Preparation and Study of Bright Orange-Yellow Long Persistent Luminescent Ca2LuScGa2Ge2O12:Pr3+ Phosphor
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ca2LuScGa2Ge2O12:xPr3+ (x = 0.003, 0.005, 0.01, 0.02, 0.03, 0.04, 0.05)
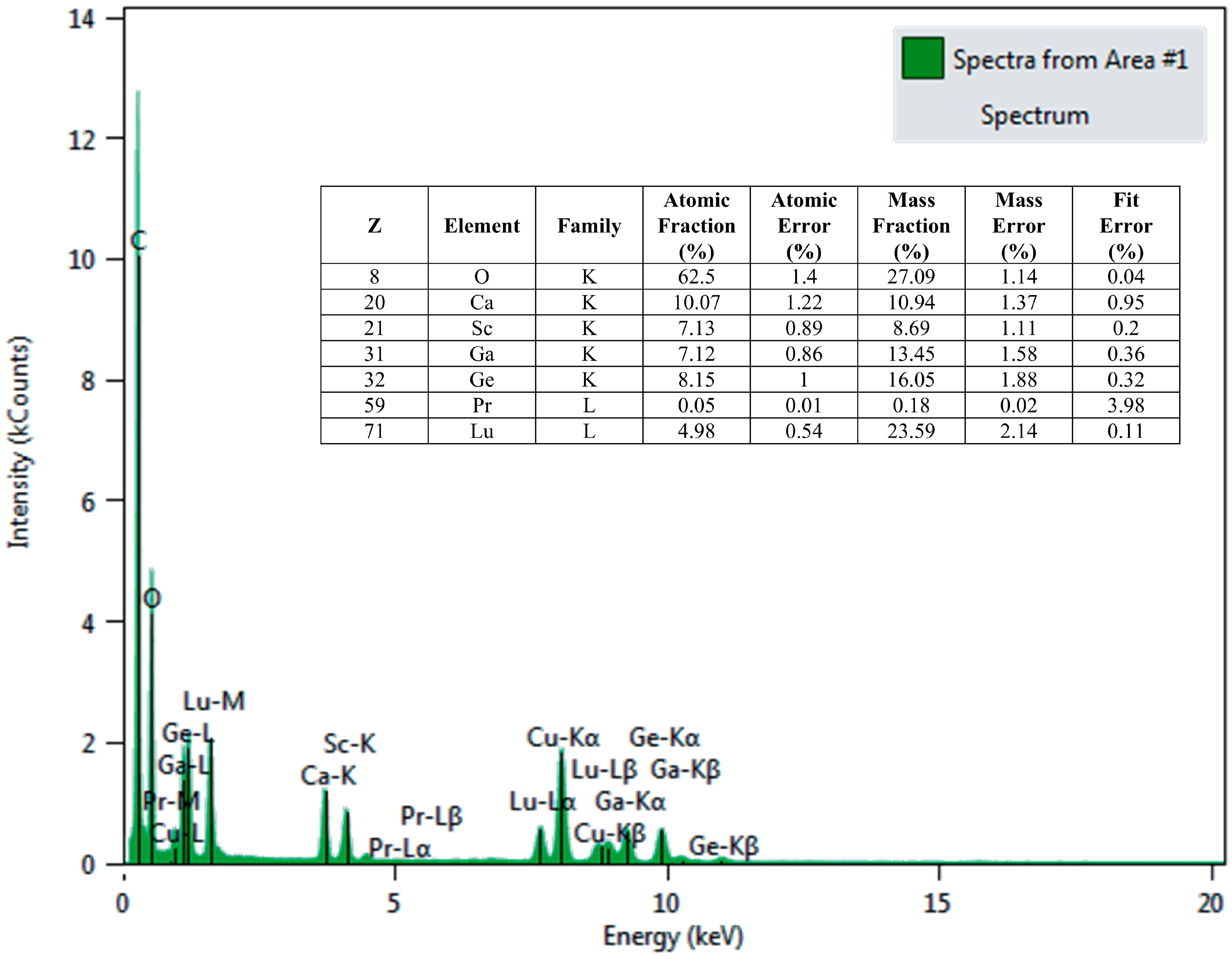
2.2. Characterizations
3. Results
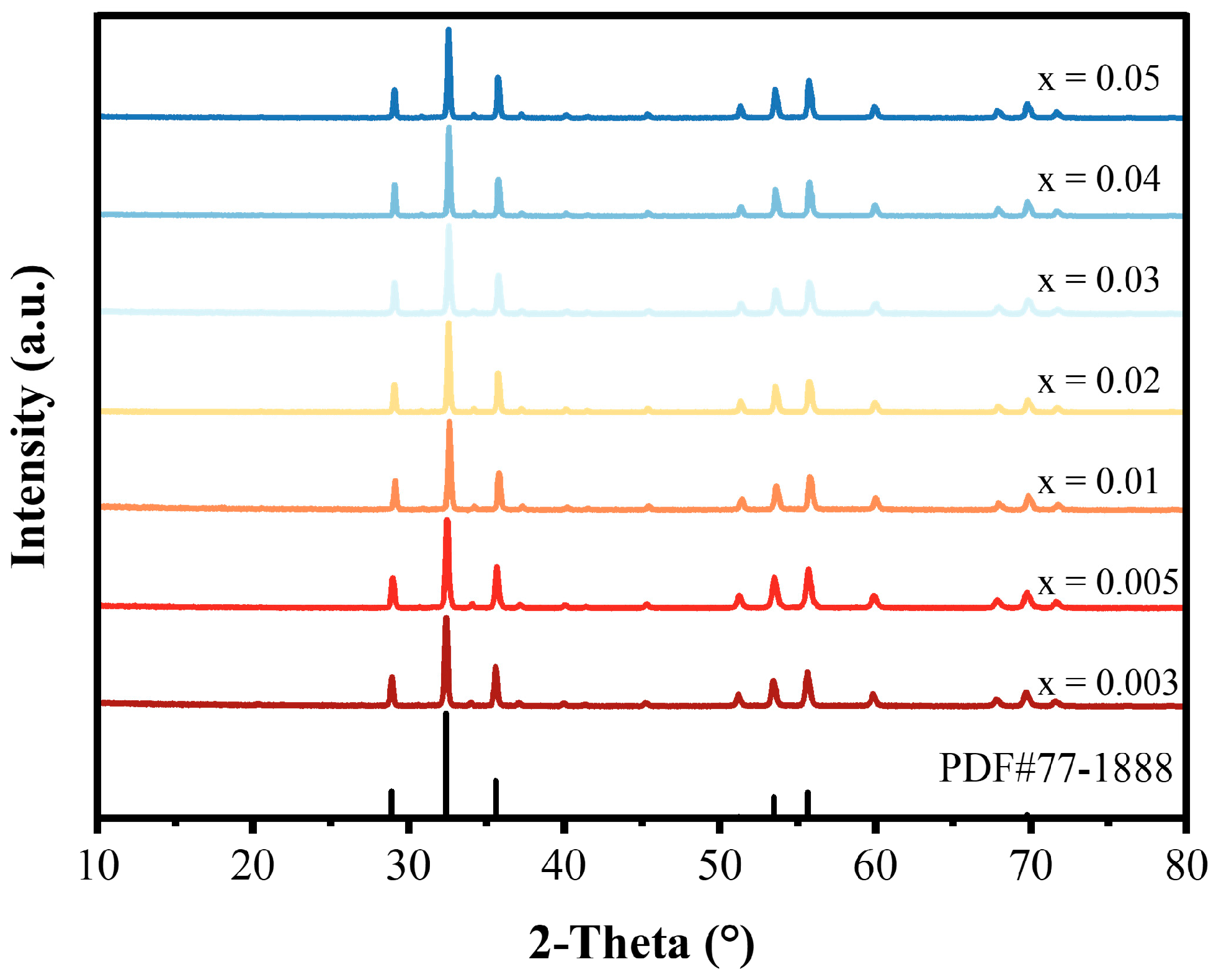
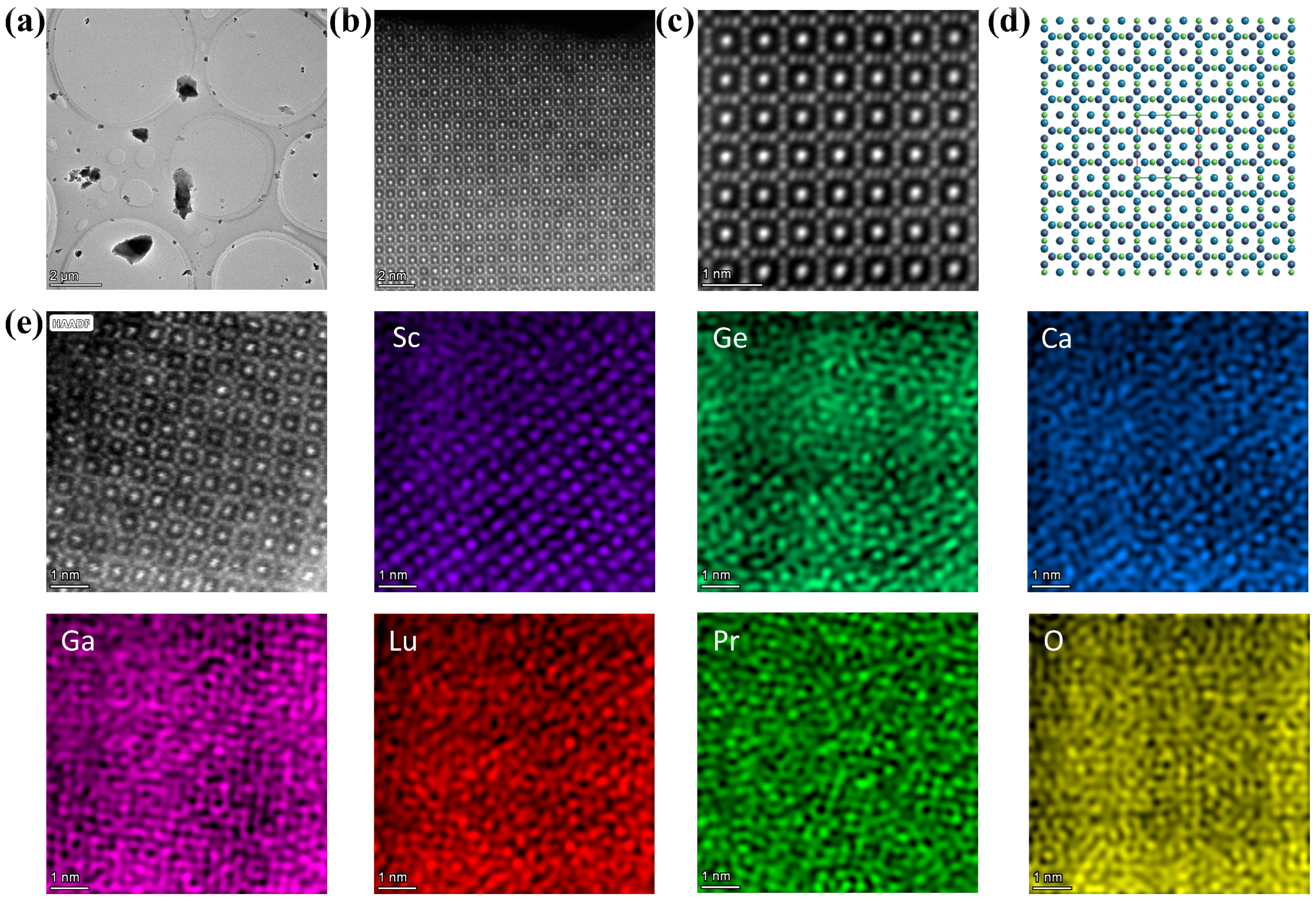
3.1. Crystal Structure
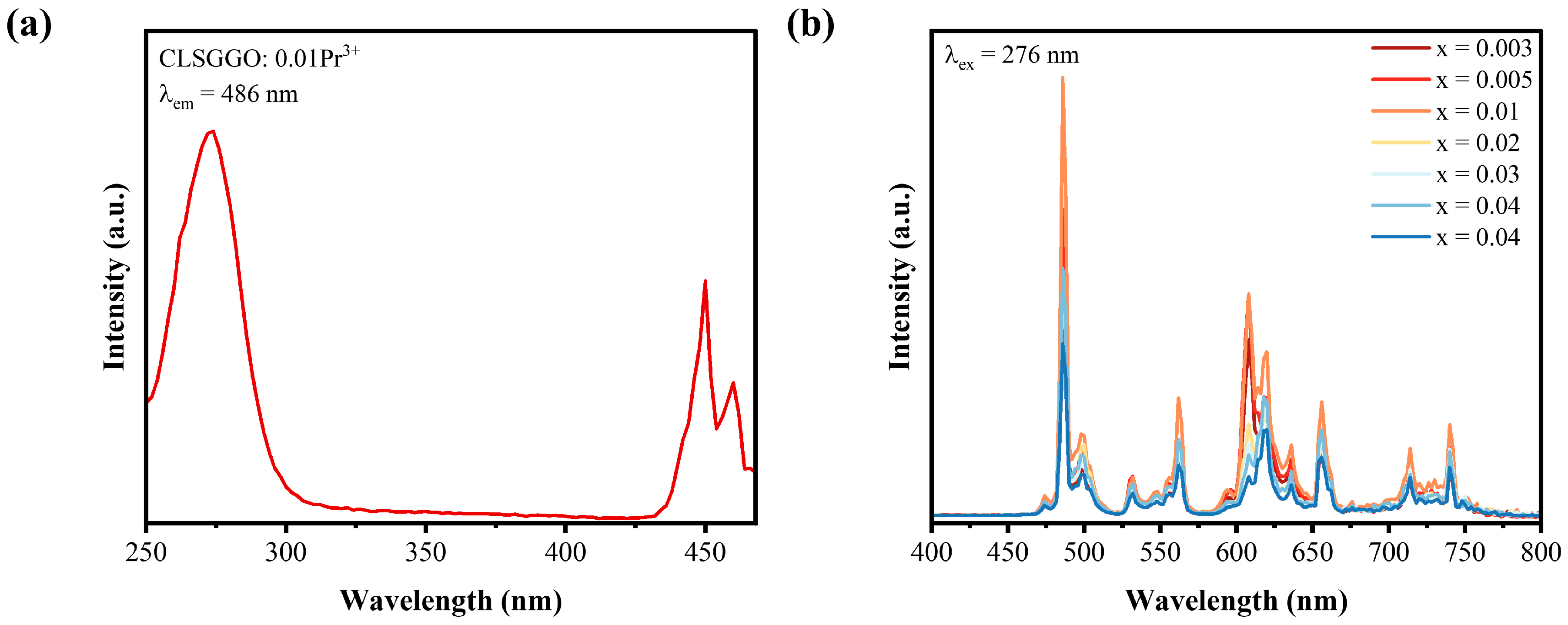
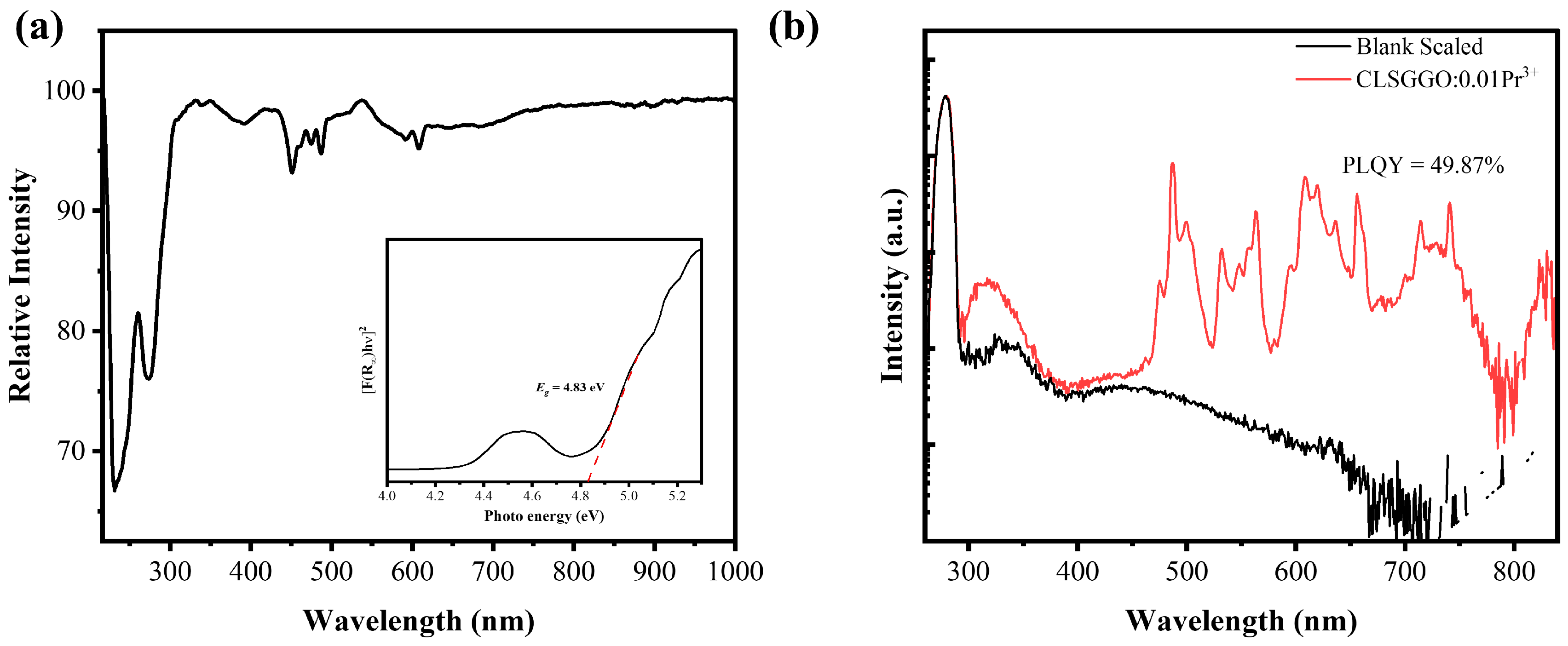
3.2. Property of Luminescence
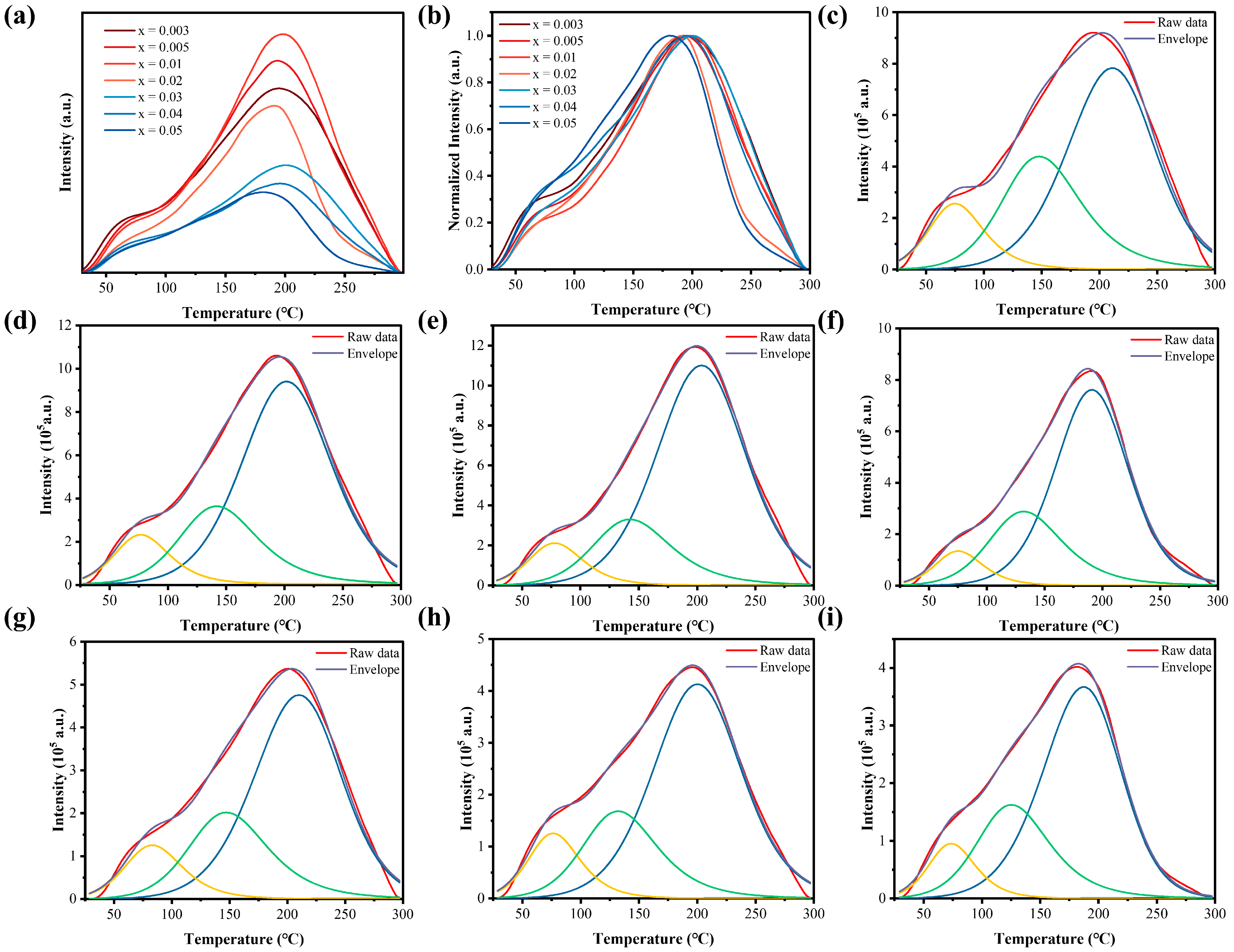
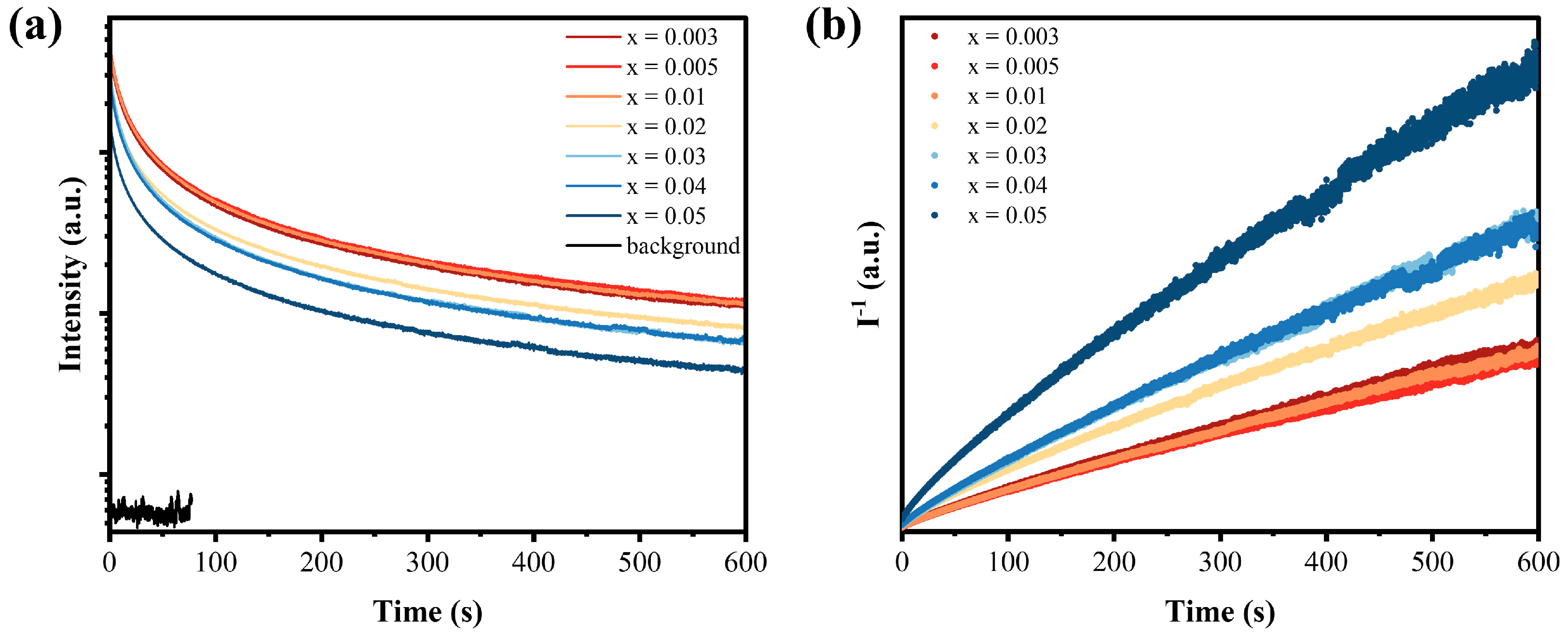
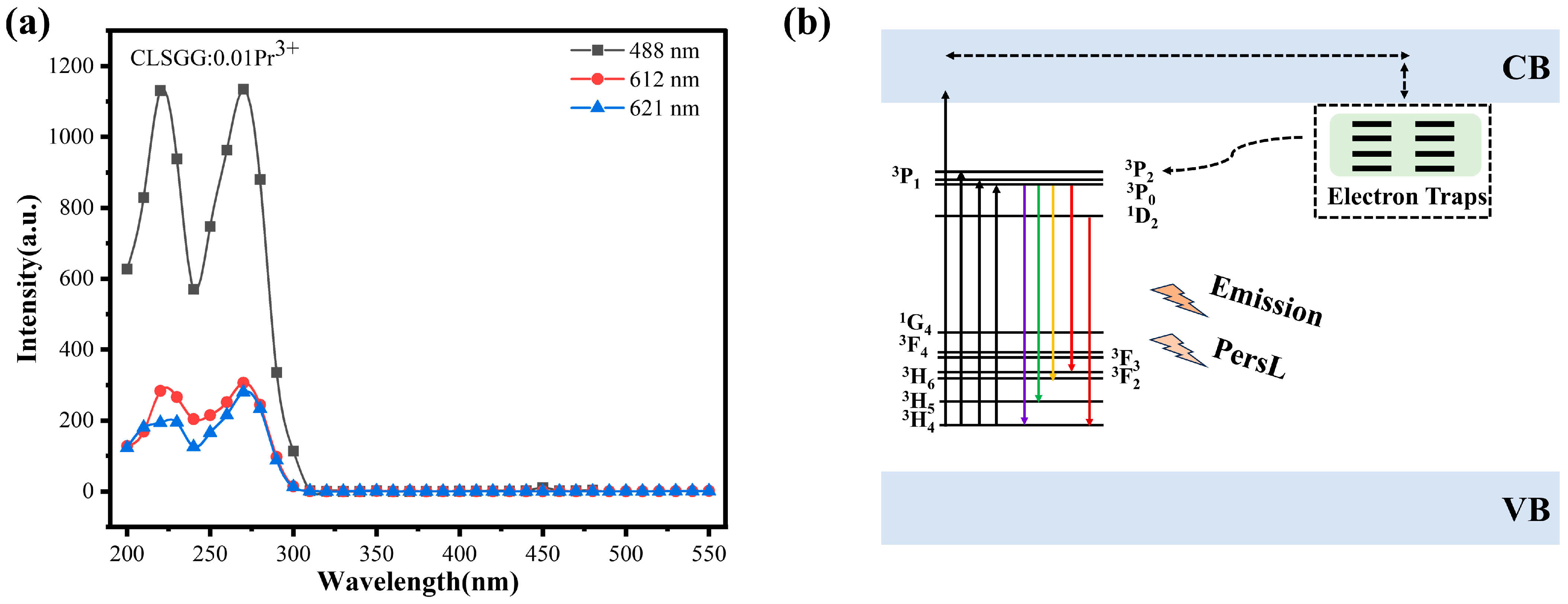
3.3. LPL Property and Trap Analysis
3.4. LPL Mechanism
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4: Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Yang, C.; Chen, X.; Mao, M.; Luo, X.; Sheng, W.; Lu, Z.; Sun, S.; He, H.; Su, W.; Song, K. Ionic Selective Occupation, Lattice Modulation and Photoluminescence Properties of Garnet Typed Orange-Yellow Phosphors for White-Light-Emitting Diodes. Ceram. Int. 2023, 49, 7683–7691. [Google Scholar] [CrossRef]
- Meng, Q.; Zhu, Q.; Li, X.; Sun, X.; Li, J. New Mg2+/Ge4+-Stabilized Gd3MgxGexAl5−2xO12:Ce Garnet Phosphor with Orange-Yellow Emission for Warm-White Leds (X = 2.0–2.5). Inorg. Chem. 2021, 60, 9773–9784. [Google Scholar] [CrossRef]
- Chen, M.; Lai, Y.; Zhou, J.; Shi, C.; Li, B.; Wu, D.; Wang, B.; Ding, J.; Jiang, X.; Wu, H.; et al. Near-Ultraviolet Excited Broadband Orange-Yellow Emitting Sr8MgIn(PO4)7:Eu2+ Phosphors for Wleds with High Color Rendering Index. Opt. Mater. 2021, 115, 111055. [Google Scholar] [CrossRef]
- Lin, T.; Fu, X.; Liu, Z.; Chen, N.; Zhang, J.; Liu, R.; Meng, W.; Zhang, H. X-ray induced long afterglow luminescence from UVC to red region in Ca2P2O7:Pr3+. J. Rare Earths 2024, 43, 1601–1606. [Google Scholar] [CrossRef]
- Bi, Y.-X.; Zhao, D.; Dai, S.-J.; Zhang, R.-J.; Yao, Q.-X. Colour change and long-persistent properties of a new phosphor YBaZn3GaO7:Bi3+/Eu3+. J. Alloy. Compd. 2024, 1003, 175693. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Huo, X.; Wang, Y.; Li, P. A Multi-Color Persistent Luminescent Phosphor Ca3Al2Ge3O12:Re3+ (Re3+ = Tb3+, Pr3+, Dy3+) for Dynamic Anti-Counterfeiting. J. Lumines. 2023, 254, 119518. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G. A long-persistent phosphor Sr3MgSi2O8−1.5xNx:Eu2+,Dy3+,Mn2+ based on white LEDs applications. J. Lumines. 2019, 211, 69–75. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Han, S.; Chen, W.; Li, G.; Wang, Y.; Wen, Y. Design, synthesis and characterization of a novel yellow long-persistent phosphor: Ca2BO3Cl:Eu2+,Dy3+. J. Mater. Chem. C 2013, 1, 3004–3011. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Zhang, D.; Chang, C. Tailoring Afterglow Properties of Polychromatic Long-Persistent LiMgxSr4−X−2y(BO3)3: y Eu2+ Phosphor by Codoping with Dy3+ or Nd3+. Ceram. Int. 2023, 49, 1336–1343. [Google Scholar] [CrossRef]
- Liang, L.; Yang, H.; Chen, Y.; Ding, Y.; Zhao, F.; Mao, Q.; Liu, M.; Zhong, J. Realizing Tunable Long Persistent Luminescent in Novel Cu2+-Doped NaGaO2 for Multi-Level Information Storage and Encryption. Adv. Opt. Mater. 2024, 12, 2401775. [Google Scholar] [CrossRef]
- Guo, A.; Zhu, Q.; Zhang, S.; Sun, X.; Li, J. A Building-Block Strategy for Dynamic Anti-Counterfeiting by Using (Ba,Sr)Ga2O4:Sm3+ New Red Persistent Luminescent Phosphor as an Important Component. Ceram. Int. 2023, 49, 4622–4630. [Google Scholar] [CrossRef]
- Vijayalakshmi, L.; Kumar, K.N.; Babu, P.R.; Palle, K.; Rosaiah, P.; Lim, J.; Ifseisi, A.A. Biocompatible Glasses Activated by Pr3+ with Tunable Luminescence and E-Band Emission for Applications in Red Phosphor, Visible Lasers, Broadband Optical Amplifiers and Greenhouse Glass. Ceram. Int. 2025, 51, 16387–16395. [Google Scholar] [CrossRef]
- Li, H.; Pang, R.; Luo, Y.; Wu, H.; Zhang, S.; Jiang, L.; Li, D.; Li, C.; Feng, J.; Zhang, H. Commendable Pr3+-Activated Ba2Ga2GeO7 Phosphor with High-Brightness White Long-Persistent Luminescence. J. Mater. Chem. C. 2019, 7, 6698–6705. [Google Scholar] [CrossRef]
- Huang, D.; Wu, G.; Liu, D.; Cheng, Z.; Dang, P.; Lian, H.; Lin, J. Defect engineering to develop green-emitting Eu2+-doped sulphide phosphor for water-resistant LED backlighting. J. Rare Earths 2025, in press. [Google Scholar] [CrossRef]
- Huang, X.; Chu, J.; Sun, J.; Wen, J.; Fang, L.; Wang, X.; Qiao, Z. Intrinsic defects and Bi3+ luminescence in Ba3Sc4O9: A first-principles study. J. Rare Earths 2025, in press. [Google Scholar] [CrossRef]
- Wang, B.; Lin, H.; Xu, J.; Chen, H.; Lin, Z.; Huang, F.; Wang, Y. Design, Preparation, and Characterization of a Novel Red Long-Persistent Perovskite Phosphor: Ca3Ti2O7:Pr3+. Inorg. Chem. 2015, 54, 11299–11306. [Google Scholar] [CrossRef]
- Lv, X.; Guo, N.; Qu, S.; Xin, Y.; Yang, M.; Shao, B.; Ouyang, R. Thermal activation induced charge transfer state absorption redshift realizes strong anti-thermal quenching in Pr3+-activated phosphor. Chem. Commun. 2024, 60, 2804–2807. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Chai, H.; Xin, Y.; Qu, S.; Guo, N. Enhanced thermally stable performance of Pr3+-doped vanadate phosphor by inhibiting the intervalence charge transfer quenching channel. Dalton Trans. 2024, 53, 4132–4138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, P.; Ding, S.; Tian, S.; Wang, Y. A Promising Route for Developing Yellow Long Persistent Luminescence and Mechanoluminescence in CaGa2O4:Pr3+,Li+. Inorg. Chem. Front. 2021, 8, 3748–3759. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, T.; Kurboniyon, M.; Wang, P.; Ma, C. Cationic Substitution-Enabled Green-Emitting Gd2-yYyLuAl4GaO12: Ce3+ Garnet Phosphors with High Quantum Efficiency and Superior Thermal Stability for Advanced Wleds. Inorg. Chem. 2025, 64, 12792–12803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Shi, Z.; Zhang, J.; Li, G.; Yu, H.; Si, W.; Zheng, H.; Zhang, L.; Su, Y. Alleviating wavelength efficiency trade-offs in Cr3+-activated garnet phosphors by high entropy engineering. J. Alloy. Compd. 2025, 1036, 181821. [Google Scholar] [CrossRef]
- Duan, J.; Bai, C.; Sun, J.; Wang, D.; Wang, Y. Highly Efficient Novel Garnet-Structured Yellow Emitting Phosphor for High Power Laser-Driven Lighting. Laser Photonics Rev. 2025, 19, 2401371. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Bai, Z. Luminescence properties and preparation of Lu3Al5O12 powder doped with Ce and Pr ions. J. Mater. Sci. Mater. Electron. 2018, 29, 10753–10761. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, J.; Zhang, X.; Hao, Z.; Liu, Y.; Luo, Y. The Energy Transfer and Effect of Doped Mg2+ in Ca3Sc2Si3O12:Ce3+, Pr3+ Phosphor for White Leds. Dalton Trans. 2014, 43, 4146–4150. [Google Scholar] [CrossRef]
- Yuan, W.; Pang, R.; Tan, T.; Wu, H.; Wang, S.; Su, J.; Wang, J.; Jiao, S.; Li, C.; Zhang, H. Tuning Emission Color and Improving the Warm-White Persistent Luminescence of Phosphor BaLu2Al2Ga2SiO12:Pr3+Via Zn2+ Co-Doping. Dalton Trans. 2021, 50, 12137–12146. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shi, Y.; Wang, H.; Zhang, Q.; Wu, T.; Yuan, Q.; Ma, K.; Li, T.; Zhou, Z.; Fang, J.; et al. Ce3+, Pr3+ Co-Doped Lu3Al5O12 Single Crystals and Ceramics: A Comparative Study. Materials 2022, 15, 9025. [Google Scholar] [CrossRef]
- Teng, Y.; Zhou, J.; Khisro, S.N.; Zhou, S.; Qiu, J. Persistent luminescence of SrAl2O4: Eu2+, Dy3+, Cr3+ phosphors in the tissue transparency window. Mater. Chem. Phys. 2014, 147, 772–776. [Google Scholar] [CrossRef]
- Sójka, M.; Runowski, M.; Woźny, P.; Carlos, L.D.; Zych, E.; Lis, S. Y2(Ge,Si)O5:Pr phosphors: Multimodal temperature and pressure sensors shaped by bandgap management. J. Mater. Chem. C 2021, 9, 13818–13831. [Google Scholar] [CrossRef]
- Wei, Y.; Dang, P.; Dai, Z.; Li, G.; Lin, J. Advances in near-Infrared Luminescent Materials without Cr3+: Crystal Structure Design, Luminescence Properties, and Applications. Chem. Mater. 2021, 33, 5496–5526. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Li, X.; Tan, T.; Wu, H.; Su, J.; Yuan, W.; Pang, R.; Jiang, L.; Li, D.; et al. Effect of Pr3+ Concentration on the Luminescent Properties of Ca2LuScGa2Ge2O12 Compound with Garnet Structure. J. Solid State Chem. 2022, 306, 122758. [Google Scholar] [CrossRef]
- Su, J.; Pang, R.; Tan, T.; Wang, S.; Chen, X.; Zhang, S.; Zhang, H. A Novel near-Infrared Emitting Sr2LuSbO6:Fe3+ Phosphor with Persistent Luminescence Performance. Adv. Optical Mater. 2024, 12, 2303187. [Google Scholar] [CrossRef]
- Xue, Z.; Ding, D.; Sima, Y.; Zhou, Z.; Dong, H.; Zhao, S.; Feng, H. Effects of two strategies on afterglow behavior of Lu2O3:Eu single crystal scintillator: Co-doping with Pr3+ and solid solution with Sc2O3. J. Rare Earths 2022, 41, 658–665. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Jiang, X.-X.; Wang, Y.-N.; Pan, X.-R.; Zhang, Y.-Y.; Liu, B.; Yang, Y.-G.; Wang, X.-P. Synthesis, structure and luminescence characteristics of La3Ga5SiO14:Pr3+ phosphors. J. Alloy. Compd. 2023, 932, 167626. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Li, Y.; Cheng, L.; Wang, Y.; Yu, H.; Chen, B. Multimodal Luminescence in Ca2InTaO6: Pr3+ Phosphors: Applications in Dynamic Anticounterfeiting and Optical Thermometry. Inorg. Chem. 2025, 64, 10653–10664. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, J.; Sheng, H.; Li, T.; Guo, L.; Ji, C.; Huang, Z.; Wen, J.; Liu, X.; Li, C.; et al. Luminescence and optical thermometry based on silico-carnotite Ca3Y2Si3O12: Pr3+ phosphor. Ceram. Int. 2022, 48, 3860–3868. [Google Scholar] [CrossRef]
- Kumari, C.; Manam, J.; Sharma, S.K. Synthesis and luminescence properties of a bluish-green-emitting Sr3LiSbO6: Pr3+ phosphor for optoelectronic applications. Ceram. Int. 2023, 50, 10535–10550. [Google Scholar] [CrossRef]
- Megharaj, T.N.; Radha Krushna, B.R.; Pruthviraj, I.S.; Sharma, S.C.; Manjunatha, K.; Wu, S.Y.; Arunakumar, R.; Komahal, F.F.; Ramakrishna, G.; Nagabhushana, H. Luminescence and structural insights of β-Ca2SiO4:Pr3+ Phosphor: Applications towards TL dosimetry and solid state lighting. Mater. Chem. Phys. 2025, 334, 130508. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, X.; Lv, X.; Chen, D.; Miao, S.; Wang, W.; Liang, Y. Multimodal luminescence in Pr3+ single-doped Li2CaSiO4 phosphor for optical information storage and anti-counterfeiting applications. Chem. Eng. J. 2023, 474, 145886. [Google Scholar] [CrossRef]
- Kang, X.; Lü, W.; Zhu, Z.; Jia, C. A Novel Blue-Light Excitable Pr3+ Doped (Sr,Ba) LaMgTao6 Phosphor for Plant Growth Lighting. J. Rare Earths. 2023, 41, 666–672. [Google Scholar] [CrossRef]
- Tian, X.; Xu, S.; Wen, J.; Zhu, L.; Ji, C.; Huang, Z.; Wang, X.; Luo, F.; Liu, X.; Lu, Y.; et al. Thermochromic Pr3+ doped CaMoO4 phosphor with diverse thermal responses for temperature sensing. Ceram. Int. 2023, 49, 27126–27137. [Google Scholar] [CrossRef]
- Long, M.; Li, C.; Li, B.; Yi, L.; Zhang, F. A Multi-Functional Phosphor Ba5La3MgAl3O15: Pr3+ with Diverse Thermal Responses for High Sensitive Temperature Sensing, Photothermochromism Indicator and Patterned Anti-Counterfeiting. Ceram. Int. 2025, 51, 22340–22350. [Google Scholar] [CrossRef]
- Kameshwaran, R.; Kumar, K.G.; Aravinth, K.; Raja, A.; Ramachandran, K.; Annalakshmi, D.O.; Bhargav, P.B.; Venthan, S.M.; Gokulkannan, K. Photoluminescence and thermoluminescence behaviour of Pr3+ doped sodium bismuth phosphate phosphors. J. Alloy. Compd. 2025, 1034, 181330. [Google Scholar] [CrossRef]
- Zheng, J.; Shen, H.; Li, Y.; Li, H.; Yue, Z. Structural and luminescent performance and optical thermometry of Pr3+ doped SrWO4 down-conversion phosphors. J. Alloy. Compd. 2023, 968, 172112. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Z.; Cao, C.; Zhang, T.; Zhang, J.; Ci, Z.; Zhao, Z.; Wang, Y. Warm-White Persistent Luminescence of Lu3Al2Ga3O12: Pr3+ Phosphor. J. Rare Earths. 2017, 35, 47–52. [Google Scholar] [CrossRef]
- Yuan, W.; Tan, T.; Wu, H.; Pang, R.; Zhang, S.; Jiang, L.; Li, D.; Wu, Z.; Li, C.; Zhang, H. Intense UV Long Persistent Luminescence Benefiting from the Coexistence of Pr3+/Pr4+ in a Praseodymium-Doped BaLu2Al2Ga2SiO12 Phosphor. J. Mater. Chem. C. 2021, 9, 5206–5216. [Google Scholar] [CrossRef]
- Yang, N.; Li, Z.; Zhou, T.; Zhang, Z.; Shi, W.; Tong, Y.; Shi, J. A Highly Sensitive Multiple-Mode Optical Thermometer Designed in Eu2+/3+ and Li+ Co-Doped Polymorphism Compound LaSc3(BO3)4. J. Adv. Ceram. 2024, 13, 821–833. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Huo, X.; Meng, X.; Wang, Y.; Suo, H.; Li, P. Anti-Counterfeiting Application of Persistent Luminescence Materials and Its Research Progress. Laser Photonics Rev. 2024, 18, 2300751. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Sun, S.; Wu, Y.; Jiang, K.; Li, S.; Lin, H. Pushing Trap-Controlled Persistent Luminescence Materials toward Multi-Responsive Smart Platforms: Recent Advances, Mechanism, and Frontier Applications. Adv. Mater. 2024, 36, 2314083. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-M.; Lin, Y.; Liu, Y.; Lou, B.; Ma, C.-G.; Zhang, H.; Wang, J. Excitation-wavelength-dependent persistent luminescence from single-component nonstoichiometric CaGaxO4:Bi for dynamic anti-counterfeiting. Light Sci. Appl. 2024, 13, 286. [Google Scholar] [CrossRef]
- Parayil, R.T.; Gupta, S.K.; Mohapatra, M. A review on defect engineered NIR persistent luminescence through transition metal ion (Cr, Mn, Fe and Ni) doping: Wider perspective covering synthesis, characterization, fundamentals and applications. Coord. Chem. Rev. 2025, 522, 216200. [Google Scholar] [CrossRef]
- Gao, D.; Du, C.; Wang, Y.; Xu, W.; Gao, W.; Pang, Q.; Wang, Y. Controllable Persistent Luminescence in Bismuth Activated Memory Phosphors by Trap Management for Artificial Intelligence Anti-Counterfeiting. J. Mater. Chem. C. 2024, 12, 19487–19497. [Google Scholar] [CrossRef]
- Chen, L.; Li, P.; Shen, G.; Kang, X.; Tang, S.; Zhang, T.; Zhu, Q. Effective electron trap regulation in near-infrared persistent phosphor of ZnAl2O4:Cr3+ for round-the-clock plant lighting. Mater. Today Chem. 2024, 40, 102204. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, H.; Shi, Q.; Qiao, J.; Cui, C.; Huang, P.; Wang, L. Investigation of a novel Pr3+-activated LiYGeO4 phosphor with red long-persistent luminescence. J. Lumines. 2024, 267, 120382. [Google Scholar] [CrossRef]
- Chen, N.; Lin, T.; Liu, Z.; Liu, R.; Zhang, J.; Fu, X.; Zhang, H. Ultraviolet and visible persistent luminescence from Sr3MgSi2O8:Pr3+. Ceram. Int. 2024, 50, 1891–1897. [Google Scholar] [CrossRef]
- Du, Q.; Ueda, J.; Tanabe, S. Influence of Trap Centers on Persistent Luminescence of Cr3+ or Yb3+ Co-Doped Y3Al2Ga3O12: Pr3+ Ceramic Phosphors. J. Am. Ceram. Soc. 2024, 107, 5524–5533. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, N.; Yue, Y.; Wang, J.; Li, Y.; Wu, Z.; Xu, S.; Bai, G. Multi-Mode Luminescence in Smart near-Infrared Cr3+/Pr3+ Codoped SrGa12O19 Phosphors Induced by Three Distinct Excitation Mechanisms. ACS Appl. Mater. Interfaces. 2024, 16, 33855–33864. [Google Scholar] [CrossRef]
- Balhara, A.; Gupta, S.K.; Ghosh, P.S.; Abraham, M.; Tyagi, M.; Yadav, A.K.; Das, S.; Sudarshan, K.; Sarkar, P.S. Unleashing the Potential of Defect Engineered Persistent Pr3+-Activated Phosphors for Multi-Dimensional Anti-Counterfeiting and X-Ray Imaging Applications. Small 2025, 2501752. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Fu, X.; Yi, J.; Lin, T.; Liu, Z.; Chen, N.; Zhang, H. X-Ray–Induced Multicolor Long Afterglow and Photostimulated Luminescence from Pr3+-Doped Sr3(PO4)2. J. Am. Ceram. Soc. 2025, 108, e20204. [Google Scholar] [CrossRef]
- Li, C.; Shen, Y.; Lai, F.; Guan, L.; Zhang, J.; Qiang, Y.; Wang, B.; Leng, Y.; Li, W.; You, W. Co-doping Sm3+ transforms Y2Ge2O7:Pr3+ into a novel orange-reddish long afterglow phosphor. J. Alloy. Compd. 2024, 1003, 175581. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Lin, H.; Poelman, D.; Zych, E. Unlocking triple-band persistent luminescence: Invisible UVC-UVB and visible red emission through structural modulation of Na2SrSi2O6:Pr3+ cyclosilicate. Mater. Horiz. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, M.; Zhou, J.; Yang, B.; Zhang, Y.; Chang, C. Rare earth Ho-induced oxygen vacancy in synergy with europium for efficient white afterglow luminescence in Sr2MgSi2O7. J. Rare Earths 2025, in press. [Google Scholar] [CrossRef]

| Sample | Tm/°C | T1/°C | T2/°C | μg | Im | E/eV | n0 |
|---|---|---|---|---|---|---|---|
| x = 0.003 | 74 | 48 | 105 | 0.54 | 255,946 | 0.694 | 1,262,805 |
| x = 0.005 | 77 | 50 | 106 | 0.52 | 228,600 | 0.700 | 1,212,912 |
| x = 0.01 | 77 | 52 | 107 | 0.55 | 211,481 | 0.700 | 1,001,847 |
| x = 0.02 | 75 | 52 | 100 | 0.52 | 133,740 | 0.696 | 615,589 |
| x = 0.03 | 83 | 54 | 113 | 0.51 | 125,653 | 0.712 | 722,050 |
| x = 0.04 | 76 | 51 | 103 | 0.52 | 126,010 | 0.698 | 618,368 |
| x = 0.05 | 74 | 50 | 98 | 0.50 | 95,399 | 0.694 | 448,016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Li, H.; Deng, R.; Zhang, S.; Zhang, H. Preparation and Study of Bright Orange-Yellow Long Persistent Luminescent Ca2LuScGa2Ge2O12:Pr3+ Phosphor. Photochem 2025, 5, 38. https://doi.org/10.3390/photochem5040038
Shi X, Li H, Deng R, Zhang S, Zhang H. Preparation and Study of Bright Orange-Yellow Long Persistent Luminescent Ca2LuScGa2Ge2O12:Pr3+ Phosphor. Photochem. 2025; 5(4):38. https://doi.org/10.3390/photochem5040038
Chicago/Turabian StyleShi, Xiaoman, Huimin Li, Ruiping Deng, Su Zhang, and Hongjie Zhang. 2025. "Preparation and Study of Bright Orange-Yellow Long Persistent Luminescent Ca2LuScGa2Ge2O12:Pr3+ Phosphor" Photochem 5, no. 4: 38. https://doi.org/10.3390/photochem5040038
APA StyleShi, X., Li, H., Deng, R., Zhang, S., & Zhang, H. (2025). Preparation and Study of Bright Orange-Yellow Long Persistent Luminescent Ca2LuScGa2Ge2O12:Pr3+ Phosphor. Photochem, 5(4), 38. https://doi.org/10.3390/photochem5040038






