Simple Summary
The combination of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is associated with significant survival benefits in select patients with peritoneal carcinomatosis. The Canadian HIPEC Collaborative Group recommends that centres complete a minimum of one procedure monthly to maintain competency and achieve good outcomes. This treatment is not currently offered in Newfoundland, Canada; thus, the aim of our study was to demonstrate that the annual patient volume justifies the feasibility of implementing a combined surgical and gynecological oncology CRS/HIPEC program in our province. A retrospective chart review confirmed a sufficient patient volume with an annual total of 31 patients eligible to receive this therapy.
Abstract
Peritoneal carcinomatosis is a common presentation found in advanced-stage gastrointestinal (GI) and gynecological cancers. Combined cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is associated with significant survival benefits for select patients. CRS/HIPEC is not currently provided in Newfoundland and Labrador (NL). The Canadian HIPEC Collaborative Group recommends that centres complete a minimum of one case monthly to maintain competency and achieve good outcomes. Thus, we aimed to demonstrate that the annual patient volume in NL justifies the feasibility of implementing a combined surgical and gynecological oncology CRS/HIPEC program. Methods: A retrospective chart review of the NL Cancer Care Registry identified patients with stage IV colorectal, appendiceal, or gastric cancer and stage III to IV epithelial ovarian cancer over a 1-year period (1 January 2020–31 December 2020) to identify the number of patients meeting the criteria for CRS/HIPEC and/or those referred out of province to receive the treatment. The results are presented as proportions and percentages. Results: Thirty-one patients were eligible to receive CRS/HIPEC during the study period (11 GI, 20 gynecological). Of the GI patients, 63% were referred out of province for the procedure. Gynecological patients underwent CRS and systemic therapy +/− outpatient intraperitoneal chemotherapy in NL. Conclusions: Allowing patients to receive this standard of care treatment near home reduces financial, social, and emotional stressors. Our results confirm a sufficient patient volume to support a combined CRS/HIPEC program in NL. The implementation of this program will require multidisciplinary collaboration, specialized training, equipment, and protocol development.
1. Introduction
Peritoneal carcinomatosis (PC) results from cancers arising primarily from the lining of the peritoneal cavity and those that have metastasized from primary sites within the abdomen. Although primary peritoneal malignancies (including mesothelioma and serous carcinoma of the peritoneum) are rare, secondary isolated spread from gastrointestinal (GI) and gynecological primaries is relatively common and results from advanced stages of colorectal, appendiceal, gastric, and ovarian cancers [1].
The presence of PC is a poor prognostic factor associated with a significant reduction in long-term survival [2,3]. Tumour histology and disease burden impact median overall survival (OS) ranging from months for gastric cancer to 5 years for epithelial ovarian cancer (EOC) [1,4]. Traditionally, patients with PC were considered to have advanced metastatic disease and were primarily treated with palliative intent, focusing on balancing survival and quality of life. However, early studies showed promising results with the introduction of a combination therapy utilizing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), leading to a paradigm shift in the management of this population [5,6,7]. Several randomized control trials and retrospective studies have since confirmed that suitable patients can achieve long-term survival outcomes with the addition of this therapy [8,9,10,11,12,13].
1.1. Colorectal Cancer
Approximately 10% of patients with colorectal cancer (CRC) present with PC at the time of diagnosis, resulting in a median survival of 15 months despite systemic therapy [2,3,11,14,15]. Multiple randomized controlled trials and retrospective studies have demonstrated the superiority of CRS and HIPEC followed by systemic chemotherapy in the oncologic outcomes of CRC when compared with systemic therapy alone [2,8,9,11,16]. Despite the recent study results questioning the benefit of adding oxaliplatin-based HIPEC to CRS and systemic therapy, the updated Canadian guidelines recommend that all patients be referred to a surgical oncologist, as a cure and extended survival remain possible [2,17].
Although there is no defined international consensus on the Peritoneal Carcinomatosis Index cut-off, the most widely accepted threshold in well or moderately differentiated disease is 20 or less, with a lower threshold of fewer than 10 for patients with poorly differentiated or signet ring pathology. Evidence supports that a lower PCI score is associated with better survival while a PCI score greater than 20 is linked to a very poor prognosis [11,17].
The objective for surgical management should be complete cytoreduction, which is achieved in approximately 80% of cases [18]. CRS should not be attempted in patients for which complete cytoreduction is impossible, as no survival benefit is associated [3]. Other generally accepted contraindications for this therapy include the presence of extra-abdominal metastases and greater than three liver metastases [19].
1.2. Gastric Cancer
Despite diagnostic advances and screening efforts, most patients with gastric cancer are diagnosed at an advanced stage and face a very poor prognosis [20]. Although no universal standard of care treatment has been established, perioperative chemotherapy and resection with extended lymphadenectomy is the preferred strategy in Canadian centres. Despite this, gastric cancer is found to have the highest rate of peritoneal disease of all digestive cancers [21]. Up to 40% of patients present with PC at the time of diagnosis [22], with an additional 5–30% confirmed during surgery [23,24]. Furthermore, 40–60% of patients develop peritoneal recurrence post-resection [21]. The peritoneal spread of gastric adenocarcinoma is associated with survival rates ranging from 2.2 to 8.8 months [23,24,25].
The role of CRS and HIPEC has been explored for patients with positive peritoneal washings alone, as well as those with macroscopic peritoneal disease without evidence of distant metastasis. Although less significant compared to other GI primaries, treatment with CRS/HIPEC has been demonstrated by multiple large studies to improve overall and recurrence-free survival in select patients [10,26,27,28]. The presenting PCI score and completeness of CRS are independent prognostic indicators. Several studies confirm that survival remains poor when the PCI is greater than 12, even with complete cytoreduction [28,29]. Although evidence supporting the use of CRS/HIPEC in the management of gastric cancer with PC is encouraging, it remains controversial and experimental. The National Comprehensive Cancer Network (NCCN) guidelines note that “HIPEC may provide a therapeutic alternative for carefully selected stage IV patients in the setting of ongoing clinical trials and is under further clinical investigation” [30]. While select patients may achieve long-term survival, the high mortality rate of this diagnosis underlines the importance of reserving patient selection to experienced institutions to ensure therapeutic benefit. These centres recommend reserving this therapy for active patients under 60 years of age, with an appropriate PCI score and small lesions; without ascites, hepatic involvement, or extraperitoneal metastases; and who have shown a positive response to neoadjuvant chemotherapy [31].
1.3. Pseudomyxoma Peritonei and Appendiceal Mucinous Neoplasms
Pseudomyxoma peritonei (PMP) is a rare peritoneal malignancy most commonly arising from mucinous neoplasms of the appendix. Appendiceal mucinous neoplasms (AMN) constitute a heterogeneous group of rare tumours accounting for less than 1% of cancers that exhibit a wide range of pathological features and biological behaviour. Among peritoneal surface malignancies, PMP of appendiceal origin generally carries the most favourable prognosis. CRS with HIPEC is established as the standard of care treatment, as it has demonstrated benefits in prolonging disease-free and overall survival [11,32,33,34].
Similar to CRC, the PCI score and completeness of cytoreduction provide prognostic information and are predictive of survival [11]. However, unlike the PC of other GI origins, high PCI scores and inability to achieve complete cytoreduction are not considered contraindications for HIPEC in this group, as patients experience favourable outcomes regardless of these thresholds [3,10,35].
Despite these positive outcomes, careful consideration of the benefit-to-risk ratio is essential. Absolute contraindications suggested by the Peritoneal Surface Oncology Group International (PSOGI) guidelines include extensive small bowel serosal involvement and mesenteric involvement causing retraction. Relative contraindications include age over 75 years, aggressive histology with PCI score > 20, involvement of the liver hilum, infiltration of the anterior pancreatic surface, ureteric obstruction, and the need for complete gastric resection [34].
1.4. Epithelial Ovarian Cancer
Of all EOC cases, approximately 75% of patients present with late-stage disease and PC, resulting in significantly reduced 5-year survival [4]. Standard treatment for advanced-stage EOC involves primary CRS followed by six cycles of IV platinum-based chemotherapy [12,36,37]. Alternatively, neoadjuvant chemotherapy can precede interval CRS followed by adjuvant chemotherapy [38]. Despite these strategies, recurrence rates remain high (70–80%).
In 2006, a phase III trial demonstrated that cold intraperitoneal (IP) chemotherapy given every three weeks via an intraperitoneal port after CRS significantly improved survival by over 1 year [39]. Building on this, HIPEC has been evaluated as an adjunct to CRS, with modern evidence supporting its use in the primary treatment setting. It is associated with improved OS, disease-free survival, and progression-free survival when implemented following neoadjuvant chemotherapy if a complete or optimal interval cytoreduction (<1 cm) is achieved [12,13,40]. A recent systematic review and meta-analysis also demonstrated its safety, confirming a similar risk for grade ≥ 3 adverse events when comparing CRS with and without HIPEC [40]. Based on these results, national agencies support CRS/HIPEC in the management of stage III and IV disease with widespread peritoneal carcinomatosis with the goal of optimal debulking (residual disease < 1 cm) [41]. Canadian guidelines recommend the consideration of HIPEC after neoadjuvant chemotherapy at the time of CRS if complete or optimal cytoreduction is achieved [1]. Similarly, the PSOGI 2022 consensus on HIPEC for EOC concluded that HIPEC at the time of interval CRS should be considered routinely [42].
Additionally, trials have explored the impact of HIPEC in recurrent EOC. In 2022, a systemic review and meta-analysis of the available literature concluded that its use in the recurrent setting did not provide a survival advantage and should continue to be considered investigational [40]. More recently, the MITO-18 (HORSE) and CHIPOR trials evaluated the efficacy of adding HIPEC to CRS in platinum-sensitive recurrent ovarian cancer; however, these studies differed in design and outcomes [43,44]. A critical difference was the timing of HIPEC; MITO-18 evaluated HIPEC before second-line chemotherapy, whereas CHIPOR evaluated HIPEC in patients who had undergone neoadjuvant chemotherapy, confirming platinum sensitivity. This distinction mirrors the findings in the first-line setting. Another key difference was patient population; MITO-18 included a higher proportion of BRCA-mutated (BRCAm) patients (43.5%) compared to CHIPOR (25%), who are known to derive less benefit from HIPEC as systemic therapies alone are often more effective in this group [45]. These differences highlight how patient selection and treatment sequencing can influence outcomes. Importantly, only CHIPOR considered OS as a primary endpoint and reported a significant improvement with the addition of HIPEC, showing a median OS of 54.3 months compared to 45.8 months in the non-HIPEC group.
With appropriate patient selection, highly trained teams, and appropriate resources, CRS/HIPEC represents a feasible treatment option and is encouraged for this population [46].
1.5. Centralization and Multidisciplinary Care
Due to the high-risk nature of CRS with HIPEC and the specialized skills, resources, and infrastructure required, this treatment is centralized within Canada. Providers and national guidelines consider CRS and HIPEC to be the domain of high-volume tertiary centres with experienced multidisciplinary teams. In 2015, the Canadian HIPEC Collaborative Group (CHICG) provided guidelines for standardizing and improving the treatment of peritoneal surface malignancies in Canada [47]. They recommend that centres perform at least one case monthly to maintain competency and achieve good outcomes. The PSOGI mirrored this recommendation, suggesting that new programs handle at least one case monthly, aiming for 20 patients annually [47]. Currently, CRS/HIPEC is offered at fewer than 15 Canadian tertiary care centres, some of which treat only GI or gynecological populations. Additionally, the QEII Health Sciences Centre in Nova Scotia is the only established program among the Atlantic provinces. Since this standard of care treatment is not offered in Newfoundland and Labrador (NL), select patients with GI primaries are referred out of province for consideration. Patients under gynecologic oncology care in NL undergo CRS with IV +/− IP chemotherapy as HIPEC is not available to them. Currently, there is no established out-of-province referral pathway for this population.
This prompted consideration for a collaborative model between surgical and gynecological oncologists with expertise in CRS/HIPEC for the management of peritoneal surface malignancies in NL. The objective of this feasibility study is to determine the combined population size of gastrointestinal and gynecological patients meeting the criteria for CRS/HIPEC in NL. We hypothesize that an appropriate annual patient volume exists to fulfill the recommendations outlined by the CHCG and PSOGI to support the implementation of the CRS/HIPEC program in our province.
2. Materials and Methods
A retrospective chart review of the NL Cancer Care Registry was conducted over a one-year period from 1 January 2020 to 31 December 2020. The review identified GI and gynecological cancer patients meeting the criteria for CRS/HIPEC and those referred out of province for this treatment. Approval was obtained by our local ethics board (HREB# 2021.137).
2.1. Gastrointestinal Population Retrospective Data Collection
Patients diagnosed with stage IV colorectal, gastric, and appendiceal cancers who developed carcinomatosis during the study period were reviewed. Patients with unresectable nodal disease, solid organ, and/or extraperitoneal metastases were excluded. Data collected included age at diagnosis, primary tumour diagnosis, histological subtype, treatment (neoadjuvant and/or adjuvant chemotherapy), geographical site of treatment, and out-of-province referral/treatment status. Eligibility for CRS/HIPEC was determined by a surgical oncologist considering PCI score, response to neo/adjuvant therapy, age, comorbidities, and functional status.
2.2. Gynecological Population Retrospective Data Collection
Patients diagnosed with stage III or IV EOC during the study period were reviewed. Disease burden, PCI score, and eligibility for CRS/HIPEC were assessed by a gynecological oncologist based on operating room records of patients treated with CRS and neoadjuvant or adjuvant chemotherapy. Exclusions included stage I/II disease, sub-optimal debulking, secondary debulking for recurrent disease, or lack of systemic chemotherapy. Data collected included patient age, histological subtype and mutation status, tumour stage, timing and extent of surgical debulking, whether a bowel resection was required, use and method of neoadjuvant and/or adjuvant chemotherapy, and the geographical site of treatment.
3. Results
3.1. Gastrointestinal Population
The Cancer Care Registry populated a list of 630 patients with a GI malignancy, of which 95 were excluded due to lack of cancer diagnosis (n = 4), other cancer diagnoses (n = 57), duplicate records (n = 28), or no records available (n = 6). There were 535 patients who met the inclusion criteria: 84.85% with colorectal cancer, 13.83% with gastric cancer, and 1.31% with appendiceal cancer. A total of 48 patients (8.9%) were diagnosed with carcinomatosis during the study period but nearly half (n = 21, 43%) of these patients developed synchronous or metachronous solid organ and/or extra-peritoneal metastatic disease and were excluded. The remaining 27 patient charts were analyzed, 11 of which were deemed eligible for CRS/HIPEC referral after reviewing surgical candidacy and the overall clinical picture in a multidisciplinary tumour board (Figure 1).
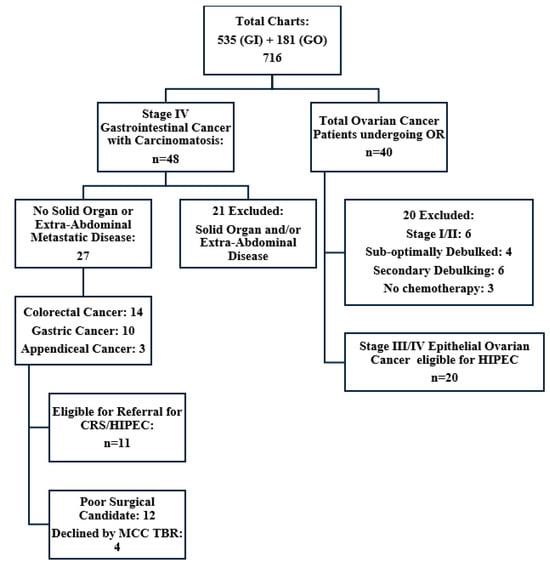
Figure 1.
Summary of retrospective chart review. GI = Gastrointestinal, GO = Gynecological Oncology, CRS/HIPEC = Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, MCC TBR = Multidisciplinary Tumour Board Rounds, OR = Operation.
The eligible population of 11 patients included seven (63.6%) colorectal cancers, one (9.1%) gastric adenocarcinoma, and three (27.3%) appendiceal cancers with a median patient age of 61 years (range 44–76). Seven patients had previously undergone a resection of their primary lesion. Three received neoadjuvant chemotherapy prior to surgery and five received adjuvant chemotherapy, with most patients (n = 7) receiving treatment at a tertiary cancer centre (Table 1 and Table 2). Seven patients were appropriately offered referral for CRS/HIPEC; however, an additional four patients identified as candidates during the review were not referred. Of those offered, two refused referral, with one quoting disinterest in travelling for medical care. Of the five patients who agreed to referral, four successfully underwent CRS/HIPEC, and one patient, who had previously undergone surgery for perforated low-grade AMN (LAMN), opted for surveillance (Figure 2).

Table 1.
Gastrointestinal population demographics.

Table 2.
Gastrointestinal population treatment characteristics.
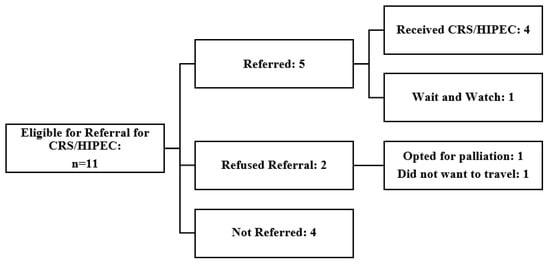
Figure 2.
Summary of outcomes for GI malignancy patients eligible for CRS and HIPEC. CRS/HIPEC = Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
3.2. Gynecological Population
The Gynecologic Oncology Department operating room records identified a total of 145 gynecologic operations and the Cancer Care Registry populated a list of an additional 36 ovarian cancer patients. This resulted in a total of 181 charts, of which 141 were excluded as those patients did not undergo surgery for a malignant ovarian neoplasm during the study period. Of the 40 patients undergoing full chart review, 15 were excluded. Four were deemed not eligible at the time of surgery due to disease burden, six due to early-stage diagnosis (stage I/II), six due to undergoing secondary debulking surgery, and three due to not receiving systemic chemotherapy as part of their treatment. We identified 20 patients diagnosed with stage III or IV primary EOC who received cytoreductive surgery with systemic therapy in NL. These patients would have been candidates to receive CRS/HIPEC at that time had it been available to them (Figure 1).
Most patients (70%) underwent interval debulking, with 30% undergoing primary debulking surgery. Optimal debulking was obtained in 45% of the cases, with complete debulking achieved for 55% of patients. The median age of the eligible patient population was 67.5 years (range 48–84). Pathology confirmed high-grade serous carcinoma in 80% of cases, with most patients found to have stage IIIC disease. Fifteen percent of patients were found to be carriers of the BRCA mutation, which is in concordance with the group studied by Classe et al. A total of 70% of patients received neoadjuvant chemotherapy, and 100% received chemotherapy in the adjuvant setting. The majority received intravenous chemotherapy, with 20% receiving IP/IV chemotherapy at our main tertiary cancer centre. Over half of these patients received their chemotherapy treatments at peripheral sites (Table 3 and Table 4).

Table 3.
Gynecological population demographics.

Table 4.
Gynecological population treatment characteristics.
3.3. Combined Population
A combined total of 31 GI and gynecological patients were deemed eligible to receive CRS/HIPEC over the one-year study period in NL.
4. Discussion
4.1. Centralization and Its Benefits
Centralization aims to steer patients to hospitals and providers meeting specific criteria to enhance the quality of care and outcomes, reduce procedure time, concentrate resources, and reduce costs. This strategy fosters the development of more skilled surgeons and teams, as well as improved systems of care. The well-established relationship between surgical volume and outcomes for complex surgical procedures supports this approach [48,49,50].
Given the relatively small and high-risk population, the specialized surgical skill set required, and the challenges in delivering HIPEC safely and cost-effectively, this treatment is best managed in dedicated centres by experienced multidisciplinary teams. Canadian guidelines recommend referring patients to an established HIPEC centre recognized and supported by academic institutions, provincial agencies, and national organizations to further education, research, and funding. These teams should be composed of surgical oncologists and/or gynecologic oncologists, anesthesiologists, perfusionists, pharmacists, nurses, supportive care professionals, residents/fellows, and pathologists. They require access to and training in intraperitoneal perfusion equipment [47].
4.2. Population Estimates and Treatment Needs
The Canadian Cancer Society expected 23,900 new colorectal cancer cases, with up to 10% (n = 2390) presenting with peritoneal carcinomatosis and about 30% of these (approximately 800 patients) expected to be eligible for HIPEC [51]. This is concordant with the approximately 9% of patients with PC in 2020 in NL. The incidence of pseudomyxoma peritonei in Canada represents approximately 30 to 40 new cases annually, with most patients eligible for CRS and HIPEC [47]. In 2020, an estimated 3100 Canadian women were diagnosed with ovarian cancer, with 70–75% (approximately 2200 patients) presenting with advanced-stage disease [52].
In 2015, the CHICG estimated that Canadian teams could treat about 200–250 new gastrointestinal patients annually [47]. Although this number has likely since grown and did not capture centres treating gynecological malignancy only, it suggests the need for additional sites to improve accessibility.
4.3. Challenges and Considerations for Newfoundland and Labrador
The previous perception of an insufficient population size to support a HIPEC program in NL is now challenged by evidence supporting new indications. In addition to patients with primary GI malignancy, there is now strong evidence supporting a select population with EOC. Currently, these patients are seldom sent out of province for HIPEC due to a lack of established collaborations with other gynecologic oncology centres. Instead, they are offered CRS with chemotherapy and the option of IV/IP chemotherapy if treated at the tertiary care centre. Geographic constraints in NL prevent gynecologic oncologists from managing IV/IP chemotherapy and potential catheter complications at peripheral sites. This lack of access affects patient survival and creates disparities in care.
Patients with PC secondary to GI primaries are currently referred to the nearest established program in Nova Scotia. While this interprovincial collaboration has been successful, it imposes significant logistical and financial burdens on patients. The duration of time away varies significantly due to the unpredictable nature of the post-operative course and the potential for prolonged hospital admission in the event of complications. Furthermore, patients may incur out-of-pocket costs between CAD 4600 to CAD 8500, despite compassionate care programs, to cover air travel, accommodations, and sustenance. These expenses, along with potential income loss for patients and their support persons during extended stays, can create financial hardship and affect treatment decisions, leading to inequity in care access.
Other considerations include the impact on the timely delivery of cancer therapy and post-operative continuity of care. Despite facilitated pathways and cooperation, the interprovincial time to referral and definitive treatment is extended, potentially negatively impacting the time-sensitive nature of certain pathologies. Additionally, postoperative care is fragmented, as patients are transferred back to NL after their surgery and early postoperative recovery in Nova Scotia for any required follow-up, management of delayed complications, adjuvant treatment, and ongoing surveillance, disrupting the continuity of care.
Moreover, the lack of provision of standard of care treatment within one’s own province can potentially affect patients’ perceptions of healthcare accessibility. Another consideration is the financial impact on the province of NL to compensate Nova Scotia for providing this service to its population. Future studies focusing on a cost analysis comparing the expenses of providing this service through out-of-province referrals versus the cost of implementing and supporting a new program in NL are indicated.
4.4. Impact of a Unique Population
Several studies have proposed the possible impact of a wide range of biomarkers relating to their diagnostic, prognostic, and therapeutic influence on the management of peritoneal surface malignancy. Research focusing on mutational alterations, microsatellite instability, circulating tumour DNA, and other biomarkers have demonstrated interesting results. However much of their impact remains unclear, warranting further research [51,53,54,55,56,57,58].
Notably, the population of NL represents a unique collection of genetic isolates supported by historic isolation and an associated founder effect, making it an invaluable resource for the study of genetic disorders [59]. The “founder effect” is a genetic phenomenon that occurs when a small group of individuals from a larger, ancestral population, establish a new population in a geographic location, often leading to a reduction or loss of genetic variation compared to the original population. This can explain why certain inherited diseases are found more frequently in some limited population groups [60]. The province of NL consists of the island of Newfoundland and remote mainland Labrador on the east coast of Canada, where previously approximately 60% of the population lived in small communities of fewer than 2500 people, with the remaining 40% in communities of fewer than 1000 inhabitants [61]. Its population has the highest incidence and mortality from both colorectal and gastric cancers in Canada, as well as the highest rate of familial CRC in the world [62,63]. For this reason, a NL CRC registry was established in order to investigate the factors responsible for these statistics. Green et al. (2007) observed that 3.7% of CRC cases in NL came from families meeting the Amsterdam II criteria and an additional 0.9% from those with familial adenomatous polyposis. An additional 43% of cases met one or more of the revised Bethesda criteria and 31% of all cases had a first-degree affected relative. When comparing the NL data with that of the province of Ontario and 13 other population-based studies worldwide, all indicators of familial risk were significantly higher in NL. Thus, they concluded that the high incidence of CRC in NL may be attributable to genetic or at least familial factors [64].
The most common inherited form of CRC that is highly prevalent in NL is hereditary non-polyposis CRC (HNPCC). In addition to CRC, HNPCC is associated with an increased risk of malignancy in a variety of other sites, including gastric and ovarian cancers. Mutations of four mismatch repair (MMR) genes account for greater than 95% of the known mutations resulting in HNPCC (MSH2, MLH1, MSH6, and PMS2). In fact, a large cohort of NL CRC patients was involved in the identification of the importance of the MSH2 mutation in HNPCC [64]. While several other MMR gene associations have been identified, their roles are not well established. A population-based study completed by Woods et al. demonstrated that a large number of NL families appearing to have a familial form of CRC cannot be explained by MMR mutations. Their results suggest that there are probable novel genetic causes of hereditary CRC in this distinct population that are not yet known [65].
Additionally, other familial genetic predispositions for malignancies managed with CRS/HIPEC exist at elevated rates in our population. Hereditary diffuse gastric cancer (HDGC) is a rare form of gastric adenocarcinoma linked to inactivating germline mutations of CDH1. HDGC accounts for 1–3% of all gastric cancers, with 25–30% of these families having germline mutations of the CDH1 gene, which confers a more than 80% lifetime risk of developing gastric cancer [66]. The province of NL has one of the largest known cohorts of CDH1 germline mutation carriers in the world and a projected incidence of gastric cancer higher than any other Canadian province [62,67,68]. A novel recurrent CHD1 mutation was initially found in NL families, with evidence implying a founder effect in this disease site as well [68].
It is well known that pathogenic variants in BRCA1 and 2 increase the lifetime risk of ovarian cancer from 1.3% in the general population to 11–44% [69,70]. A population study completed in NL in 2023 identified 276 BRCA mutation carriers [71]. The frequency of BRCAm observed in this cohort and their recurrence among multiple families is consistent with NL’s known ancestry and suggests evidence for multiple founder effects [59]. In 2024, the Canadian Cancer Society expected 40 cases of ovarian cancer in NL, with a mortality rate of 80%; thus, identifying and understanding the impact of these BRCA mutations on hereditary ovarian cancer in NL is critical [62].
Given that the true impact of these biomarkers on the CRS/HIPEC population remains unclear, data obtained from our population represents a wealth of future research potential. With the establishment of a combined peritoneal surface malignancy group in NL, we plan to participate in a Pan-Canadian Peritoneal Database along with other HIPEC centres to allow for national data sharing. In turn, we can assess whether outcomes differ as a result of the unique findings within our population compared to others.
4.5. Combining Expertise for Better Outcomes
The CHICG suggests that new teams handle at least one case per month, referring patients to established centres if this number is not met [46]. Although NL has a smaller gastrointestinal population requiring HIPEC, combining this with gynecological oncology cases confirms sufficient annual cases to meet this threshold. Combining these specialty-specific backgrounds and perspectives creates a synergistic skillset in pelvic and upper abdominal surgery necessary for complete CRS. This collaboration enhances surgical care, patient selection, and perioperative care, significantly benefiting patients. Evidence from other centres supports that collaborative effort between surgical and gynecological oncologists with expertise in CRS results in improved patient selection and high rates of complete cytoreduction without compromising patient safety [72].
4.6. Identified Gaps and Recommendations
This review recognized gaps in current care, notably the non-referral of eligible gastrointestinal patients from peripheral non-academic centres, indicating a need for ongoing education regarding referral indications. The need for proper documentation and management during original presentations in the periphery was also identified as an area of improvement, as this promotes more favourable future oncologic outcomes [17]. This includes taking intra-operative biopsies, calculations and documentation of PCI scores, and avoidance of unnecessary dissection in the setting of acute presentations such as bowel obstruction or perforation. Moving forward, creating accessible communication and access channels with a carcinomatosis team is crucial for facilitating discussions and dealing with questions as they arise.
4.7. Developing a HIPEC Program
Establishing a HIPEC program requires institutional support, a dedicated and appropriately trained team, adequate resources and equipment, and audit systems. Our centre has published a review encompassing evidence-based information to guide the development of a successful HIPEC program. This includes an assessment of the preoperative, anesthetic, surgical, and postoperative considerations, as well as a review of perfusion equipment requirements and training [46].
4.8. Limitations
This study used data over a one-year period to provide an estimate of the annual population size, which may not fully capture annual variability.
Notably, the data collection period captured the first year of the COVID-19 pandemic, which may have resulted in reduced case numbers. Fear of infection risk in healthcare facilities, government-enforced restrictions, and the overwhelming impacts on the healthcare system may have resulted in delayed clinical assessment, surveillance investigations, and/or pathology processing, affecting diagnosis. Consequently, it is possible that the number of cases may be underestimated.
Furthermore, our selection excluded all gastrointestinal patients with documented liver metastases. Given that many centres may offer this therapy to patients with low-volume resectable liver disease, we may have underestimated the eligible population size.
Finally, given that our institution currently does not have an established collaborative carcinomatosis program involving both Surgical Oncologists and Gynecologic Oncologists, there may be differences in data collection between the two groups.
Despite these limitations, our methodology provides a framework approach that can be applied universally to other populations of interest in the assessment of feasibility for centralized procedures.
5. Conclusions
CRS with HIPEC is a safe and effective treatment for managing PC from both GI and gynecological malignancies. Giving patients the opportunity to receive standard of care treatment closer to home is important and reduces financial, social, and emotional stressors. Collaboration between surgical and gynecological oncology has been demonstrated to benefit this patient population. Our results confirm a sufficient annual combined patient volume to support a collaborative program and maintain surgical competency in the unique population of NL; implementation will require multidisciplinary collaboration, specialized training, infrastructure, and protocol development.
Author Contributions
Conceptualization, K.H. (Kala Hickey), S.G., J.N., D.P., A.M. and P.P.; methodology, K.H. (Kala Hickey), S.G., J.N., A.M. and D.P.; formal analysis, K.H. (Kala Hickey), S.G. and J.N.; investigation, K.H. (Kala Hickey), Z.B., K.H. (Kaitlyn Harding) and H.Y.; data curation, K.H. (Kala Hickey), Z.B., K.H. (Kaitlyn Harding) and H.Y.; writing—original draft preparation, K.H. (Kala Hickey), S.G. and J.N.; writing—review and editing, K.H. (Kala Hickey), S.G. and J.N.; supervision, J.N. and D.P.; funding acquisition, D.P. and J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Approval was obtained by a local ethics board (HREB# 2021.137). This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Board of Memorial University (HREB# 2021.137).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMN | Appendiceal mucinous neoplasm |
| BRCAm | BRCA mutated |
| CHICG | Canadian HIPEC Collaborative Group |
| CRC | Colorectal Cancer |
| CRS | Cytoreductive Surgery |
| EOC | Epithelial Ovarian Cancer |
| GO | Gynecological Oncology |
| HDGC | Hereditary diffuse gastric cancer |
| HIPEC | Hyperthermic Intraperitoneal Chemotherapy |
| HNPCC | Hereditary non-polyposis colorectal cancer |
| IP | Intraperitoneal |
| IV | Intravenous |
| MMR | Mismatch repair |
| NL | Newfoundland |
| OR | Operation |
| OS | Overall survival |
| PC | Peritoneal carcinomatosis |
| PCI | Peritoneal carcinomatosis Index |
| PMP | Pseudomyxoma peritonei |
| PSOGI | Peritoneal Surface Oncology Group |
References
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery: A Clinical Practice Guideline. Curr. Oncol. 2020, 27, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Topgül, K.; Çetinkaya, M.B.; Arslan, N.Ç.; Gül, M.K.; Çan, M.; Gürsel, M.F.; Erdem, D.; Malazgirt, Z. Cytoreductive surgery (SRC) and hyperthermic intraperitoneal chemotherapy (HIPEC) for treatment of peritoneal carcinomatosis: Our initial experience and technical details. Turk. J. Surg. 2015, 31, 138–147. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, R.H.; Gianola, F.J.; Speyer, J.C.; Wesley, R.; Barofsky, I.; Meyers, C.E. Prospective, Randomized Trial of Intravenous Versus Intraperitoneal 5-Fluorouracil in Patients with Advanced Primary Colon or Rectal Cancer. Surgery 1985, 98, 414–422. Available online: https://europepmc.org/article/med/3898450 (accessed on 8 July 2024). [PubMed]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Epithelial appendiceal neoplasms. Cancer J. 2009, 15, 225–235. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Goéré, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef]
- Morris, D.L. Peritonectomy HIPEC-contemporary results, indications. Chin. J. Cancer Res. 2013, 25, 373–374. [Google Scholar] [CrossRef]
- Nassabein, R.; Younan, R.; Loungarath, R.; Mercier, F.; Dagbert, F.; Aubin, F.; Ayoub, J.P.; Tehfé, M. A Canadian single-centre experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for abdominal malignancies. Can. J. Surg. 2022, 65, E342–E351. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.; Hermans, R.H.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.Y.; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374. [Google Scholar] [CrossRef]
- Klaver, Y.L.B.; Simkens, L.H.J.; Lemmens, V.E.P.P.; Teerenstra, S.; Bleichrodt, R.P.; de Hingh, I.H.J.T.; Punt, C.J.A. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur. J. Surg. Oncol. 2012, 38, 617–623. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.B.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.B.; Lemmens, V.E.P.P.; Creemers, G.J.; Rutten, H.J.T.; Nienhuijs, S.W.; de Hingh, I.H.J.T. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Ann. Oncol. 2011, 22, 2250–2256. [Google Scholar] [CrossRef]
- Brind’amour, A.; Dubé, P.; Tremblay, J.F.; Soucisse, M.L.; Mack, L.; Bouchard-Fortier, A.; McCart, J.A.; Govindarajan, A.; Dischof, D.; Hasse, E.; et al. Canadian Guidelines on the Management of Colorectal Peritoneal Metastases. Curr. Oncol. 2020, 27, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yan, T.D.; Black, D.; Morris, D.L. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2009, 16, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Benizri, E.; Pocard, M.; Ducreux, M.; Boige, V.; Lasser, P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur. J. Surg. Oncol. 2006, 32, 632–636. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Winer, J.H. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer. Cancers 2019, 11, 1662. [Google Scholar] [CrossRef]
- Khan, H.; Johnston, F.M. Current role for cytoreduction and HIPEC for gastric cancer with peritoneal disease. J. Surg. Oncol. 2022, 125, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef]
- Saito, H.; Kihara, K.; Kuroda, H.; Matsunaga, T.; Tatebe, S.; Ikeguchi, M. Surgical outcomes for gastric cancer patients with intraperitoneal free cancer cell, but no macroscopic peritoneal metastasis. J. Surg. Oncol. 2011, 104, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Brucher, B.L.D.M.; Piso, P.; Verwaal, V.; Esquivel, J.; Derraco, M.; Yonemura, Y.; Gonzalez-Moreno, S.; Pelz, J.; Königarainer, A.; Ströhlein, M.; et al. Peritoneal carcinomatosis: Cytoreductive surgery and HIPEC—Overview and basics. Cancer Investig. 2012, 30, 209–224. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T.; Isawa, E.; Sumida, M.; Ohkubo, H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997, 79, 884–891. [Google Scholar]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 2.2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434 (accessed on 8 July 2024).
- Aziz, M.B.; Napoli, R.D. Hyperthermic Intraperitoneal Chemotherapy; Published online 31 July 2023; StatPearls: Treasure Island, FL, USA, 2023; Available online: https://www-ncbi-nlm-nih-gov.qe2a-proxy.mun.ca/books/NBK570563/ (accessed on 8 July 2024).
- Turner, K.M.; Hanna, N.N.; Zhu, Y.; Jain, A.; Kesmodel, S.B.; Switzer, R.A.; Taylor, L.M.; Alexander, H.R. Assessment of Neoadjuvant Chemotherapy on Operative Parameters and Outcome in Patients with Peritoneal Dissemination from High-Grade Appendiceal Cancer. Ann. Surg. Oncol. 2013, 20, 1068–1073. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and Long-Term Outcome Data of Patients With Pseudomyxoma Peritonei From Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.H.J.T.; Speeten, K.V.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, D.J.; Wick, M.R.; Dehner, L.P.; Mills, S.E.; Swanson, P.E.; Clarke, R.E. Peritoneal malignant mesothelioma versus serous papillary adenocarcinoma. A histochemical and immunohistochemical comparison. Am. J. Surg. Pathol. 1989, 13, 659–670. [Google Scholar] [CrossRef]
- Desai, A.; Xu, J.; Aysola, K.; Qin, Y.; Okali, C.; Hariprasad, R.; Chinemerem, U.; Gates, C.; Reddy, A.; Danner, O.; et al. Epithelial ovarian cancer: An overview. World J. Transl. Med. 2014, 3, 1. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Ovarian Cancer Continue Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 2.2024. In NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®); NCCN: Philadelphia, PA, USA, 2025. [Google Scholar]
- Bhatt, A.; Glehen, O.; Zivanovic, O.; Brennan, D.; Nadeau, C.; Van Driel, W.; Bakrin, N. The 2022 PSOGI international consensus on HIPEC regimens for peritoneal Malignancies: Epithelial ovarian cancer. Ann. Surg. Oncol. 2023, 30, 8115–8137. [Google Scholar] [CrossRef]
- Fagotti, A.; Costantini, B.; Fanfani, F.; Giannarelli, D.; De Iaco, P.; Chiantera, V.; Mandato, V.; Giorda, G.; Aletti, G.; Greggi, S.; et al. Hyperthermic Intraperitoneal Chemotherapy in Platinum-Sensitive Recurrent Ovarian Cancer: A Randomized Trial on Survival Evaluation (HORSE; MITO-18). J. Clin. Oncol. 2025, 43, 852–860. [Google Scholar] [CrossRef]
- Classe, J.M.; Meeus, P.; Hudry, D.; Wernert, R.; Guenet, F.; Marchal, F.; Houvenaeghel, G.; Bats, A.S.; Lecuru, F.; Ferron, G.; et al. Hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer (CHIPOR): A randomised, open-label, phase 3 trial. Lancet Oncol. 2024, 25, 1551–1562. [Google Scholar] [CrossRef]
- Koole, S.N.; Schouten, P.C.; Hauke, J.; Kluin, R.J.C.; Nederlof, P.; Richters, L.K.; Krebsbach, G.; Sikorska, K.; Alkemade, M.; Opdam, M.; et al. Effect of HIPEC according to HRD/BRCAwt genomic profile in stage III ovarian cancer: Results from the phase III OVHIPEC trial. Int. J. Cancer 2022, 151, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Neveu, J.; Tremblay, E.; Mercier, F.; Garneau, S.; Cormier, B. Developing a hyperthermic intraperitoneal chemotherapy (HIPEC) gynecologic oncology program: A Canadian experience. Int. J. Gynecol. Cancer 2023, 33, 1957–1965. [Google Scholar] [CrossRef]
- Dubé, P.; Sideris, L.; Law, C.; Mack, L.; Hasse, E.; Giacomantonio, C.; Govindarajan, A.; Krzyzanowska, M.K.; Major, P.; McConnell, Y.; et al. Guidelines on the use of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal surface malignancy arising from colorectal or appendiceal neoplasms. Curr. Oncol. 2015, 22, e100–e112. [Google Scholar] [CrossRef]
- Balzano, G.; Guarneri, G.; Pecorelli, N.; Paiella, S.; Rancoita, P.M.V.; Bassi, C.; Falconi, M. Modelling centralization of pancreatic surgery in a nationwide analysis. Br. J. Surg. 2020, 107, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Sheetz, K.H.; Dimick, J.B.; Nathan, H. Centralization of High-Risk Cancer Surgery Within Existing Hospital Systems. J. Clin. Oncol. 2019, 37, 3234–3242. [Google Scholar] [CrossRef]
- Vonlanthen, R.; Lodge, P.; Barkun, J.S.; Farges, O.; Rogiers, X.; Soreide, K.; Kehlet, H.; Reynolds, J.V.; Käser, S.A.; Naredi, P.; et al. Toward a Consensus on Centralization in Surgery. Ann. Surg. 2018, 268, 712–724. [Google Scholar] [CrossRef] [PubMed]
- el Asmar, A.; Delcourt, M.; Kamden, L.; Khaled, C.; Bohlok, A.; Moreau, M.; Sclafani, F.; Donckier, V.; Liberale, G. Prognostic Value of Preoperative Serological Biomarkers in Patients Undergoing Curative-Intent Cytoreductive Surgery for Colorectal Cancer Peritoneal Metastases. Ann. Surg. Oncol. 2023, 30, 1863–1869. [Google Scholar] [CrossRef]
- Hurry, M.; Hassan, S.; Seung, S.J.; Walton, R.N.; Elnoursi, A.; McGee, J.D. Real-World Treatment Patterns, Survival, and Costs for Ovarian Cancer in Canada: A Retrospective Cohort Study Using Provincial Administrative Data. J. Health Econ. Outcomes Res. 2021, 8, 114. [Google Scholar] [CrossRef]
- Solomon, D.; Leigh, N.; Bekhor, E.; Feferman, Y.; Dhorajiya, P.; Feingold, D.; Hofstedt, M.; Aycart, S.N.; Golas, B.J.; Sarpel, D.; et al. The role of molecular biomarkers in outcomes and patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal metastases of colorectal origin. Surgeon 2021, 19, e379–e385. [Google Scholar] [CrossRef]
- Hulshof, E.C.; Lurvink, R.J.; Caserta, N.; de Hingh, I.H.J.T.; van Wezel, T.; Böhringer, S.; Swen, J.J.; Gelderblom, H.; Guchelaar, H.J.; Deenen, M.J. Identification of pharmacogenetic biomarkers for efficacy of cytoreductive surgery plus hyperthermic intraperitoneal mitomycin C in patients with colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2020, 46, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Koumpa, F.S.; Xylas, D.; Konopka, M.; Galea, D.; Veselkov, K.; Antoniou, A.; Mehta, A.; Mirnezami, R. Colorectal Peritoneal Metastases: A Systematic Review of Current and Emerging Trends in Clinical and Translational Research. Gastroenterol. Res. Pract. 2019, 2019, 5180895. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, T.Y.; Liu, J.L.; Ou, Y.C.; Lee, L.W.; Hung, C.H.; Lee, C.P.; Lung, J. Association between cytokines and progression-free survival in ovarian cancer following CRS/HIPEC treatment. J. Ovarian Res. 2025, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Ducoulombier, S.; Golfier, F.; Colomban, O.; Benayoun, D.; Bolze, P.A.; Tod, M.; You, B. Modeling CA-125 during neoadjuvant chemotherapy for predicting optimal cytoreduction and relapse risk in ovarian cancer. Anticancer. Res. 2017, 37, 6879–6886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738. [Google Scholar] [CrossRef]
- Zhai, G.; Zhou, J.; Woods, M.O.; Green, J.S.; Parfrey, P.; Rahman, P.; Green, R.C. Genetic structure of the Newfoundland and Labrador population: Founder effects modulate variability. Eur. J. Hum. Genet. 2016, 24, 1063–1070. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. Founder Effect. Available online: https://www.genome.gov/genetics-glossary/Founder-Effect (accessed on 20 March 2025).
- Bear, J.C.; Nemec, T.F.; Kennedy, J.C.; Marshall, W.H.; Power, A.A.; Kolonel, V.M.; Burke, G.B. Persistent genetic isolation in outport Newfoundland. Am. J. Med. Genet. 1987, 27, 807–830. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2023; Canadian Cancer Society: Toronto, ON, Canada, 2023; ISSN 0835-2976. Available online: https://cc-arcc.ca/canadian-cancer-statistics-2023/ (accessed on 20 March 2025).
- Parfrey, P.S.; Dicks, E.; Parfrey, O.; McNicholas, P.J.; Noseworthy, H.; Woods, M.O.; Negriin, C.; Green, J. Evaluation of a population-based approach to familial colorectal cancer. Clin. Genet. 2017, 91, 672–682. [Google Scholar] [CrossRef]
- Green, R.C.; Green, J.S.; Buehler, S.K.; Robb, J.D.; Daftary, D.; Gallinger, S.; McLaughlin, J.R.; Parfrey, P.S.; Younghusband, H.B. Very high incidence of familial colorectal cancer in Newfoundland: A comparison with Ontario and 13 other population-based studies. Fam. Cancer 2007, 6, 53–62. [Google Scholar] [CrossRef]
- Woods, M.O.; Hyde, A.J.; Curtis, F.K.; Stuckless, S.; Green, J.S.; Pollett, A.F.; Robb, J.D.; Green, R.C.; Croitoru, M.E.; Careen, A.; et al. High frequency of hereditary colorectal cancer in Newfoundland likely involves wovel susceptibility genes. Clin. Cancer Res. 2005, 11, 6853–6861. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Hardwick, R.; Huntsman, D.; Carneiro, F.; Guilford, P.; Blair, V.; Chung, D.C.; Norton, J.; Ragunath, K.; Van Krieken, J.H.; et al. Hereditary diffuse gastric cancer: Updated consensus guidelines for clinical management and directions for future research. J. Med. Genet. 2010, 47, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, P.C.; MacMillan, A.; Huntsman, D.; Kaurah, P.; Carneiro, F.; Wen, X.; Kwan, A.; Boone, D.; Bursey, F.; Fernandez, B.; et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): The Newfoundland experience with 23 patients. Ann. Surg. Oncol. 2009, 16, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Kaurah, P.; MacMillan, A.; Boyd, N.; Senz, J.; De Luca, A.; Chun, N.; Suriano, G.; Zaor, S.; Van Manen, L.; Gilpin, C.; et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007, 297, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.A.; et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. J. Am. Med. Assoc. 2015, 313, 1347–1361. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Roebothan, A.; Smith, K.N.; Seal, M.; Etchegary, H.; Dawson, L. Specialty Care and Counselling about Hereditary Cancer Risk Improves Adherence to Cancer Screening and Prevention in Newfoundland and Labrador Patients with BRCA1/2 Pathogenic Variants: A Population-Based Retrospective Cohort Study. Curr. Oncol. 2023, 30, 9367–9381. [Google Scholar] [CrossRef]
- Nikiforchin, A.; Sardi, A.; King, M.C.; Baron, E.; Lopez-Ramirez, F.; Falla-Zuniga, L.F.; Barakat, P.; Iugai, S.; Pawlikowski, K.; Nieroda, C.; et al. Collaborative expertise of gynecological and surgical oncologists in managing advanced epithelial ovarian cancer. Eur. J. Surg. Oncol. 2024, 50, 107948. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).