Racial Diversity in the Decline in Hepatocellular Carcinoma and Increasing Age at Diagnosis in a Primarily African American Medical Center Population
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Overview
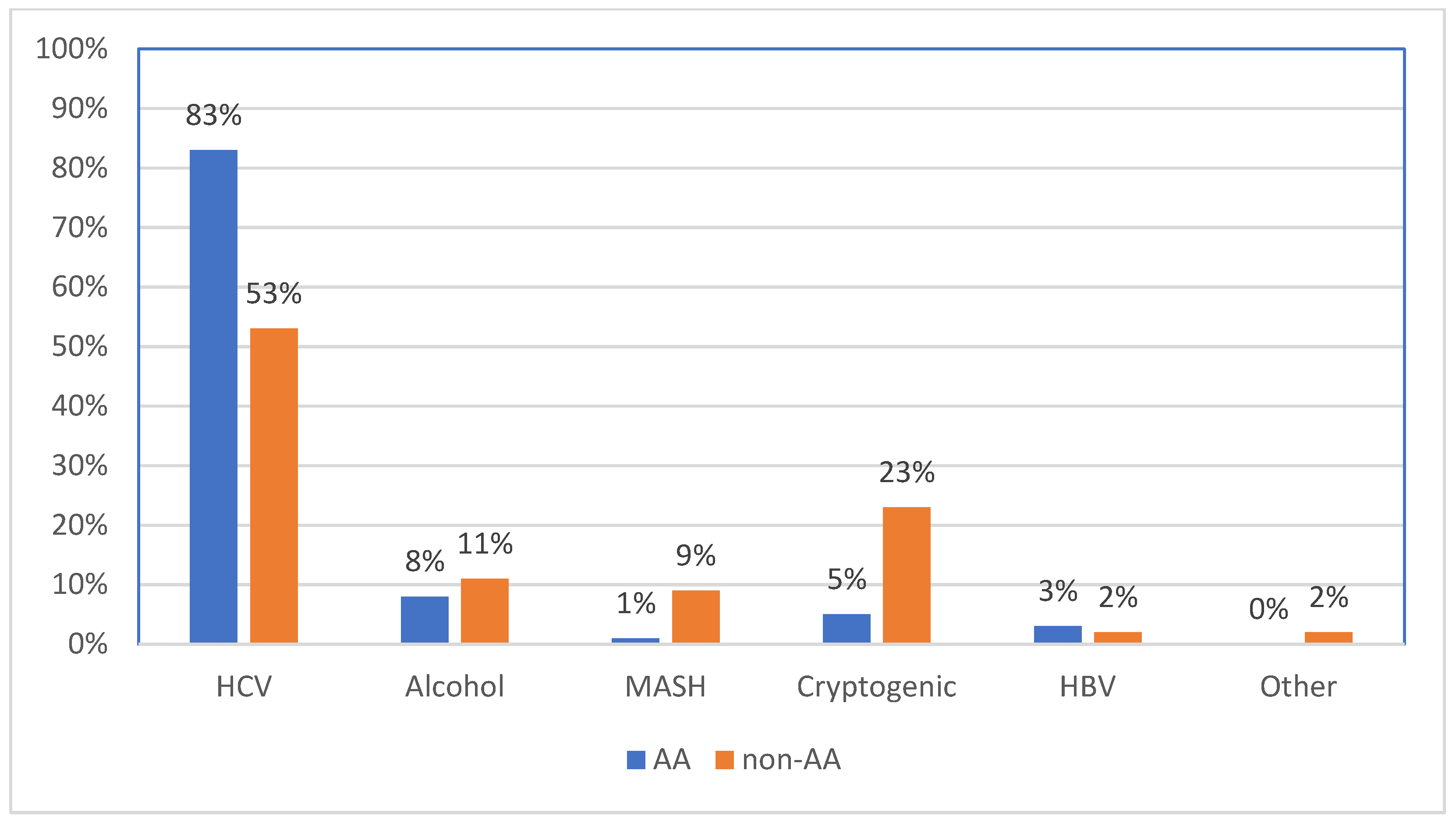
3.2. Risk Factor Distribution
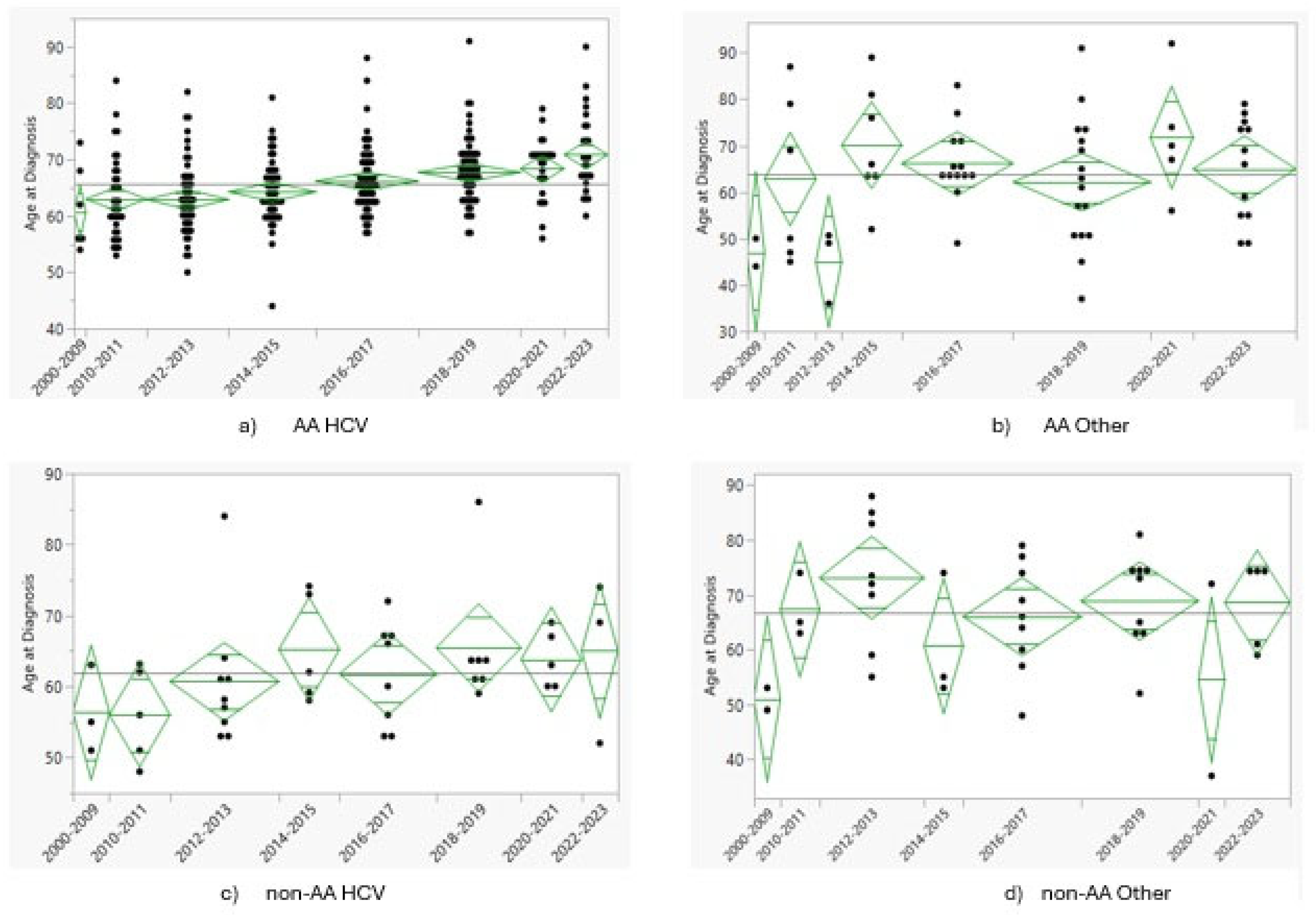
3.3. Temporal Trends
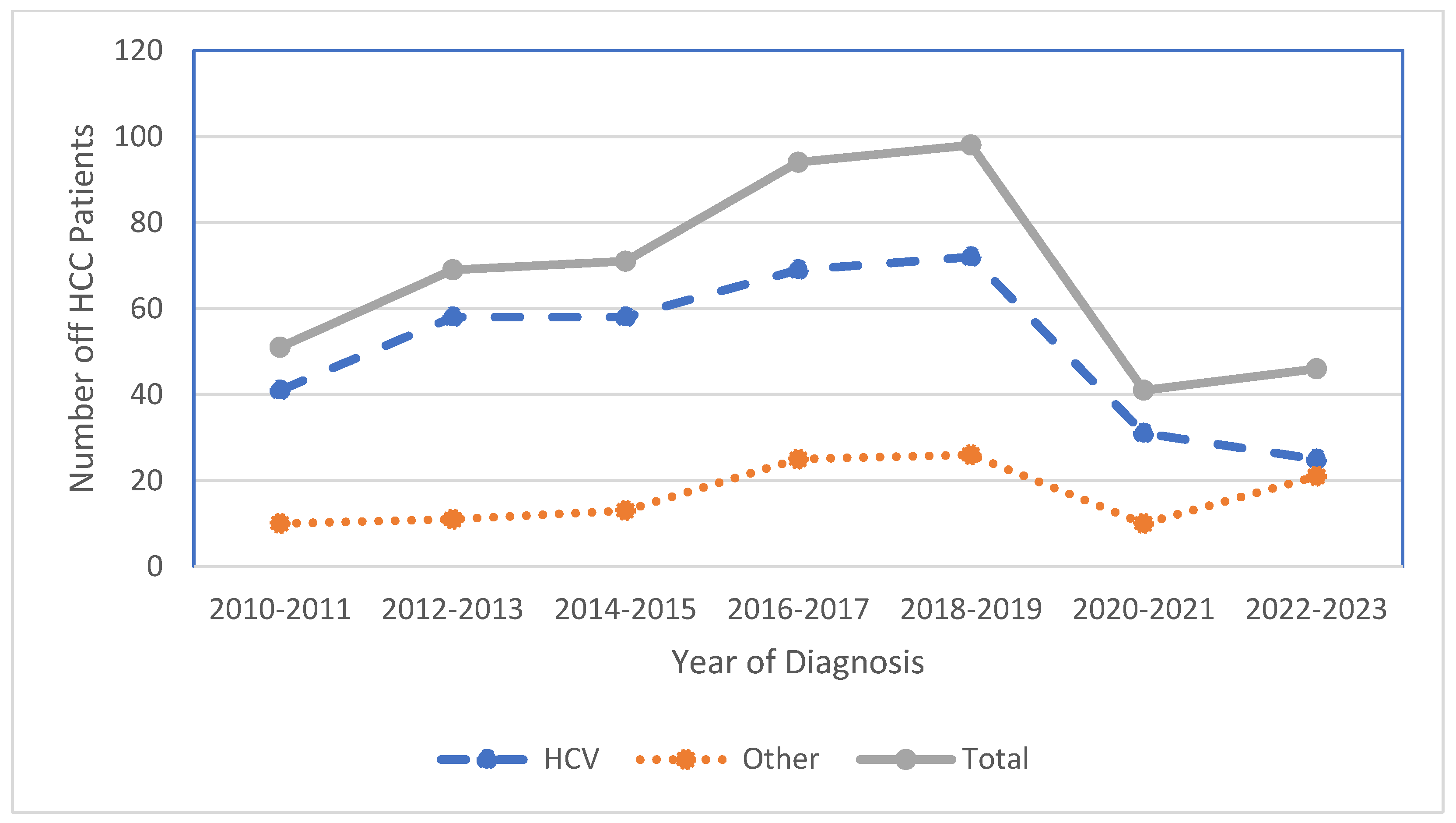
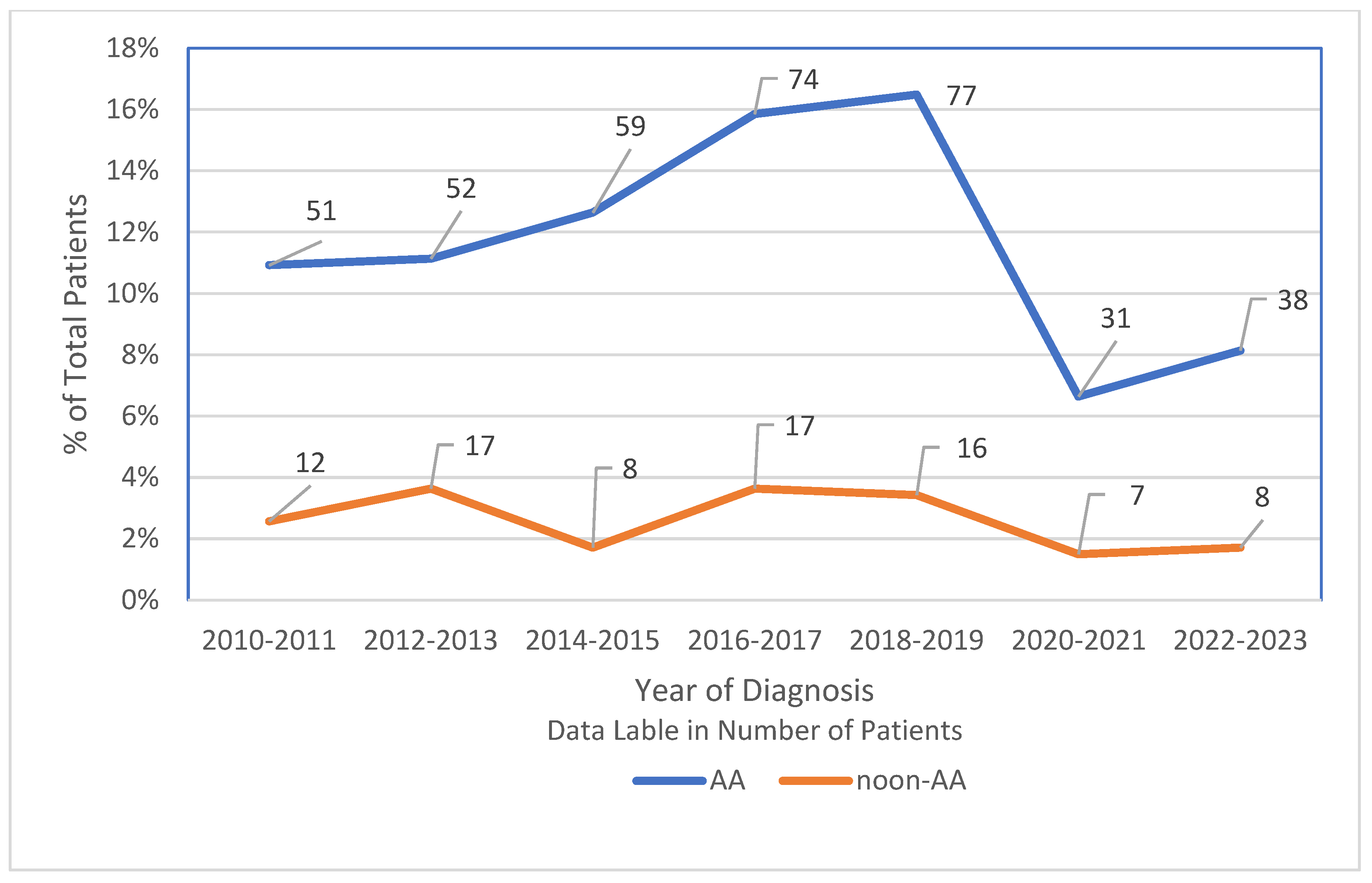
3.4. Shifts in HCC Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, S.; Cai, J.; Yang, Z.; He, X.; Xing, Z.; Zu, J.; Xie, E.; Henry, L.; Chong, C.R.; John, E.M.; et al. Trends in hepatocellular carcinoma mortality rates in the US and projections through 2040. JAMA Netw. Open 2024, 7, e2445525. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 18, 2650–2666. [Google Scholar] [CrossRef]
- Bisceglie, A.M.; Lyra, A.C.; Schwartz, M.; Reddy, R.K.; Martin, P.; Gores, G.; Lok, A.S.F.; Hussain, K.B.; Gish, R.; Thiel, D.H.; et al. Hepatitis C-related hepatocellular carcinoma in the United States: Influence of ethnic status. Am. J. Gastroenterol. 2003, 98, 2060–2063. [Google Scholar] [CrossRef]
- Ajayi, F.; Jan, J.; Singal, A.G.; Rich, N.E. Racial and sex disparities in hepatocellular carcinoma in the USA. Curr. Hepatol. Rep. 2020, 19, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.R.; Karande, G.; Gudur, A.; Garud, A.; Patil, M.S.; Patil, S. Recent trends in liver cancer: Epidemiology, risk factors, and diagnostic techniques. Cureus 2024, 16, e72239. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E. Changing epidemiology of hepatocellular carcinoma within the United States and worldwide. Surg. Oncol. Clin. N. Am. 2023, 33, 1–12. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Ganesan, P.; Kulik, L.M. Hepatocellular carcinoma. Clin. Liver Dis. 2022, 27, 85–102. [Google Scholar] [CrossRef]
- Alawyia, B.; Constantinou, C. Hepatocellular Carcinoma: A Narrative Review on Current Knowledge and Future Prospects. Curr. Treat. Options Oncol. 2023, 24, 711–724. [Google Scholar] [CrossRef]
- Jiang, J.; Shiels, M.S.; Rivera, D.; Ghany, M.G.; Engels, E.A.; O’Brien, T.R. Trends in hepatocellular carcinoma and viral hepatitis treatment in older Americans. PLoS ONE 2024, 19, e0307746. [Google Scholar] [CrossRef]
- Ji, F.; Yeo, Y.H.; Wei, M.T.; Ogawa, E.; Enomoto, M.; Lee, D.H.; Iio, E.; Lubel, J.; Wang, W.; Wei, B.; et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 473–485. [Google Scholar] [CrossRef]
- Dang, H.; Yeo, Y.H.; Yasuda, S.; Huang, C.; Iio, E.; Landis, C.; Jun, D.W.; Enomoto, M.; Ogawa, E.; Tsai, P.; et al. Cure with Interferon-Free Direct-Acting antiviral is associated with increased survival in patients with hepatitis C Virus-Related hepatocellular carcinoma from both East and west. Hepatology 2019, 71, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Itani, M.I.; Farah, B.; Wasvary, M.; Wadehra, A.; Wilson, T.; Rutledge, B.; Naylor, P.; Beal, E.W.; Mutchnick, M. Impact of DAA treatment for HCV on hepatocellular carcinoma in a predominately African American population. J. Gastrointest. Cancer 2024, 55, 1324–1332. [Google Scholar] [CrossRef]
- Samant, H.; Kohli, K.; Patel, K.; Shi, R.; Jordan, P.; Morris, J.; Schwartz, A.; Alexander, J.S. Clinical Presentation of Hepatocellular Carcinoma in African Americans vs. Caucasians: A Retrospective Analysis. Pathophysiology 2021, 28, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Estevez, J.; Yang, J.D.; Leong, J.; Nguyen, P.; Giama, N.H.; Zhang, N.; Ali, H.A.; Lee, M.-H.; Cheung, R.; Roberts, L.; et al. Clinical Features Associated with Survival Outcome in African-American Patients with Hepatocellular Carcinoma. Am. J. Gastroenterol. 2018, 114, 80–88. [Google Scholar] [CrossRef]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.D.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology 2019, 157, 1253–1263.e2. [Google Scholar] [CrossRef]
- Leal, C.; Strogoff-De-Matos, J.; Theodoro, C.; Teixeira, R.; Perez, R.; Guaraná, T.; De Tarso Pinto, P.; Guimarães, T.; Artimos, S. Incidence and Risk Factors of Hepatocellular Carcinoma in Patients with Chronic Hepatitis C Treated with Direct-Acting Antivirals. Viruses 2023, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Awad, M.H.; Gal-Tanamy, M.; Yu, M.L. Unmet needs in the post-direct-acting antivirals era: The risk and molecular mechanisms of hepatocellular carcinoma after hepatitis C virus eradication. Clin Mol Hepatol. 2024, 30, 326. [Google Scholar] [CrossRef]
- Fiehn, F.; Beisel, C.; Binder, M. Hepatitis C virus and hepatocellular carcinoma: Carcinogenesis in the era of direct-acting antivirals. Curr Opin Virol. 2024, 67, 10142. [Google Scholar] [CrossRef]
- Lockart, I.; Yeo, M.G.H.; Hajarizadeh, B.; Dore, G.J.; Danta, M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatology 2022, 76, 139–154. [Google Scholar] [CrossRef]
- Kew, M.C. Hepatocellular carcinoma with and without cirrhosis. Gastroenterology 1989, 97, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Vutien, P.; Kim, N.J.; Moon, A.M.; Johnson, K.M.; Berry, K.; Green, P.K.; Ioannou, G.N. Hepatocellular carcinoma risk decreases as time accrues following hepatitis C virus eradication. Aliment Pharmacol Ther. 2023, 59, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Mezzacappa, C.; Kim, N.J.; Vutien, P.; Kaplan, D.E.; Ioannou, G.N.; Taddei, T.H. Screening for Hepatocellular Carcinoma and Survival in Patients With Cirrhosis After Hepatitis C Virus Cure. JAMA Netw Open 2024, 7, e2420963. [Google Scholar] [CrossRef] [PubMed]
- Leyh, C.; Coombes, J.D.; Schmidt, H.H.; Canbay, A.; Manka, P.P.; Best, J. MASLD-Related HCC—Update on Pathogenesis and current treatment options. J. Pers. Med. 2024, 14, 370. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Jones, P.D.; Medina, H.; Cranford, H.M.; Koru-Sengul, T.; Bungum, T.; Wong, R.; Kobetz, E.N.; McGlynn, K.A. Incidence of etiology-specific hepatocellular carcinoma: Diverging trends and significant heterogeneity by race and ethnicity. Clin. Gastroenterol. Hepatol. 2023, 22, 562–571.e8. [Google Scholar] [CrossRef]
- Lekakis, V.; Papatheodoridis, G.V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2023, 122, 3–10. [Google Scholar] [CrossRef]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.H.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022, 34, 969–977.e2. [Google Scholar] [CrossRef]
- Zeng, R.W.; Ong, C.E.Y.; Ong, E.Y.H.; Chung, C.H.; Lim, W.H.; Xiao, J.; Danpanichkul, P.; Law, J.H.; Syn, N.; Chee, D.; et al. Global prevalence, clinical characteristics, surveillance, treatment allocation, and outcomes of Alcohol-Associated hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2024, 22, 2394–2402.e15. [Google Scholar] [CrossRef]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2022, 20, 37–49. [Google Scholar] [CrossRef]
- Costentin, C.E.; Minoves, M.; Kotzki, S.; Farges, O.; Goutté, N.; Decaens, T.; Bailly, S. Alcohol-related hepatocellular carcinoma is a heterogenous condition: Lessons from a latent class analysis. Liver Int. 2022, 42, 1638–1647. [Google Scholar] [CrossRef]
- Reggidori, N.; Bucci, L.; Santi, V.; Stefanini, B.; Lani, L.; Rampoldi, D.; Ghittoni, G.; Farinati, F.; Masotto, A.; Stefanini, B.; et al. Landscape of alcohol-related hepatocellular carcinoma in the last 15 years highlights the need to expand surveillance programs. JHEP Rep. 2023, 5, 100784. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef] [PubMed]
| Race | Number | % of Total | Age (Years) | Gender (M) | Etiology (%HCV) | HCV (%Not Treated) | Surveillance | Tumor Size (>3 cm) |
|---|---|---|---|---|---|---|---|---|
| AA | 282 | 49 | 65 | 72% | 81% | 85% | 11% | 85% |
| Non-AA | 86 | 18 | 64 | 71% | 52% | 84% | 22% | 78% |
| Unknown | 16 | 3 | 67 | 72% | 56% | 78% | 13% | 67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudagh, G.; Alnasart, A.; Abou Chaer, K.; Naylor, P.; Mutchnick, M. Racial Diversity in the Decline in Hepatocellular Carcinoma and Increasing Age at Diagnosis in a Primarily African American Medical Center Population. Onco 2025, 5, 30. https://doi.org/10.3390/onco5030030
Boudagh G, Alnasart A, Abou Chaer K, Naylor P, Mutchnick M. Racial Diversity in the Decline in Hepatocellular Carcinoma and Increasing Age at Diagnosis in a Primarily African American Medical Center Population. Onco. 2025; 5(3):30. https://doi.org/10.3390/onco5030030
Chicago/Turabian StyleBoudagh, Gabriel, Ahmad Alnasart, Kenan Abou Chaer, Paul Naylor, and Milton Mutchnick. 2025. "Racial Diversity in the Decline in Hepatocellular Carcinoma and Increasing Age at Diagnosis in a Primarily African American Medical Center Population" Onco 5, no. 3: 30. https://doi.org/10.3390/onco5030030
APA StyleBoudagh, G., Alnasart, A., Abou Chaer, K., Naylor, P., & Mutchnick, M. (2025). Racial Diversity in the Decline in Hepatocellular Carcinoma and Increasing Age at Diagnosis in a Primarily African American Medical Center Population. Onco, 5(3), 30. https://doi.org/10.3390/onco5030030





