The Feasibility of Whole-Body Vibration Training as an Approach to Improve Health in Autistic Adults
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Participants
2.3. Procedures
2.4. Measures
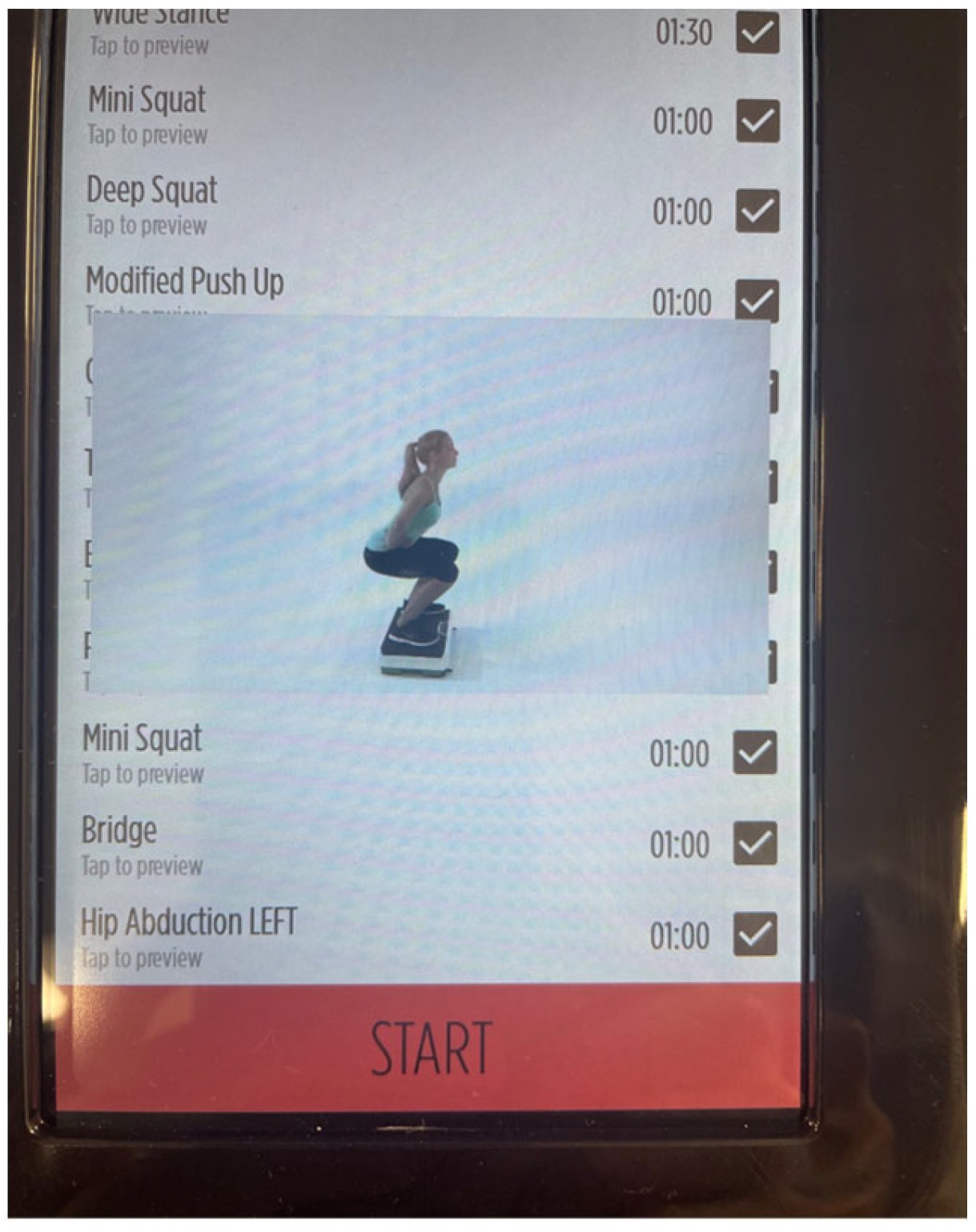
2.5. WBV Intervention
2.6. Statistical Analyses
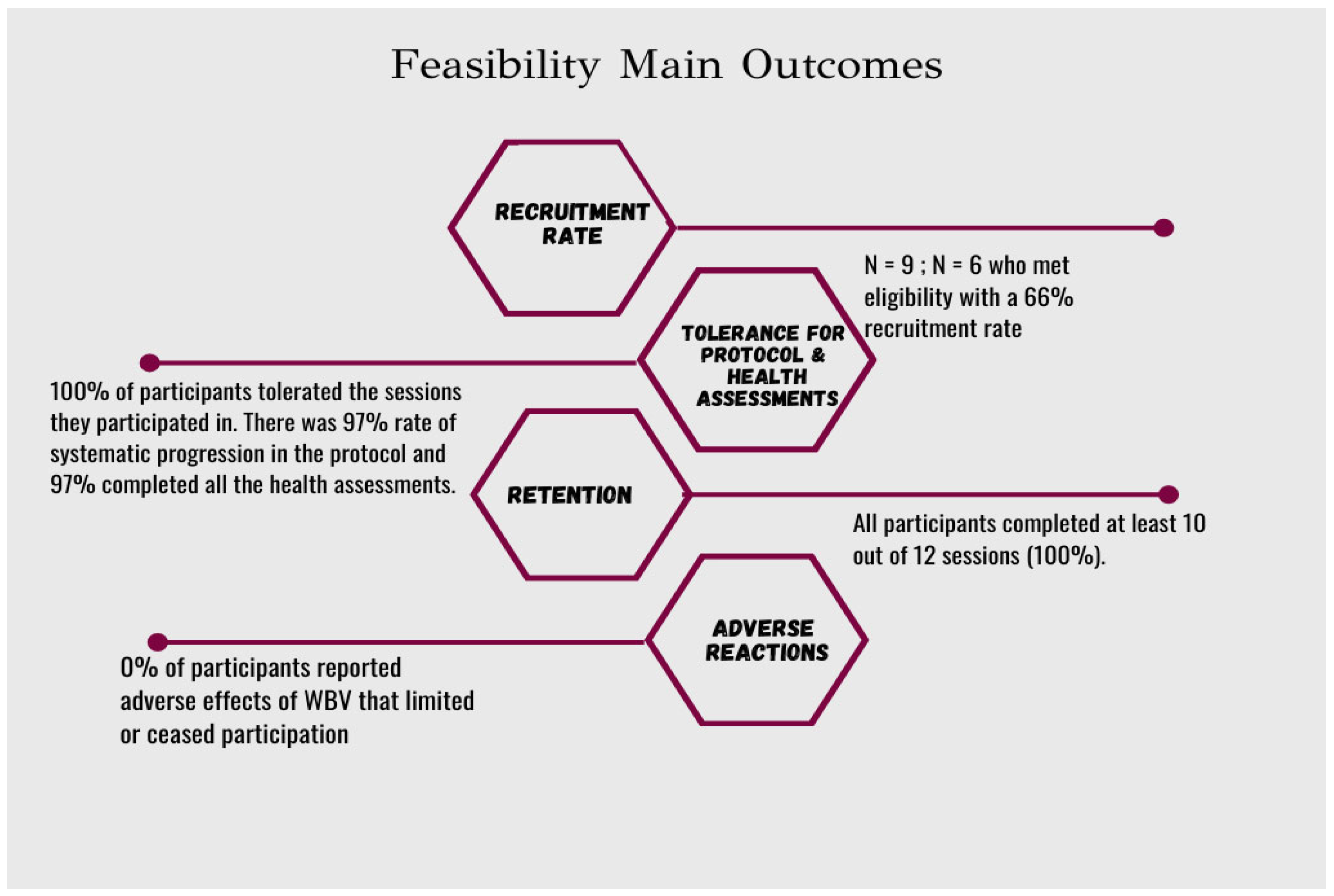
3. Results
Qualitative Feedback from Study Participants
4. Discussion
4.1. Feasibility of WBV Training in an ASD Population
4.2. Cardiovascular Biomarker and Muscular Strength Outcomes
4.3. Strengths and Limitations
4.4. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 2 December 2021).
- Centers for Disease Control and Prevention. Heart Disease Facts. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 27 September 2021).
- Bilder, D.; Botts, E.L.; Smith, K.R.; Pimentel, R.; Farley, M.; Viskochil, J.; McMahon, W.M.; Block, H.; Ritvo, E.; Ritvo, R.A.; et al. Excess mortality and causes of death in autism spectrum disorders: A follow up of the 1980s Utah/UCLA autism epidemiologic study. J. Autism Dev. Disord. 2013, 43, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Mouridsen, S.E.; Brønnum-Hansen, H.; Rich, B.; Isager, T. Mortality and causes of death in autism spectrum disorders: An update. Autism 2008, 12, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Chomistek, A.K.; Manson, J.E.; Stefanick, M.L.; Lu, B.; Sands-Lincoln, M.; Going, S.B.; Garcia, L.; Allison, M.A.; Sims, S.T.; LaMonte, M.J.; et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: Results from the Women’s Health Initiative. J. Am. Coll. Cardiol. 2013, 61, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Sr.; Pencina, M.J.; Massaro, J.M.; Coady, S. Cardiovascular disease risk assessment: Insights from Framingham. Glob. Heart 2013, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Broder-Fingert, S.; Brazauskas, K.; Lindgren, K.; Iannuzzi, D.; Van Cleave, J. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad. Pediatr. 2014, 14, 408–414. [Google Scholar] [CrossRef]
- Corbett, B.A.; Muscatello, R.A.; Horrocks, B.K.; Klemencic, M.E.; Tanguturi, Y. Differences in Body Mass Index (BMI) in Early adolescents with autism spectrum disorder compared to youth with typical development. J. Autism Dev. Disord. 2021, 51, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Columna, L.; Russo, N.; Myers, B.A.; Ashby, C.E.; Norris, M.L.; Barreira, T.V. Brief Report: Physical Activity, Body mass index and arterial stiffness in children with autism spectrum disorder: Preliminary findings. J. Autism Dev. Disord. 2018, 48, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Gil, R.; Wong, A.; Hooshmand, S.; Park, S.Y.; Vicil, F.; Sanchez-Gonzalez, M.A. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens. Res. 2012, 35, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.; Block, M.E.; Bishop, J.C.; McIntire, B. Physical activity in young adults with autism spectrum disorder: Parental perceptions of barriers and facilitators. Autism 2018, 23, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.E.; Thomas, E.A.; Rynders, C.; Holliman, B.D.; Perreira, C.; Ostendorf, D.M.; Catenacci, V.A. Improving lifestyle obesity treatment during the COVID-19 pandemic and beyond: New challenges for weight management. Obes. Sci. Pract. 2021, 8, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Hallett, R. Physical Activity for Autistic Adults: Recommendations for a Shift in Approach. Autism Adulthood. 2019, 1, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.N.; Tomaszewski, B.T.; Hume, K.A. Step It Up: Increasing Physical Activity for Adults With Autism Spectrum Disorder and Intellectual Disability Using Supported Self-Management and Fitbit Technology. Focus Autism Other Dev. Disabilities 2022, 37, 146–157. [Google Scholar] [CrossRef]
- Shields, N.; Willis, C.; Imms, C.; McKenzie, G.; van Dorsselaer, B.; Bruder, A.M.; Kennedy, R.A.; Bhowon, Y.; Southby, A.; Prendergast, L.A.; et al. Feasibility of scaling-up a community-based exercise program for young people with disability. Disabil. Rehabil. 2022, 44, 1669–1681. [Google Scholar] [CrossRef]
- Chanou, K.; Gerodimos, V.; Karatrantou, K.; Jamurtas, A. Whole-body vibration and rehabilitation of chronic diseases: A review of the literature. J. Sports Sci. Med. 2012, 11, 187–200. [Google Scholar]
- Park, S.Y.; Son, W.M.; Kwon, O.S. Effects of whole-body vibration training on body composition, skeletal muscle strength, and cardiovascular health. J. Exerc. Rehabil. 2015, 11, 289–295. [Google Scholar] [CrossRef]
- Fischer, M.; Vialleron, T.; Laffaye, G.; Fourcade, P.; Hussein, T.; Chèze, L.; Deleu, P.A.; Honeine, J.L.; Yiou, E.; Delafontaine, A. Long-term effects of whole-body vibration on human gait: A systematic review and meta-analysis. Front. Neurol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Matute-Llorente, A.; González-Agüero, A.; Gómez-Cabello, A.; Vicente-Rodríguez, G.; Casajús Mallén, J.A. Effect of whole-body vibration therapy on health-related physical fitness in children and adolescents with disabilities: A systematic review. J. Adolesc. Health 2014, 54, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Pin, T.W.; Butler, P.B.; Purves, S. Use of whole-body vibration therapy in individuals with moderate severity of cerebral palsy- A feasibility study. BMC Neurol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Saquetto, M.B.; Pereira, F.F.; Queiroz, R.S.; da Silva, C.M.; Conceição, C.S.; Gomes Neto, M. Effects of whole-body vibration on muscle strength, bone mineral content and density, and balance and body composition of children and adolescents with Down syndrome: A systematic review. Osteoporos. Int. 2018, 29, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Bemben, D.; Stark, C.; Taiar, R.; Bernardo-Filho, M. Relevance of Whole-Body Vibration Exercises on Muscle Strength/Power and Bone of Elderly Individuals. Dose Response 2018, 16, 1559325818813066. [Google Scholar] [CrossRef] [PubMed]
- Ruhde, L.; Hulla, R. An overview of the effects of whole-body vibration on individuals with cerebral palsy. J. Pediatr. Rehabil. Med. 2022, 15, 193–210. [Google Scholar] [CrossRef]
- Nowak-Lis, A.; Nowak, Z.; Gabrys, T.; Szmatlan-Gabrys, U.; Batalik, L.; Knappova, V. The Use of Vibration Training in Men after Myocardial Infarction. Int. J. Environ. Res. Public Health 2022, 19, 3326. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Oliveira, A.C.; Lacerda, A.C.R.; de Souza, A.L.C.; Santos, L.M.D.M.; da Fonseca, S.F.; Dos Santos, J.M.; Ribeiro, V.G.C.; Leite, H.R.; Figueiredo, P.H.S.; Fernandes, J.S.C.; et al. Acute whole-body vibration exercise promotes favorable handgrip neuromuscular modifications in rheumatoid arthritis: A cross-over randomized clinical. BioMed Res. Int. 2021, 2021, 9774980. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.D.; Palombo, K.T.M.; Feland, J.B.; Blotter, J.D. Effects of whole-body vibration on flexibility and stiffness: A literature review. Int. J. Exerc. Sci. 2019, 12, 735–747. [Google Scholar]
- Osawa, Y.; Oguma, Y.; Ishii, N. The effects of whole-body vibration on muscle strength and power: A meta-analysis. J. Musculoskelet. Neuronal Interact. 2013, 13, 380–390. [Google Scholar] [PubMed]
- Li, K.; Cho, Y.; Chen, R. The effect of whole-body vibration on proprioception and motor function for individuals with moderate Parkinson disease: A single-blind randomized controlled trial. Hilton C, ed. Occup. Ther. Int. 2021, 2021, 9441366. [Google Scholar] [CrossRef] [PubMed]
- Marín-Cascales, E.; Alcaraz, P.; Ramos-Campo, D.; Martinez-Rodriguez, A.; Chung, L.; Rubio-Arias, J. Whole-body vibration training and bone health in postmenopausal women: A systematic review and meta-analysis. Medicine 2018, 97, e11918. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.; Liao, L.R.; Kwok, T.C.; Pang, M.Y. The effect of vertical whole-body vibration on lower limb muscle activation in elderly adults: Influence of vibration frequency, amplitude and exercise. Maturitas 2016, 88, 59–64. [Google Scholar] [CrossRef]
- Severino, G.; Sanchez-Gonzalez, M.; Walters-Edwards, M.; Nordvall, M.; Chernykh, O.; Adames, J.; Wong, A. Whole-body vibration training improves heart rate variability and body fat percentage in obese Hispanic postmenopausal women. J. Aging Phys. Act. 2017, 25, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Alvarez-Alvarado, S.; Kinsey, A.W.; Figueroa, A. Whole-Body vibration exercise therapy improves cardiac autonomic function and blood pressure in obese pre- and stage 1 hypertensive postmenopausal women. J. Altern. Complement. Med. 2016, 22, 970–976. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of whole-body vibration on motor impairments in patients with neurological disorders: A systematic review. Am. J. Phys. Med. Rehabil. 2019, 98, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Tsai, C.H.; Lu, M.K.; Tseng, H.C.; Lu, G.; Liu, B.L.; Lin, H.C. The neuromuscular responses in patients with Parkinson’s disease under different conditions during whole-body vibration training. BMC Complement. Med. Ther. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Santos-Filho, S.D.; Cameron, M.; Bernardo-Filho, M. Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis: A systematic review. Mult. Scler. Int. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-D.; Chen, S.; Tsou, Y.-A. Effects of whole-body vibration and balance training on female athletes with chronic ankle instability. J. Clin. Med. 2021, 10, 2380. [Google Scholar] [CrossRef] [PubMed]
- González-Agüero, A.; Matute-Llorente, Á.; Gómez-Cabello, A.; Casajús, J.A.; Vicente-Rodríguez, G. Effects of whole-body vibration training on body composition in adolescents with down syndrome. Res. Dev. Disabil. 2013, 34, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.A. Effect of Whole-Body Vibration Training on Standing Balance and Muscle Strength in Children with Down Syndrome. Am. J. Phys. Med. Rehabil. 2015, 94, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Duquette, S.A.; Guiliano, A.M.; Starmer, D.J. Whole body vibration and cerebral palsy: A systematic review. J. Can. Chiropr. Assoc. 2015, 59, 245–252. [Google Scholar] [PubMed]
- Havercamp, S.M.; Tassé, M.J.; Navas, P.; Benson, B.A.; Allain, D.; Manickam, K. Exploring the weight and health status of adults with down syndrome. J. Educ. Train. Stud. 2017, 5, 97. [Google Scholar] [CrossRef]
- El-Kotob, R.; Giangregorio, L.M. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Study 2018, 4, 137. [Google Scholar] [CrossRef] [PubMed]
- Orsmond, G.I.; Cohn, E.S. The distinctive features of a feasibility study. OTJR Occup. Particip. Health 2015, 35, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; de Sousa, N.M.F.; Prestes, J.; da Cunha Nascimento, D.; Ernesto, C.; Falk Neto, J.H.; Kennedy, M.D.; Voltarelli, F.A. Is Perceived Exertion a Useful Indicator of the Metabolic and Cardiovascular Responses to a Metabolic Conditioning Session of Functional Fitness? Sports 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Wall Sit Test. Testsforsports. Available online: https://testsforsports.com/strength/wall-sit-test (accessed on 25 May 2019).
- Lancaster, G.A.; Thabane, L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot. Feasibility Stud. 2019, 5, 114. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; PAFS Consensus Group. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Statistical notes for clinical researchers: Nonparametric statistical methods: 1. Nonparametric methods for comparing two groups. Restor. Dent. Endod. 2014, 39, 235–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liguori, G. ACSMs Guidelines for Exercise Testing and Prescription; Wolters Kluwers: Philadelphia, PA, USA, 2022; ISBN 9781975150181. [Google Scholar]
- Scheerer, N.E.; Curcin, K.; Stojanoski, B.; Anagnostou, E.; Nicolson, R.; Kelley, E.; Georgiades, S.; Liu, X.; Stevenson, R.A. Exploring sensory phenotypes in autism spectrum disorder. Mol. Autism 2021, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Bressel, E.; Gibbons, M.W.; Samaha, A. Effect of whole-body vibration on stereotypy of young children with autism. Case Rep. 2011, 2011, bcr0220113834. [Google Scholar] [CrossRef] [PubMed]
- Besag, F.M. Epilepsy in patients with autism: Links, risks, and treatment challenges. Neuropsychiatr. Dis. Treat. 2017, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]



| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Make up Week e | |
|---|---|---|---|---|---|---|---|
| Hertz (Hz) | S1: 5–7 S2: 6–7 | S1: 8–15 S2: 8–15 | S1: 6–18 S2: 6–18 | S1: 6–24 c S2: 6–24 c | S1: 12–24 S2: 12–24 | S1: 12–25 d S2: 12–25 d | S1: 12–25 |
| Number of exercises | S1: 1 S2: 1 | S1: 12 S2: 12 | S1: 15 S2: 15 | S1: 15 S2: 15 | S1: 14–15 S2: 14–15 | S1: 10–14 d S2: 10–14 d | S1: 14 |
| Total time (minutes) | S1: 10 S2: 10 | S1: 21 S2: 21 | S1: 23 S2: 23 | S1: 23–26 c S2: 23–26 c | S1: 23–26 S2. 23–26 | S1: 19–24 S2: 19–24 | S1:24 |
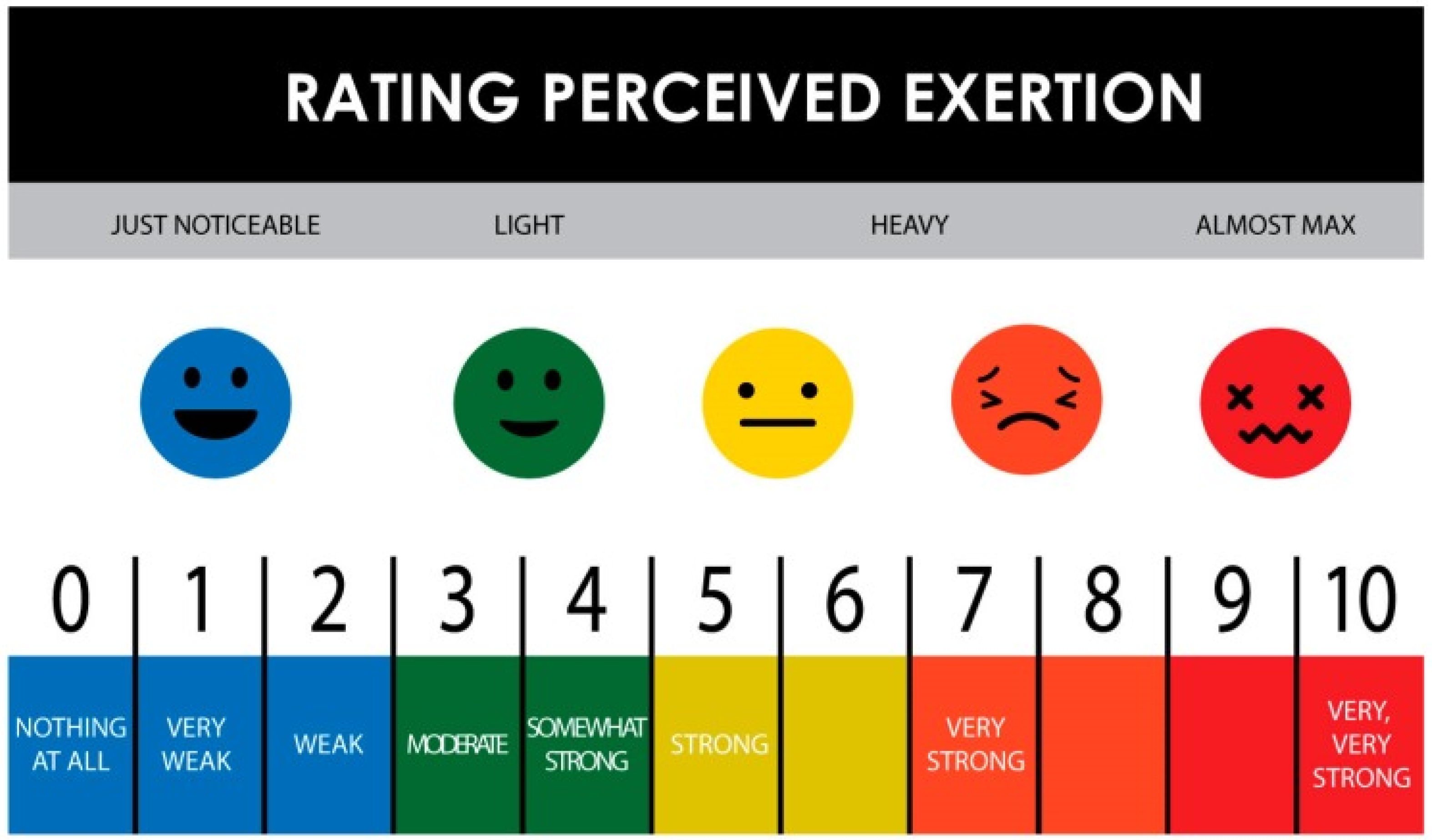
| Rating of Perceived exertion (RPE: 1–10) | S1: 1–3.5 S2: 0–8 a | S1: 1–6 b S2: 1–7 | S1: 2–6 S2: 1–9 | S1: 2–7 S2: 2–7 | S1: 2–7 S2: 2–7 | S1: 1–7 S2: 1–8 f | S1: 7 |
| Baseline (Pre) | Post-Intervention | Pre-Percentiles (50th) Median | Post-Percentiles (50th) Median | |
|---|---|---|---|---|
| Body mass (kg) | 73.41 ± (15.71) | 73.13 ± (13.93) | 71.37 | 72.96 |
| BMI (kg/m2) | 25.16 ± (4.30) | 25.07 ± (3.70) | 21.59 | 21.93 |
| Body Composition (% BF) | 26.46 ± (7.97) | 27.24 ± (8.32) | 29.80 | 30.00 |
| WHR a | 0.848 ± (0.080) | 0.826 ± (0.086) | 0.846 | 0.795 |
| Leg Strength (s) b | 20.55 ± (15.06) | 28.46 ± (12.16) | 13.35 | 29.89 |
| Systolic BP (mmHg) | 119.83 ± (13.72) | 123.33 ± (8.52) | 117.0 | 118.5 |
| Diastolic BP (mmHg) | 76.83 ± (8.86) | 76.67 ± (6.74) | 76.0 | 78.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allnutt, A.; Pappa, S.; Nordvall, M. The Feasibility of Whole-Body Vibration Training as an Approach to Improve Health in Autistic Adults. Disabilities 2024, 4, 429-443. https://doi.org/10.3390/disabilities4030027
Allnutt A, Pappa S, Nordvall M. The Feasibility of Whole-Body Vibration Training as an Approach to Improve Health in Autistic Adults. Disabilities. 2024; 4(3):429-443. https://doi.org/10.3390/disabilities4030027
Chicago/Turabian StyleAllnutt, Amy, Sara Pappa, and Michael Nordvall. 2024. "The Feasibility of Whole-Body Vibration Training as an Approach to Improve Health in Autistic Adults" Disabilities 4, no. 3: 429-443. https://doi.org/10.3390/disabilities4030027
APA StyleAllnutt, A., Pappa, S., & Nordvall, M. (2024). The Feasibility of Whole-Body Vibration Training as an Approach to Improve Health in Autistic Adults. Disabilities, 4(3), 429-443. https://doi.org/10.3390/disabilities4030027






