Determining the Spectral Characteristics of Fynbos Wetland Vegetation Species Using Unmanned Aerial Vehicle Data
Abstract
1. Introduction
2. Materials and Methods
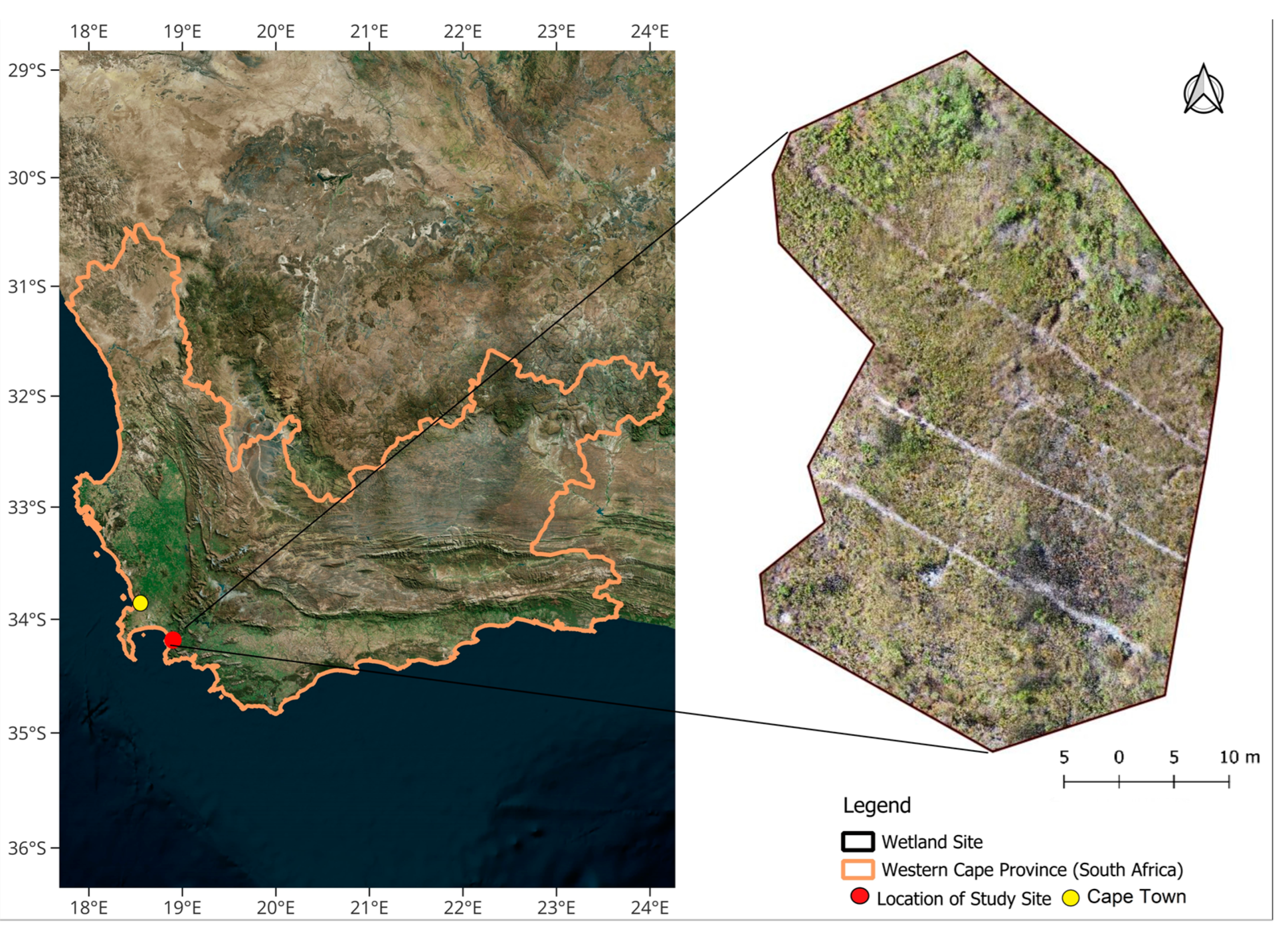
2.1. Study Area
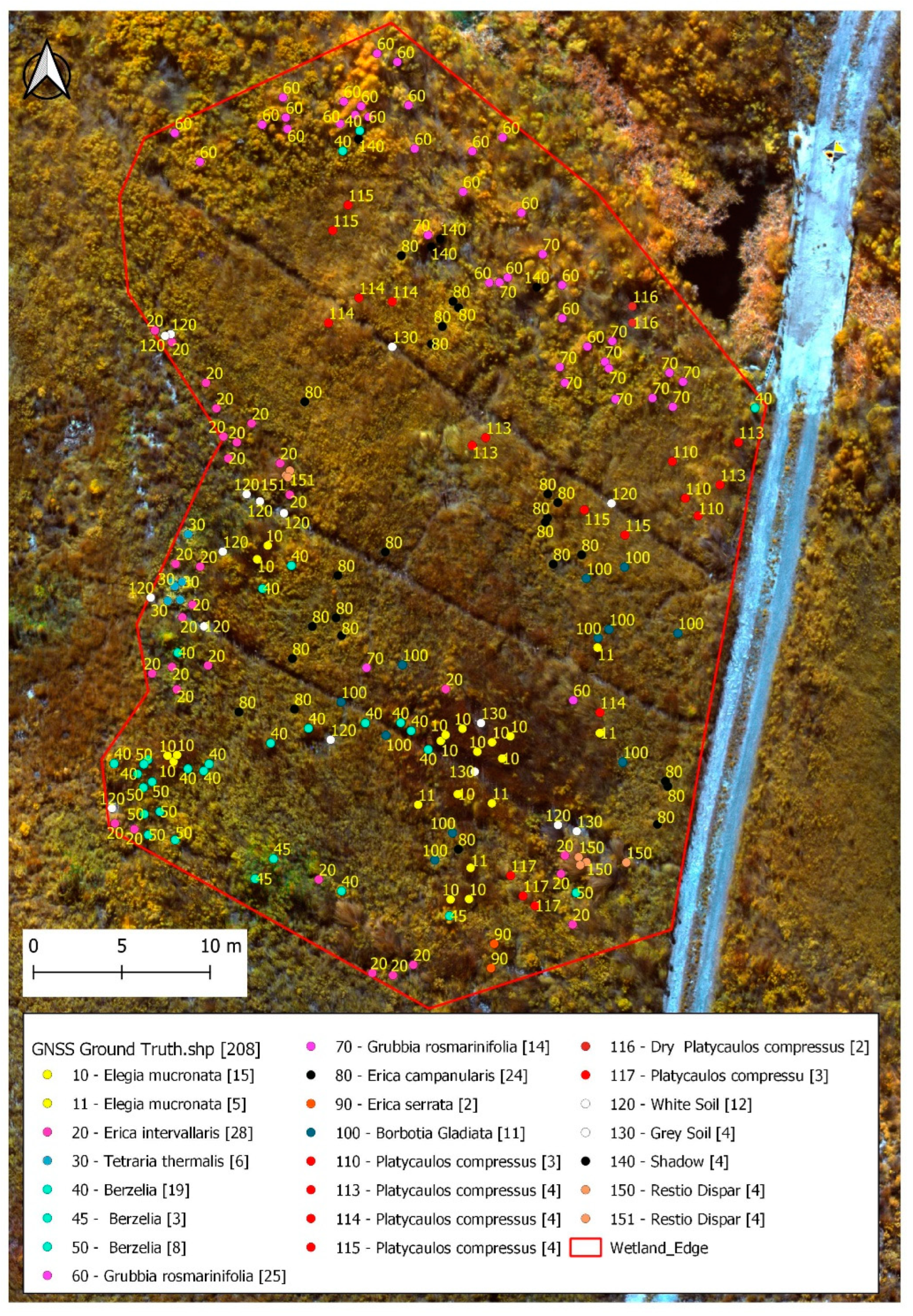
2.2. Identifying the Dominant Plant Species
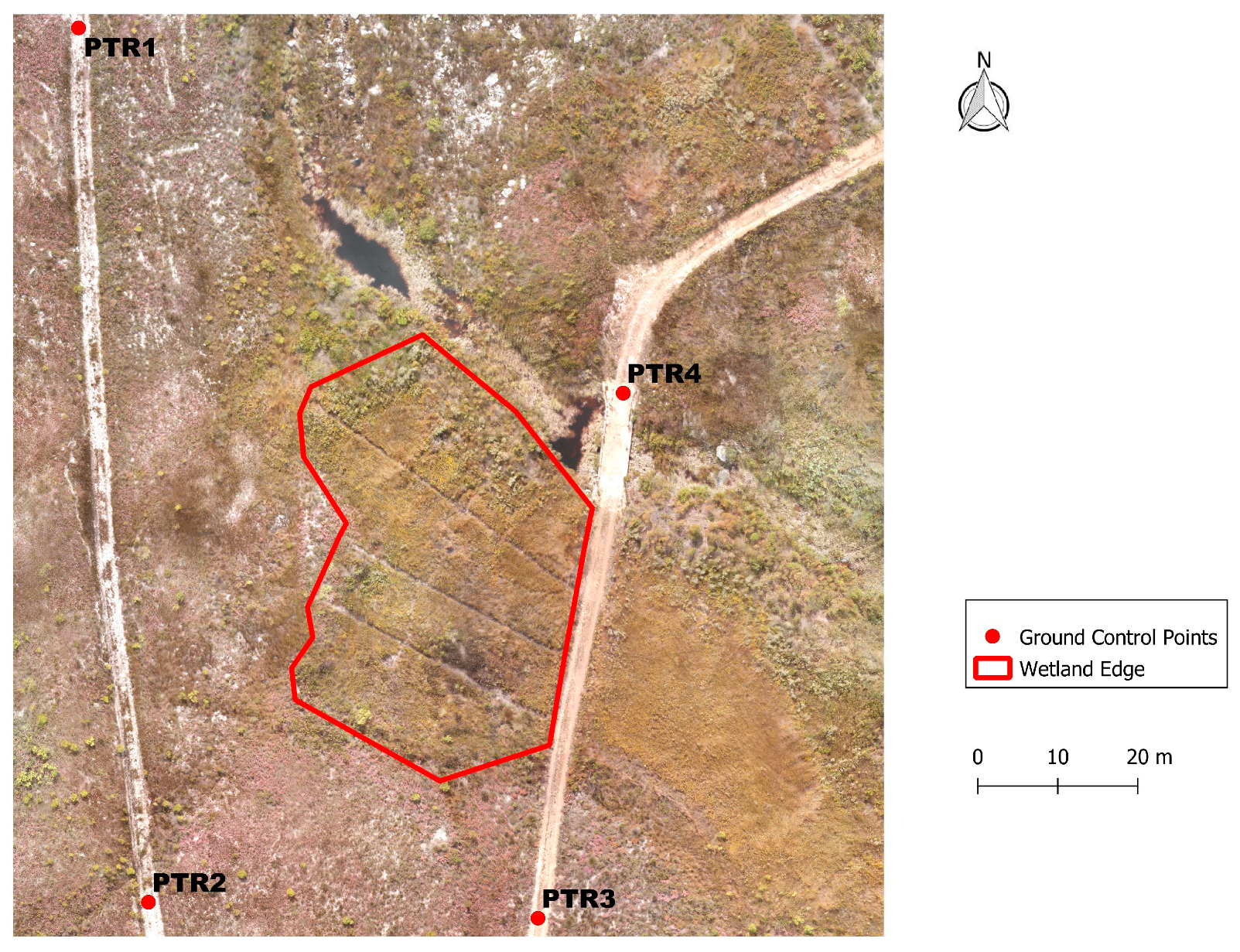
2.3. Creating Photogrammetric Ground Control Points
2.4. Acquiring UAV Data
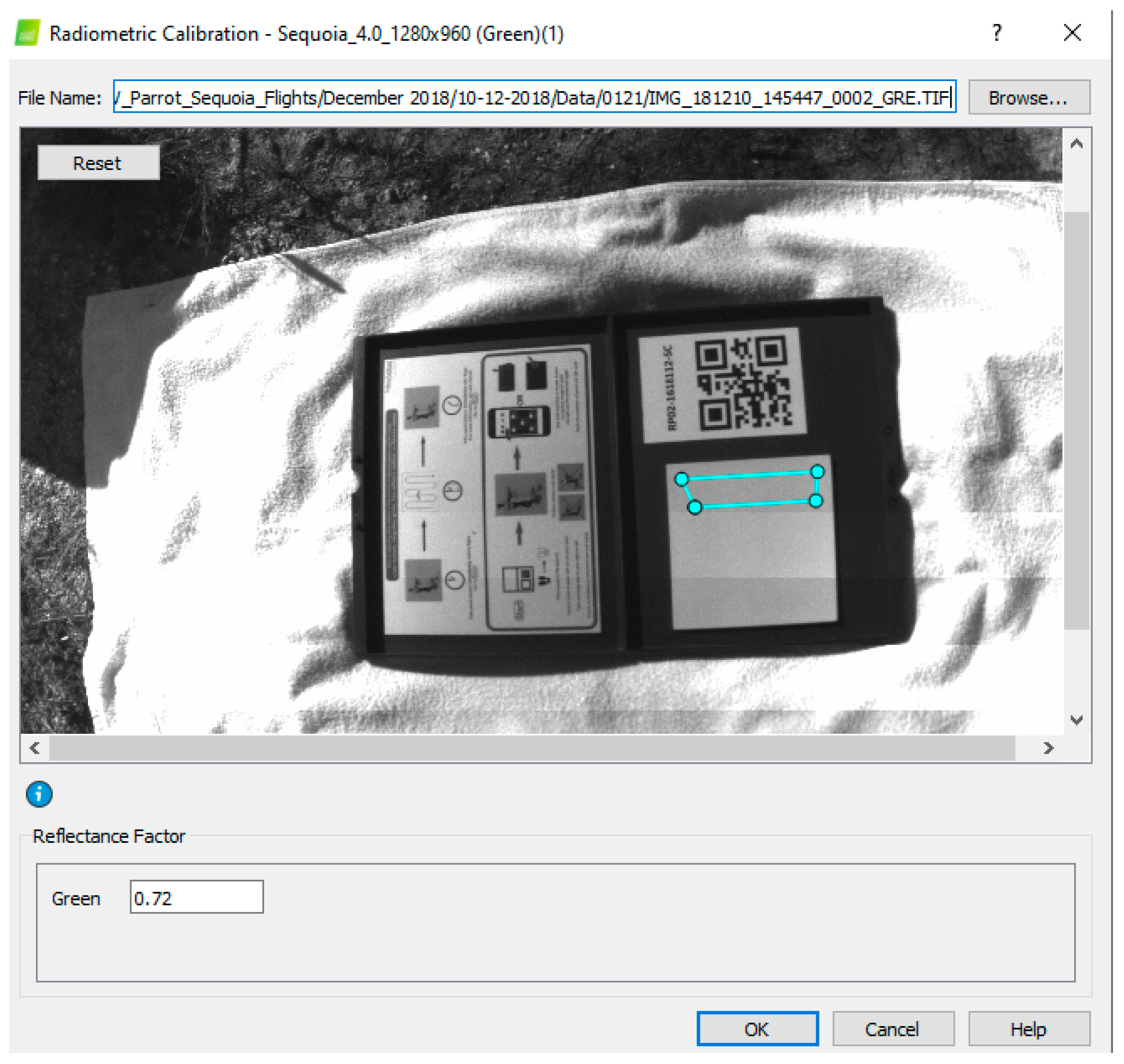
2.5. Processing UAV Data
2.6. Processing Ground Truth Data and Spectral Curves
2.7. Classification of the Wetland Species
3. Results and Discussion
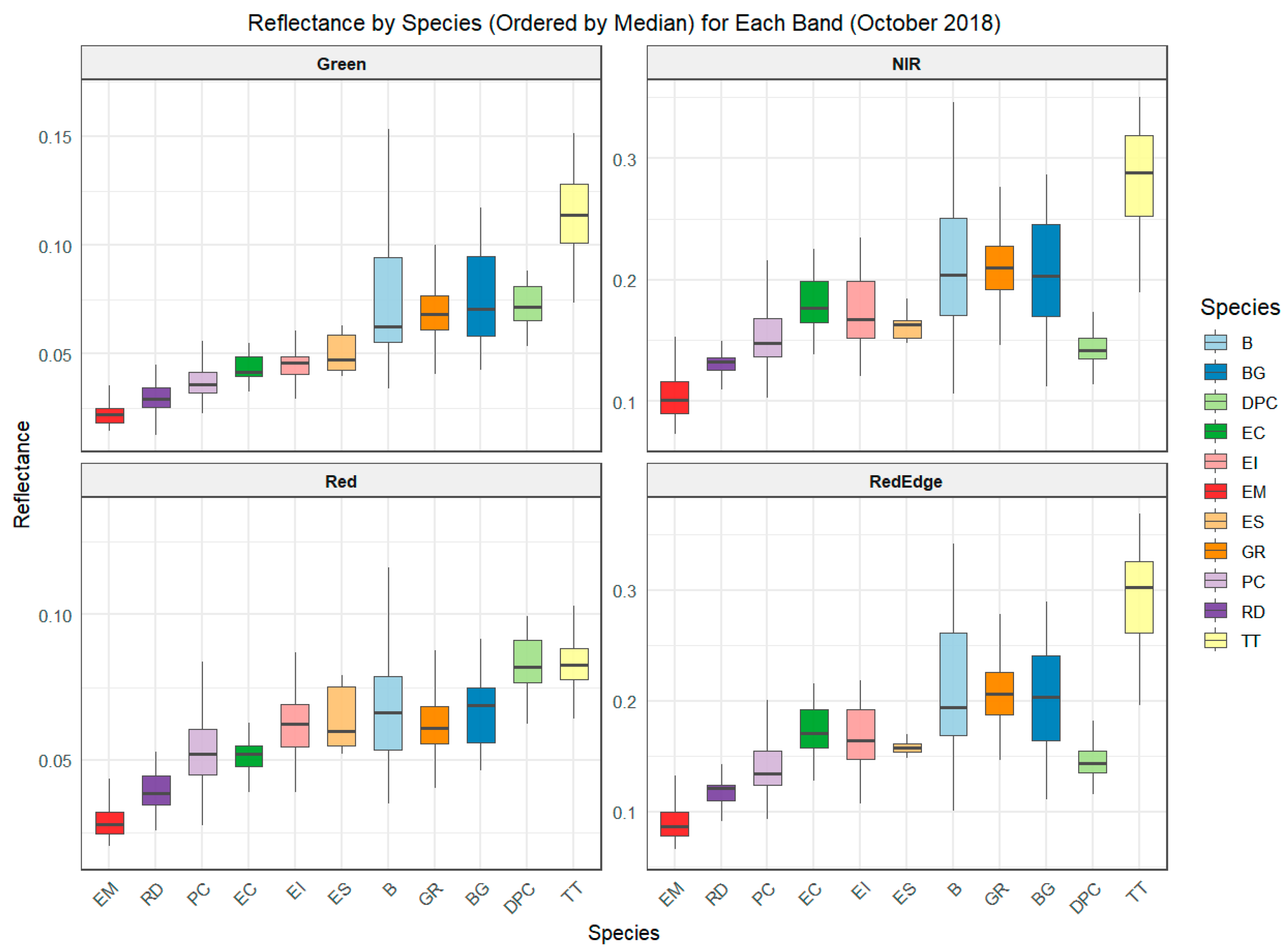
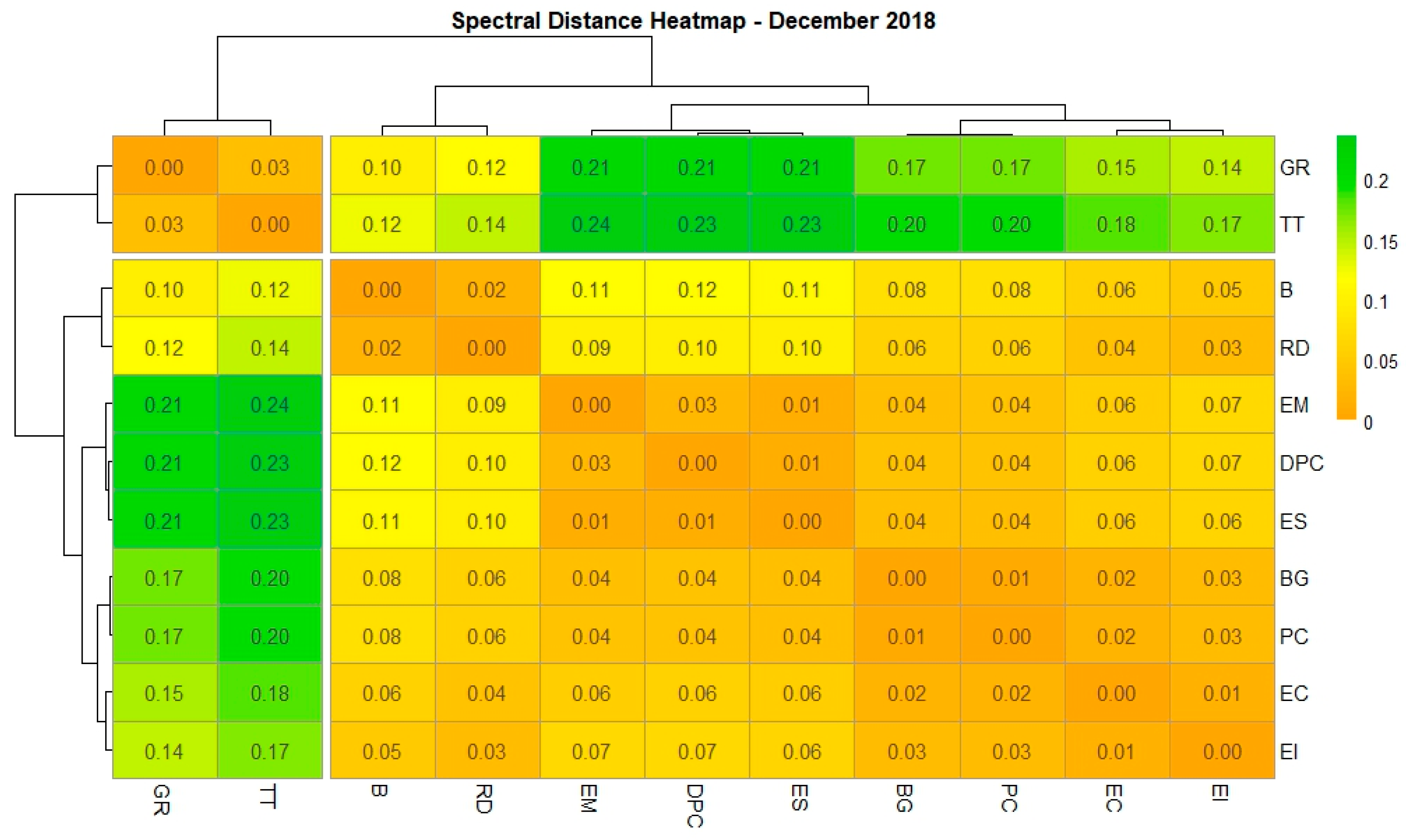
3.1. Differentiating Between Wetland Species Based on Their Spectral Signatures
3.2. Inter-Seasonal Foliar Spectral Variations in the Species
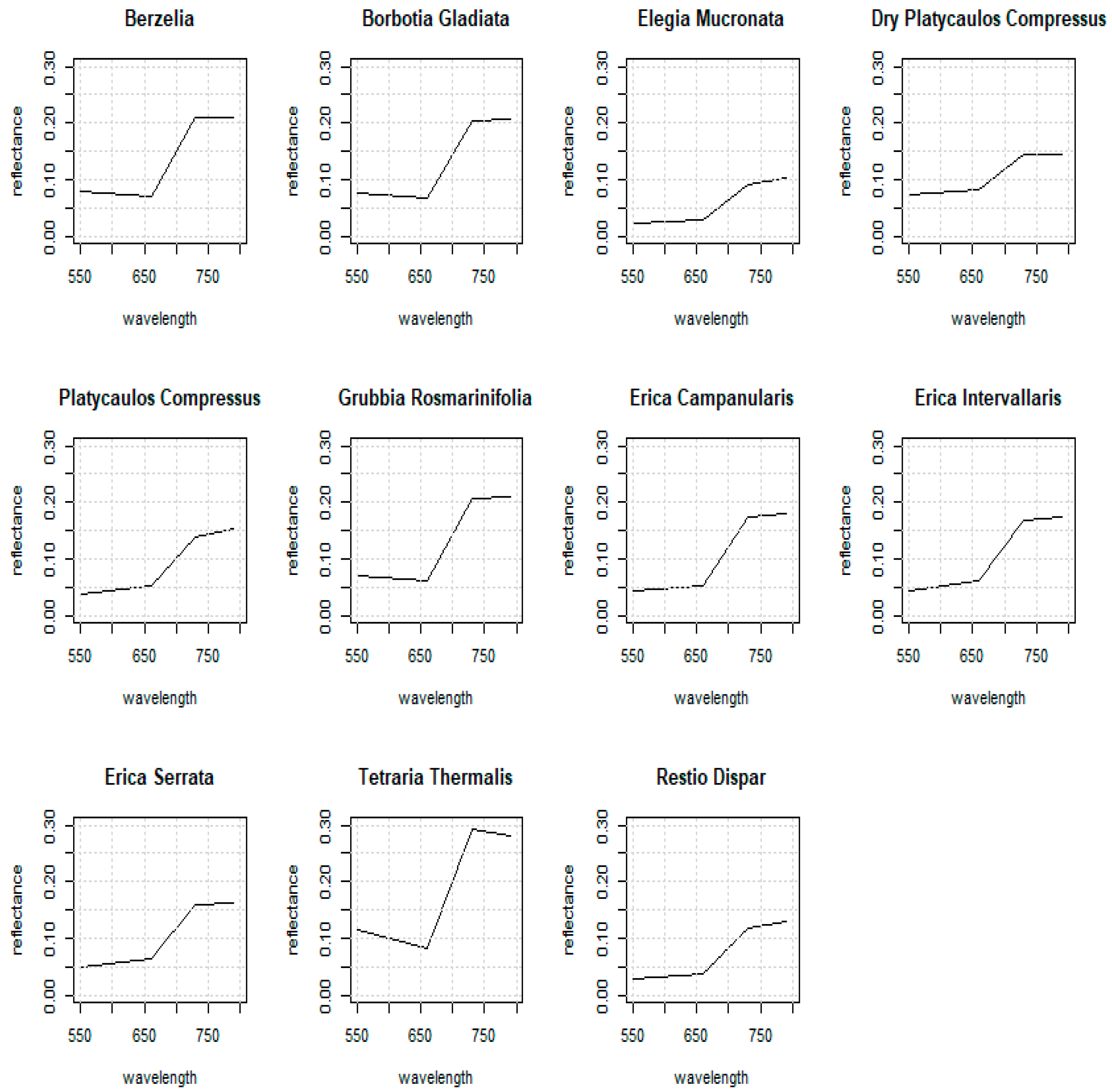
3.2.1. Berzelia lanuginosa
3.2.2. Borbotia gladiata
3.2.3. Grubbia rosmarinifolia
3.2.4. Tetraria thermalis
3.2.5. Erica intervallaris
3.2.6. Erica serrata
3.2.7. Elegia mucronata
3.2.8. Platycaulos compressus
3.2.9. Restio dispar
3.2.10. Erica campanularis (and Restio leptostachyus)
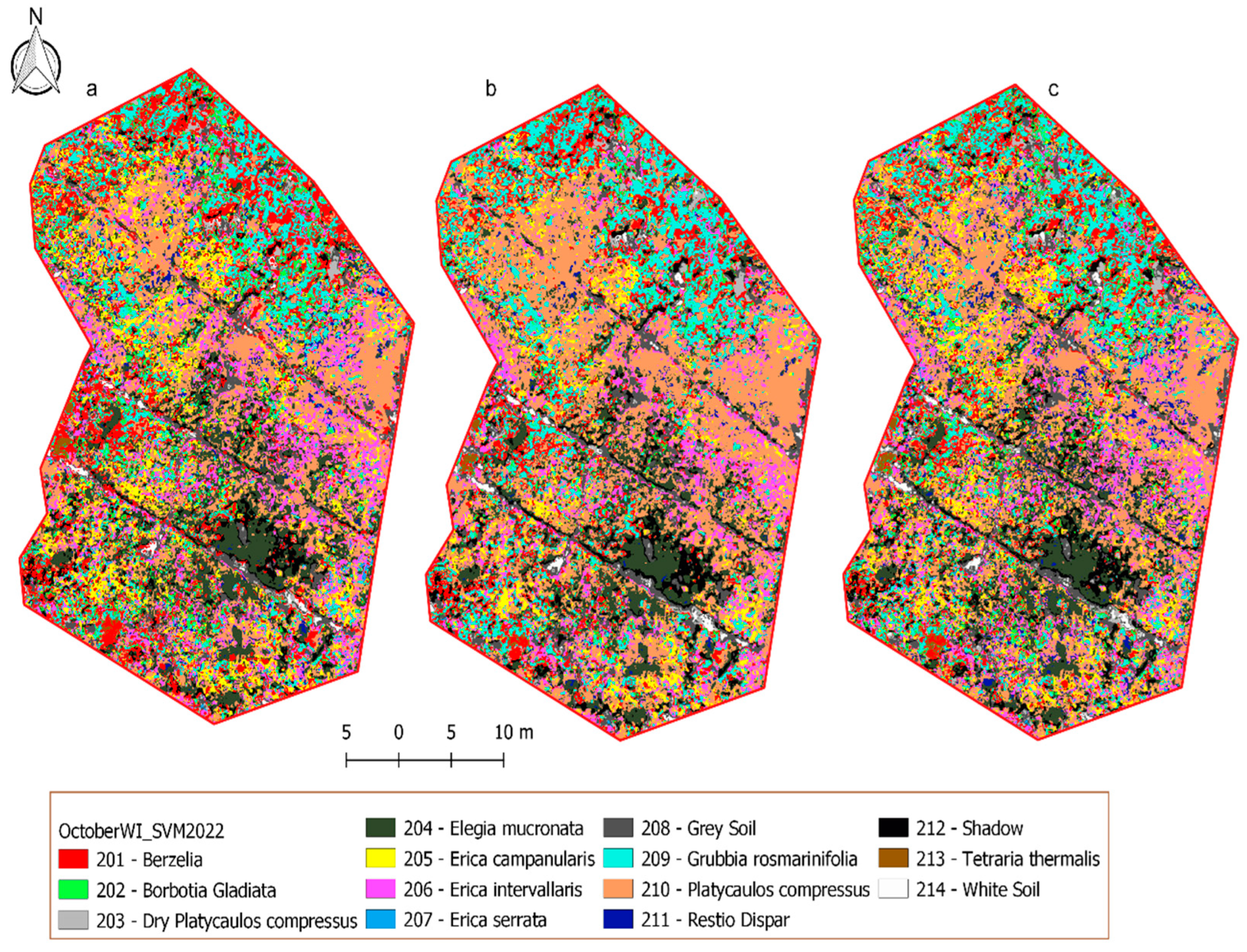
3.3. The Classification of the Wetland Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turpie, J.K.; Heydenrych, B.J.; Lamberth, S.J. Economic Value of Terrestrial and Marine Biodiversity in the Cape Floristic Region: Implications for Defining Effective and Socially Optimal Conservation Strategies. Biol. Conserv. 2003, 112, 233–251. [Google Scholar] [CrossRef]
- Bek, D.; O’grady, K. The Scale, Structure and Sustainability of the Wild Fynbos Harvesting Supply Chain in the Cape Floral Kingdom; Coventry University: Coventry, UK, 2018. [Google Scholar]
- Bossi, L. Mapping Cape Fynbos Vegetation with the Aid of Landsat Imagery. Veld Flora 1984, 70, 31–33. [Google Scholar]
- Helme, N.A.; Trinder-Smith, T.H. The Endemic Flora of the Cape Peninsula, South Africa. S. Afr. J. Bot. 2006, 72, 205–210. [Google Scholar] [CrossRef]
- Rebelo, A.G.; Boucher, C.; Helme, N. Fynbos Biome. In The Vegetation of South Africa, Lesotho and Swaziland; Mucina, L., Rutherford, M.C., Eds.; SANBI: Pretoria, South Africa, 2006; pp. 53–219. [Google Scholar]
- Wittridge, H.-M. Integrated Reserve Management Plan—Steenbras Nature Reserve; City of Cape Town: Cape Town, South Africa, 2011. [Google Scholar]
- Ewart-Smith, J.; Boucher, C. Baseline Assessment of Ecosystems Potentially Impacted by The Well-Field Development at Steenbras; Freshwater Consulting Group: Cape Town, South Africa, 2019. [Google Scholar]
- West, S.; Cairns, R.; Schultz, L. What Constitutes a Successful Biodiversity Corridor? A Q-Study in the Cape Floristic Region, South Africa. Biol. Conserv. 2016, 198, 183–192. [Google Scholar] [CrossRef]
- Topp, E.N.; Loos, J.; Martín-López, B. Decision-Making for Nature’s Contributions to People in the Cape Floristic Region: The Role of Values, Rules and Knowledge. Sustain. Sci. 2022, 17, 739–760. [Google Scholar] [CrossRef]
- Van Wilgen, B.W. The Evolution of Fire and Invasive Alien Plant Management Practices in Fynbos. S. Afr. J. Sci. 2009, 105, 335–342. [Google Scholar] [CrossRef]
- van Wilgen, B. Plantation Forestry and Invasive Pines in the Cape Floristic Region: Towards Conflict Resolution. S. Afr. J. Sci. 2015, 111, 1–2. [Google Scholar] [CrossRef]
- Kraaij, T.; Baard, J.A.; Rikhotso, D.R.; Cole, N.S.; Van Wilgen, B.W. Assessing the Effectiveness of Invasive Alien Plant Management in a Large Fynbos Protected Area. Bothalia 2017, 47, a2105. [Google Scholar] [CrossRef]
- Rebelo, A.G.; Holmes, P.M.; Dorse, C.; Wood, J. Impacts of Urbanization in a Biodiversity Hotspot: Conservation Challenges in Metropolitan Cape Town. S. Afr. J. Bot. 2011, 77, 20–35. [Google Scholar] [CrossRef]
- Kingsford, R.T.; Basset, A.; Jackson, L. Wetlands: Conservation’s Poor Cousins. Aquat. Conserv. 2016, 26, 892–916. [Google Scholar] [CrossRef]
- Sieben, E.J.J.; Glen, R.P.; van Deventer, H.; Dayaram, A. The Contribution of Wetland Flora to Regional Floristic Diversity across a Wide Range of Climatic Conditions in Southern Africa. Biodivers. Conserv. 2021, 30, 575–596. [Google Scholar] [CrossRef]
- Adam, E.; Mutanga, O. Spectral Discrimination of Papyrus Vegetation (Cyperus Papyrus L.) in Swamp Wetlands Using Field Spectrometry. ISPRS J. Photogramm. Remote Sens. 2009, 64, 612–620. [Google Scholar] [CrossRef]
- Gallant, A. The Challenges of Remote Monitoring of Wetlands. Remote Sens. 2015, 7, 10938–10950. [Google Scholar] [CrossRef]
- Harris, B.L.; Taylor, C.M.; Weedon, G.P.; Talib, J.; Dorigo, W.; van der Schalie, R. Satellite-Observed Vegetation Responses to Intraseasonal Precipitation Variability. Geophys. Res. Lett. 2022, 49, e2022GL099635. [Google Scholar] [CrossRef]
- Mishra, D.R.; Ghosh, S.; Hladik, C.; O’Connell, J.L.; Cho, H.J. Wetland Mapping Methods and Techniques Using Multisensor, Multiresolution Remote Sensing: Successes and Challenges. In Remote Sensing Handbook; Thenkabail, P.S., Ed.; Taylor and Francis: New York, NY, USA, 2015; Volume 3, pp. 191–226. ISBN 0780312406. [Google Scholar]
- Ozer, E.; Leloglu, U.M. Wetland Spectral Unmixing Using Multispectral Satellite Images. Geocarto Int. 2022, 37, 15754–15777. [Google Scholar] [CrossRef]
- Jiang, J.; Johansen, K.; Tu, Y.-H.; McCabe, M.F. Multi-Sensor and Multi-Platform Consistency and Interoperability between UAV, Planet CubeSat, Sentinel-2, and Landsat Reflectance Data. GIsci Remote Sens. 2022, 59, 936–958. [Google Scholar] [CrossRef]
- Fu, B.; Li, S.; Lao, Z.; Yuan, B.; Liang, Y.; He, W.; Sun, W.; He, H. Multi-Sensor and Multi-Platform Retrieval of Water Chlorophyll a Concentration in Karst Wetlands Using Transfer Learning Frameworks with ASD, UAV, and Planet CubeSate Reflectance Data. Sci. Total Environ. 2023, 901, 165963. [Google Scholar] [CrossRef]
- Islam, M.K.; Simic Milas, A.; Abeysinghe, T.; Tian, Q. Integrating UAV-Derived Information and WorldView-3 Imagery for Mapping Wetland Plants in the Old Woman Creek Estuary, USA. Remote Sens. 2023, 15, 1090. [Google Scholar] [CrossRef]
- Park, S.-I.; Hwang, Y.-S.; Lee, J.-J.; Um, J.-S. Evaluating Operational Potential of UAV Transect Mapping for Wetland Vegetation Survey. J. Coast. Res. 2021, 114. [Google Scholar] [CrossRef]
- Van Alphen, R.; Rains, K.C.; Rodgers, M.; Malservisi, R.; Dixon, T.H. UAV-Based Wetland Monitoring: Multispectral and Lidar Fusion with Random Forest Classification. Drones 2024, 8, 113. [Google Scholar] [CrossRef]
- Wang, C.; Pavelsky, T.M.; Kyzivat, E.D.; Garcia-Tigreros, F.; Podest, E.; Yao, F.; Yang, X.; Zhang, S.; Song, C.; Langhorst, T.; et al. Quantification of Wetland Vegetation Communities Features with Airborne AVIRIS-NG, UAVSAR, and UAV LiDAR Data in Peace-Athabasca Delta. Remote Sens. Environ. 2023, 294, 113646. [Google Scholar] [CrossRef]
- Ramjukadh, C.-L. Are Wetland Plant Communities in the Cape Flora Influenced by Environmental and Land-Use Changes? Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014. [Google Scholar]
- Duncan, P.; Lewarne, M. Using Remote Sensing and GIS Techniques to Detect Changes to the Prince Alfred Hamlet Conservation Area in the Western Cape, South Africa. ISPRS—Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, XLI-B7, 475–481. [Google Scholar] [CrossRef]
- Thamaga, K.H.; Dube, T.; Shoko, C. Evaluating the Impact of Land Use and Land Cover Change on Unprotected Wetland Ecosystems in the Arid-Tropical Areas of South Africa Using the Landsat Dataset and Support Vector Machine. Geocarto Int. 2022, 37, 10344–10365. [Google Scholar] [CrossRef]
- Hope, A.; Albers, N.; Bart, R. Characterizing Post-Fire Recovery of Fynbos Vegetation in the Western Cape Region of South Africa Using MODIS Data. Int. J. Remote Sens. 2012, 33, 979–999. [Google Scholar] [CrossRef]
- Wilson, A.M.; Latimer, A.M.; Silander, J.A. Climatic Controls on Ecosystem Resilience: Postfire Regeneration in the Cape Floristic Region of South Africa. Proc. Natl. Acad. Sci. USA 2015, 112, 9058–9063. [Google Scholar] [CrossRef] [PubMed]
- van Blerk, J.J.; West, A.G.; Smit, J.; Altwegg, R.; Hoffman, M.T. UAVs Improve Detection of Seasonal Growth Responses during Post-Fire Shrubland Recovery. Landsc. Ecol. 2022, 37, 3179–3199. [Google Scholar] [CrossRef]
- Beckett, H. Remote-Sensing of Water Stress in Fynbos Vegetation. Honours Thesis, University of Cape Town, Cape Town, South Africa, 2010. [Google Scholar]
- Jovanovic, N.; Garcia, C.L.; Bugan, R.D.H.; Teich, I.; Rodriguez, C.M.G. Validation of Remotely-Sensed Evapotranspiration and NDWI Using Ground Measurements at Riverlands, South Africa. Water SA 2014, 40, 211–220. [Google Scholar] [CrossRef]
- Skowno, A.L.; Jewitt, D.; Slingsby, J.A. Rates and Patterns of Habitat Loss across South Africa’s Vegetation Biomes. S. Afr. J. Sci. 2021, 117, 1–6. [Google Scholar] [CrossRef]
- Mtengwana, B.; Dube, T.; Mudereri, B.T.; Shoko, C. Modeling the Geographic Spread and Proliferation of Invasive Alien Plants (IAPs) into New Ecosystems Using Multi-Source Data and Multiple Predictive Models in the Heuningnes Catchment, South Africa. GIsci Remote Sens. 2021, 58, 483–500. [Google Scholar] [CrossRef]
- Mtengwana, B.; Dube, T.; Mkunyana, Y.P.; Mazvimavi, D. Use of Multispectral Satellite Datasets to Improve Ecological Understanding of the Distribution of Invasive Alien Plants in a Water-Limited Catchment, South Africa. Afr. J. Ecol. 2020, 58, 709–718. [Google Scholar] [CrossRef]
- Shoko, C.; Mutanga, O.; Dube, T. Remotely Sensed Characterization of Acacia Longifolia Invasive Plants in the Cape Floristic Region of the Western Cape, South Africa. J. Appl. Remote Sens. 2020, 14, 044511. [Google Scholar] [CrossRef]
- Duncan, P.; Podest, E.; Esler, K.J.; Geerts, S.; Lyons, C. Mapping Invasive Herbaceous Plant Species with Sentinel-2 Satellite Imagery: Echium Plantagineum in a Mediterranean Shrubland as a Case Study. Geomatics 2023, 3, 328–344. [Google Scholar] [CrossRef]
- Seymour, D. Wetland Ecotones: Testing Remote Sensing Techniques to Map Ecotones in A Fynbos Embedded Wetland; Stellenbosch University: Stellenbosch, South Africa, 2022. [Google Scholar]
- van Blerk, J.J.; Cramer, M.D.; West, A.G.; Musker, S.D.; Verboom, G.A. The Contribution of Hydric Habitats to the Richness of the Cape Fynbos Flora. Divers. Distrib. 2025, 31, e13962. [Google Scholar] [CrossRef]
- Ndebele, N.E.; Grab, S.; Hove, H. Wet Season Rainfall Characteristics and Temporal Changes for Cape Town, South Africa, 1841–2018. Clim. Past. 2022, 18, 2463–2482. [Google Scholar] [CrossRef]
- Roffe, S.J.; Steinkopf, J.; Fitchett, J.M. South African Winter Rainfall Zone Shifts: A Comparison of Seasonality Metrics for Cape Town from 1841–1899 and 1933–2020. Theor. Appl. Climatol. 2022, 147, 1229–1247. [Google Scholar] [CrossRef]
- Martínez-Carricondo, P.; Agüera-Vega, F.; Carvajal-Ramírez, F.; Mesas-Carrascosa, F.J.; García-Ferrer, A.; Pérez-Porras, F.J. Assessment of UAV-Photogrammetric Mapping Accuracy Based on Variation of Ground Control Points. Int. J. Appl. Earth Obs. Geoinf. 2018, 72, 1–10. [Google Scholar] [CrossRef]
- Ntuli, S.; Forbes, A. Classification of 3D UAS-SfM Point Clouds in the Urban Environment. S. Afr. J. Geomat. 2023, 12, 190–205. [Google Scholar] [CrossRef]
- Onwudinjo, K.C.; Smit, J. Estimating the Performance of Multi-Rotor Unmanned Aerial Vehicle Structure-from-Motion (UAVsfm) Imagery in Assessing Homogeneous and Heterogeneous Forest Structures: A Comparison to Airborne and Terrestrial Laser Scanning. S. Afr. J. Geomat. 2022, 11, 65–83. [Google Scholar] [CrossRef]
- Zeng, C.; Richardson, M.; King, D.J. The Impacts of Environmental Variables on Water Reflectance Measured Using a Lightweight Unmanned Aerial Vehicle (UAV)-Based Spectrometer System. ISPRS J. Photogramm. Remote Sens. 2017, 130, 217–230. [Google Scholar] [CrossRef]
- Tmušić, G.; Manfreda, S.; Aasen, H.; James, M.R.; Gonçalves, G.; Ben-Dor, E.; Brook, A.; Polinova, M.; Arranz, J.J.; Mészáros, J.; et al. Current Practices in UAS-Based Environmental Monitoring. Remote Sens. 2020, 12, 1001. [Google Scholar] [CrossRef]
- Delavarpour, N.; Koparan, C.; Nowatzki, J.; Bajwa, S.; Sun, X. A Technical Study on UAV Characteristics for Precision Agriculture Applications and Associated Practical Challenges. Remote Sens. 2021, 13, 1204. [Google Scholar] [CrossRef]
- Cramer, M.; Przybilla, H.-J.; Zurhorst, A. UAV Cameras: Overview and Geometric Calibration Benchmark. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2017, XLII-2/W6, 85–92. [Google Scholar] [CrossRef]
- Przybilla, H.-J.; Bäumker, M.; Luhmann, T.; Hastedt, H.; Eilers, M. Interaction Between Direct Georeferencing, Control Point Configuration and Camera Self-Calibration for RTK-Based UAV Photogrammetry. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2020, XLIII-B1-2020, 485–492. [Google Scholar] [CrossRef]
- Sai, S.S.; Tjahjadi, M.E.; Rokhmana, C.A. Geometric Accuracy Assessments of Orthophoto Production From UAV Aerial Images. In Proceedings of the 1st International Conference on Geodesy, Geomatics, and Land Administration 2019, Semarang, Indonesia, 24–25 July 2019; KnE Engineering: Semarang, Indonesia, 2019; pp. 333–344. [Google Scholar] [CrossRef]
- Nex, F.; Remondino, F. UAV for 3D Mapping Applications: A Review. Appl. Geomat. 2014, 6, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Rupnik, E.; Meynard, C.; Thom, C.; Pierrot-Deseilligny, M. Simulation and Analysis of Photogrammetric UAV Image Blocks—Influence of Camera Calibration Error. Remote Sens. 2019, 12, 22. [Google Scholar] [CrossRef]
- Harwin, S.; Lucieer, A.; Osborn, J. The Impact of the Calibration Method on the Accuracy of Point Clouds Derived Using Unmanned Aerial Vehicle Multi-View Stereopsis. Remote Sens. 2015, 7, 11933–11953. [Google Scholar] [CrossRef]
- Mathews, A. A Practical UAV Remote Sensing Methodology to Generate Multispectral Orthophotos for Vineyards: Estimation of Spectral Reflectance. Int. J. Appl. Geospat. Res. 2015, 6, 65–87. [Google Scholar] [CrossRef]
- Cao, S.; Danielson, B.; Clare, S.; Koenig, S.; Campos-Vargas, C.; Sanchez-Azofeifa, A. Radiometric Calibration Assessments for UAS-Borne Multispectral Cameras: Laboratory and Field Protocols. ISPRS J. Photogramm. Remote Sens. 2019, 149, 132–145. [Google Scholar] [CrossRef]
- Daniels, L.; Eeckhout, E.; Wieme, J.; Dejaegher, Y.; Audenaert, K.; Maes, W.H. Identifying the Optimal Radiometric Calibration Method for UAV-Based Multispectral Imaging. Remote Sens. 2023, 15, 2909. [Google Scholar] [CrossRef]
- Cubero-Castan, M.; Schneider-Zapp, K.; Bellomo, M.; Shi, D.; Rehak, M.; Strecha, C. Assessment of The Radiometric Accuracy in A Target Less Work Flow Using Pix4D Software. In Proceedings of the 2018 9th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Amsterdam, The Netherlands, 23–26 September 2018; IEEE: New York, NY, USA, 2018; pp. 1–4. [Google Scholar]
- Schneider-Zapp, K.; Cubero-Castan, M.; Shi, D.; Strecha, C. A New Method to Determine Multi-Angular Reflectance Factor from Lightweight Multispectral Cameras with Sky Sensor in a Target-Less Workflow Applicable to UAV. Remote Sens. Environ. 2019, 229, 60–68. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, Y.; An, Z.; Zhang, H.; Han, Y.; Zhao, Z.; Li, F.; Zhang, C.; Hou, C. Assessing Radiometric Calibration Methods for Multispectral UAV Imagery and the Influence of Illumination, Flight Altitude and Flight Time on Reflectance, Vegetation Index and Inversion of Winter Wheat AGB and LAI. Comput. Electron. Agric. 2024, 219, 108821. [Google Scholar] [CrossRef]
- Avtar, R.; Suab, S.A.; Syukur, M.S.; Korom, A.; Umarhadi, D.A.; Yunus, A.P. Assessing the Influence of UAV Altitude on Extracted Biophysical Parameters of Young Oil Palm. Remote Sens. 2020, 12, 3030. [Google Scholar] [CrossRef]
- Guo, Y.; Senthilnath, J.; Wu, W.; Zhang, X.; Zeng, Z.; Huang, H. Radiometric Calibration for Multispectral Camera of Different Imaging Conditions Mounted on a UAV Platform. Sustainability 2019, 11, 978. [Google Scholar] [CrossRef]
- Shetty, S.; Gupta, P.K.; Belgiu, M.; Srivastav, S.K. Assessing the Effect of Training Sampling Design on the Performance of Machine Learning Classifiers for Land Cover Mapping Using Multi-Temporal Remote Sensing Data and Google Earth Engine. Remote Sens. 2021, 13, 1433. [Google Scholar] [CrossRef]
- Dimov, D. Classification of Remote Sensing Time Series and Similarity Metrics for Crop Type Verification. J. Appl. Remote Sens. 2022, 16, 024519. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Jiang, J.; Pan, X.; Huang, X. Spatio-Temporal-Text Fusion for Hierarchical Multi-Label Crop Classification Based on Time-Series Remote Sensing Imagery. Int. J. Appl. Earth Obs. Geoinf. 2025, 139, 104471. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Guo, Y.; Chen, X.; Zhou, R.; Liu, W. A Novel Remote Sensing Image Enhancement Method, the Pseudo-Tasseled Cap Transformation: Taking Buildings and Roads in GF-2 as an Example. Appl. Sci. 2023, 13, 6585. [Google Scholar] [CrossRef]
- Yu, L.; Luo, X.; Croft, H.; Rogers, C.A.; Chen, J.M. Seasonal Variation in the Relationship between Leaf Chlorophyll Content and Photosynthetic Capacity. Plant Cell Environ. 2024, 47, 3953–3965. [Google Scholar] [CrossRef]
- Connelly, L.M. Introduction to Analysis of Variance (ANOVA). MEDSURG Nurs. 2021, 30, 218. [Google Scholar] [CrossRef]
- Fraser, D.A.S. The P-Value Function and Statistical Inference. Am. Stat. 2019, 73, 135–147. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Sheng, C.; Xu, J.; Wu, L. A Review of Wetland Remote Sensing. Sensors 2017, 17, 777. [Google Scholar] [CrossRef] [PubMed]
- Mahdianpari, M.; Granger, J.E.; Mohammadimanesh, F.; Salehi, B.; Brisco, B.; Homayouni, S.; Gill, E.; Huberty, B.; Lang, M. Meta-Analysis of Wetland Classification Using Remote Sensing: A Systematic Review of a 40-Year Trend in North America. Remote Sens. 2020, 12, 1882. [Google Scholar] [CrossRef]
- Mahdavi, S.; Salehi, B.; Granger, J.; Amani, M.; Brisco, B.; Huang, W. Remote Sensing for Wetland Classification: A Comprehensive Review. GIsci Remote Sens. 2018, 55, 623–658. [Google Scholar] [CrossRef]
- Thanh Noi, P.; Kappas, M. Comparison of Random Forest, k-Nearest Neighbor, and Support Vector Machine Classifiers for Land Cover Classification Using Sentinel-2 Imagery. Sensors 2017, 18, 18. [Google Scholar] [CrossRef]
- Judah, A.; Hu, B. The Integration of Multi-Source Remotely-Sensed Data in Support of the Classification of Wetlands. Remote Sens. 2019, 11, 1537. [Google Scholar] [CrossRef]
- Farda, N.M. Multi-Temporal Land Use Mapping of Coastal Wetlands Area Using Machine Learning in Google Earth Engine. IOP Conf. Ser. Earth Environ. Sci. 2017, 98, 012042. [Google Scholar] [CrossRef]
- Okolie, C.; Adeleke, A.; Mills, J.; Smit, J.; Maduako, I.; Bagheri, H.; Komar, T.; Wang, S. Assessment of Explainable Tree-Based Ensemble Algorithms for the Enhancement of Copernicus Digital Elevation Model in Agricultural Lands. Int. J. Image Data Fusion. 2024, 15, 430–460. [Google Scholar] [CrossRef]
- Raczko, E.; Zagajewski, B. Comparison of Support Vector Machine, Random Forest and Neural Network Classifiers for Tree Species Classification on Airborne Hyperspectral APEX Images. Eur. J. Remote Sens. 2017, 50, 144–154. [Google Scholar] [CrossRef]
- Boateng, E.Y.; Otoo, J.; Abaye, D.A. Basic Tenets of Classification Algorithms K-Nearest-Neighbor, Support Vector Machine, Random Forest and Neural Network: A Review. J. Data Anal. Inf. Process. 2020, 8, 341–357. [Google Scholar] [CrossRef]
- Rummell, A.J.; Leon, J.X.; Borland, H.P.; Elliott, B.B.; Gilby, B.L.; Henderson, C.J.; Olds, A.D. Watching the Saltmarsh Grow: A High-Resolution Remote Sensing Approach to Quantify the Effects of Wetland Restoration. Remote Sens. 2022, 14, 4559. [Google Scholar] [CrossRef]
- Fallah, M.; Reza, A.; Zefrehei, P.; Aliakbar Hedayati, S.; Bagheri, T. Comparison of Temporal and Spatial Patterns of Water Quality Parameters in Anzali Wetland (Southwest of the Caspian Sea) Using Support Vector Machine Model. Casp. J. Environ. Sci. 2021, 19, 95–104. [Google Scholar] [CrossRef]
- Magidi, J.; Bangira, T.; Kelepile, M.; Shoko, M. Land Use and Land Cover Changes in Notwane Watershed, Bot-swana, Using Extreme Gradient Boost (XGBoost) Machine Learning Algorithm. Afr. Geogr. Rev. 2024, 1–21. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C.; Merzlyak, M.N. Nondestructive Estimation of Anthocyanin Content in Grapevine Leaves. Am. J. Enol. Vitic. 2009, 60, 87–92. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A. Sensitivity to Foliar Anthocyanin Content of Vegetation Indices Using Green Reflectance. IEEE Geosci. Remote Sens. Lett. 2011, 8, 464–468. [Google Scholar] [CrossRef]
- Bayle, A.; Carlson, B.; Thierion, V.; Isenmann, M.; Choler, P. Improved Mapping of Mountain Shrublands Using the Sentinel-2 Red-Edge Band. Remote Sens. 2019, 11, 2807. [Google Scholar] [CrossRef]
- Rwanga, S.S.; Ndambuki, J.M. Accuracy Assessment of Land Use/Land Cover Classification Using Remote Sensing and GIS. Int. J. Geosci. 2017, 8, 611–622. [Google Scholar] [CrossRef]
- Story, M.; Congalton, R.G. Remote Sensing Brief Accuracy Assessment: A User’s Perspective. Photogramm. Eng. Remote Sens. 1986, 52, 397–399. [Google Scholar]
- Olofsson, P.; Foody, G.M.; Herold, M.; Stehman, S.V.; Woodcock, C.E.; Wulder, M.A. Good Practices for Estimating Area and Assessing Accuracy of Land Change. Remote Sens. Environ. 2014, 148, 42–57. [Google Scholar] [CrossRef]
- Barsi, Á.; Kugler, Z.; László, I.; Szabó, G.; Abdulmutalib, H.M. Accuracy Dimensions in Remote Sensing. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2018, XLII–3, 61–67. [Google Scholar] [CrossRef]






| Plant Name | Plant Family | Average Height | Leaves |
|---|---|---|---|
| Berzelia lanuginosa | Bruniaceae | 1.5 m | Small and narrow in whorls |
| Bobartia gladiata | Iridaceae | 0.8 m | Rigid ensiform |
| Elegia mucronata | Restionaceae | 2.0 m | Stout erect sheaths |
| Erica campanularis | Ericaceae | 0.7 m | Small needle-like |
| Erica intervallaris | Ericaceae | 0.7 m | Incurved, erect squarrose |
| Erica serrata | Ericaceae | 0.7 m | Serrated edges |
| Grubbia rosmarinifolia | Grubbiaceae | 1.3 m | Glossy narrow lanceolate |
| Platycaulos compressus | Restionaceae | 0.5 m | Long and narrow |
| Restio dispar | Restionaceae | 1.0 m | Reed like tufts |
| Restio leptostachyus | Restionaceae | 0.5 m | Feathery plume-like spikelets |
| Tetraria thermalis | Cyperaceae | 0.4 m | Drooping sword-shaped |
| Date | Time | Sensor | Season |
|---|---|---|---|
| 4 October 2018 | 10 h 45 | Parrot Sequoia | Late Spring |
| 10 December 2018 | 14 h 57 | Parrot Sequoia | Early Summer |
| Bands | Centre Wavelength (nm) | Bandwidth (nm) | Reflectance Factor |
|---|---|---|---|
| Green | 550 | 40 | 0.72 |
| Red | 660 | 40 | 0.73 |
| Red Edge | 735 | 10 | 0.72 |
| Near-infrared | 790 | 40 | 0.69 |
| Classes | B | BG | DPC | EM | EC | EI | ES | GR | PC | RD | TT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RF—Overall Accuracy [%] = 87.4% Kappa = 0.85 | |||||||||||
| PA [%] | 85.1 | 83.8 | 88.0 | 94.7 | 88.3 | 89.7 | 35.4 | 74.8 | 93.0 | 47.3 | 87.2 |
| UA [%] | 66.7 | 85.3 | 92.9 | 92.8 | 73.2 | 83.5 | 57.5 | 90.2 | 96.9 | 60.7 | 82.7 |
| Kappa | 0.63 | 0.85 | 0.93 | 0.92 | 0.72 | 0.82 | 0.57 | 0.88 | 0.96 | 0.6 | 0.83 |
| SVM—Overall Accuracy [%] = 83.6% Kappa = 0.81 | |||||||||||
| PA [%] | 89.7 | 85.3 | 78.5 | 96.0 | 87.9 | 84.5 | 42.1 | 67.6 | 85.8 | 46.5 | 96.3 |
| UA [%] | 66.4 | 75.8 | 94.6 | 90.5 | 72.9 | 76.4 | 73.7 | 93.0 | 96.6 | 53.1 | 80.9 |
| Kappa | 0.62 | 0.75 | 0.94 | 0.89 | 0.70 | 0.74 | 0.73 | 0.92 | 0.96 | 0.52 | 0.81 |
| KNN—Overall Accuracy [%] = 85.5% Kappa = 0.83 | |||||||||||
| PA [%] | 85.8 | 89.0 | 77.9 | 93.6 | 90.1 | 88.9 | 53.1 | 74.6 | 86.4 | 65.9 | 76.2 |
| UA [%] | 72.2 | 74.5 | 96.3 | 92.3 | 74.2 | 76.5 | 69.6 | 93.0 | 96.3 | 57.4 | 74.5 |
| Kappa | 0.69 | 0.74 | 0.96 | 0.91 | 0.72 | 0.74 | 0.69 | 0.92 | 0.95 | 0.56 | 0.74 |
| Classes | B | BG | DPC | EM | EC | EI | ES | GR | PC | RD | TT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RF—Overall Accuracy [%] = 88.0% Kappa = 0.86 | |||||||||||
| PA [%] | 33.5 | 69.1 | 100.0 | 100.0 | 96.2 | 86.9 | 31.6 | 65.6 | 99.2 | 64.5 | 100.0 |
| UA [%] | 13.2 | 75.0 | 100.0 | 92.5 | 75.0 | 84.0 | 100.0 | 95.7 | 95.0 | 100.0 | 100.0 |
| Kappa | 0.11 | 0.74 | 1.00 | 0.92 | 0.73 | 0.83 | 1.00 | 0.95 | 0.94 | 1.00 | 1.00 |
| SVM—Overall Accuracy [%] = 61.9% Kappa = 0.56 | |||||||||||
| PA [%] | 95.6 | 37.4 | 21.1 | 100.0 | 73.2 | 46.8 | 33.7 | 64.8 | 92.2 | 3.2 | 6.1 |
| UA [%] | 33.8 | 22.2 | 100.0 | 90.2 | 80.0 | 73.9 | 85.7 | 96.0 | 93.0 | 100.0 | 100.0 |
| Kappa | 0.21 | 0.19 | 1.00 | 0.90 | 0.78 | 0.70 | 0.86 | 0.95 | 0.92 | 1.00 | 1.00 |
| KNN—Overall Accuracy [%] = 85.7% Kappa = 0.83 | |||||||||||
| PA [%] | 69.8 | 18.7 | 100.0 | 100.0 | 92.3 | 79.8 | 54.8 | 77.2 | 93.9 | 22.0 | 100.0 |
| UA [%] | 53.8 | 12.5 | 100.0 | 75.5 | 81.3 | 68.6 | 60.0 | 96.9 | 90.9 | 100.0 | 100.0 |
| Kappa | 0.51 | 0.09 | 1.00 | 0.74 | 0.80 | 0.67 | 0.59 | 0.97 | 0.89 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musungu, K.; Shoko, M.; Smit, J. Determining the Spectral Characteristics of Fynbos Wetland Vegetation Species Using Unmanned Aerial Vehicle Data. Geomatics 2025, 5, 17. https://doi.org/10.3390/geomatics5020017
Musungu K, Shoko M, Smit J. Determining the Spectral Characteristics of Fynbos Wetland Vegetation Species Using Unmanned Aerial Vehicle Data. Geomatics. 2025; 5(2):17. https://doi.org/10.3390/geomatics5020017
Chicago/Turabian StyleMusungu, Kevin, Moreblessings Shoko, and Julian Smit. 2025. "Determining the Spectral Characteristics of Fynbos Wetland Vegetation Species Using Unmanned Aerial Vehicle Data" Geomatics 5, no. 2: 17. https://doi.org/10.3390/geomatics5020017
APA StyleMusungu, K., Shoko, M., & Smit, J. (2025). Determining the Spectral Characteristics of Fynbos Wetland Vegetation Species Using Unmanned Aerial Vehicle Data. Geomatics, 5(2), 17. https://doi.org/10.3390/geomatics5020017








