl, L.; Herder, C.; Buyken, A. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef]
- Santos-Pereira, M.; Pereira, S.C.; Rebelo, I.; Spadella, M.A.; Oliveira, P.F.; Alves, M.G. Decoding the Influence of Obesity on Prostate Cancer and Its Transgenerational Impact. Nutrients 2023, 15, 4858. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, C.; Yu, F.; Yuan, D.; Wang, W.; Jiao, K.; Yang, S.; Zhang, Y.; Wang, Y.; Liu, L. Association of Total Dietary Intake of Sugars with Prostate-Specific Antigen (PSA) Concentrations: Evidence from the National Health and Nutrition Examination Survey (NHANES), 2003–2010. BioMed Res. Int. 2021, 2021, 4140767. [Google Scholar] [CrossRef]
- Miles, F.L.; Neuhouser, M.L.; Zhang, Z.F. Concentrated Sugars and Incidence of Prostate Cancer in a Prospective Cohort. Br. J. Nutr. 2018, 120, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Khaled, K.; Almilaji, O. Prostate Cancer and Dietary Sugar Intake: A Systematic Review and Meta-Analysis. 2025. Available online: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251039770 (accessed on 15 May 2025).
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A Tool to Assess Risk of Bias in Non-Randomised Follow-up Studies of Exposure Effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Makarem, N.; Bandera, E.V.; Lin, Y.; Jacques, P.F.; Hayes, R.B.; Parekh, N. Consumption of Sugars, Sugary Foods, and Sugary Beverages in Relation to Adiposity-Related Cancer Risk in the Framingham Offspring Cohort (1991–2013). Cancer Prev. Res. 2018, 11, 347–358. [Google Scholar] [CrossRef]
- Trudeau, K.; Rousseau, M.-C.; Barul, C.; Csizmadi, I.; Parent, M.-É. Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada. Nutrients 2020, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Sonestedt, E.; Gullberg, B.; Ahlgren, G.; Bjartell, A.; Wallström, P.; Wirfält, E. Dietary Intakes of Carbohydrates in Relation to Prostate Cancer Risk: A Prospective Study in the Malmö Diet and Cancer Cohort. Am. J. Clin. Nutr. 2012, 96, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Chazelas, E.; Srour, B.; Desmetz, E.; Kesse-Guyot, E.; Julia, C.; Deschamps, V.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Sugary Drink Consumption and Risk of Cancer: Results from NutriNet-Santé Prospective Cohort. BMJ 2019, 366, l2408. [Google Scholar] [CrossRef]
- Hasan, N.; Yazdanpanah, O.; Khaleghi, B.; Benjamin, D.J.; Kalebasty, A.R. The Role of Dietary Sugars in Cancer Risk: A Comprehensive Review of Current Evidence. Cancer Treat. Res. Commun. 2024, 43, 100876. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef]
- Zhai, L.; Cheng, S.; Zhang, D. Dietary Carbohydrate and Prostate Cancer Risk: A Meta-Analysis. Nutr. Cancer 2015, 67, 594–602. [Google Scholar] [CrossRef]
- Shikany, J.M.; Flood, A.P.; Kitahara, C.M.; Hsing, A.W.; Meyer, T.E.; Willcox, B.J.; Redden, D.T.; Ziegler, R.G. Dietary Carbohydrate, Glycemic Index, Glycemic Load, and Risk of Prostate Cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) Cohort. Cancer Causes Control 2011, 22, 995–1002. [Google Scholar] [CrossRef]
- Kanehara, R.; Katagiri, R.; Goto, A.; Yamaji, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Tsugane, S. Sugar Intake and Colorectal Cancer Risk: A Prospective Japanese Cohort Study. Cancer Sci. 2023, 114, 2584–2595. [Google Scholar] [CrossRef]
- Chan, J.M.; Wang, F.; Holly, E.A. Sweets, Sweetened Beverages, and Risk of Pancreatic Cancer in a Large Population-Based Case–Control Study. Cancer Causes Control 2009, 20, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Weber, A.L.; Walker, A.H.; Stefflova, K.; Tran, T.V.; Spangler, E.; Chang, B.-L.; Zeigler-Johnson, C.M. Context-Dependent Effects of Genome-Wide Association Study Genotypes and Macroenvironment on Time to Biochemical (Prostate Specific Antigen) Failure after Prostatectomy. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2115–2123. [Google Scholar] [CrossRef]
- Ni Raghallaigh, H.; Eeles, R. Genetic Predisposition to Prostate Cancer: An Update. Fam. Cancer 2021, 21, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L. The Etiology of Prostate Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK571322/ (accessed on 5 May 2025).
- Arroyo-Quiroz, C.; Brunauer, R.; Alavez, S. Sugar-Sweetened Beverages and Cancer Risk: A Narrative Review. Nutr. Cancer 2022, 74, 3077–3095. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive Intake of Sugar: An Accomplice of Inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef]
- Rippe, J.; Angelopoulos, T. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef]
- Khaled, K.; Hundley, V.; Almilaji, O.; Koeppen, M.; Tsofliou, F. A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK. Nutrients 2020, 12, 2921. [Google Scholar] [CrossRef]
- Khaled, K.; Tsofliou, F.; Hundley, V.A. A Structural Equation Modelling Approach to Examine the Mediating Effect of Stress on Diet in Culturally Diverse Women of Childbearing Age. Nutrients 2024, 16, 3354. [Google Scholar] [CrossRef]
| PEO | Keywords with Boolean Operators and Truncation |
|---|---|
| Population (Men) | M#n OR male OR males OR “adult m#n” OR “human males” OR “middle-aged m#n” OR “elderly m#n” |
| Exposure (Sugar) | sugar* OR “dietary sugar*” OR “added sugar*” OR “free sugar*” OR sucrose OR fructose OR glucose OR “sugar-sweetened beverage*” OR “soft drink*” OR soda OR “sugary drink*” OR “sweetened food*” OR “high-sugar diet*” OR “sugar-rich diet*” |
| Outcome (Prostate Cancer) | “prostate cancer” OR “prostatic cancer” OR “prostate neoplasm*” OR “prostatic neoplasm*” OR “prostate carcinoma” OR “prostate malignanc*” OR “prostate tumor*” OR “prostate adenocarcinoma” |
| Combined Search | (men OR male OR males) AND (sugar* OR “added sugar*” OR “sugar-sweetened beverage*” OR soda) AND (“prostate cancer” OR “prostate neoplasm*” OR “prostate tumor*”) |
| Author, Year | Country | Study Design | Age Range or Mean Age | Number of Participants | Participants in Study |
|---|---|---|---|---|---|
| Liu et al., 2021 [11] | USA | CS | Mean age: 58.1 (± 13.6) | 6403 | Men aged >40 years from the NHANES study with no history of malignancy. |
| Trudeau et al., 2020 [17] | Canada | CC | Mean age: Cases: 64 years Control: 65 years | Cases (n = 1919) Controls (n = 1991) | Cases enrolled from 7–9 French language hospitals in Montreal. Controls enrolled randomly from Quebec’s permanent electoral list of French electors |
| Chazelas et al., 2019 [19] | France | LG (9 years follow-up) | Mean age: At baseline: 46.9 At cancer diagnosis: 58.5 | 291 | Adults from the French NutriNet-Sante Cohort not previously diagnosed with any type of cancer. |
| Makarem et al., 2018 [16] | USA | LG (22 years follow-up) | 26–84 (55.4) | 157 | Adults from the Framingham Offspring Cohort. |
| Miles et al., 2018 [12] | USA | LG (9 years follow-up) | 55–74 years | 1996 | Men from the general population from the PLCO Cancer Screening Trial |
| Drake et al., 2012 [18] | Sweden | LG (15 years follow-up) | 45–73 (58.5) | 817 | Cases of prostate cancer diagnosed between 1992 and 2009 from the Malmo Diet and Cancer Cohort Study |
| Author, Year | Type of Sugar Measured | Sugar Intake Assessment Tool | Prostate Cancer Risk Assessment Methods | Confounding Factors |
|---|---|---|---|---|
| Liu et al., 2021 [11] | Total dietary sugar intake | USDA AMPM | Hybritech PSA method | Age, SES, BMI, smoking, and history of diabetes, hypertension, and coronary heart disease/stroke. |
| Trudeau et al., 2020 [17] | Western Sweet and Beverage intake | 63-item FFQ |
| Age, ethnicity, education, family history of prostate cancer, timing of last screening. |
| Chazelas et al., 2019 [19] | Sugary drinks | Three 24-dietary records | Medical records and ICD-10 | Age, sex, energy intake, smoking, family history of cancer and diabetes, BMI, physical activity. |
| Makarem et al., 2018 [16] |
| Semi-quantitative 126-item Harvard FFQ | Medical and pathology records | Age, sex, smoking, alcohol, energy intake, BMI, waist circumference, chronic diseases (CVD and diabetes) history, physical activity, antioxidant use. |
| Miles et al., 2018 [12] | Concentrated sugars (sugar sweetened beverages, desserts, and fruit juices) | DHQ FFQ |
| Study center, age, race, education, smoking, BMI, history of diabetes and prostate cancer, number PSA screens over the previous three years, energy intake (kcal/day), red and processed meat (g/day), fruit (servings/day), and vegetables (servings/day). |
| Drake et al., 2012 [18] |
|
|
| Age, BMI, waist circumference, alcohol intake, selenium intake, calcium intake, smoking, educational level, physical activity, diabetes diagnosis, history of cardiovascular event, born in Sweden, past food habit change, and energy intake. |
| Author, Year | Association Between Sugar Intake and Prostatic Cancer | Association Direction | |||
|---|---|---|---|---|---|
| β [95% CI] (p-Value) in Fully Adjusted Multivariable Weighted Linear Regression | Hazard Ratio [CI] (p-Value) | OR [CI] (p-Value) in Adjusted Logistic Regression | |||
| Cross-sectional study | |||||
| Liu et al., 2021 [11] | For each additional 1 g of sugar intake, the PSA concentrations were increased by 0.003 ng/mL [0.001–0.005] (0.0029) (log2-transformed) | ↑ | |||
| Case–control study | |||||
| Trudeau et al., 2020 [17] | 1.35 [1.10–1.66] (0.002) ‡ | ↑ | |||
| Longitudinal studies | |||||
| Chazelas et al., 2019 [19] | 1.1 [0.92–1.31] (0.31) * | ⇌ | |||
| Makarem et al., 2018 [16] | Sugary foods ^ | 1.00 [0.62–1.62] (p > 0.05) | ⇌ | ||
| Sugary drinks ^ | 1.36 [0.88–2.09] (p > 0.05) | ⇌ | |||
| Miles et al., 2018 [12] | Sugar-sweetened beverages ^ | 1.21 [1.06–1.39] (p < 0.01) | ↑ | ||
| Fruit juices ^ | 1.07 [0.94–1.22] (p > 0.05) | ⇌ | |||
| Desserts ^ | 0.95 [0.83–1.10] (p > 0.05) | ⇌ | |||
| Drake et al., 2012 [18] | Monosaccharides | 1.18 [0.92–1.52] (0.59) | ⇌ | ||
| Sucrose | 0.9 [0.71–1.15] (0.83) | ⇌ | |||
| Sugar-sweetened beverages | 1.13 [0.92–1.38] (0.22) | ⇌ | |||
| Cakes and biscuits | 1.21 [0.94–1.56] (0.23) | ⇌ | |||
| Sweets and sugar | 0.93 [0.73–1.19] (0.63) | ⇌ | |||
| Fruit juices | 0.99 [0.81–1.22] (0.62) | ⇌ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaled, K.; Jardaly, H.; Almilaji, O. Prostate Cancer and Dietary Sugar Intake: A Systematic Review. Onco 2025, 5, 31. https://doi.org/10.3390/onco5030031
Khaled K, Jardaly H, Almilaji O. Prostate Cancer and Dietary Sugar Intake: A Systematic Review. Onco. 2025; 5(3):31. https://doi.org/10.3390/onco5030031
Chicago/Turabian StyleKhaled, Karim, Hala Jardaly, and Orouba Almilaji. 2025. "Prostate Cancer and Dietary Sugar Intake: A Systematic Review" Onco 5, no. 3: 31. https://doi.org/10.3390/onco5030031
APA StyleKhaled, K., Jardaly, H., & Almilaji, O. (2025). Prostate Cancer and Dietary Sugar Intake: A Systematic Review. Onco, 5(3), 31. https://doi.org/10.3390/onco5030031






