Photocatalytic Degradation of Methylene Blue by Magnetic Opal/Fe3O4 Colloidal Crystals under Visible Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the SiO2-Fe3O4 Composites
2.2. Evaluation of the Photocatalytic Activity
2.3. XRD Characterization
3. Results and Discussion
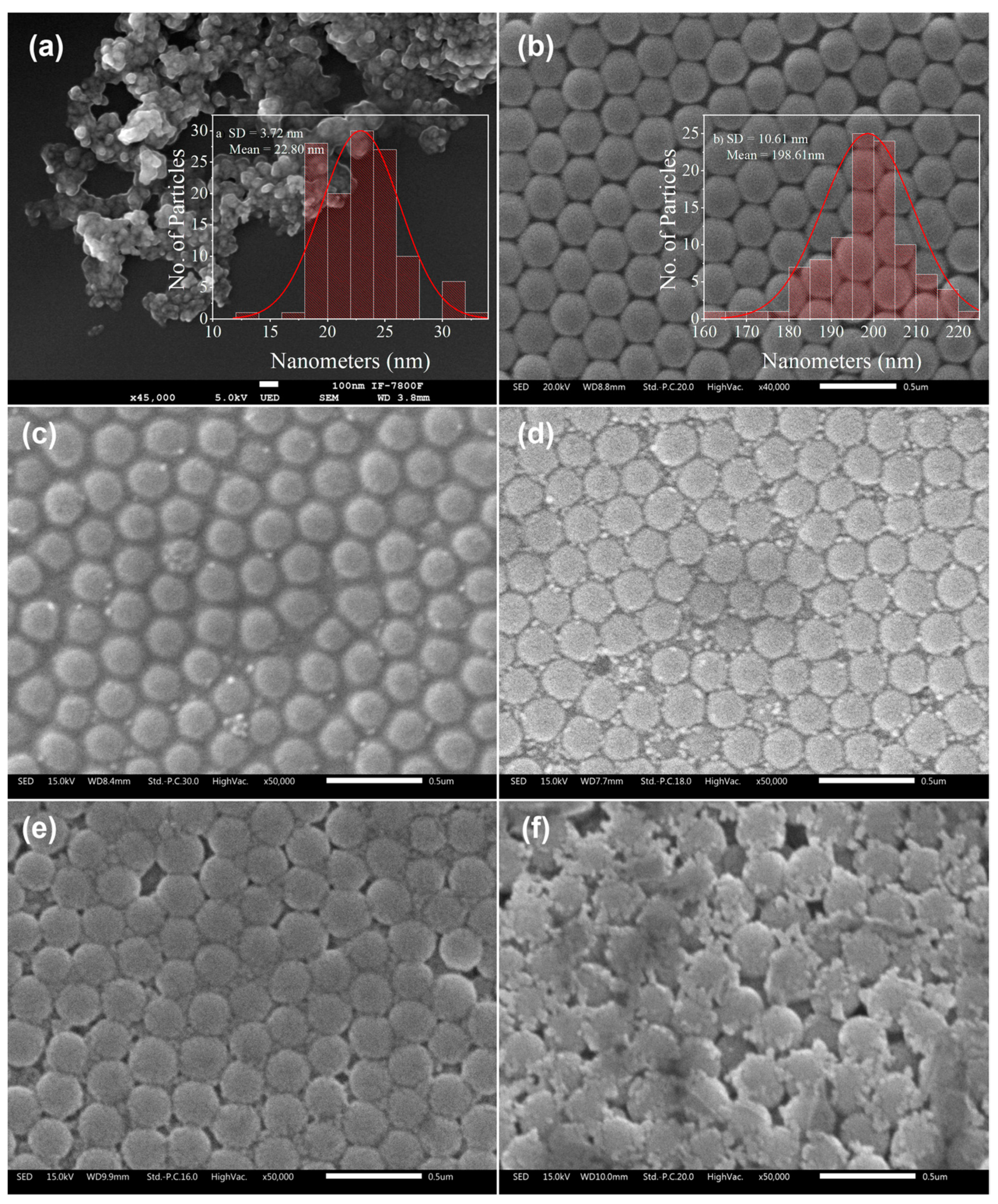
3.1. Morphologic Analysis
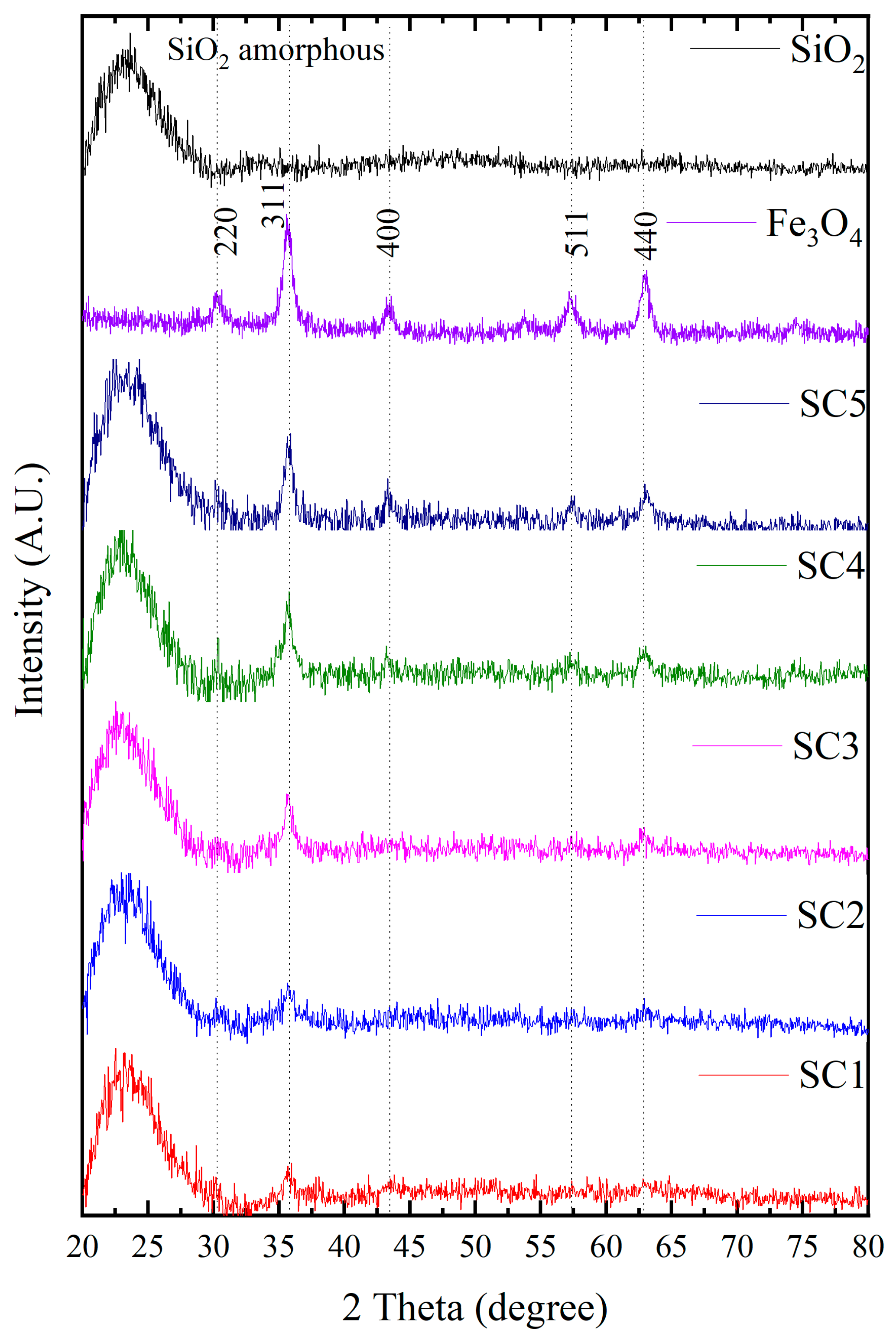
3.2. XRD Measurements
3.3. FTIR Measurements
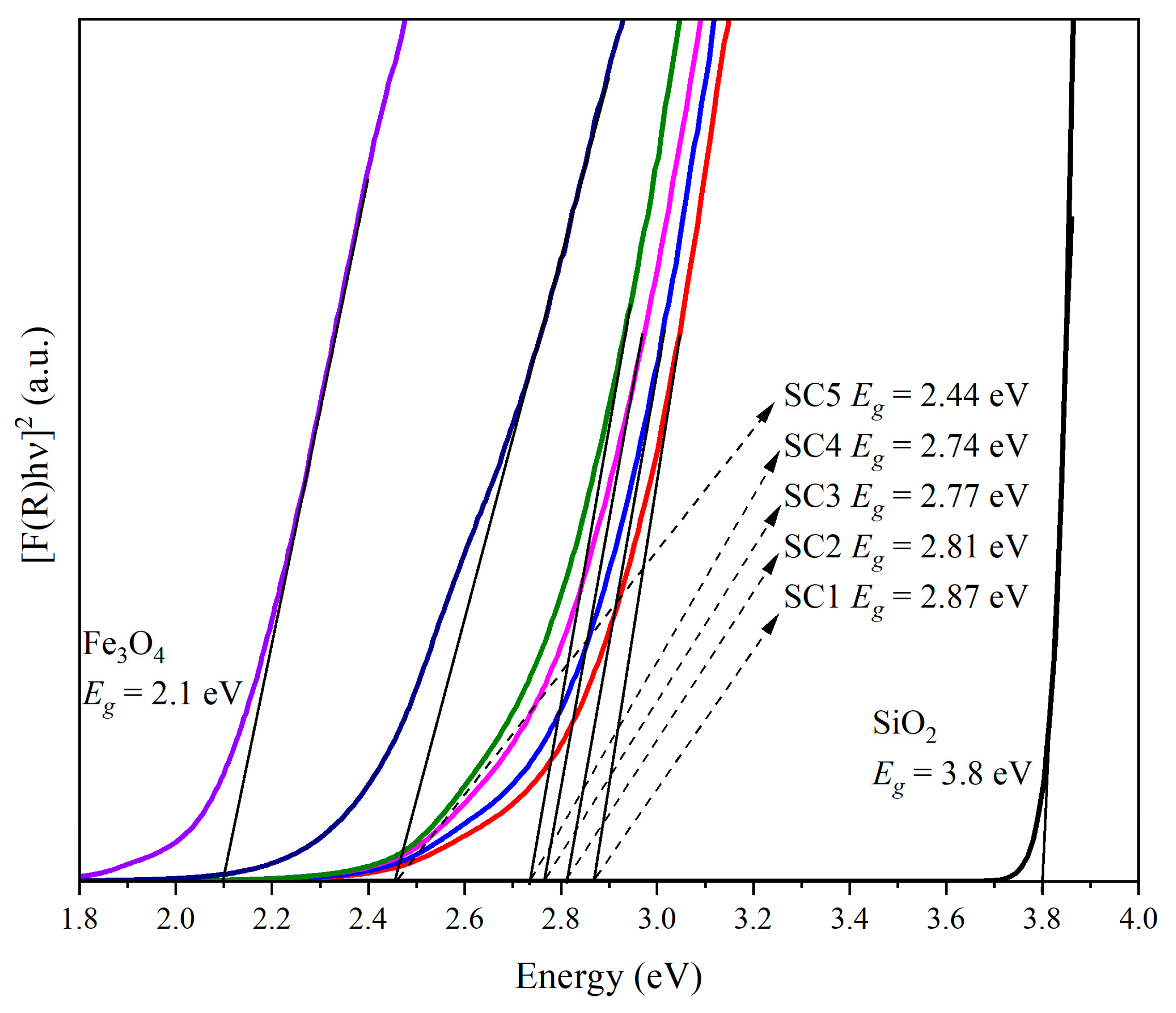
3.4. Bandgap Determination
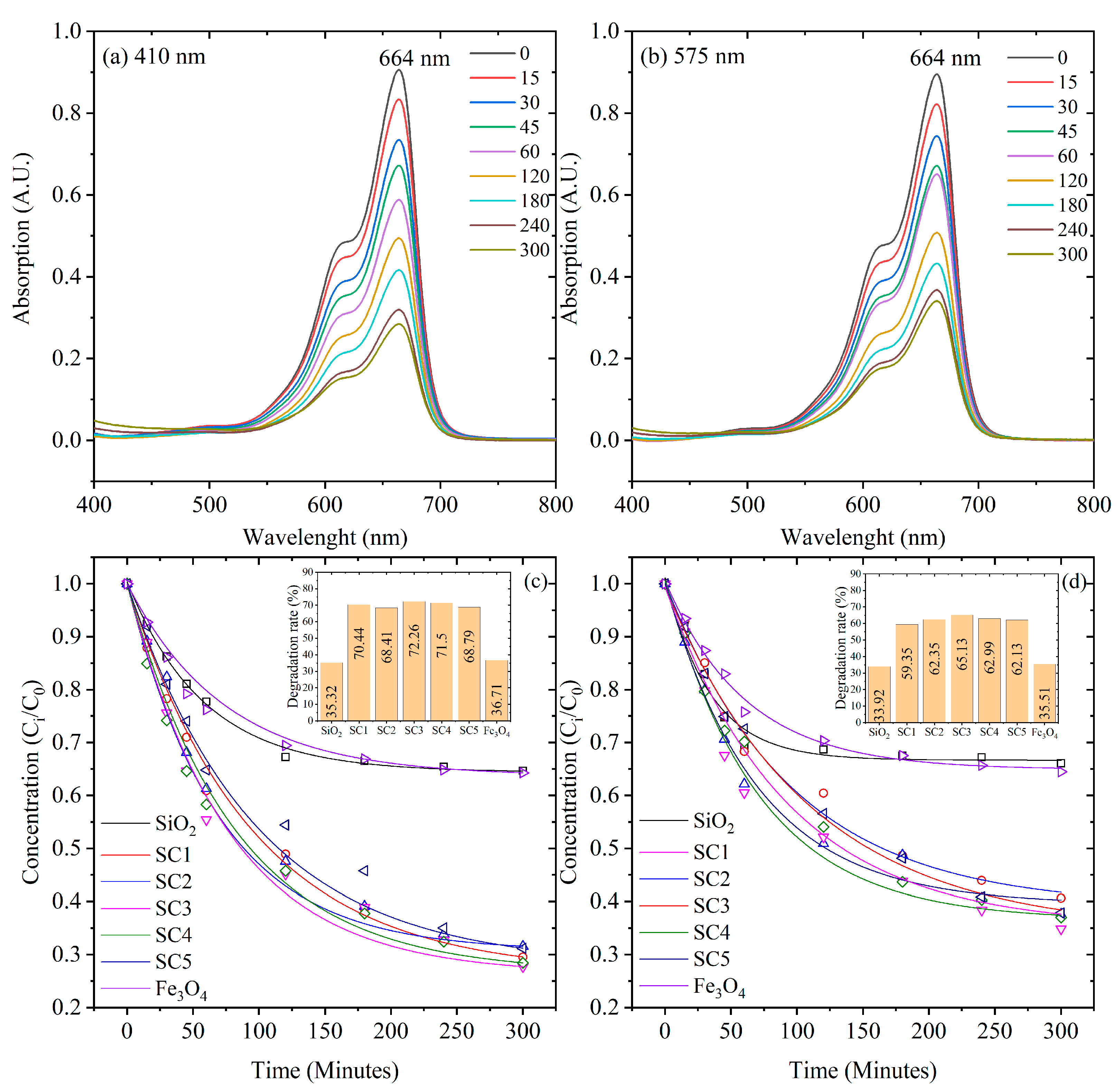
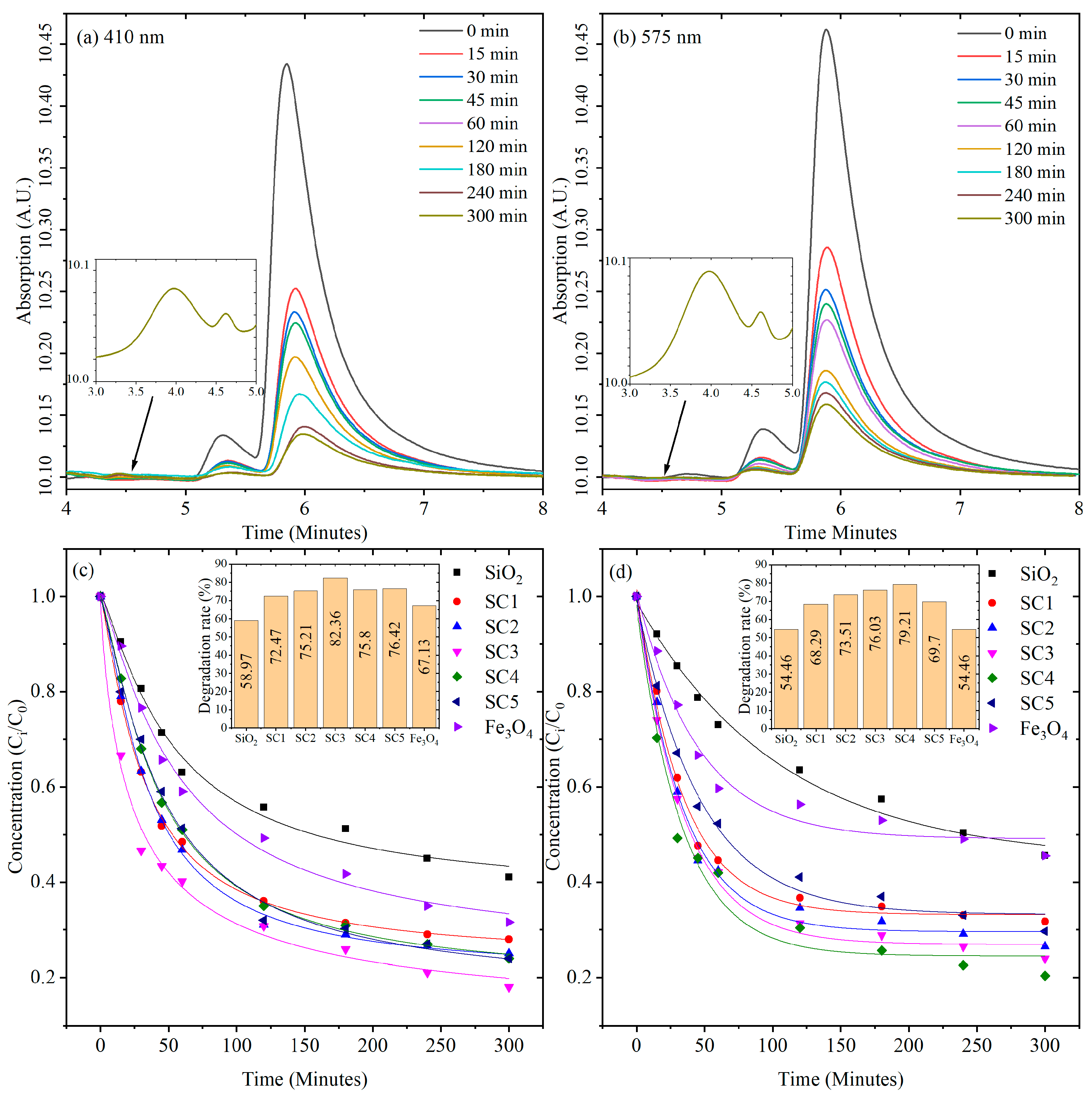
3.5. Photocatalytic Activity under Limited O2
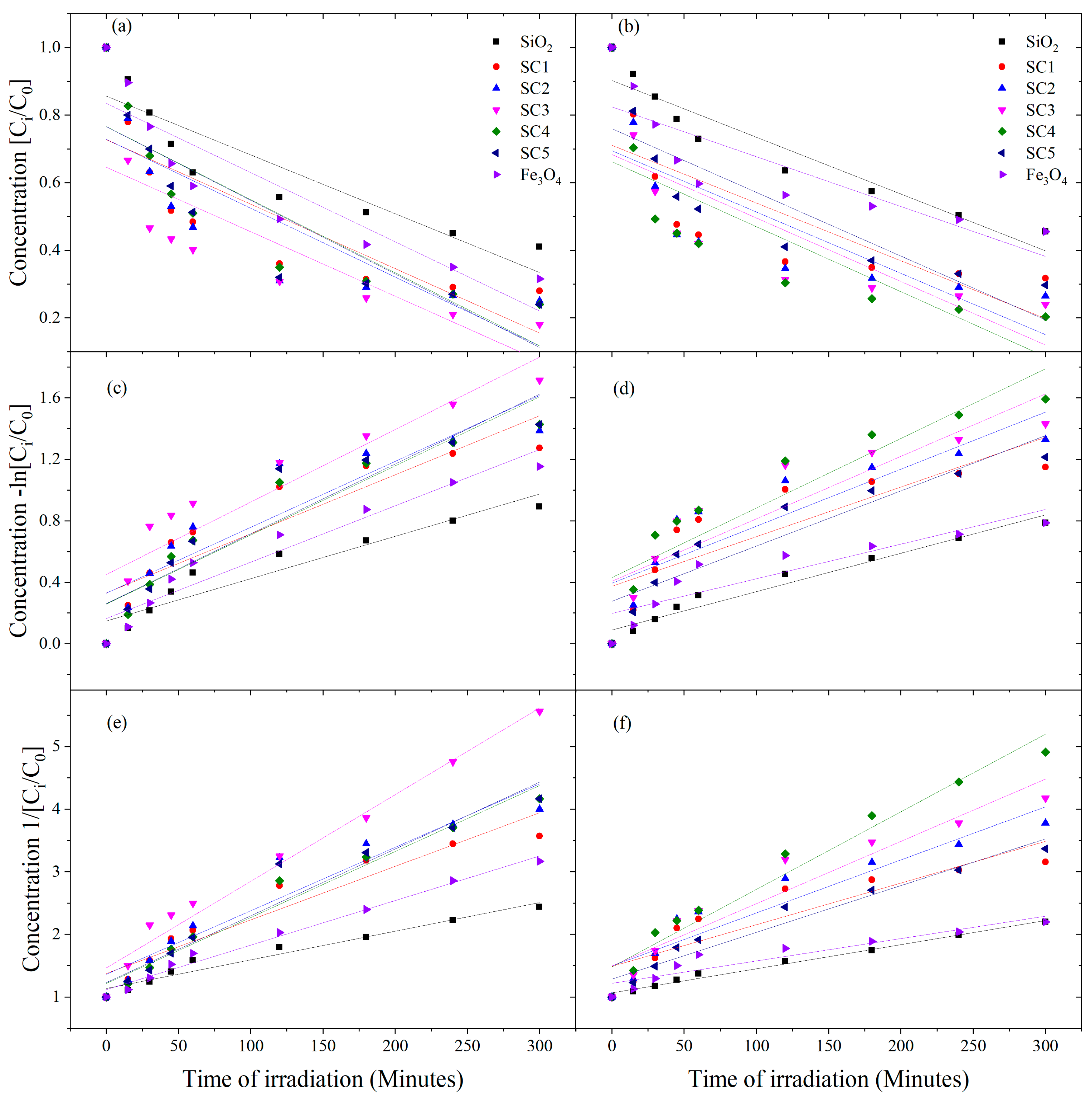
3.6. Kinetic Model Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohrabnezhad, S. Study of catalytic reduction and photodegradation of methylene blue by heterogeneous catalyst. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 81, 228–235. [Google Scholar] [CrossRef]
- El-Kemary, M.; Abdel-Moneam, Y.; Madkour, M.; El-Mehasseb, I. Enhanced photocatalytic degradation of Safranin-O by heterogeneous nanoparticles for environmental applications. J. Lumin. 2011, 131, 570–576. [Google Scholar] [CrossRef]
- Kamat, P.V.; Huehn, R.; Nicolaescu, R. A “Sense and Shoot” Approach for Photocatalytic Degradation of Organic Contaminants in Water. J. Phys. Chem. B 2002, 106, 788–794. [Google Scholar] [CrossRef]
- Yang, J.L.; An, S.J.; Park, W.I.; Yi, G.-C.; Choi, W. Photocatalysis Using ZnO Thin Films and Nanoneedles Grown by Metal-Organic Chemical Vapor Deposition. Adv. Mater. 2004, 16, 1661–1664. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Elabiad, M.A.; Mohamed, A.A. Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100719. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Madima, N.; Mishra, S.B.; Inamuddin, I.; Mishra, A.K. Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ. Chem. Lett. 2020, 18, 1169–1191. [Google Scholar] [CrossRef]
- Soleimani, M.; Ghasemi, J.B.; Ziarani, G.M.; Karimi-Maleh, H.; Badiei, A. Photocatalytic degradation of organic pollutants, viral and bacterial pathogens using titania nanoparticles. Inorg. Chem. Commun. 2021, 130, 108688. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Deshpande, B.; Agrawal, P.; Yenkie, M.; Dhoble, S. Prospective of nanotechnology in degradation of waste water: A new challenges. Nano-Struct. Nano-Objects 2020, 22, 100442. [Google Scholar] [CrossRef]
- Fei, L.; Bilal, M.; Qamar, S.A.; Imran, H.M.; Riasat, A.; Jahangeer, M.; Ghafoor, M.; Ali, N.; Iqbal, H.M. Nano-remediation technologies for the sustainable mitigation of persistent organic pollutants. Environ. Res. 2022, 211, 113060. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Gomez-Maldonado, D.; Whitehead, D.C.; Yang, M.; Peresin, M.S. Comparison between nanocellu-lose-polyethylenimine composites synthesis methods towards multiple water pollutants removal: A review. Int. J. Biol. Macromol. 2023, 232, 123342. [Google Scholar]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Chang, P.R.; Yu, J.; Ma, X.; Anderson, D.P. Polysaccharides as stabilizers for the synthesis of magnetic nanoparticles. Carbohydr. Polym. 2011, 83, 640–644. [Google Scholar] [CrossRef]
- Santhosh, C.; Malathi, A.; Dhaneshvar, E.; Bhatnagar, A.; Grace, A.N.; Madhavan, J. Iron Oxide Nanomaterials for Water Purification. In Micro and Nano Technologies, Nanoscale Materials in Water Purification, 1st ed.; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 431–446. [Google Scholar]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, F.; He, M.; Song, M.; Ma, Z.; Shi, W.; Yan, Y.; Lan, J.; Li, F.; Xiao, P. Microwave synthesis of a novel magnetic imprinted TiO2 photocatalyst with excellent transparency for selective photodegradation of enrofloxacin hydrochloride residues solution. Chem. Eng. J. 2014, 249, 15–26. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase Photocatalysis: A Review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, K.; Qi, Y.; Sheng, J.; Liu, Z. One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J. Mater. Chem. 2010, 20, 1799–1805. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, H.; Sun, D.D. Facile fabrication of porous chitosan/TiO2/Fe3O4microspheres with multifunction for water purifications. New J. Chem. 2011, 35, 137–140. [Google Scholar] [CrossRef]
- Johnson, B.F.G. Nanoparticles in Catalysis. Top. Catal. 2003, 24, 147–159. [Google Scholar] [CrossRef]
- Xiong, L.; Sun, W.; Yang, Y.; Chen, C.; Ni, J. Heterogeneous photocatalysis of methylene blue over titanate nanotubes: Effect of adsorption. J. Colloid Interface Sci. 2011, 356, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Carmona-Carmona, A.; Palomino-Ovando, M.; Hernández-Cristobal, O.; Sánchez-Mora, E.; Toledo-Solano, M. Synthesis and characterization of magnetic opal/Fe3O4 colloidal crystal. J. Cryst. Growth 2017, 462, 6–11. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Razo, D.A.S.; Pallavidino, L.; Garrone, E.; Geobaldo, F.; Descrovi, E.; Chiodoni, A.; Giorgis, F. A version of Stöber synthesis enabling the facile prediction of silica nanospheres size for the fabrication of opal photonic crystals. J. Nanopart. Res. 2008, 10, 1225–1229. [Google Scholar] [CrossRef]
- Cui, Y.-H.; Li, X.-Y.; Chen, G. Electrochemical degradation of bisphenol A on different anodes. Water Res. 2009, 43, 1968–1976. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. A Green and Facile Approach for Synthesis of Magnetite Nanoparticles. Nanosci. Nanotechnol. 2012, 2, 208–213. [Google Scholar] [CrossRef]
- Andrade, L.; Fabris, J.D.; Ardisson, J.D.; Valente, M.A.; Ferreira, J.M.F. Effect of Tetramethylammonium Hydroxide on Nucleation, Surface Modification and Growth of Magnetic Nanoparticles. J. Nanomater. 2012, 2012, 454759. [Google Scholar] [CrossRef]
- Ruso, J.M.; Gravina, A.N.; D’Elía, N.L.; Messina, P.V. Highly efficient photoluminescence of SiO2 and Ce–SiO2 microfibres and microspheres. Dalton Trans. 2013, 42, 7991–8000. [Google Scholar] [CrossRef] [PubMed]
- Bachan, N.; Asha, A.; Jeyarani, W.J.; Kumar, D.A.; Shyla, J.M. A Comparative Investigation on the Structural, Optical and Electrical Properties of SiO2–Fe3O4 Core–Shell Nanostructures with Their Single Components. Acta Met. Sin. Engl. Lett. 2015, 28, 1317–1325. [Google Scholar] [CrossRef]
- Yang, S.; He, H.; Wu, D.; Chen, D.; Liang, X.; Qin, Z.; Fan, M.; Zhu, J.; Yuan, P. Decolorization of methylene blue by heterogeneous Fenton reaction using Fe3−xTixO4 (0≤x≤0.78) at neutral pH values. Appl. Catal. B Environ. 2009, 89, 527–535. [Google Scholar] [CrossRef]
- Zuo, R.; Du, G.; Zhang, W.; Liu, L.; Liu, Y.; Mei, L.; Li, Z. Photocatalytic Degradation of Methylene Blue Using TiO2 Impregnated Diatomite. Adv. Mater. Sci. Eng. 2014, 2014, 170148. [Google Scholar] [CrossRef]
- Luong, N.H.; Phu, N.D.; Hai, N.H.; Thuy, N.T.D. Surface Modification of SiO2-Coated FePt Nanoparticles with Amino Groups. E-J. Surf. Sci. Nanotechnol. 2011, 9, 536–538. [Google Scholar] [CrossRef][Green Version]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K.; Kuvarega, A.T. Fabrication of magnetic recoverable Fe3O4/TiO2 heterostructure for photocatalytic degradation of rhodamine B dye. Inorg. Chem. Commun. 2022, 145, 109966. [Google Scholar] [CrossRef]
- Peña-Flores, J.I.; Sánchez-Mora, E.; Gervacio-Arciniega, J.J.; Toledo-Solano, M.; Márquez-Beltrán, C. Effect of light on the wettability properties of films made by the assembly of SiO2 and α−Fe2O3 nanoparticles. J. Adhes. Sci. Technol. 2021, 35, 386–405. [Google Scholar] [CrossRef]
- Yuan, Z.-H.; You, W.; Jia, J.-H.; Zhang, L.-D. Optical Absorption Red Shift of Capped ZnFe 2 O 4 Nanoparticle. Chin. Phys. Lett. 1998, 15, 535–536. [Google Scholar] [CrossRef]
- Ali, A.M.; Emanuelsson, E.A.; Patterson, D.A. Photocatalysis with nanostructured zinc oxide thin films: The relationship between morphology and photocatalytic activity under oxygen limited and oxygen rich conditions and evidence for a Mars Van Krevelen mechanism. Appl. Catal. B Environ. 2010, 97, 168–181. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Yang, W.; Liu, J. Synthesis of Magnetic Fe3O4@hierarchical Hollow Silica Nanospheres for Efficient Removal of Methylene Blue from Aqueous Solutions. Ind. Eng. Chem. Res. 2014, 53, 10629–10636. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Karthikeyan, C.; Kumar, G.G.; Kim, A.R.; Yoo, D.J. One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 256–264. [Google Scholar] [CrossRef]
- Imran, M.; Alam, M.; Hussain, S.; Ali, M.A.; Shkir, M.; Mohammad, A.; Ahamad, T.; Kaushik, A.; Irshad, K. Highly photocatalytic active r-GO/Fe3O4 nanocomposites development for enhanced photocatalysis application: A facile low-cost preparation and characterization. Ceram. Int. 2021, 47, 31973–31982. [Google Scholar] [CrossRef]
- Martin, N.; Leprince-Wang, Y. HPLC-MS and UV–Visible Coupled Analysis of Methylene Blue Photodegradation by Hydrothermally Grown ZnO Nanowires. Phys. Status Solidi (a) 2021, 218, 2100532. [Google Scholar] [CrossRef]
- Lagrergen, S. Zur theorie der sogenannten adsorption gelöster stoffe kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and Modelling Studies of Activated Carbon Produced from Rubber-Seed Shell Using KOH for CO2 Adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef]
- de Jesús Ruíz-Baltazar, Á.; Reyes-López, S.Y.; Mondragón-Sánchez, M.d.L.; Robles-Cortés, A.I.; Pérez, R. Eco-friendly synthesis of Fe3O4 nanoparticles: Evaluation of their catalytic activity in methylene blue degradation by kinetic adsorption models. Results Phys. 2018, 12, 989–995. [Google Scholar] [CrossRef]
- Sanguino, A.; Diaz-Uribe, C.; Duran, F.; Vallejo, W.; Guzman, L.; Ruiz, D.; Puello, E.; Quiñones, C.; Schott, E.; Zarate, X. Photocatalytic Degradation of Methylene Blue under Visible Light Using TiO2 Thin Films Impregnated with Porphyrin and Anderson-Type Polyoxometalates (Cu and Zn). Catalysts 2022, 12, 1169. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Saquib, M.; Muneer, M. TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dye. Pigment. 2003, 56, 37–49. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Augugliaro, V.; Baiocchi, C.; Prevot, A.B.; García-López, E.; Loddo, V.; Malato, S.; Marcí, G.; Palmisano, L.; Pazzi, M.; Pramauro, E. Azo-dyes photocatalytic degradation in aqueous suspension of TiO2 under solar irradiation. Chemosphere 2002, 49, 1223–1230. [Google Scholar] [CrossRef]
- Das, A.K.; Layek, R.K.; Kim, N.H.; Jung, D.; Lee, J.H. Reduced graphene oxide (RGO)-supported NiCo2O4nanoparticles: An electrocatalyst for methanol oxidation. Nanoscale 2014, 6, 10657–10665. [Google Scholar] [CrossRef] [PubMed]
- Corriu, R.; Leclercq, D.; Lefèvre, P.; Mutin, P.; Vioux, A. Preparation of monolithic gels from silicon halides by a non-hydrolytic sol-gel process. J. Non-Cryst. Solids 1992, 146, 301–303. [Google Scholar] [CrossRef]
- Tamura, H.; Mita, K.; Tanaka, A.; Ito, M. Mechanism of Hydroxylation of Metal Oxide Surfaces. J. Colloid Interface Sci. 2001, 243, 202–207. [Google Scholar] [CrossRef]
- Kim, T.G.; An, G.S.; Han, J.S.; Hur, J.U.; Park, B.G.; Choi, S.-C. Synthesis of Size Controlled Spherical Silica Nanoparticles via Sol-Gel Process within Hydrophilic Solvent. J. Korean Ceram. Soc. 2017, 54, 49–54. [Google Scholar] [CrossRef]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of Zeta Potential and Particle Size on the Stability of SiO2 Nanospheres as Carrier for Ultrasound Imaging Contrast Agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Valadi, K.; Gharibi, S.; Maleki, A. Synergistic photocatalytic effect between green LED light and Fe3O4/ZnO-modified natural pumice: A novel cleaner product for degradation of methylene blue. Mater. Res. Bull. 2020, 130, 110946. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO nanocomposite with highly performance photocatalytic activity for degradation methylene blue under visible light irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Alamo-Nole, L.; Bailon-Ruiz, S.; Luna-Pineda, T.; Perales-Perez, O.; Roman, F.R. Photocatalytic activity of quantum dot–magnetite nanocomposites to degrade organic dyes in the aqueous phase. J. Mater. Chem. A 2013, 1, 5509. [Google Scholar] [CrossRef]
- Khan, B.; Nawaz, M.; Waseem, M.; Hussain, R.; Arif, S.; Price, G.J.; Haq, S.; Rehman, W. Adsorption of methylene blue onto size controlled magnetite nanoparticles. Mater. Res. Express 2019, 6, 095511. [Google Scholar] [CrossRef]
- Roque-Malherbe, R. The Physical Chemistry of Materials: Energy and Environmental Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 2; pp. 64–65. [Google Scholar]
- Pierre, V.C.; Allen, M.J. (Eds.) Contrast Agents for MRI: Experimental Methods; The Royal Society of Chemistry: London, UK, 2017. [Google Scholar] [CrossRef]
- Xing, Z.; Tian, J.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X. Two-dimensional hybrid mesoporous Fe2O3–graphene nanostructures: A highly active and reusable peroxidase mimetic toward rapid, highly sensitive optical detection of glucose. Biosens. Bioelectron. 2014, 52, 452–457. [Google Scholar] [CrossRef]
- Carvalho, H.W.; Hammer, P.; Pulcinelli, S.H.; Santilli, C.V.; Molina, E.F. Improvement of the photocatalytic activity of magnetite by Mn-incorporation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 181, 64–69. [Google Scholar] [CrossRef]
- Chang, S.; Lin, K.-Y.A.; Lu, C. Efficient adsorptive removal of Tetramethylammonium hydroxide (TMAH) from water using graphene oxide. Sep. Purif. Technol. 2014, 133, 99–107. [Google Scholar] [CrossRef]


| Sample | Volume of Fe3O4 (mL) | Mass of Fe3O4 (g) | Weight of Fe3O4 (wt%) |
|---|---|---|---|
| SiO2 | 0.00 | 0.0000 | 0.000 |
| Fe3O4 | 30.0 | 0.4004 | 100.00 |
| SC1 | 0.50 | 0.0067 | 1.402 |
| SC2 | 0.75 | 0.0099 | 1.680 |
| SC3 | 1.00 | 0.0133 | 2.030 |
| SC4 | 1.50 | 0.0199 | 7.728 |
| SC5 | 2.00 | 0.0266 | 24.400 |
| Kinetic Parameters at λ = 410 nm | ||||||
|---|---|---|---|---|---|---|
| Zero Order Kinetics [C] = [C0] − k1t | First Order Kinetics ln[C] = ln[C0] − k2t | Second Order Kinetics 1/[C] = 1/[C0] + k3t | ||||
| Catalysts | k1 | R2 | k2 | R2 | k3 | R2 |
| SiO2 | −0.00105 ± 2.255 × 10−4 | 0.76 | −0.00135 ± 2.6295 × 10−4 | 0.79 | 0.00176 ± 3.11295 × 10−4 | 0.82 |
| SC1 | −0.0021 ± 3.5195 × 10−4 | 0.84 | −0.00397 ± 3.8081 × 10−4 | 0.94 | 0.00823 ± 2.67124 × 10−4 | 0.99 |
| SC2 | −0.00216 ± 3.456 × 10−4 | 0.85 | −0.00387 ± 3.9465 × 10−4 | 0.93 | 0.00752 ± 3.81192 × 10−4 | 0.98 |
| SC3 | −0.00214 ± 3.780 × 10−4 | 0.82 | −0.00403 ± 4.1775 × 10−4 | 0.93 | 0.00835 ± 3.58456 × 10−4 | 0.99 |
| SC4 | −0.00218 ± 3.171 × 10−4 | 0.87 | −0.00401 ± 3.307 × 10−4 | 0.95 | 0.00804 ± 2.20321 × 10−4 | 0.99 |
| SC5 | −0.00217 ± 2.648 × 10−4 | 0.91 | −0.00382 ± 2.39 × 10−4 | 0.97 | 0.00731 ± 2.50768 × 10−4 | 0.99 |
| Fe3O4 | −0.00104 ± 2.232 × 10−4 | 0.76 | −0.00135 ± 2.537 × 10−4 | 0.80 | 0.00176 ± 2.89849 × 10−4 | 0.84 |
| Kinetic Parameters at λ = 575 nm | ||||||
| Zero Order Kinetics [C] = [C0] − k1t | First Order Kinetics ln[C] = ln[C0] − k2t | Second Order Kinetics 1/[C] = 1/[C0] + k3t | ||||
| Catalysts | k1 | R2 | k2 | R2 | k3 | R2 |
| SiO2 | −8.5180 × 10−4 ± 2.81 × 10−4 | 0.57 | −0.0010 ± 3.34582 × 10−4 | 0.60 | 0.0014 ± 4.02627 × 10−4 | 0.63 |
| SC1 | −0.0018 ± 2.5831 × 10−4 | 0.88 | −0.0029 ± 2.69812 × 10−4 | 0.95 | 0.00496 ± 2.49154 × 10−4 | 0.98 |
| SC2 | −0.0018 ± 3.1983 × 10−4 | 0.83 | −0.0030 ± 3.65326 × 10−4 | 0.91 | 0.00541 ± 3.92235 × 10−4 | 0.96 |
| SC3 | −0.0019 ± 3.371 × 10−4 | 0.83 | −0.0033 ± 3.73482 × 10−4 | 0.92 | 0.00617 ± 3.49054 × 10−4 | 0.98 |
| SC4 | −0.0019 ± 2.7962 × 10−4 | 0.88 | −0.0033 ± 3.05304 × 10−4 | 0.94 | 0.00584 ± 3.00699 × 10−4 | 0.98 |
| SC5 | −0.002 ± 2.3981 × 10−4 | 0.91 | −0.0032 ± 2.33959 × 10−4 | 0.96 | 0.00561 ± 1.68955 × 10−4 | 0.99 |
| Fe3O4 | −0.0010 ± 2.1253 × 10−4 | 0.78 | −0.0013 ± 2.40785 × 10−4 | 0.82 | 0.00176 ± 2.73892 × 10−4 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Carmona, A.J.; Mora, E.S.; Flores, J.I.P.; Márquez-Beltrán, C.; Castañeda-Antonio, M.D.; González-Reyna, M.A.; Barrera, M.C.; Misaghian, K.; Lugo, J.E.; Toledo-Solano, M. Photocatalytic Degradation of Methylene Blue by Magnetic Opal/Fe3O4 Colloidal Crystals under Visible Light Irradiation. Photochem 2023, 3, 390-407. https://doi.org/10.3390/photochem3040024
Carmona-Carmona AJ, Mora ES, Flores JIP, Márquez-Beltrán C, Castañeda-Antonio MD, González-Reyna MA, Barrera MC, Misaghian K, Lugo JE, Toledo-Solano M. Photocatalytic Degradation of Methylene Blue by Magnetic Opal/Fe3O4 Colloidal Crystals under Visible Light Irradiation. Photochem. 2023; 3(4):390-407. https://doi.org/10.3390/photochem3040024
Chicago/Turabian StyleCarmona-Carmona, Abraham Jorge, Enrique Sánchez Mora, Jesús Ivan Peña Flores, César Márquez-Beltrán, María Dolores Castañeda-Antonio, Marlén Alexis González-Reyna, María Concepción Barrera, Khashayar Misaghian, Jesús Eduardo Lugo, and Miller Toledo-Solano. 2023. "Photocatalytic Degradation of Methylene Blue by Magnetic Opal/Fe3O4 Colloidal Crystals under Visible Light Irradiation" Photochem 3, no. 4: 390-407. https://doi.org/10.3390/photochem3040024
APA StyleCarmona-Carmona, A. J., Mora, E. S., Flores, J. I. P., Márquez-Beltrán, C., Castañeda-Antonio, M. D., González-Reyna, M. A., Barrera, M. C., Misaghian, K., Lugo, J. E., & Toledo-Solano, M. (2023). Photocatalytic Degradation of Methylene Blue by Magnetic Opal/Fe3O4 Colloidal Crystals under Visible Light Irradiation. Photochem, 3(4), 390-407. https://doi.org/10.3390/photochem3040024






