Ultrafast Excited State Dynamics of a Verdazyl Diradical System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Spectroscopy Methods
2.4. Computational Methods
3. Results
3.1. Steady-State Absorption Spectroscopy
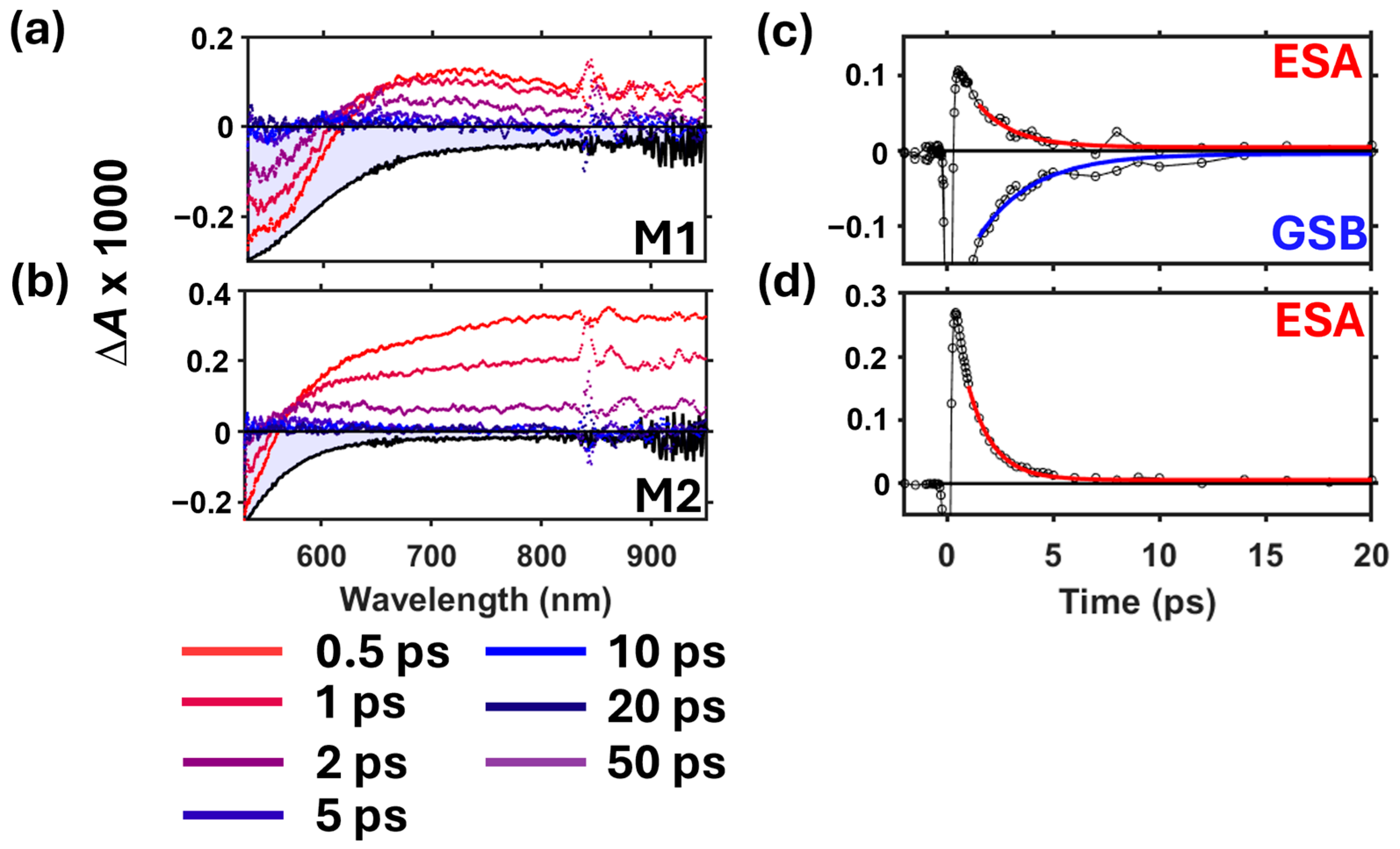
3.2. Broadband Transient Absorption Spectroscopy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnan, F.; Dhindsa, J.S.; Anghel, M.; Bazylewski, P.; Fanchini, G.; Gilroy, J.B. A Divergent Strategy for the Synthesis of Redox-Active Verdazyl Radical Polymers. Polym. Chem. 2021, 12, 2786–2797. [Google Scholar] [CrossRef]
- McKinnon, S.D.J.; Patrick, B.O.; Lever, A.B.P.; Hicks, R.G. Verdazyl Radicals as Redox-Active, Non-Innocent, Ligands: Contrasting Electronic Structures as a Function of Electron-Poor and Electron-Rich Ruthenium Bis(β-Diketonate) Co-Ligands. Chem. Commun. 2010, 46, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.J.; Wu, J.-Z.; Judd, M.; Rousset, E.; Korb, M.; Moggach, S.A.; Cox, N.; Low, P.J. The Syntheses, Structures and Spectroelectrochemical Properties of 6-Oxo-Verdazyl Derivatives Bearing Surface Anchoring Groups. J. Mater. Chem. C 2022, 10, 1896–1915. [Google Scholar] [CrossRef]
- Kunz, S.; Bui, T.T.; Emmel, D.; Janek, J.; Henkensmeier, D.; Schröder, D. Aqueous Redox Flow Cells Utilizing Verdazyl Cations Enabled by Polybenzimidazole Membranes. ChemSusChem 2024. [Google Scholar] [CrossRef]
- Shil, S.; Bhattacharya, D.; Misra, A.; Bytautas, L. Antiaromatic Molecules as Magnetic Couplers: A Computational Quest. J. Phys. Chem. A 2024, 128, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Ratera, I.; Veciana, J. Playing with Organic Radicals as Building Blocks for Functional Molecular Materials. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef]
- Polo, V.; Alberola, A.; Andres, J.; Anthony, J.; Pilkington, M. Towards Understanding of Magnetic Interactions within a Series of Tetrathiafulvalene–π Conjugated-Verdazyl Diradical Cation System: A Density Functional Theory Study. Phys. Chem. Chem. Phys. 2008, 10, 857–864. [Google Scholar] [CrossRef]
- Bodzioch, A.; Obijalska, E.; Jakubowski, R.; Celeda, M.; Gardias, A.; Trzybiński, D.; Tokarz, P.; Szczytko, J.; Woźniak, K.; Kaszyński, P. Electronic and Magnetic Interactions in 6-Oxoverdazyl Diradicals: Connection through N(1) vs. C(3) Revisited. J. Org. Chem. 2024, 89, 6306–6321. [Google Scholar] [CrossRef]
- Yeo, H.; Debnath, S.; Krishnan, B.P.; W. Boudouris, B. Radical Polymers in Optoelectronic and Spintronic Applications. RSC Appl. Polym. 2024, 2, 7–25. [Google Scholar] [CrossRef]
- Brook, D.J.R.; Lynch, V.; Conklin, B.; Fox, M.A. Spin Delocalization in the Copper(I) Complexes of Bis (Verdazyl) Diradicals. J. Am. Chem. Soc. 1997, 119, 5155–5162. [Google Scholar] [CrossRef]
- Poh, Y.R.; Morozov, D.; Kazmierczak, N.P.; Hadt, R.G.; Groenhof, G.; Yuen-Zhou, J. Alternant Hydrocarbon Diradicals as Optically Addressable Molecular Qubits. J. Am. Chem. Soc. 2024, 146, 15549–15561. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.D.; Huff, J.S.; Barclay, M.S.; Wilson, C.K.; Barcenas, G.; Duncan, K.M.; Ketteridge, M.; Obukhova, O.M.; Krivoshey, A.I.; Tatarets, A.L.; et al. Intramolecular Charge Transfer and Ultrafast Nonradiative Decay in DNA-Tethered Asymmetric Nitro- and Dimethylamino-Substituted Squaraines. J. Phys. Chem. A 2023, 127, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Choppella, S.; Paramasivam, G.; Sambasivam, S.; Ravva, M.K. Understanding the Stability of π-Conjugated Diradicaloid Organic Molecules. J. Electron. Mater. 2023, 52, 1681–1690. [Google Scholar] [CrossRef]
- Koivisto, B.D.; Hicks, R.G. The Magnetochemistry of Verdazyl Radical-Based Materials. Coord. Chem. Rev. 2005, 249, 2612–2630. [Google Scholar] [CrossRef]
- Rota, J.-B.; Le Guennic, B.; Robert, V. Toward Verdazyl Radical-Based Materials: Ab Initio Inspection of Potential Organic Candidates for Spin-Crossover Phenomenon. Inorg. Chem. 2010, 49, 1230–1237. [Google Scholar] [CrossRef]
- Haller, B.C.; Chambers, D.; Cheng, R.; Chemistruck, V.; Hom, T.F.; Li, Z.; Nguyen, J.; Ichimura, A.; Brook, D.J.R. Oxidation of Electron Donor-Substituted Verdazyls: Building Blocks for Molecular Switches. J. Phys. Chem. A 2015, 119, 10750–10760. [Google Scholar] [CrossRef]
- Brook, D.J.R.; Yee, G.T. Radical–Radical Interaction through a Saturated Link: Methylenebis-6-Oxoverdazyl. J. Org. Chem. 2006, 71, 4889–4895. [Google Scholar] [CrossRef]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef]
- Buchwalter, S.L.; Closs, G.L. Electron Spin Resonance and CIDNP Studies on 1,3-Cyclopentadiyls. A Localized 1,3 Carbon Biradical System with a Triplet Ground State. Tunneling in Carbon-Carbon Bond Formation. J. Am. Chem. Soc. 1979, 101, 4688–4694. [Google Scholar] [CrossRef]
- Alam, M.M.; Sato, M.; Watanabe, A.; Akasaka, T.; Ito, O. Photochemical Reactions between C60 and Aromatic Thiols. Protonation of C60 via Photoinduced Electron Transfer. J. Phys. Chem. A 1998, 102, 7447–7451. [Google Scholar] [CrossRef]
- Baldovi, H.G.; Krüger, M.; Reinsch, H.; Alvaro, M.; Stock, N.; Garcia, H. Transient Absorption Spectroscopy and Photochemical Reactivity of CAU-8. J. Mater. Chem. C 2015, 3, 3607–3613. [Google Scholar] [CrossRef]
- Son, M.; Park, K.H.; Shao, C.; Würthner, F.; Kim, D. Spectroscopic Demonstration of Exciton Dynamics and Excimer Formation in a Sterically Controlled Perylene Bisimide Dimer Aggregate. J. Phys. Chem. Lett. 2014, 5, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, E.; Sunny, J.; Hariharan, M. Excimer Evolution Hampers Symmetry-Broken Charge-Separated States. Chem. Sci. 2022, 13, 10824–10835. [Google Scholar] [CrossRef] [PubMed]
- Alías-Rodríguez, M.; De Graaf, C.; Huix-Rotllant, M. Ultrafast Intersystem Crossing in Xanthone from Wavepacket Dynamics. J. Am. Chem. Soc. 2021, 143, 21474–21477. [Google Scholar] [CrossRef]
- Mundt, R.; Villnow, T.; Ziegenbein, C.T.; Gilch, P.; Marian, C.; Rai-Constapel, V. Thioxanthone in Apolar Solvents: Ultrafast Internal Conversion Precedes Fast Intersystem Crossing. Phys. Chem. Chem. Phys. 2016, 18, 6637–6647. [Google Scholar] [CrossRef]
- Aloïse, S.; Ruckebusch, C.; Blanchet, L.; Réhault, J.; Buntinx, G.; Huvenne, J.-P. The Benzophenone S1(n,Π*) → T1(n,Π*) States Intersystem Crossing Reinvestigated by Ultrafast Absorption Spectroscopy and Multivariate Curve Resolution. J. Phys. Chem. A 2008, 112, 224–231. [Google Scholar] [CrossRef]
- Weinert, C.; Wezisla, B.; Lindner, J.; Vöhringer, P. Ultrafast Primary Processes of the Stable Neutral Organic Radical, 1,3,5-Triphenylverdazyl, in Liquid Solution. Phys. Chem. Chem. Phys. 2015, 17, 13659–13671. [Google Scholar] [CrossRef]
- Sadhukhan, T.; Datta, A.; Datta, S.N. Quantum Chemical Investigation of Light-Activated Spin State Change in Pyrene Coupled to Oxoverdazyl Radical Center. J. Phys. Chem. A 2015, 119, 9414–9424. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Lang, B.; Rosspeintner, A.; Vauthey, E. Ultrafast Elementary Photochemical Processes of Organic Molecules in Liquid Solution. Chem. Rev. 2017, 117, 10826–10939. [Google Scholar] [CrossRef]
- Shimizu, D.; Sotome, H.; Miyasaka, H.; Matsuda, K. Optically Distinguishable Electronic Spin-Isomers of a Stable Organic Diradical. ACS Cent. Sci. 2024, 10, 890–898. [Google Scholar] [CrossRef]
- Brook, D.J.R. Coordination Chemistry of Verdazyl Radicals. Comments Inorg. Chem. 2015, 35, 1–17. [Google Scholar] [CrossRef]
- Brook, D.J.R.; Fox, H.H.; Lynch, V.; Fox, M.A. Structural Indicators of Electronic Interaction in the 1,1’,5,5’-Tetramethyl-6,6’-Dioxo-3,3’-Biverdazyl Diradical. J. Phys. Chem. 1996, 100, 2066–2071. [Google Scholar] [CrossRef]
- Günaydın-Şen, Ö.; Chen, P.; Fosso-Tande, J.; Allen, T.L.; Cherian, J.; Tokumoto, T.; Lahti, P.M.; McGill, S.; Harrison, R.J.; Musfeldt, J.L. Magnetoelectric Coupling in 4,4′-Stilbenedinitrene. J. Chem. Phys. 2013, 138, 204716. [Google Scholar] [CrossRef]
- Dorris, A.L.; Umar, A.R.; Grieco, C. Ultrabroadband Near-Infrared Transient Absorption Spectrometer with Simultaneous 900–2350 Nm Detection. Appl. Spectrosc. 2024. [Google Scholar] [CrossRef]
- Umar, A.R.; Dorris, A.L.; Kotadiya, N.B.; Giebink, N.C.; Collier, G.S.; Grieco, C. Probing Polaron Environment in a Doped Polymer via the Photoinduced Stark Effect. J. Phys. Chem. C 2023, 127, 9498–9508. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21 + G Basis Set for First-Row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilizaion of AB Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Miertus, S.; Tomasi, J. Approximate Evaluations of the Electrostatic Free Energy and Internal Energy Changes in Solution Processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Pascual-ahuir, J.L.; Silla, E.; Tuñon, I. GEPOL: An improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J. Comput. Chem. 1994, 15, 1127–1138. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum Solvation Models: A New Approach to the Problem of Solute’s Charge Distribution and Cavity Boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cammi, R.; Mennucci, B.; Tomasi, J. Second-Order Møller—Plesset Analytical Derivatives for the Polarizable Continuum Model Using the Relaxed Density Approach. J. Phys. Chem. A 1999, 103, 9100–9108. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF Version of the PCM Solvation Method: An Overview of a New Method Addressed to Study Molecular Solutes at the QM Ab Initio Level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of Solvent Effects in Isotropic and Anisotropic Dielectrics and in Ionic Solutions with a Unified Integral Equation Method: Theoretical Bases, Computational Implementation, and Numerical Applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Lipparini, F.; Scalmani, G.; Mennucci, B.; Cancès, E.; Caricato, M.; Frisch, M.J. A Variational Formulation of the Polarizable Continuum Model. J. Chem. Phys. 2010, 133, 014106. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous Surface Charge Polarizable Continuum Models of Solvation. I. General Formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Caricato, M. Absorption and Emission Spectra of Solvated Molecules with the EOM–CCSD–PCM Method. J. Chem. Theory Comput. 2012, 8, 4494–4502. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Cammi, R.; Mennucci, B.; Tomasi, J. Fast Evaluation of Geometries and Properties of Excited Molecules in Solution: A Tamm-Dancoff Model with Application to 4-Dimethylaminobenzonitrile. J. Phys. Chem. A 2000, 104, 5631–5637. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Time-Dependent Density Functional Theory for Molecules in Liquid Solutions. J. Chem. Phys. 2001, 115, 4708–4717. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2019. [Google Scholar]
- Sulzer, P. Intensitätsverteilung eines Kontinuierlichen Absorptionsspektrums in Abhängigkeit von Wellenzahl und Temperatur. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 1952. [Google Scholar]
- Schalk, O.; Yang, J.-P.; Hertwig, A.; Hippler, H.; Unterreiner, A.N. Vibrational Cooling in the Liquid Phase Studied by Ultrafast Investigations of Cycloheptatriene. Mol. Phys. 2009, 107, 2159–2167. [Google Scholar] [CrossRef][Green Version]
- Beckwith, J.S.; Rumble, C.A.; Vauthey, E. Data Analysis in Transient Electronic Spectroscopy—An Experimentalist’s View. Int. Rev. Phys. Chem. 2020, 39, 135–216. [Google Scholar] [CrossRef]
- Marazzi, M.; Mai, S.; Roca-Sanjuán, D.; Delcey, M.G.; Lindh, R.; González, L.; Monari, A. Benzophenone Ultrafast Triplet Population: Revisiting the Kinetic Model by Surface-Hopping Dynamics. J. Phys. Chem. Lett. 2016, 7, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.; Morlet-Savary, F.; Jacques, P.; Fouassier, J.P. Solvent Dependence of the Intersystem Crossing Kinetics of Thioxanthone. Chem. Phys. 2000, 255, 335–346. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Triplet State. Its Radiative and Nonradiative Properties. Acc. Chem. Res. 1968, 1, 8–16. [Google Scholar] [CrossRef]







| Sample/Solvent | N/103 | τ (ps) | c/103 (Offset) |
|---|---|---|---|
| M1/butanol | 0.13 ± 0.01 (ESA) 2 −0.037 ± 0.007 (GSB) 3 | 1.7 ± 0.1 (ESA) 1.7 ± 0.3 (GSB) | 0.0047 ± 0.0008 (ESA) 0.0028 ± 0.0008 (GSB) |
| M1/hexane | 0.154 ± 0.001 (ESA) −0.367 ± 0.003 (GSB) | 4.06 ± 0.05 (ESA) 4.84 ± 0.06 (GSB) | 0.0070 ± 0.0003 (ESA) 0.0024 ± 0.0008 (GSB) |
| M2/butanol | 0.345 ± 0.006 (ESA) | 1.18 ± 0.02 (ESA) | 0.0057 ± 0.0004 (ESA) |
| M2/hexane | 0.591 ± 0.008 (ESA) | 1.56 ± 0.02 (ESA) | 0.0027 ± 0.0004 (ESA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, C.; Pawłowski, F.; Brook, D.J.R.; Grieco, C. Ultrafast Excited State Dynamics of a Verdazyl Diradical System. Photochem 2024, 4, 404-416. https://doi.org/10.3390/photochem4040025
Clark C, Pawłowski F, Brook DJR, Grieco C. Ultrafast Excited State Dynamics of a Verdazyl Diradical System. Photochem. 2024; 4(4):404-416. https://doi.org/10.3390/photochem4040025
Chicago/Turabian StyleClark, Caitlyn, Filip Pawłowski, David J. R. Brook, and Christopher Grieco. 2024. "Ultrafast Excited State Dynamics of a Verdazyl Diradical System" Photochem 4, no. 4: 404-416. https://doi.org/10.3390/photochem4040025
APA StyleClark, C., Pawłowski, F., Brook, D. J. R., & Grieco, C. (2024). Ultrafast Excited State Dynamics of a Verdazyl Diradical System. Photochem, 4(4), 404-416. https://doi.org/10.3390/photochem4040025







