An Exploratory Randomised Trial of a Self-Managed Home-Based Exaggerated Spatial Cueing Intervention for Handwriting in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Participants
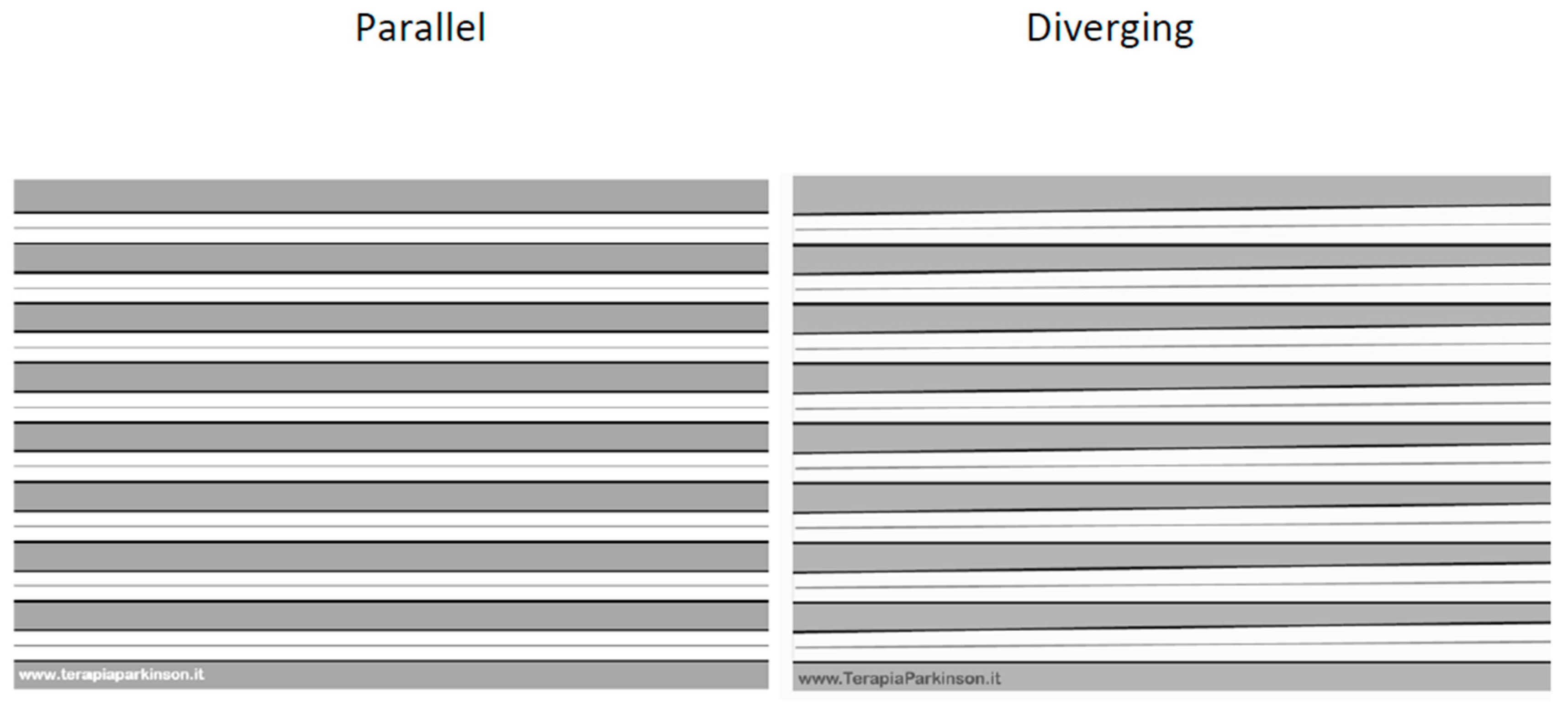
2.4. Intervention
2.4.1. Parallel Group
2.4.2. Diverging Group
2.5. Assessment
2.6. Outcomes
2.6.1. Impairment
2.6.2. Function
2.6.3. Intervention Fidelity
2.7. Analysis
3. Results
3.1. Recruitment and Participant Flow
3.2. Intervention Fidelity
3.3. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disability Language/Terminology Positionality Statement
Abbreviations
| IRQ | Inter Quartile Range |
| SD | Standard deviation |
| 95% CI | 95% Confidence Intervals |
| d | Cohens d |
| OR | Odds Ratio |
| UREC | University Research Ethics committee |
| CONSORT | Consolidated Standards of Reporting Trials |
| TIDieR | Template for Intervention Description and Replication |
| UK | United Kingdom |
| EPTC | European Parkinson’s Therapy Centre |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| mm | millimeters |
| LMM | Linear Mixed Models |
Appendix A
References
- Loewenstein, D.A.; Amigo, E.; Duara, R.; Guterman, A.; Hurwitz, D.; Berkowitz, N.; Wilkie, F.; Weinberg, G.; Black, B.; Gittelman, B.; et al. A New Scale for the Assessment of Functional Status in Alzheimer’s Disease and Related Disorders. J. Gerontol. 1989, 44, P114–P121. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.W.; Ounpraseuth, S.; Okun, M.S.; Gray, V.; Schwankhaus, J.; Metzer, W.S. Micrographia and related deficits in Parkinson’s disease: A cross-sectional study. BMJ Open 2012, 2, e000628. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N.; Alva-Diaz, C.; Morán-Mariños, C.; Mejía-Rojas, K.; Lira, D.; Montesinos, R.; Herrera-Pérez, E.; Castro-Suárez, S.; Bardales, Y. Factors associated with depression in patients with Parkinson’s disease A multicenter study in Lima, Peru. Dement. Neuropsychol. 2018, 12, 292–298. [Google Scholar] [CrossRef]
- Collett, J.; Brusco, N.; Cordell, N.; Cockroft, A.; Lawrie, S.; Coe, S.; Reed, A.; Dawes, H. Lost employment potential and supporting people with Parkinson’s to stay in work: Insights from a Pan European cross-sectional survey. Disabil. Rehabil. 2022, 45, 832–839. [Google Scholar] [CrossRef]
- Foster, E.R.; Carson, L.G.; Archer, J.; Hunter, E.G. Occupational Therapy Interventions for Instrumental Activities of Daily Living for Adults With Parkinson’s Disease: A Systematic Review. Am. J. Occup. Ther. 2021, 75. [Google Scholar] [CrossRef]
- Nackaerts, E.; Heremans, E.; Vervoort, G.; Smits-Engelsman, B.C.; Swinnen, S.P.; Vandenberghe, W.; Bergmans, B.; Nieuwboer, A. Relearning of Writing Skills in Parkinson’s Disease After Intensive Amplitude Training. Mov. Disord. 2016, 31, 1209–1216. [Google Scholar] [CrossRef]
- Vorasoot, N.; Termsarasab, P.; Thadanipon, K.; Pulkes, T. Effects of handwriting exercise on functional outcome in Parkinson disease: A randomized controlled trial. J. Clin. Neurosci. 2020, 72, 298–303. [Google Scholar] [CrossRef]
- Collett, J.; Franssen, M.; Winward, C.; Izadi, H.; Meaney, A.; Mahmoud, W.; Bogdanovic, M.; Tims, M.; Wade, D.; Dawes, H. A long-term self-managed handwriting intervention for people with Parkinson’s disease: Results from the control group of a phase II randomized controlled trial. Clin. Rehabil. 2017, 31, 1636–1645. [Google Scholar] [CrossRef]
- De Vleeschhauwer, J.; Nackaerts, E.; D’cRuz, N.; Vandendoorent, B.; Micca, L.; Vandenberghe, W.; Nieuwboer, A. Associations between resting-state functional connectivity changes and prolonged benefits of writing training in Parkinson’s disease. J. Neurol. 2022, 269, 4696–4707. [Google Scholar] [CrossRef]
- Gardoni, A.; Sarasso, E.; Agosta, F.; Filippi, M.; Corbetta, D. Rehabilitative interventions for impaired handwriting in people with Parkinson’s disease: A scoping review. Neurol. Sci. 2023, 44, 2667–2677. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Hallett, M.; Feng, T.; Hou, Y.; Chan, P. Neural correlates underlying micrographia in Parkinson’s disease. Brain 2015, 139, 144–160. [Google Scholar] [CrossRef]
- Nackaerts, E.; Vervoort, G.; Heremans, E.; Smits-Engelsman, B.C.; Swinnen, S.P.; Nieuwboer, A. Relearning of writing skills in Parkinson’s disease: A literature review on influential factors and optimal strategies. Neurosci. Biobehav. Rev. 2013, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Heremans, E.; Broeder, S.; Nieuwboer, A.; Bekkers, E.M.; Ginis, P.; Janssens, L.; Nackaerts, E. When motor control gets out of hand: Speeding up triggers freezing in the upper limb in Parkinson’s disease. Park. Relat. Disord. 2019, 64, 163–168. [Google Scholar] [CrossRef]
- Tucha, O.; Mecklinger, L.; Thome, J.; Reiter, A.; Alders, G.L.; Sartor, H.; Naumann, M.; Lange, K.W. Kinematic analysis of dopaminergic effects on skilled handwriting movements in Parkinson’s disease. J. Neural Transm. 2005, 113, 609–623. [Google Scholar] [CrossRef]
- Glickstein, M.; Stein, J. Paradoxical movement in Parkinson’s disease. Trends Neurosci. 1991, 14, 480–482. [Google Scholar] [CrossRef]
- Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. [Google Scholar] [CrossRef]
- Heremans, E.; Nackaerts, E.; Broeder, S.; Vervoort, G.; Swinnen, S.P.; Nieuwboer, A. Handwriting Impairments in People With Parkinson’s Disease and Freezing of Gait. Neurorehabilit. Neural Repair 2016, 30, 911–919. [Google Scholar] [CrossRef]
- Miner, D.G.; Aron, A.; DiSalvo, E. Therapeutic effects of forced exercise cycling in individuals with Parkinson’s disease. J. Neurol. Sci. 2020, 410, 116677. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
- Smits, E.J.; Tolonen, A.J.; Cluitmans, L.; van Gils, M.; Conway, B.A.; Zietsma, R.C.; Leenders, K.L.; Maurits, N.M. Standardized Handwriting to Assess Bradykinesia, Micrographia and Tremor in Parkinson’s Disease. PLoS ONE 2014, 9, e97614. [Google Scholar] [CrossRef]
- Chen, H.; Cohen, P.; Chen, S. How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Commun. Stat.-Simul. Comput. 2010, 39, 860–864. [Google Scholar] [CrossRef]
- Blank, R.; Miller, V.; Von Voss, H.; Von Kries, R. Effects of age on distally and proximally generated drawing movements: A kinematic analysis of school children and adults. Dev. Med. Child Neurol. 1999, 1999. 41, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Planton, S.; Jucla, M.; Roux, F.-E.; Démonet, J.-F. The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 2013, 49, 2772–2787. [Google Scholar] [CrossRef]
- Ashby, F.G.; Ennis, J.M.; Spiering, B.J. A neurobiological theory of automaticity in perceptual categorization. Psychol. Rev. 2007, 114, 632–656. [Google Scholar] [CrossRef]
- Waldschmidt, J.G.; Ashby, F.G. Cortical and striatal contributions to automaticity in information-integration catego-rization. Neuroimage 2011, 56, 1791–1802. [Google Scholar] [CrossRef]
- Wu, T.; Liu, J.; Zhang, H.; Hallett, M.; Zheng, Z.; Chan, P. Attention to Automatic Movements in Parkinson’s Disease: Modified Automatic Mode in the Striatum. Cereb. Cortex 2014, 25, 3330–3342. [Google Scholar] [CrossRef]
- Broeder, S.; Vandendoorent, B.; Hermans, P.; Nackaerts, E.; Verheyden, G.; Meesen, R.; de Xivry, J.-J.O.; Nieuwboer, A. Transcranial direct current stimulation enhances motor learning in Parkinson’s disease: A randomized controlled trial. J. Neurol. 2023, 270, 3442–3450. [Google Scholar] [CrossRef]
- Gardoni, A.; Sarasso, E.; Basaia, S.; Corbetta, D.; Zenere, L.; Grassi, A.; Canu, E.; Castelnovo, V.; Sibilla, E.; Malcangi, M.; et al. Handwriting, touchscreen dexterity and bradykinesia measures in Parkinson’s disease: A feature selection study. J. Neurol. 2025, 272, 389. [Google Scholar] [CrossRef]
- Vanbellingen, T.; Nyffeler, T.; Nigg, J.; Janssens, J.; Hoppe, J.; Nef, T.; Müri, R.M.; van Wegen, E.E.; Kwakkel, G.; Bohlhalter, S. Home based training for dexterity in Parkinson’s disease: A randomized controlled trial. Park. Relat. Disord. 2017, 41, 92–98. [Google Scholar] [CrossRef]
- Llamas-Velasco, S.; Ferreiro, C.R.; Fuertes, Á.G.; Tell, P.G.; Blanco-Palmero, V.A.; Martín-Jimenez, P.; Martínez, D.A.P.; Méndez-Guerrero, A. Home calligraphic exercises as manual dexterity training in patients with Parkinson’s disease: A pilot feasibility study. Disabil. Rehabil. 2023, 46, 870–874. [Google Scholar] [CrossRef] [PubMed]

| Demographics | Diverging | Parallel | Difference |
|---|---|---|---|
| n = 20 | n = 22 | ||
| Age (years) | 66.2 ± 7.2 | 66.7 ± 0.8.7 | p = 0.827 |
| Progressive micrograpthia (Y:N) | 15:5 | 16:6 | p = 0.867 |
| L-Dopa (Y:N) | 14:6 | 18:4 | p = 0.369 |
| Gender (M:F) | 11:9 | 14:8 | p = 0.569 |
| Years since diagnosis | 9.0 ± 6.3 | 5.3 ± 4.2 | p = 0.034 |
| Hoen Yahr | 2 (2–2.5) | 2 (1–3) | p = 0.890 |
| el sequence | |||
| Speed (s) | 93.2 ± 53.8 | 101.5 ± 77.2 | p = 0.708 |
| Amplitude (mm) | 5.4 ± 1.7 | 5.0 ± 1.6 | p = 0.467 |
| Ratio (%) | 103.2 ± 38.3 | 92.5 ± 25.3 | p = 0.337 |
| Dual Task el sequence | |||
| Speed (s) | 145.1 ± 106.9 | 175.2 ± 167.8 | p = 0.543 |
| Amplitude (mm) | 5.1 ± 2.0 | 4.8 ± 1.7 | p = 0.639 |
| Ratio (%) | 109.7 ± 35.6 | 119.0 ± 36.6 | p = 0.463 |
| Handwriting | |||
| Pangram speed (s) | 75.0 ± 56.2 | 47.2 ± 15.6 | p = 0.057 |
| ‘Lascia’ (mm) | 5.5 ± 0.15 | 5.3 ± 1.4 | p = 0.330 |
| ‘Lascia’ ratio (%) | 91.5 ± 17.0 | 90.1 ± 23.2 | p = 0.848 |
| Free writing words | 65 ± 39 | 80 ± 34 | p = 0.098 |
| Free writing legible words | 50 ± 36 | 68 ± 32 | p = 0.054 |
| UPDRS 2.7 | 2 (1–4) | 2 (1–3) | p = 0.650 |
| Impairment | Pre-Post | Diverging | Parallel | Between Group | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Weeks | Baseline | 6 Weeks | |||||||||||||||||||
| d, (95% CI), p | EMM ± Standard Error | EMM ± Standard Error | EMM ± Standard Error | EMM ± Standard Error | d (95% CI), p | |||||||||||||||||
| el sequence | ||||||||||||||||||||||
| Speed (s) | −0.90 (−1.41: −0.38), 0.001 | 93.2 ± 14.2 | 76.6 ± 14.5 | 101.5 ± 13.8 | 82 ± 14.2 | 0.11 (−0.54: 0.75), 0.904 | ||||||||||||||||
| Amplitude (mm) | 1.07 (0.49: 1.66), <0.001 | 5.4 ± 0.4 | 6.4 ± 0.4 | 5.0 ± 0.4 | 5.6 ± 0.4 | 0.34 (−0.35: 1.02), 0.404 | ||||||||||||||||
| Ratio (%) | 0.27 (−0.22: 0.77), 0.279 | 103.2 ± 10.4 | 92.32 ± 7.3 | 92.5 ± 6.6 | 91.5 ± 7.1 | −0.28 (−0.38: 0.95), 0.497 | ||||||||||||||||
| Dual-Task el sequence | ||||||||||||||||||||||
| speed (s) | −0.72 (−1.24: −0.21), 0.09 | 145.1 ± 31.3 | 124.1 ± 32.0 | 175.2 ± 29.5 | 135.4 ± 30.1 | 0.25 (−0.43: 0.92), 0.597 | ||||||||||||||||
| Amplitude (mm) | 0.86 (0.35: 1.37), 0.002 | 5.1 ± 0.5 | 5.7 ± 0.5 | 4.8 ± 0.5 | 5.3 ± 0.5 | 0.27 (−0.40: 0.93), 0.539 | ||||||||||||||||
| Ratio (%) | 0.07 (−0.42: 0.56), 0.779 | 109.7 ± 7.6 | 115.4 ± 8.4 | 119.0 ± 7.4 | 108.9 ± 8.1 | 0.26 (−0.41: 0.92), 0.570 | ||||||||||||||||
| Pangram | ||||||||||||||||||||||
| Speed (s) | 0.08 (−0.57: 0.72), 0.812 | 75.0 ± 9.8 | 78.1 ± 9.9 | 47.2 ± 9.7 | 51.7 ± 9.8 | 0.48 (−0.00: 0.96), 0.032 | ||||||||||||||||
| ‘Lascia’ (mm) | 0.80 (0.30: 1.29), 0.003 | 5.5 ± 0.4 | 5.8 ± 0.4 | 5.3 ± 0.4 | 6.1 ± 0.4 | −0.26 (−0.38: 0.91), 0.529 | ||||||||||||||||
| ‘Lascia’ ratio (%) | 0.28 (−0.20: 0.77), 0.260 | 91.5 ± 4.9 | 97.7 ± 5.5 | 90.1 ± 4.8 | 92.9 ± 5.2 | 0.15 (−0.50: 0.81), 0.814 | ||||||||||||||||
| Free writing | ||||||||||||||||||||||
| Number of words | 0.03 (−0.47: 0.53), 0.910 | 65 ± 28 | 71 ± 29 | 80 ± 8 | 75 ± 8 | 0.34 (−0.28: 0.97), 0.275 | ||||||||||||||||
| Number of legible words | 0.16 (−0.30: 0.63), 0.497 | 50 ± 8 | 57 ± 8 | 68 ± 7 | 65 ± 8 | 0.40 (−0.24: 1.03), 0.227 | ||||||||||||||||
| Perceived difficulties | OR, (95% CI), p | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | OR, (95% CI), p |
| % response each level | −3.6 (−12.6: −1.0), 0.047 | 5 | 21 | 32 | 16 | 19 | 13 | 33 | 40 | 13 | 0 | 0 | 45 | 25 | 20 | 10 | 11 | 53 | 24 | 6 | 6 | 0.7 (0.1: 4.1), 0.723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreoli, D.; Reed, A.; Coe, S.; Dawes, H.; Collett, J. An Exploratory Randomised Trial of a Self-Managed Home-Based Exaggerated Spatial Cueing Intervention for Handwriting in Parkinson’s Disease. Disabilities 2025, 5, 93. https://doi.org/10.3390/disabilities5040093
Andreoli D, Reed A, Coe S, Dawes H, Collett J. An Exploratory Randomised Trial of a Self-Managed Home-Based Exaggerated Spatial Cueing Intervention for Handwriting in Parkinson’s Disease. Disabilities. 2025; 5(4):93. https://doi.org/10.3390/disabilities5040093
Chicago/Turabian StyleAndreoli, Daria, Alex Reed, Shelly Coe, Helen Dawes, and Johnny Collett. 2025. "An Exploratory Randomised Trial of a Self-Managed Home-Based Exaggerated Spatial Cueing Intervention for Handwriting in Parkinson’s Disease" Disabilities 5, no. 4: 93. https://doi.org/10.3390/disabilities5040093
APA StyleAndreoli, D., Reed, A., Coe, S., Dawes, H., & Collett, J. (2025). An Exploratory Randomised Trial of a Self-Managed Home-Based Exaggerated Spatial Cueing Intervention for Handwriting in Parkinson’s Disease. Disabilities, 5(4), 93. https://doi.org/10.3390/disabilities5040093






