Intratumoral/Peritumoral Herpes Simplex Virus-1 Mutant HSV1716 in Pediatric Patients with Refractory or Recurrent High-Grade Gliomas: A Report of the Pediatric Brain Tumor Consortium
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility
2.3. Treatment
2.4. Required Observations
2.5. Outcome Measures
2.6. Statistical Design and Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Toxicity and Survival
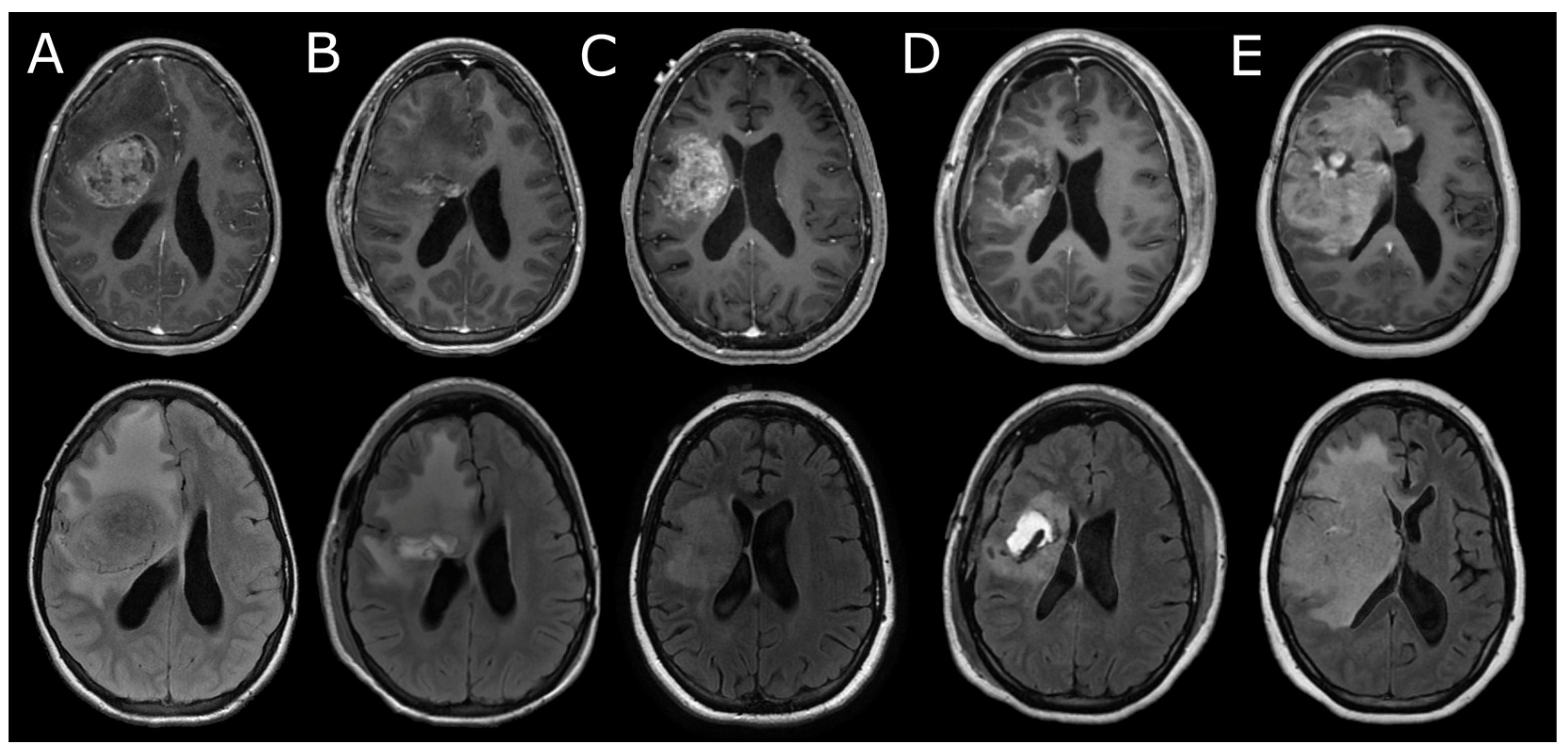
3.3. Neuroimaging
3.4. Viral Immune Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varn, F.S.; Johnson, K.C.; Martinek, J.; Huse, J.T.; Nasrallah, M.P.; Wesseling, P.; Cooper, L.A.D.; Malta, T.M.; Wade, T.E.; Sabedot, T.S.; et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 2022, 185, 2184–2199.e16. [Google Scholar] [CrossRef] [PubMed]
- Kalathoor, S.; Rajendran, S.; Canella, A.; Raval, R.; Cripe, T.P.; Mardis, E.R.; Rajappa, P. Myeloid cell heterogeneity in the tumor microenvironment and therapeutic implications for childhood central nervous system (CNS) tumors. J. Neuroimmunol. 2022, 374, 578009. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Burford, A.; Molinari, V.; Jones, D.T.W.; Izquierdo, E.; Brouwer-Visser, J.; Giangaspero, F.; Haberler, C.; Pietsch, T.; Jacques, T.S.; et al. Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial. Cancer Cell 2018, 33, 829–842.e5. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncology 2017, 19, 153–161. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Wang, Y.; Guo, Y.; Xing, B.; Ma, W. Classification of pediatric gliomas based on immunological profiling: Implications for immunotherapy strategies. Mol. Ther. Oncolytics 2021, 20, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, C.; Choi, J.; Alexandrescu, S.; Zimmerman, M.A.; Cooney, T.M.; Chordas, C.; Clymer, J.; Chi, S.; Yeo, K.K. Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: A single institution experience. J. Neurooncol. 2020, 149, 113–122. [Google Scholar] [CrossRef]
- Wei, X.; Meel, M.H.; Breur, M.; Bugiani, M.; Hulleman, E.; Phoenix, T.N. Defining tumor-associated vascular heterogeneity in pediatric high-grade and diffuse midline gliomas. Acta Neuropathol. Commun. 2021, 9, 142. [Google Scholar] [CrossRef]
- Detta, A.; Harland, J.; Hanif, I.; Brown, S.M.; Cruickshank, G. Proliferative activity and in vitro replication of HSV1716 in human metastatic brain tumours. J. Gene Med. 2003, 5, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Harland, J.; MacLean, A.R.; Podlech, J.; Clements, J.B. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J. Gen. Virol. 1994, 75 Pt 9, 2367–2377. [Google Scholar] [CrossRef]
- Harland, J.; Papanastassiou, V.; Brown, S.M. HSV1716 persistence in primary human glioma cells in vitro. Gene Ther. 2002, 9, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J.; et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Harrow, S.; Papanastassiou, V.; Harland, J.; Mabbs, R.; Petty, R.; Fraser, M.; Hadley, D.; Patterson, J.; Brown, S.M.; Rampling, R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: Safety data and long-term survival. Gene Ther. 2004, 11, 1648–1658. [Google Scholar] [CrossRef]
- Papanastassiou, V.; Rampling, R.; Fraser, M.; Petty, R.; Hadley, D.; Nicoll, J.; Harland, J.; Mabbs, R.; Brown, M. The potential for efficacy of the modified (ICP 34.5−) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002, 9, 398–406. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Gröbner, N.S.; Worst, C.B.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, A.V.; Johann, D.P.; Balasubramanian, P.G.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Persson, L.M.; Douglas, M.A.; Alvaro, F.; Faridi, P.; Larsen, R.M.; Alonso, M.M.; Vitanza, A.N.; Dun, D.M. The intrinsic and microenvironmental features of diffuse midline glioma: Implications for the development of effective immunotherapeutic treatment strategies. Neuro-Oncology 2022, 24, 1408–1422. [Google Scholar] [CrossRef]
- Wang, S.S.; Bandopadhayay, P.; Jenkins, R.M. Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol. 2019, 40, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, A.W.K.; Sawaya, R.; Weinberg, S.J.; Prabhu, S.S.; Rao, G.; Fuller, N.G.; Aldape, D.K.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Gujar, S.; Pol, J.G.; Kroemer, G. Heating it up: Oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies. OncoImmunology 2018, 7, e14421692018. [Google Scholar] [CrossRef]
- Benencia, F.; Courreges, M.C.; Fraser, N.W.; Coukos, G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol. Ther. 2008, 7, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Totsch, S.K.; Schlappi, C.; Kang, K.D.; Ishizuka, A.S.; Lynn, G.M.; Fox, B.; Beierle, E.A.; Whitley, R.J.; Markert, J.M.; Gillespie, G.Y.; et al. Oncolytic herpes simplex virus immunotherapy for brain tumors: Current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene 2019, 38, 6159–6171. [Google Scholar] [CrossRef]
- Ling, L.A.; Solomon, H.I.; Landivar, M.A.; Nakashima, H.; Woods, K.J.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, S.A.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, E.J.; Beaubier, N.; Bolognesi, P.D.; Friedman, H.A.; Friedman, S.H.; Mcsherry, F.; Muscat, M.A.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-M.; Saha, D. The Current State of Oncolytic Herpes Simplex Virus for Glioblastoma Treatment. Oncolytic Virotherapy 2021, 10, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Winder, N.; Atkinson, E.; Al-Janabi, H.; Allen, R.J.; Hughes, R.; Moamin, M.; Louie, R.; Evans, D.; Hutchinson, M.; et al. Macrophages Mediate the Antitumor Effects of the Oncolytic Virus HSV1716 in Mammary Tumors. Mol. Cancer Ther. 2021, 20, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Mineta, T.; Rabkin, S.D.; Yazaki, T.; Hunter, W.D.; Martuza, R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995, 1, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Landi, D.; Brown, M.C.; Friedman, H.S.; McLendon, R.; Herndon, J.E., 2nd; Buckley, E.; Bolognesi, D.P.; Lipp, E.; Schroeder, K.; et al. Recombinant polio-rhinovirus immunotherapy for recurrent paediatric high-grade glioma: A phase 1b trial. Lancet Child. Adolesc. Health 2023, 7, 471–478. [Google Scholar] [CrossRef]
- Pérez-Larraya, G.J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Hauschild, A.; Mehnert, J.M.; Long, G.V. Double Trouble: Immunotherapy Doublets in Melanoma-Approved and Novel Combinations to Optimize Treatment in Advanced Melanoma. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 745–766. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Milhem, M.M.; Minor, D.; Hamid, O.; Li, A.; Chen, L.; Chastain, M.; Gorski, K.S.; Anderson, A.; Chou, J.; et al. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J. Clin. Oncol. 2016, 34, 2619–2626. [Google Scholar] [CrossRef]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Minn, J.A. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2023, 9, 122–139. [Google Scholar] [CrossRef]
- Garcia-Moure, M.; Laspidea, V.; Gupta, S.; Gillard, A.G.; Khatua, S.; Parthasarathy, A.; He, J.; Lang, F.F.; Fueyo, J.; Alonso, M.M.; et al. The emerging field of viroimmunotherapy for pediatric brain tumors. Neuro-Oncology 2024, 26, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.R.; Johnson, B.D.; Balko, M.J. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.M.; Zdioruk, M.; Nowicki, O.M.; Griffith, M.A.; Aguilar, E.; Aguilar, K.L.; Guzik, W.B.; Barone, F.; Tak, P.P.; Tabatabai, G.; et al. Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models. J. Immunother. Cancer 2022, 10, e0033682022. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.S.; Pennell, A.N. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Maslov, V.D.; Tawagi, K.; Kc, M.; Simenson, V.; Yuan, H.; Parent, C.; Bamnolker, A.; Goel, R.; Blake, Z.; Matrana, R.M.; et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J. Immunother. Cancer 2021, 9, e002261. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Dahlberg, E.S.; Adeni, A.; Sholl, M.L.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients With Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]

| Patient 1 | Patient 2 | |
|---|---|---|
| Age (years) | ||
| At diagnosis | 9 | 14.3 |
| At study entry | 12.5 | 16.6 |
| Ethnicity | Non-Hispanic | Non-Hispanic |
| Race | White | White |
| Diagnosis | Recurrent glioblastoma, right frontal lobe | Recurrent glioblastoma, right frontal lobe |
| Lansky performance score at study entry | 100 | 80 |
| Patient | Time From Surgery (Days) | HSV IgG (IV, 0.89 or Less = Not Detected; 0.90–1.09 = Indeterminate; 1.10 or Greater = Detected) | HSV IgM (IV, 0.89 or Less = Not Detected; 0.90–1.09 = Indeterminate; 1.10 or Greater = Detected) | ANC (K/mcL, 1.8–8.0) | ALC (K/mcL, 1.5–6.5) | NLR | Dexamethasone Dose |
|---|---|---|---|---|---|---|---|
| 1 | −2 | 0.09 | 0.33 | 2.4 | 1.73 | 1.4 | 0 |
| 1 | 0 | NA | NA | 7.24 | 1.13 | 6.4 | 4 mg every 6 h |
| 1 | 1 | NA | NA | 6.48 | 0.9 | 7.2 | 4 mg every 6 h |
| 1 | 2 | NA | NA | 6.54 | 1.44 | 4.5 | 4 mg every 6 h |
| 1 | 28 | 0.53 | 1.3 | 1.57 | 1.09 | 1.4 | 0 |
| 1 | 56 | NA | NA | 8.74 | 0.56 | 15.6 | 0 |
| 1 | 84 | NA | NA | 5.37 | 0.55 | 9.8 | 0 |
| 2 | −10 | 0.51 | 0.52 | 16.69 | 2.57 | 6.5 | 4 mg every 8 h |
| 2 | 0 | NA | NA | NA | NA | NA | 4 mg every 6 h |
| 2 | 1 | NA | NA | 11.54 | 1.78 | 6.5 | 4 mg every 6 h |
| 2 | 2 | NA | NA | 8.93 | 1.86 | 4.8 | 4 mg every 6 h |
| 2 | 3 | NA | NA | 7.95 | 7.95 | 1 | 4 mg every 6 h |
| 2 | 4 | NA | NA | 4.42 | 1.22 | 3.6 | 4 mg every 6 h |
| 2 | 28 | 1.44 | 3.79 | 4.07 | 2.29 | 1.8 | 1 mg every 8 h |
| 2 | 57 | 5.03 | 3.39 | 9.9 | 2.11 | 4.7 | 2 mg every 8 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, A.Y.; Hummel, T.R.; Cripe, T.; Fouladi, M.; Pollack, I.F.; Mitchell, D.; Young Poussaint, T.; Onar-Thomas, A.; Pillay-Smiley, N.; DeWire-Schottmiller, M.; et al. Intratumoral/Peritumoral Herpes Simplex Virus-1 Mutant HSV1716 in Pediatric Patients with Refractory or Recurrent High-Grade Gliomas: A Report of the Pediatric Brain Tumor Consortium. Onco 2025, 5, 1. https://doi.org/10.3390/onco5010001
Mochizuki AY, Hummel TR, Cripe T, Fouladi M, Pollack IF, Mitchell D, Young Poussaint T, Onar-Thomas A, Pillay-Smiley N, DeWire-Schottmiller M, et al. Intratumoral/Peritumoral Herpes Simplex Virus-1 Mutant HSV1716 in Pediatric Patients with Refractory or Recurrent High-Grade Gliomas: A Report of the Pediatric Brain Tumor Consortium. Onco. 2025; 5(1):1. https://doi.org/10.3390/onco5010001
Chicago/Turabian StyleMochizuki, Aaron Y., Trent R. Hummel, Timothy Cripe, Maryam Fouladi, Ian F. Pollack, Duane Mitchell, Tina Young Poussaint, Arzu Onar-Thomas, Natasha Pillay-Smiley, Mariko DeWire-Schottmiller, and et al. 2025. "Intratumoral/Peritumoral Herpes Simplex Virus-1 Mutant HSV1716 in Pediatric Patients with Refractory or Recurrent High-Grade Gliomas: A Report of the Pediatric Brain Tumor Consortium" Onco 5, no. 1: 1. https://doi.org/10.3390/onco5010001
APA StyleMochizuki, A. Y., Hummel, T. R., Cripe, T., Fouladi, M., Pollack, I. F., Mitchell, D., Young Poussaint, T., Onar-Thomas, A., Pillay-Smiley, N., DeWire-Schottmiller, M., & Stevenson, C. B. (2025). Intratumoral/Peritumoral Herpes Simplex Virus-1 Mutant HSV1716 in Pediatric Patients with Refractory or Recurrent High-Grade Gliomas: A Report of the Pediatric Brain Tumor Consortium. Onco, 5(1), 1. https://doi.org/10.3390/onco5010001






