Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field
Abstract
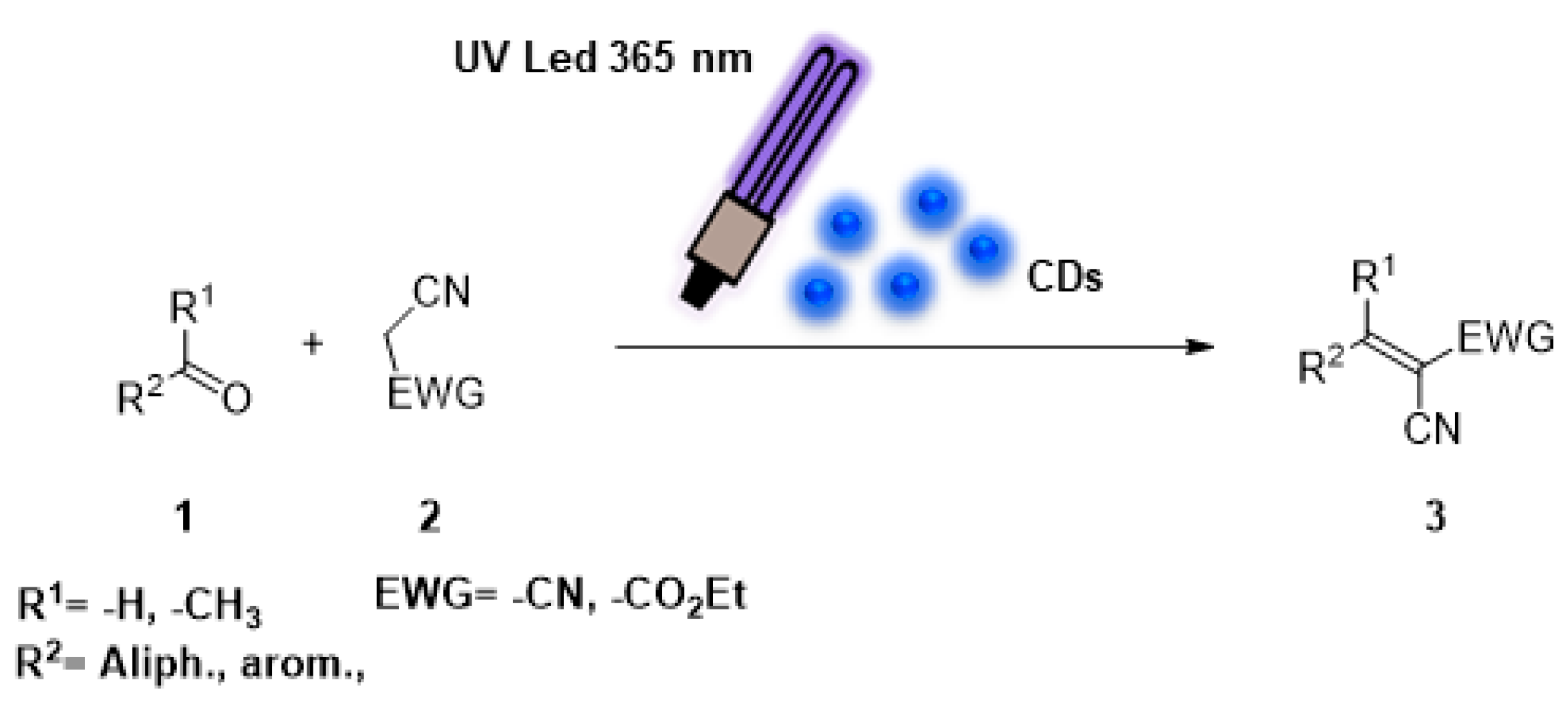
1. Introduction
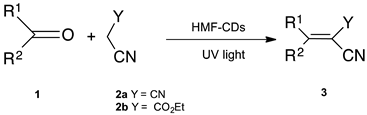
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. Photochemical Setup
3.3. General Procedures for the Synthesis of 5HMF-Derived CDs
3.4. General Procedure for the Synthesis of 3ab–pa
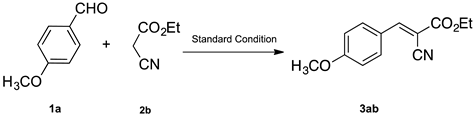
3.4.1. Ethyl(E)-2-cyano-3-(4-methoxyphenyl)acrylate (3ab)
3.4.2. Ethyl(E)-2-cyano-3-phenylacrylate (3bb)
3.4.3. Ethyl(E)-2-cyano-3-(p-tolyl)acrylate (3cb)
3.4.4. Ethyl(E)-2-cyano-3-(4-nitrophenyl)acrylate (3db)
3.4.5. Ethyl(E)-2-cyano-3-(4-(trifluoromethyl)phenyl)acrylate (3eb)
3.4.6. Ethyl(E)-2-cyano-3-(4-fluorophenyl)acrylate (3fb)
3.4.7. Ethyl(E)-3-(4-chlorophenyl)-2-cyanoacrylate (3gb)
3.4.8. Ethyl(E)-2-cyano-3-(naphthalen-2-yl)acrylate (3hb)
3.4.9. Ethyl(E)-2-cyano-3-(furan-3-yl)acrylate (3kb)
3.4.10. Ethyl(E)-2-cyano-3-cyclohexylacrylate (3ib)
3.4.11. Ethyl(E)-2-cyano-5,9-dimethyldeca-2,8-dienoate (3jb)
3.4.12. Ethyl(E)-2-cyano-3-(pyridin-4-yl)acrylate (3lb)
3.4.13. Ethyl 2-cyano-2-cyclopentylideneacetate (3mb)
3.4.14. 2-(1-phenylethylidene)malononitrile (3na)
3.4.15. 2-(1-(4-bromophenyl)ethylidene)malononitrile (3pa)
3.5. Reusability of the Catalyst
3.6. Scale Up Procedure
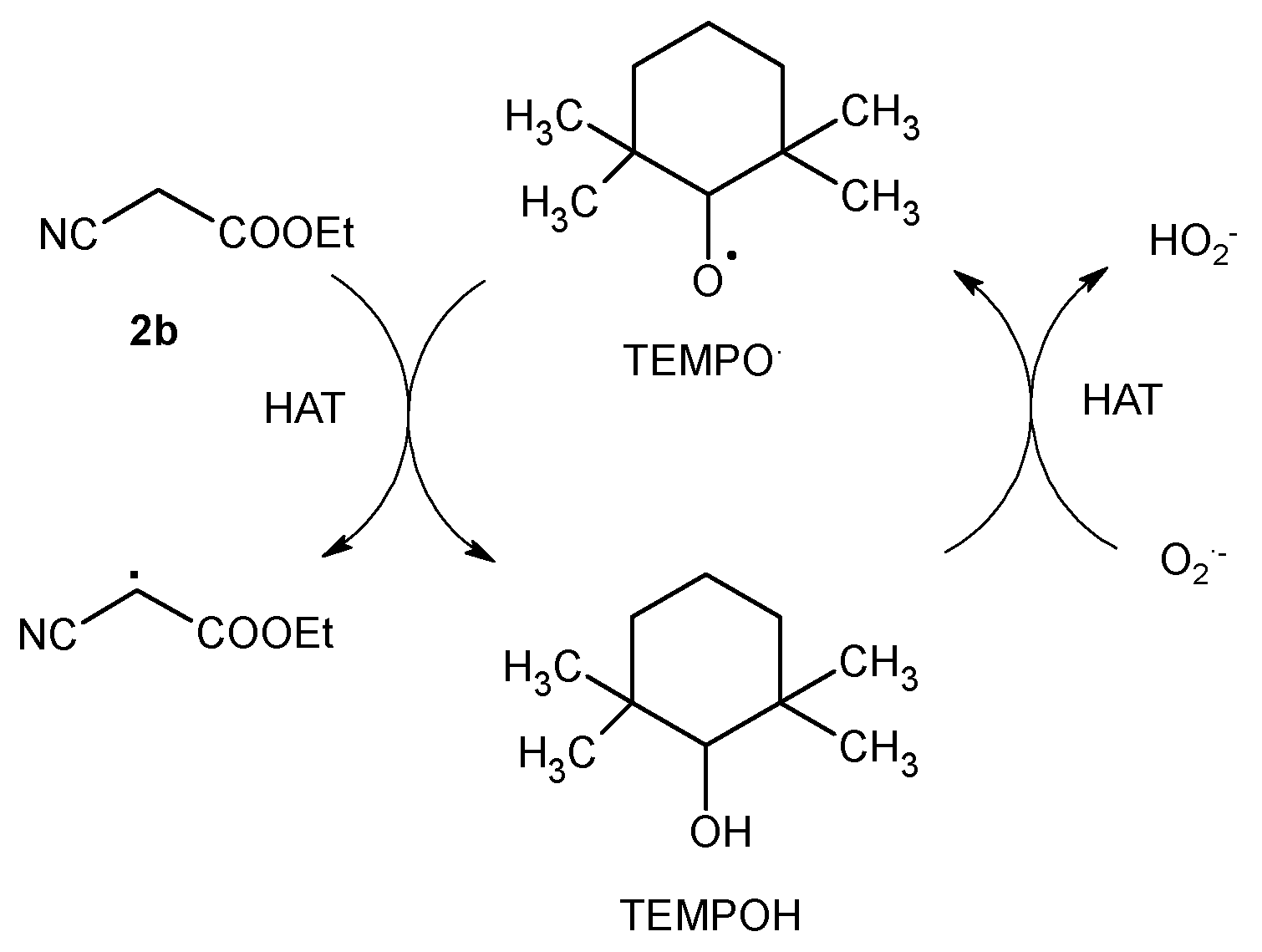
3.7. Mechanistic Investigations
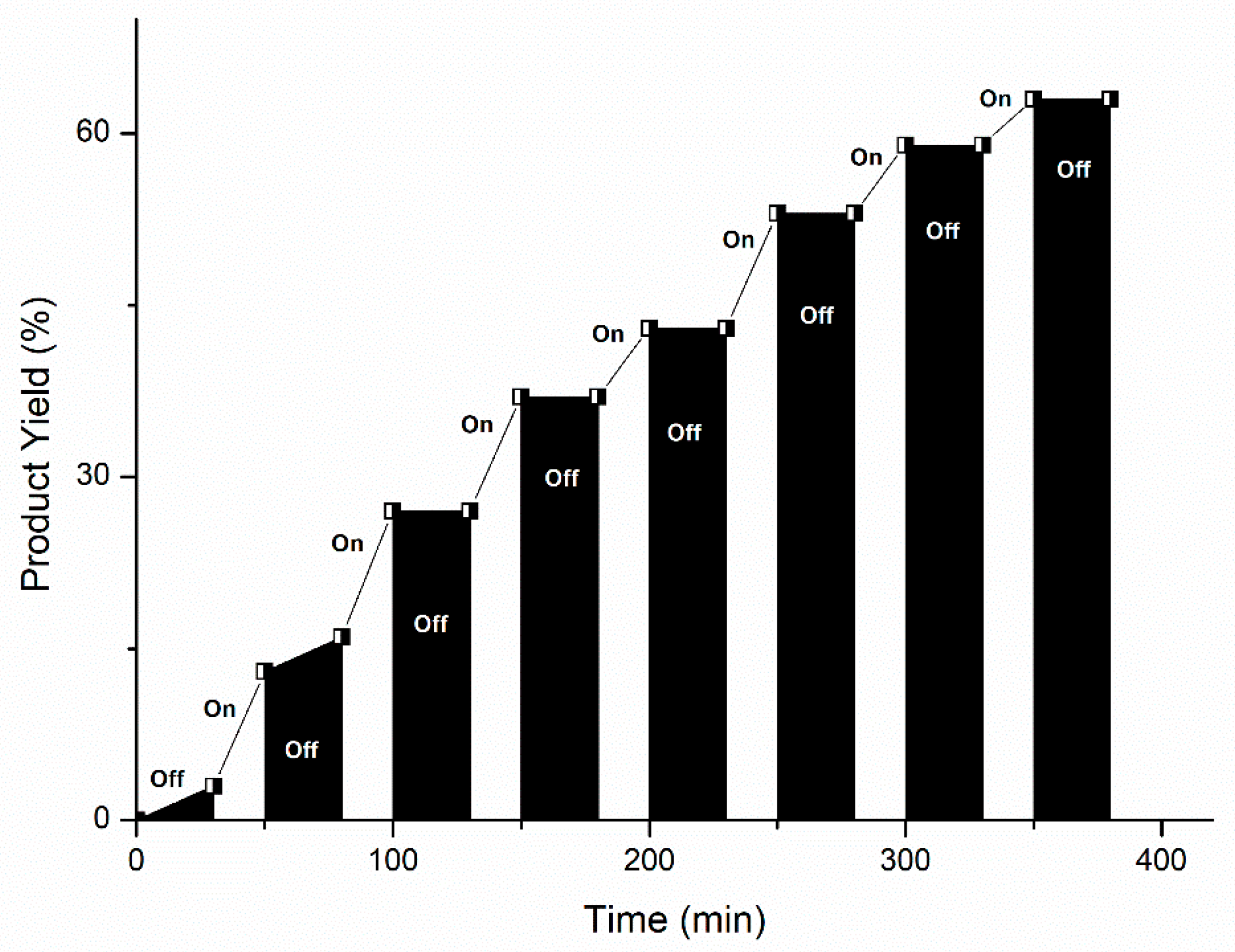
3.8. Light On–Off Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Michelin, C.; Hoffmann, N. Photosensitization and photocatalysis—Perspectives in organic synthesis. ACS Catal. 2018, 8, 12046–12055. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Maity, P.P.; Ganguly, S.; Ghosh, S.; Baral, J.; Bose, M.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Das, A.K.; et al. Biocompatible carbon dots derived from κ-carrageenan and phenyl boronic acid for dual modality sensing platform of sugar and its anti-diabetic drug release behavior. Int. J. Biol. Macromol. 2019, 132, 316–329. [Google Scholar] [CrossRef]
- Shahraki, H.S.; Ahmad, A.; Bushra, R. Green carbon dots with multifaceted applications—Waste to wealth strategy. Flat Chem. 2022, 31, 100310. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Khairol Anuar, N.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Bakar, N.F. A review on multifunctional carbon dots synthesized from biomass waste: Design/fabrication, characterization and applications. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]
- Gao, Y.; Ge, L.; Xu, H.; Davey, K.; Zheng, Y.; Qiao, S.-Z. Electrocatalytic refinery of biomass-based 5-hydroxymethylfurfural to fine chemicals. ACS Catal. 2023, 13, 11204–11231. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y.; Yao, Y.; Wei, X.; Yue, T.; Meng, J. Formation of 5-hydroxymethylfurfural in industrial-scale apple juice concentrate processing. Food Control 2019, 102, 56–68. [Google Scholar] [CrossRef]
- Bressi, V.; Chiarotto, I.; Ferlazzo, A.; Celesti, C.; Michenzi, C.; Len, T.; Iannazzo, D.; Neri, G.; Espro, C. Voltammetric sensor based on waste-derived carbon nanodots for enhanced detection of nitrobenzene. ChemElectroChem 2023, 10, e202300004. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Prato, M. Carbon dots as nano-organocatalysts for synthetic applications. ACS Catal. 2020, 10, 8090–8105. [Google Scholar] [CrossRef]
- Michenzi, C.; Espro, C.; Bressi, V.; Celesti, C.; Vetica, F.; Salvitti, C.; Chiarotto, I. Electrochemical bottom-up synthesis of biomass-derived carbon dots for promoting Knoevenagel condensation. Mol. Catal. 2023, 544, 113182. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.; Garg, A.K.; Dalal, C.; Anand, S.R.; Sunkar, S.K.; Sonker, A.K.; Westman, G. Visible-light-promoted photocatalytic applications of carbon dots: A review. ACS Appl. Nano Mater. 2022, 5, 3087–3109. [Google Scholar] [CrossRef]
- Munir, S.; Dionysiou, D.D.; Khan, S.B.; Shah, S.M.; Adhikari, B.; Shah, A. Development of photocatalysts for selective and efficient organic transformations. J. Photochem. Photobiol. B 2015, 148, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Ravelli, D.; Protti, S.; Basso, A. Photoinduced multicomponent reactions. Angew. Chem. Int. Ed. 2016, 55, 15476–15484. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, Q.; Tao, S.; Xia, C.; Liu, C.; Liu, P.; Yang, B. Carbon dots based photoinduced reactions: Advances and perspective. Adv. Sci. 2023, 10, 2207621. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 25, 3774–3776. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Melchiorre, P. Chemistry glows green with photoredox catalysis. Nat. Commun. 2020, 11, 803. [Google Scholar] [CrossRef]
- Wang, W.; Luo, M.; Yao, W.; Ma, M.; Pullarkat, S.A.; Xu, L.; Leung, P.-H. Catalyst-free and solvent-free cyanosilylation and Knoevenagel condensation of aldehydes. ACS Sustain. Chem. Eng. 2019, 7, 1718–1722. [Google Scholar] [CrossRef]
- Kotnik, T.; Žerjav, G.; Pintar, A.; Žagar, E.; Kovačič, S. Highly porous poly(arylene cyano-vinylene) beads derived through the Knoevenagel condensation of the Oil-in-Oil-in-Oil double emulsion templates. ACS Macro Lett. 2021, 10, 1248–1253. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Liu, B.; Gao, A.; Cao, J.; Yu, Y.; Hei, X. A fluorescent conjugated polymer photocatalyst based on the Knoevenagel polycondensation for hydrogen production. New J. Chem. 2019, 43, 7093–7098. [Google Scholar] [CrossRef]
- Gambacorta, G.; Sharley, J.S.; Baxendale, I.R. A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries. Belstein J. Org. Chem. 2021, 17, 1181–1312. [Google Scholar] [CrossRef]
- Dieter, M.Z.; Freshwater, S.L.; Solis, W.A.; Nebert, D.W.; Dalton, T.P. Tryphostin AG879, a tyrosine kinase inhibitor: Prevention of transcriptional activation of the electrophile and the aromatic hydrocarbon response elements. Biochem. Pharmacol. 2001, 61, 215–225. [Google Scholar] [CrossRef]
- Beutler, U.; Fuenfschilling, P.C.; Steinkemper, A. An improved manufacturing process for the antimalaria drug coartem. Part II. Org. Process Res. Dev. 2007, 11, 341–345. [Google Scholar] [CrossRef]
- Tokala, R.; Bora, D.; Shankara, N. Contribution of Knoevenagel condensation products toward the development of anticancer agents: An updated review. ChemMedChem 2022, 17, e202100736. [Google Scholar] [CrossRef]
- Magaji, B.; Singh, P.; Skelton, A.A.; Martincigh, B.S. Synthesis, photostability and antibacterial activity of a series of symmetrical α,β-unsaturated ketones as potential UV filters. J. Photochem. Photobiol. A 2023, 445, 115018. [Google Scholar] [CrossRef]
- Dalessandro, E.V.; Collin, H.P.; Valle, M.S.; Pliego, J.R., Jr. Mechanism and free energy profile of base catalyzed Knoevenagel condensation reaction. RSC Adv. 2016, 6, 57803–57810. [Google Scholar] [CrossRef]
- Pandolfi, F.; Feroci, M.; Chiarotto, I. Role of anion and cation in the 1-methyl-3-butyl imidazolium ionic liquids BMImX: The Knoevenagel condensation. ChemistrySelect 2018, 3, 4745–4749. [Google Scholar] [CrossRef]
- Salvitti, C.; Pepi, F.; Managò, M.; Bortolami, M.; Michenzi, C.; Chiarotto, I.; Troiani, A.; de Petris, G. Free N-heterocyclic carbenes from Brønsted acidic ionic liquids: Direct detection by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2022, 36, e9338. [Google Scholar] [CrossRef] [PubMed]
- Salvitti, C.; Chiarotto, I.; Pepi, F.; Troiani, A. Charge-tagged N-heterocyclic carbenes (NHCs): Revealing the hidden side of NHC-catalysed reactions through electrospray ionization mass spectrometry. ChemPlusChem 2021, 86, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; de Petris, G.; Pepi, F.; Garzoli, S.; Salvitti, C.; Rosi, M.; Ricci, A. Base-assisted conversion of protonated D-fructose to 5-HMF: Searching for gas-phase green models. ChemistryOpen 2019, 8, 1190–1198. [Google Scholar] [CrossRef]
- Salvitti, C.; Bortolami, M.; Chiarotto, I.; Troiani, A.; de Petris, G. The Knoevenagel condensation catalysed by ionic liquids: A mass spectrometric insight into the reaction mechanism. New J. Chem. 2021, 45, 17787–17795. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ratti, R.; Phoon, B.L.; Batagarawa, S.M.; Din, I.U.; Selvaraj, M.; Ramalingam, R.J. A review of the recent progress on heterogeneous catalysts for Knoevenagel condensation. Dalton Trans. 2021, 50, 4445–4469. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.-N.; Li, J.-M.; Liu, N.; Zhang, Z.-H. Visible-light initiated one-pot, three-component synthesis of 2-amino-4H-pyran-3,5-dicarbonitrile derivatives. ACS Comb. Sci. 2019, 21, 685–691. [Google Scholar] [CrossRef]
- Das, A.; Justin Thomas, K.R. Rose bengal photocatalyzed Knoevenagel condensation of aldehydes and ketones in aqueous medium. Green Chem. 2022, 24, 4952–4957. [Google Scholar] [CrossRef]
- Cosentino, F.; Michenzi, C.; Di Noi, A.; Salvitti, C.; Pepi, F.; de Petris, G.; Chiarotto, I.; Troiani, A. Photo-activated Carbon dots (CDs) as catalysts in the Knoevenagel condensation: A mechanistic study by dual-mode monitoring via ESI-MS. ChemPlusChem 2024, 89, e202400174. [Google Scholar] [CrossRef]
- Han, Y.; Huang, H.; Zhang, H.; Liu, Y.; Han, X.; Liu, R.; Li, H.; Kang, Z. Carbon quantum dots with photoenhanced hydrogen-bond catalytic activity in aldol condensations. ACS Catal. 2014, 4, 781–787. [Google Scholar] [CrossRef]
- Pei, X.; Xiong, D.; Wang, H.; Gao, S.; Zhang, X.; Zhang, S.; Wang, J. Reversible phase transfer of carbon dots between an organic phase and aqueous solution triggered by CO2. Angew. Chem. Int. Ed. 2018, 57, 3687–3691. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Lakshmanan, A.; Vinitha, G.; Ramalingam, G.; Rameshkumar, P. Facile preparation of high fluorescent carbon quantum dots from orange waste peels for nonlinear optical applications. Luminescence 2020, 35, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Capisciano, V.; Giacalone, F.; Gruttadauria, M. Is a Catalyst Always Needed? The Case of the Knoevenagel Reaction with Malononitrile. ChemCatChem 2022, 14, e202200696. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Zhou, J.; Wang, Y.; Lang, X. Anchoring dye onto 1D Nb2O5 in cooperation with TEMPO for the selective photocatalytic aerobic oxidation of amines. Chem. Eng. J. 2021, 426, 131418. [Google Scholar] [CrossRef]
- Ito, T.; Seidel, F.W.; Jin, X.; Nozaki, K. TEMPO as a hydrogen atom transfer catalyst for aerobic dehydrogenation of activated alkanes to alkenes. J. Org. Chem. 2022, 87, 12733–12740. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Gajaganti, S.; Bajpai, S.; Srivastava, V.; Singh, S. An efficient, room temperature, oxygen radical anion (O2•−) mediated, one-pot, and multicomponent synthesis of spirooxindoles. Can. J. Chem. 2017, 95, 1296–1302. [Google Scholar] [CrossRef]
- Hojatti, M.; Kresge, A.J.; Wang, W.H. Cyanocarbon acid. Direct evidence that their ionization is not an encounter-controlled process and rationalization of the unusual solvent isotope effects. J. Am. Chem. Soc. 1987, 109, 4023–4028. [Google Scholar] [CrossRef]
- Zhang, X.M.; Yang, D.L.; Liu, Y.C.; Chen, W.; Cheng, J.L. Kinetics and mechanism of the reactions of o-and p-nitrohalobeszenes with the sodium salt of ethyl cyanoacetate carbanion: A non-chain radical nucleophilic substitution mechanism. Res. Chem. Intermed. 1989, 11, 281–300. [Google Scholar] [CrossRef]
- Roschangar, F.; Sheldonb, R.A.; Senanayakea, C.H. Overcoming barriers to green chemistry in the pharmaceutical industry—the Green Aspiration LevelTM concept. Green Chem. 2015, 17, 752–768. [Google Scholar] [CrossRef]
- Michenzi, C.; Proietti, A.; Rossi, M.; Espro, C.; Bressi, V.; Vetica, F.; Simonis, B.; Chiarotto, I. Carbon nanodots from orange peel waste as fluorescent probes for detecting nitrobenzene. RSC Sustain. 2024, 2, 933–942. [Google Scholar] [CrossRef]
- Naskar, K.; Maity, S.; Maity, H.S.; Sinha, C.K. A reusable efficient green catalyst of 2D Cu-MOF for the click and Knoevenagel reaction. Molecules 2021, 26, 5296. [Google Scholar] [CrossRef] [PubMed]
- Kalar, P.L.; Jain, K.; Agrawal, S.; Khan, S.; Vishwakarma, R.; Shivhare, A.; Deshmukh, M.M.; Das, K. Green synthesis of electrophilic alkenes using a magnesium catalyst under aqueous conditions and mechanistic insights by density functional theory. J. Org. Chem. 2023, 88, 16829–16844. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Mukhtar, S.; Alsharif, M.A.; Alahmdi, M.I.; Ahmed, N. PhI(OAc)2 mediated an efficient Knoevenagel reaction and their synthetic application for coumarin derivatives. Tetrahedron Lett. 2017, 58, 3183–3187. [Google Scholar] [CrossRef]
- Meng, G.; Gao, S.; Liu, Y.; Zhang, L.; Song, C.; Huang, K. Amino-and sulfo-bifunctionalized hyper-crosslinked organic nanotube frameworks as efficient catalysts for one-pot cascade reactions. New J. Chem. 2019, 43, 2269–2273. [Google Scholar] [CrossRef]
- Jain, K.; Chaudhuri, S.; Pal, K.; Das, K. The Knoevenagel condensation using quinine as an organocatalyst under solvent-free conditions. New J. Chem. 2019, 43, 1299–1304. [Google Scholar] [CrossRef]
- Nicholson, W.; Howard, J.; Magri, G.; Seastram, A.; Khan, A.; Morrill, L.C.; Richards, E.; Browne, D. Ball-milling-enabled reactivity of manganese metal. Angew. Chem. Int. Ed. 2021, 60, 23128–23133. [Google Scholar] [CrossRef]
- Wu, D.P.; Ou, W.; Huang, P.Q. Ir-catalyzed chemoselective reductive condensation reactions of tertiary amides with active methylene compounds. Org. Lett. 2022, 24, 5366–5371. [Google Scholar] [CrossRef]
- Javad Kalbasi, R.; Khojastegi, A. Hierarchically pore structure poly 2-(dimethyl amino) ethyl methacrylate/Hi-ZSM-5: A novel acid–base Bi-functional catalyst as heterogeneous platform for a tandem reaction. Catalysis Lett. 2018, 148, 958–971. [Google Scholar] [CrossRef]
- Yamakoshi, H.; Shibata, D.; Bando, K.; Kajimoto, S.; Kohyama, A.; Egoshi, S.; Dodo, K.; Iwabuchi, Y.; Sodeoka, M.; Fujita, K.; et al. Ratiometric analysis of reversible thia-Michael reactions using nitrile-tagged molecules by Raman microscopy. Chem. Commun. 2023, 59, 14563–14566. [Google Scholar] [CrossRef]
- Vilela, G.G.; dos Santos Silva, W.F.; de Melo Batista, V.; Rocha Silva, L.; Maus, H.; Hammerschmidt, S.J.; Crisóstomo Bezerra Costa, C.A.; da Silva Moura, O.F.; Duarte de Freitas, J.; Lobo Coelho, G.; et al. Fragment-based design of α-cyanoacrylates and α-cyanoacrylamides targeting Dengue and Zika NS2B/NS3 proteases. New J. Chem. 2022, 46, 20322–20346. [Google Scholar] [CrossRef]
- Krech, A.; Yakimchyk, V.; Jarg, T.; Kananovich, D.; Ošeka, M. Ring-opening coupling reaction of cyclopropanols with electrophilic alkenes enabled by decatungstate as photoredox catalyst. Adv. Synth. Catal. 2024, 366, 91–100. [Google Scholar] [CrossRef]
- Hayashi, Y.; Han, X.; Mori, N. Secondary amine-mediated domino reaction for the synthesis of substituted quinolines from dicyanoalkenes and enynals. Chem. Eur. J. 2023, 29, e202301093. [Google Scholar] [CrossRef] [PubMed]



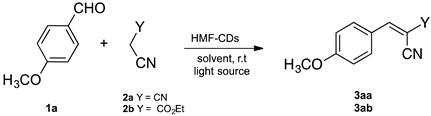 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst Loading (mg) | 2 | Solvent | Time (min) | Light Source | Yields (%) 3aa/3ab |
| 1 | 10 | 2a | H2O | 30 | ambient light | 55 |
| 2 | 10 | 2a | H2O | 30 | white light b | 49 |
| 3 | 10 | 2a | H2O | 30 | UV light c | 76 |
| 4 | 10 | 2a | H2O/EtOH d | 30 | UV light c | 98 |
| 5 | 10 | 2a | H2O | 30 | dark | 52 |
| 6 | - | 2a | H2O | 30 | UV light c | Trace |
| 7 | 10 | 2b | H2O | 30 | ambient light | ND |
| 8 | 10 | 2b | H2O | 30 | UV light c | Trace |
| 9 | 10 | 2b | H2O/EtOH d | 30 | UV light c | 25 |
| 10 | 10 | 2b | H2O/EtOH d | 150 | UV light c | 80 |
| 11 | 10 | 2b | H2O/EtOH d | 30 | dark | ND |
| 12 | - | 2b | H2O/EtOH d | 30 | UV light c | ND |
| 13 | 5 | 2b | H2O/EtOH d | 30 | UV light c | 15 |
 | ||
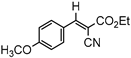 3ab, 40% | 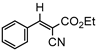 3bb, 8% |  3cb, 50% |
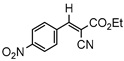 3db, 80% | 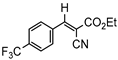 3eb, 60% | 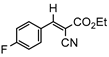 3fb, 93% |
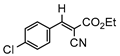 3gb, 81% | 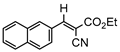 3hb, 54% | 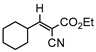 3ib, 21% 80 min |
 3jb, 23%, 80 min | 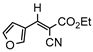 3kb, 60% | 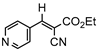 3lb, <99% |
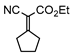 3mb, 13% isolated, 5 h |  3na, 25% isolated, 11 h | 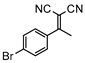 3pa, 26% isolated, 11 h |
 | ||
|---|---|---|
| Entry | Conditions a | Yield (%) 3ab |
| 1 | Standard | 40 |
| 2 | Solvent free | 48 b |
| 3 | H2O | ND |
| 4 | Dark | Trace |
| 5 | No catalyst | 3 |
| 6 | N2 | 19 |
| 7 | p-benzoquinone | 25 |
| 8 | TEMPO | 71 |
| 9 | AgNO3 | Trace |
| 10 | HCO2H | Trace |
| Entry | Conditions | Yield 3ab (%) |
|---|---|---|
| 1 | No catalyst | ND |
| 2 | Only CDs | Trace |
| 3 | Only UV LED | Trace |
| 4 | UV LED + TEMPO (20% mmol) | ND |
| 5 | Standard a | 40 |
| 6 | UV LED + CDs + TEMPO (20% mmol) | 45 |
| 7 | UV LED + CDs + TEMPO (2 eq) | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michenzi, C.; Scaramuzzo, F.; Salvitti, C.; Pepi, F.; Troiani, A.; Chiarotto, I. Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field. Photochem 2024, 4, 361-376. https://doi.org/10.3390/photochem4030022
Michenzi C, Scaramuzzo F, Salvitti C, Pepi F, Troiani A, Chiarotto I. Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field. Photochem. 2024; 4(3):361-376. https://doi.org/10.3390/photochem4030022
Chicago/Turabian StyleMichenzi, Cinzia, Francesca Scaramuzzo, Chiara Salvitti, Federico Pepi, Anna Troiani, and Isabella Chiarotto. 2024. "Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field" Photochem 4, no. 3: 361-376. https://doi.org/10.3390/photochem4030022
APA StyleMichenzi, C., Scaramuzzo, F., Salvitti, C., Pepi, F., Troiani, A., & Chiarotto, I. (2024). Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field. Photochem, 4(3), 361-376. https://doi.org/10.3390/photochem4030022









