Assessing Prognosis: Factors Influencing Outcomes in Hospitalized Lung Cancer
Simple Summary
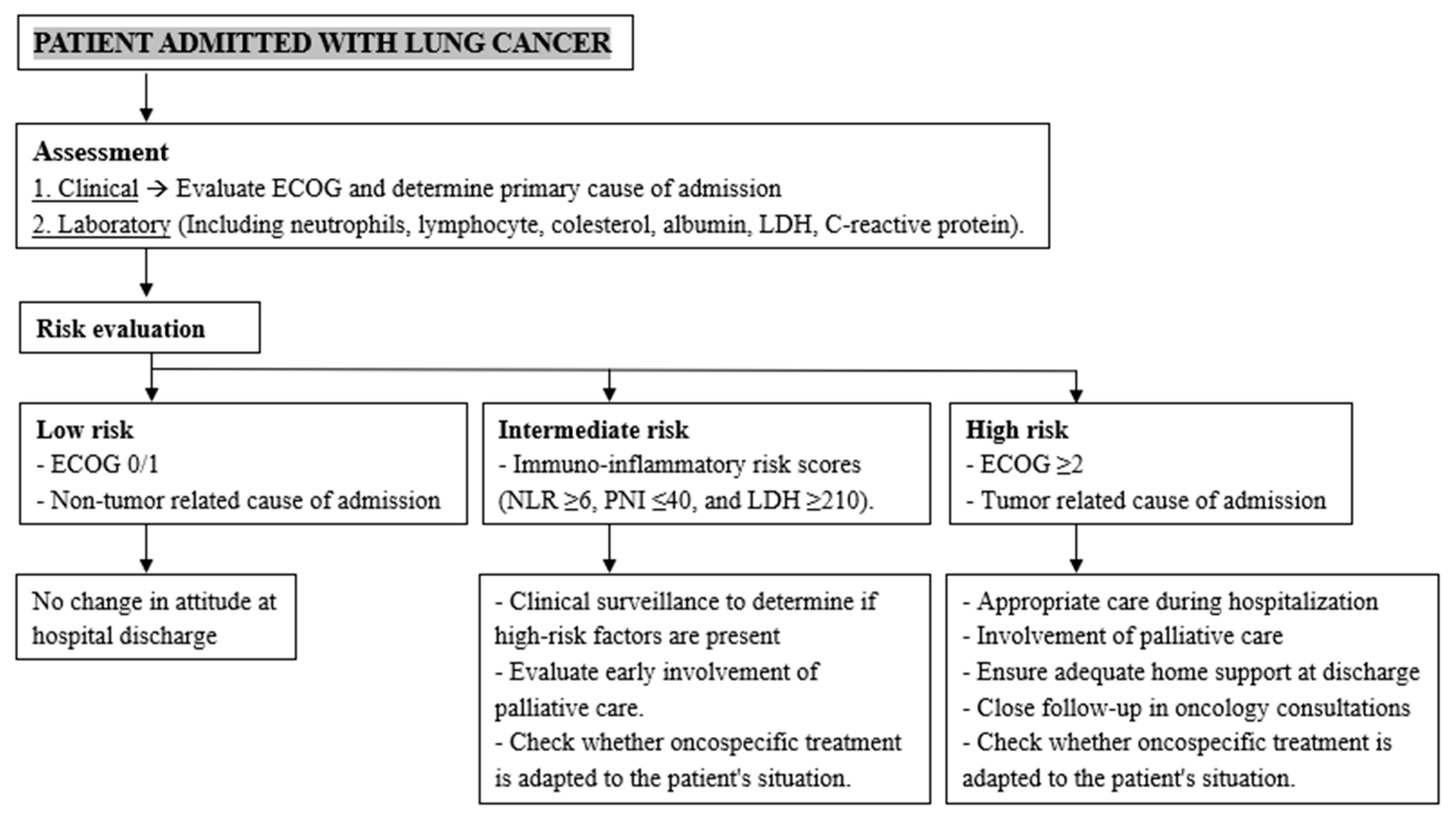
Abstract
1. Introduction
- Neutrophil/lymphocyte (NLR) = neutrophils/lymphocytes [15];
- Prognostic nutritional index (PNI) = albumin (g/dL) × 10 + lymphocytes/µL × 0.005 [16];
- Glasgow Prognostic Score Modified (mGPS): 1 point if C-reactive protein (PCR) (mg/L) > 10 → 2 points if CRP (mg/L) > 10 and albumin (g/dL) < 3.5 [17];
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Analytical Parameters and Immuno-Nutritional/Inflammatory Scores
3.3. ICIs in Hospitalized Patients
3.4. Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Komici, K.; Bencivenga, L.; Navani, N.; D’Agnano, V.; Guerra, G.; Bianco, A.; Rengo, G.; Perrotta, F. Frailty in Patients with Lung Cancer: A Systematic Review and Meta-Analysis. Chest 2022, 162, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. Impact of comorbidity on lung cancer survival. Int. J. Cancer 2003, 103, 792–802. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Ragin, C.C.; Belani, C.P.; Oton, A.B.; Gooding, W.E.; Taioli, E.; Ramalingam, S.S. Lung cancer in elderly patients: An analysis of the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2007, 25, 5570–5577. [Google Scholar] [CrossRef]
- Planas, M.; Álvarez-Hernández, J.; León-Sanz, M.; Celaya-Pérez, S.; Araujo, K.; García de Lorenzo, A.; on behalf of the PREDyCES® researchers. Prevalence of hospital malnutrition in cancer patients: A sub-analysis of the PREDyCES® study. Support. Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Geneen, L.J.; Buchanan, D.; Guthrie, B.; Smith, B.H. Factors associated with unscheduled care use by cancer decedents: A systematic review with narrative synthesis. BMJ Support. Palliat. Care 2024, 14, e50–e57. [Google Scholar] [CrossRef]
- Rocque, G.B.; Barnett, A.E.; Illig, L.C.; Eickhoff, J.C.; Bailey, H.H.; Campbell, T.C.; Stewart, J.A.; Cleary, J.F. Inpatient hospitalization of oncology patients: Are we missing an opportunity for end-of-life care? J. Oncol. Pract. 2013, 9, 51–54. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Handley, N.R.; Schuchter, L.M.; Bekelman, J.E. Best Practices for Reducing Unplanned Acute Care for Patients with Cancer. J. Oncol. Pract. 2018, 14, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Buckle, P.; Dolan, R.; Feliu, J.; Hui, D.; Laird, B.J.A.; Maltoni, M.; Moine, S.; Morita, T.; Nabal, M.; et al. Prognostic evaluation in patients with advanced cancer in the last months of life: ESMO Clinical Practice Guideline. ESMO Open 2023, 8, 101195. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Jaiyesimi, I.A.; Leighl, N.B.; Ismaila, N.; Alluri, K.; Florez, N.; Gadgeel, S.; Masters, G.; Schenk, E.L.; Schneider, B.J.; Sequist, L.; et al. Therapy for Stage IV Non–Small Cell Lung Cancer with Driver Alterations: ASCO Living Guideline, Version 2023.3. J. Clin. Oncol. 2024, 42, e1–e22. [Google Scholar] [CrossRef]
- Jaiyesimi, I.A.; Leighl, N.B.; Ismaila, N.; Alluri, K.; Florez, N.; Gadgeel, S.; Masters, G.; Schenk, E.L.; Schneider, B.J.; Sequist, L.; et al. Therapy for Stage IV Non–Small Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline, Version 2023.3. J. Clin. Oncol. 2024, 42, e23–e43. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Burke, K.; Cox, T.R.; Guise, T.; Jamal-Hanjani, M.; Janowitz, T.; Kaplan, R.; Lee, R.; Swanton, C.; Vander Heiden, M.G.; et al. Why do patients with cancer die? Nat. Rev. Cancer 2024, 24, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Schulz, C.; Prabhash, K.; Kowalski, D.; Szczesna, A.; Han, B.; Rittmeyer, A.; Talbot, T.; Vicente, D.; Califano, R.; et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): A phase 3, global, multicentre, open-label, randomised controlled study. Lancet 2023, 402, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Morabito, A.; Cavanna, L.; Luciani, A.; Maione, P.; Bonanno, L.; Filipazzi, V.; Leo, S.; Cinieri, S.; Ciardiello, F.; et al. Cisplatin-Based First-Line Treatment of Elderly Patients with Advanced Non-Small-Cell Lung Cancer: Joint Analysis of MILES-3 and MILES-4 Phase III Trials. J. Clin. Oncol. 2018, 36, 2585–2592. [Google Scholar] [CrossRef]
- Lund, C.M.; Vistisen, K.K.; Olsen, A.P.; Bardal, P.; Schultz, M.; Dolin, T.G.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: A randomised trial (GERICO). Br. J. Cancer 2021, 124, 1949–1958. [Google Scholar] [CrossRef]
- Hall, P.S.; Swinson, D.; Cairns, D.A.; Waters, J.S.; Petty, R.; Allmark, C.; Ruddock, S.; Falk, S.; Wadsley, J.; Roy, R.; et al. Efficacy of Reduced-Intensity Chemotherapy with Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients with Advanced Gastroesophageal Cancer: The GO2 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 869–877. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Rich, D.Q.; Seplaki, C.; Lund, J.L.; Flannery, M.; Culakova, E.; Magnuson, A.; Wells, M.; Tylock, R.; Mohile, S.G. Primary Treatment Modification and Treatment Tolerability Among Older Chemotherapy Recipients with Advanced Cancer. JAMA Netw. Open 2024, 7, e2356106. [Google Scholar] [CrossRef]
- Leonetti, A.; Peroni, M.; Agnetti, V.; Pratticò, F.; Manini, M.; Acunzo, A.; Marverti, F.; Sulas, S.; Rapacchi, E.; Mazzaschi, G.; et al. Thirty-day mortality in hospitalised patients with lung cancer: Incidence and predictors. BMJ Support. Palliat. Care 2023, 14, e2003–e2010. [Google Scholar] [CrossRef]
- Morgan, H.K.; Hodgson, L.; Baldock, E.; Doffman, S.R. P195 Outcomes in emergency admissions with lung cancer: A 1-year perspective from a teaching hospital. Thorax 2011, 66, A146–A147. [Google Scholar] [CrossRef]
- Mieras, A.; Pasman, H.R.W.; Onwuteaka-Philipsen, B.D.; Dingemans, A.-M.M.C.; Kok, E.V.; Cornelissen, R.; Jacobs, W.; van den Berg, J.-W.; Welling, A.; Bogaarts, B.A.H.A.; et al. Is In-Hospital Mortality Higher in Patients with Metastatic Lung Cancer Who Received Treatment in the Last Month of Life? A Retrospective Cohort Study. J. Pain Symptom Manag. 2019, 58, 805–811. [Google Scholar] [CrossRef]
- Liapikou, A.; Petras, P.; Panagiotarakou, M.; Anastasopoulos, A.; Toumbis, M. Causes of hospitalization in patients with lung cancer. Eur. Respir. J. 2017, 50, PA4245. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Vickerstaff, V.; Kalpakidou, A.; Todd, C.; Griffiths, J.; Keeley, V.; Spencer, K.; Buckle, P.; Finlay, D.; Omar, R.Z. Prognostic tools or clinical predictions: Which are better in palliative care? PLoS ONE 2021, 16, e0249763. [Google Scholar] [CrossRef] [PubMed]
- CDC, U.S. Cancer Statistics Lung Cancer Stat Bite. Available online: https://www.cdc.gov/united-states-cancer-statistics/publications/lung-cancer-stat-bite.html (accessed on 24 November 2024).
- Consonni, D.; Pierobon, M.; Gail, M.H.; Rubagotti, M.; Rotunno, M.; Goldstein, A.; Goldin, L.; Lubin, J.; Wacholder, S.; Caporaso, N.E.; et al. Lung Cancer Prognosis Before and After Recurrence in a Population-Based Setting. JNCI J. Natl. Cancer Inst. 2015, 107, djv059. [Google Scholar] [CrossRef]
- Morita, T.; Tsunoda, J.; Inoue, S.; Chihara, S. The Palliative Prognostic Index: A scoring system for survival prediction of terminally ill cancer patients. Support. Care Cancer 1999, 7, 128–133. [Google Scholar] [CrossRef]
- Maltoni, M.; Nanni, O.; Pirovano, M.; Scarpi, E.; Indelli, M.; Martini, C.; Monti, M.; Arnoldi, E.; Piva, L.; Ravaioli, A.; et al. Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J. Pain Symptom Manag. 1999, 17, 240–247. [Google Scholar] [CrossRef]
- Barbot, A.-C.; Mussault, P.; Ingrand, P.; Tourani, J.-M. Assessing 2-month clinical prognosis in hospitalized patients with advanced solid tumors. J. Clin. Oncol. 2008, 26, 2538–2543. [Google Scholar] [CrossRef]
- Feliu, J.; Jiménez-Gordo, A.M.; Madero, R.; Rodríguez-Aizcorbe, J.R.; Espinosa, E.; Castro, J.; Acedo, J.D.; Martínez, B.; Alonso-Babarro, A.; Molina, R.; et al. Development and validation of a prognostic nomogram for terminally ill cancer patients. J. Natl. Cancer Inst. 2011, 103, 1613–1620. [Google Scholar] [CrossRef]
- Mirallas, O.; Cullell, B.M.; Navarro, V.; Pedrola, A.; Cano, K.S.V.; Recuero-Borau, J.; Salva, C.; Valbuena, D.E.L.; Puerto, D.A.G.; Beltran, J.S.; et al. 2144P The PRognostic Oncologic Plantology (PROP) website tool predicts 30-day mortality in hospitalized cancer patients on treatment. Ann. Oncol. 2023, 34, S1116–S1117. [Google Scholar] [CrossRef]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer cachexia: A nutritional or a systemic inflammatory syndrome? Br. J. Cancer 2022, 127, 379–382. [Google Scholar] [CrossRef]
- Numico, G.; Cristofano, A.; Mozzicafreddo, A.; Cursio, O.E.; Franco, P.; Courthod, G.; Trogu, A.; Malossi, A.; Cucchi, M.; Sirotovà, Z.; et al. Hospital Admission of Cancer Patients: Avoidable Practice or Necessary Care? PLoS ONE 2015, 10, e0120827. [Google Scholar] [CrossRef]


| Grade | Performance Status |
|---|---|
| 0 | Fully active, able to maintain pre-disease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities; active for more than 50% of waking hours |
| 3 | Capable of only limited self-care; confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled; cannot carry out any self-care; totally confined to bed or chair |
| 5 | Dead |
| Parameter | Normal | Light Undernutrition | Moderate Undernutrition | Severe Undernutrition |
|---|---|---|---|---|
| Albumin (g/dL) | 3.5–4.5 (0) | 3.0–3.49 (2) | 2.5–2.9 (4) | <2.5 (6) |
| Lymphocytes/µL | >1600 (0) | 1200–1599 (1) | 800–1199 (2) | <800 (3) |
| Cholesterol (mg/dL) | >180 (0) | 140–180 (1) | 100–139 (2) | <100 (3) |
| Screening Total Score | 0–1 | 2–4 | 5–8 | 9–12 |
| Baseline Characteristics | N = 158 |
|---|---|
| Age (years)—Median (range) | 68 (27–89) |
| Sex—n (%) | |
| - Male | 105 (66.5%) |
| - Female | 53 (33.5%) |
| Stage—n (%) | |
| - I | 2 (1.3%) |
| - II | 4 (2.5%) |
| - III | 18 (11.4%) |
| - IV | 134 (84.8%) |
| Histology—n (%) | |
| - Non-small cell | 127 (80.4%) |
| * Adenocarcinoma | 76 (48.1%) |
| * Squamous cell | 35 (22.2%) |
| * Undifferentiated | 14 (8.9%) |
| * Giant cell | 2 (1.3%) |
| - Small cell | 29 (18.4%) |
| - Other (salivary gland, mesothelioma) | 2 (1.2%) |
| Driver mutation—n (%) | 28 (17.7%) |
| - EFGR | 17 (10.8%) |
| - ALK | 4 (2.5%) |
| - ROS | 1 (0.6%) |
| - KRAS | 5 (3.2%) |
| - BRAF | 1 (0.6%) |
| Previous surgery—n (%) | 13 (8.2%) |
| Previous radiotherapy—n (%) | 56 (35.4%) |
| Systemic treatment at admission—n (%) | 133 (84.1%) |
| - (Neo-)adjuvant | 9 (5.7%) |
| - First line | 74 (46.8%) |
| - Second line | 25 (15.8%) |
| - Third line | 17 (10.8%) |
| - Fourth line | 8 (5.1%) |
| Has received ICI—n (%) | 92 (58.2%) |
| - At the moment of admission | 61 (38.6%) |
| - Previously | 31 (19.6%) |
| ECOG at admission—n (%) | |
| - 0 | 8 (5.1%) |
| - 1 | 44 (27.8%) |
| - 2 | 52 (32.9%) |
| - 3 | 43 (27.2%) |
| - 4 | 11 (7.0%) |
| Main cause of admission recorded—n (%) | |
| - Tumor-related | 68 (43%) |
| * Start of oncospecific treatment | * 6 (3.8%) |
| - Infection | 51 (32%) |
| - Immune-related adverse events | 12 (7.6%) |
| - Non-infectious chemotherapy-related | 5 (3.2%) |
| - Heart failure | 4 (2.5%) |
| Fever present on admission—n (%) | 50 (31.6%) |
| Variable | |
|---|---|
| Hb (g/dL)—median (range) | 12.1 (6.4–17.3) |
| NLR—median (range) | 12.4 (0.2–256.6) |
| - NLR ≥ 6—n (%) | 90 (57%) |
| Albumin (g/dL)—median (range) | 7.6 (2.4–8.1) |
| Cholesterol (mg/dL)—median (range) | 159 (66–315) |
| CRP (mg/L)—median (range) | 114 (0.5–315) |
| LDH (UI/L)—median (range) | 410 (110–4124) |
| - LDH ≥ 210—n (%) | 109 (69%) |
| PNI—median (range) | 42.3 (24.1–87.5) |
| - PNI ≤ 40—n (%) | 51 (32%) |
| mGPS—n (%) | |
| - 0 points | 10 (6.3%) |
| - 1 point | 84 (53.2%) |
| - 2 points | 43 (27.2%) |
| CONUT—n (%) | |
| - Normal | 17 (10.7%) |
| - Light undernutrition | 73 (46.2%) |
| - Moderate undernutrition | 32 (20.3%) |
| - Severe undernutrition | 8 (5.1%) |
| Article | Population |
|---|---|
| Morita et al., 1999 [36] | PPI: ECOG, oral intake, edema, dyspnea at rest, and delirium |
| Maltoni et al., 1999 [37] | PaP Score: dyspnea, anorexia, KPS, CPS, and leukocytes |
| Barbot et al., 2008 [38] | KPS, number of metastatic sites, albumin, and LDH |
| Feliu et al., 2011 [39] | ECOG, LDH, albumin, lymphocytes, and time from initial diagnosis to diagnosis of terminal disease |
| Leonetti et al., 2023 [28] | BRASS |
| Mirallas et al., 2023 [40] | ECOG, LDH, albumin, neutrophils, lung metastases, and treatment response at admission |
| Our series | Admission for tumor-related causes and ECOG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-López, J.; Gutiérrez-Sainz, L.; Jiménez-Bou, D.; Ruíz-Gutiérrez, I.; Navas-Jiménez, C.; Alonso-Eiras, J.I.; García-Zamarriego, Á.; Sánchez-Cabrero, D.; Ruíz-Giménez, L.; Pertejo-Fernández, A.; et al. Assessing Prognosis: Factors Influencing Outcomes in Hospitalized Lung Cancer. Onco 2024, 4, 458-470. https://doi.org/10.3390/onco4040032
Peña-López J, Gutiérrez-Sainz L, Jiménez-Bou D, Ruíz-Gutiérrez I, Navas-Jiménez C, Alonso-Eiras JI, García-Zamarriego Á, Sánchez-Cabrero D, Ruíz-Giménez L, Pertejo-Fernández A, et al. Assessing Prognosis: Factors Influencing Outcomes in Hospitalized Lung Cancer. Onco. 2024; 4(4):458-470. https://doi.org/10.3390/onco4040032
Chicago/Turabian StylePeña-López, Jesús, Laura Gutiérrez-Sainz, Diego Jiménez-Bou, Icíar Ruíz-Gutiérrez, Carmen Navas-Jiménez, Jorge Ignacio Alonso-Eiras, Álvaro García-Zamarriego, Darío Sánchez-Cabrero, Leticia Ruíz-Giménez, Ana Pertejo-Fernández, and et al. 2024. "Assessing Prognosis: Factors Influencing Outcomes in Hospitalized Lung Cancer" Onco 4, no. 4: 458-470. https://doi.org/10.3390/onco4040032
APA StylePeña-López, J., Gutiérrez-Sainz, L., Jiménez-Bou, D., Ruíz-Gutiérrez, I., Navas-Jiménez, C., Alonso-Eiras, J. I., García-Zamarriego, Á., Sánchez-Cabrero, D., Ruíz-Giménez, L., Pertejo-Fernández, A., Villamayor-Sánchez, J., Cruz-Castellanos, P., Higuera-Gómez, O., & de Castro, J. (2024). Assessing Prognosis: Factors Influencing Outcomes in Hospitalized Lung Cancer. Onco, 4(4), 458-470. https://doi.org/10.3390/onco4040032





