Bismuth Vanadate-Nanostructured Graphite Electrodes for Rhodamine B Photoelectrochemical Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BVO Nanoparticles
2.3. Preparation of BVO@C Electrode
Factorial Planning
2.4. Materials Characterization
2.5. Electrochemical Characterization
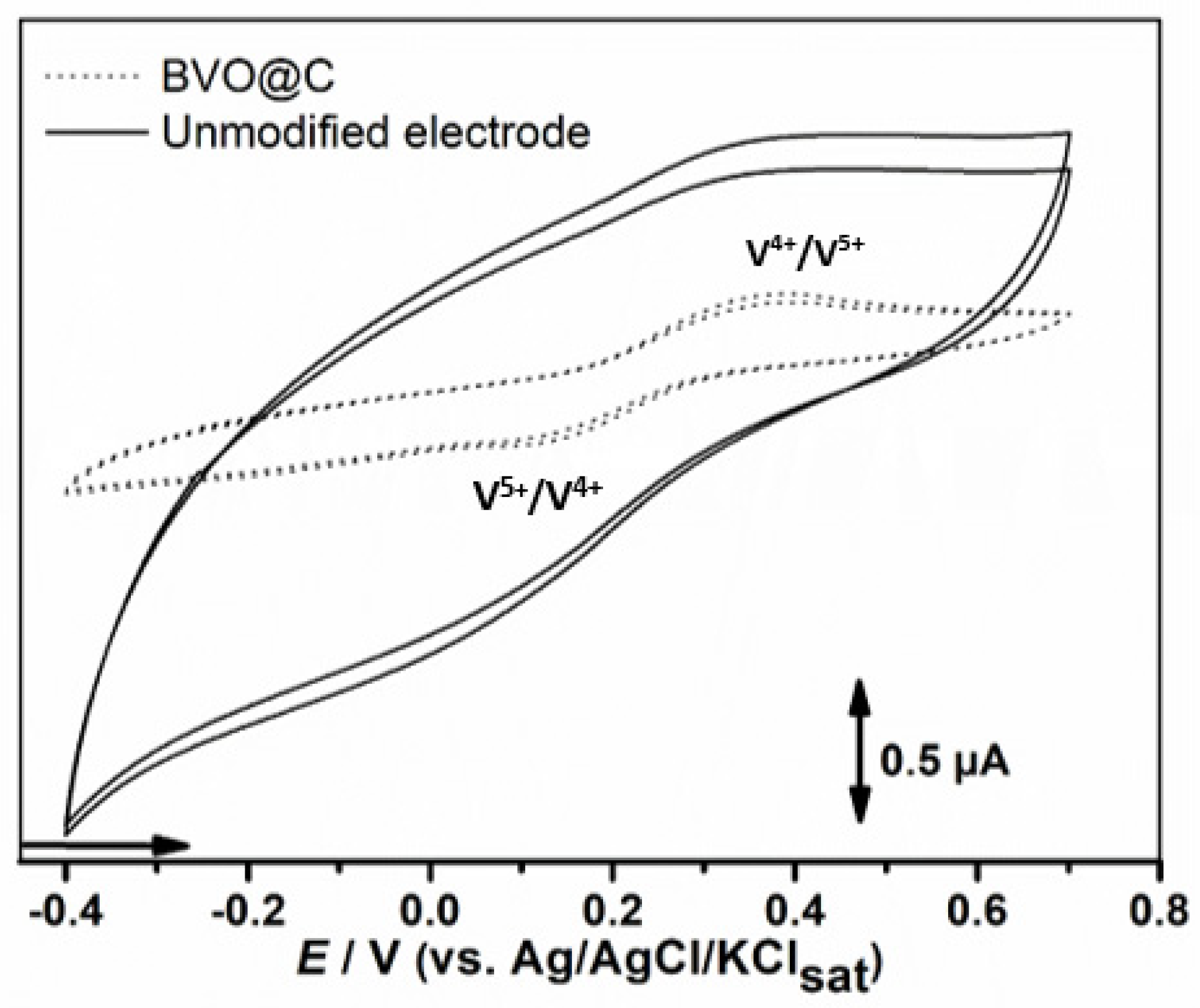
2.5.1. Voltammetric Assays
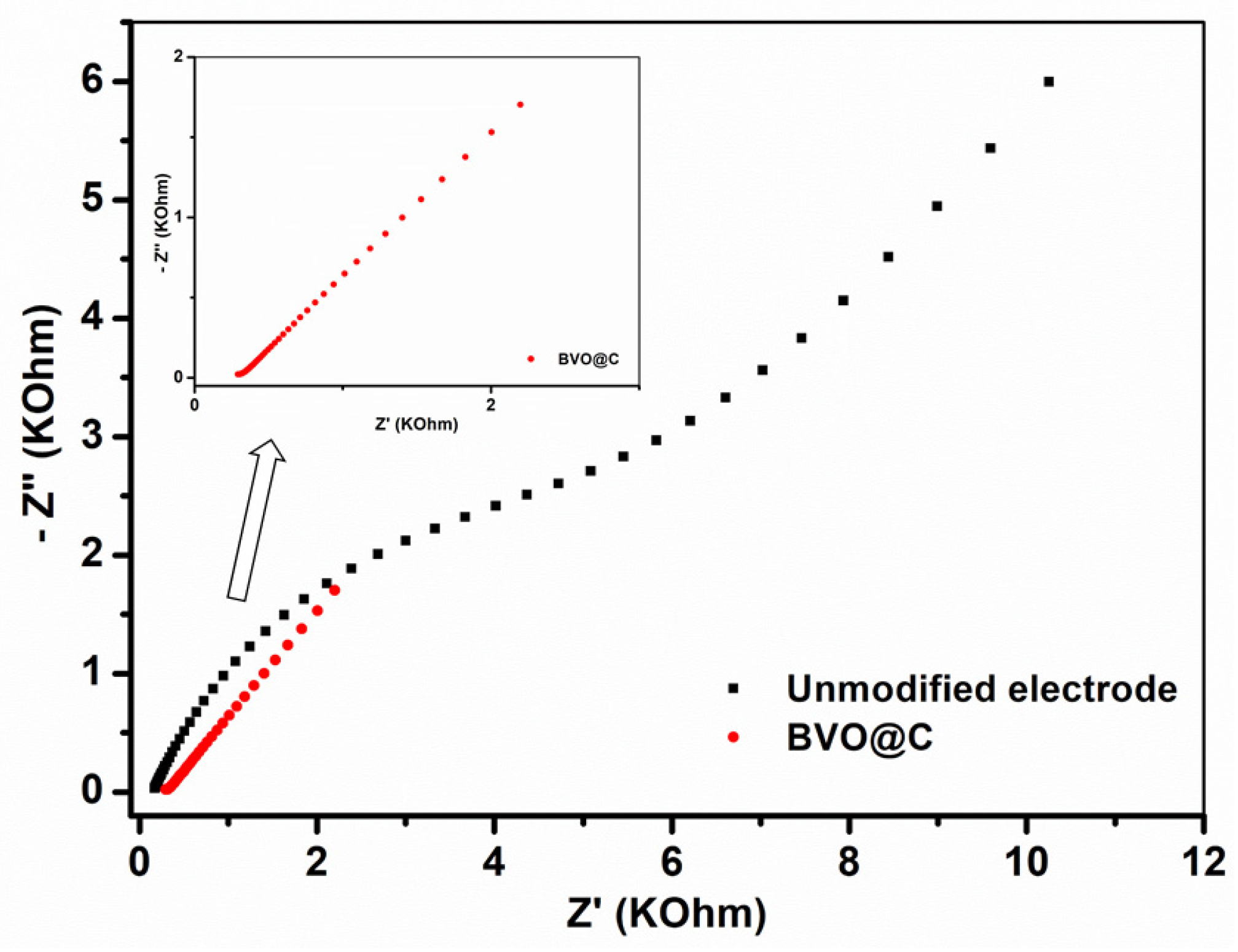
2.5.2. EIS Characterization
2.6. Photoelectrocatalytic (PEC) and Elecrocatalytic (EC) Systems
3. Results and Discussion
3.1. Factorial Planning
3.2. Characterization of BVO@C
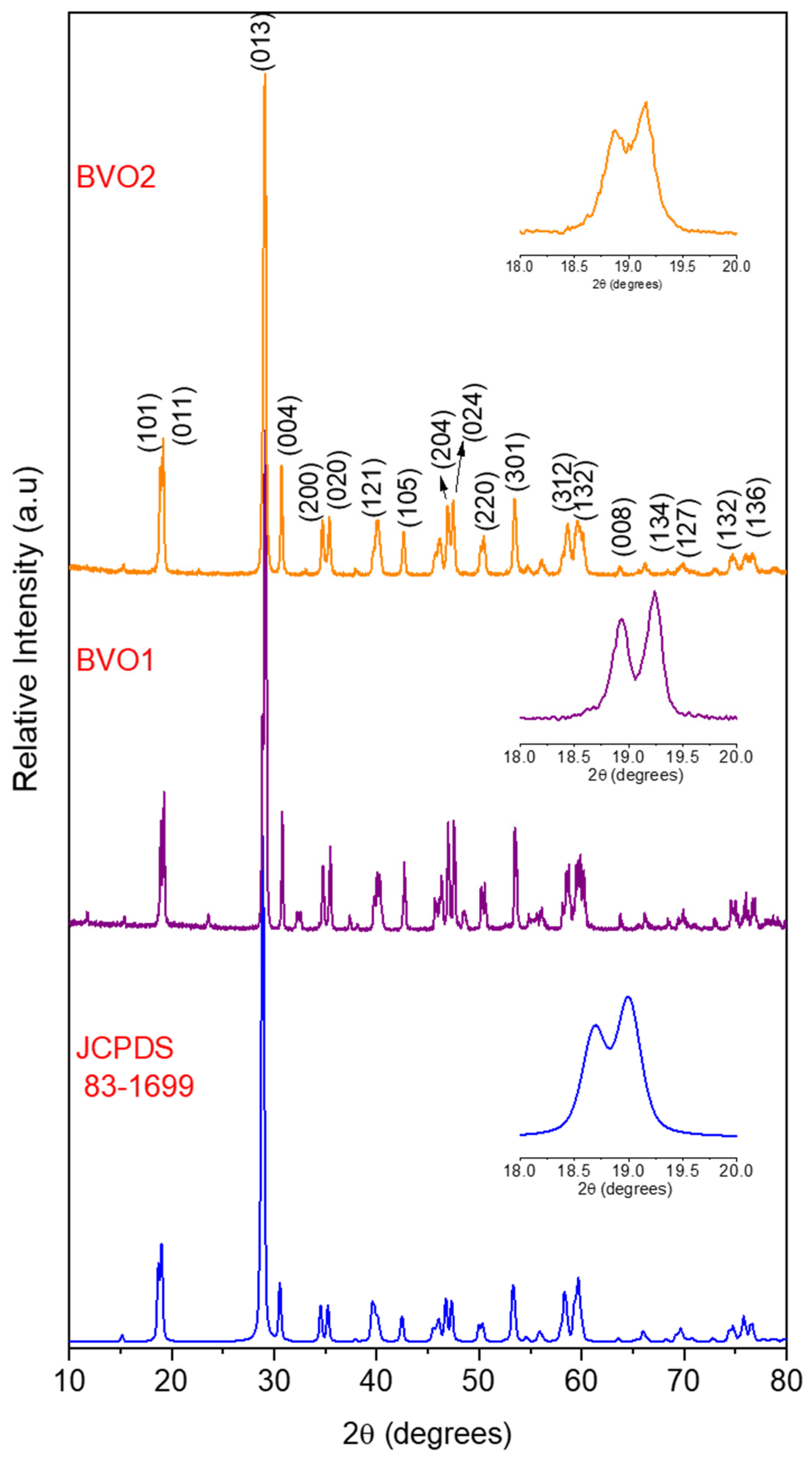
3.2.1. X-ray Diffraction (XRD)
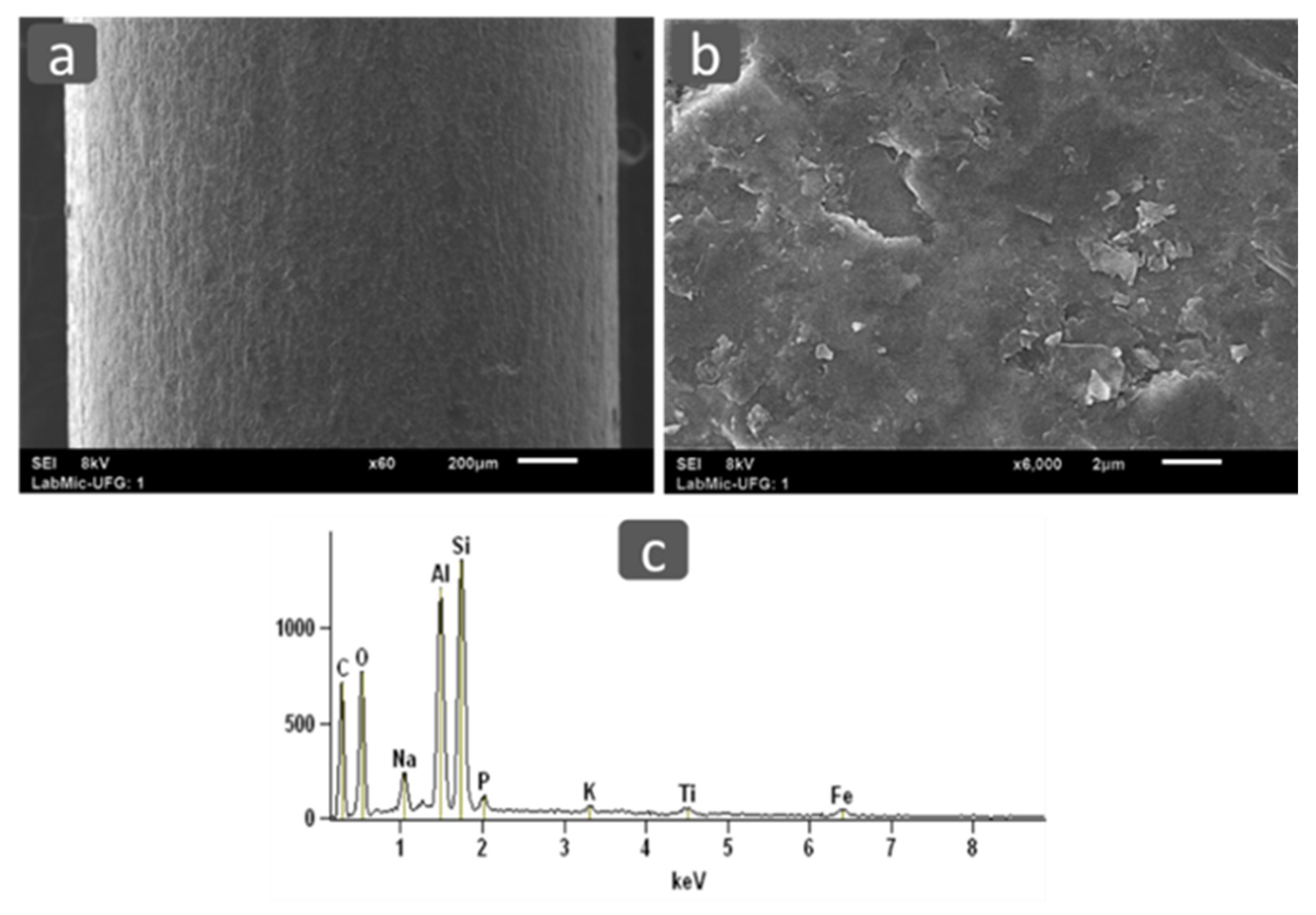
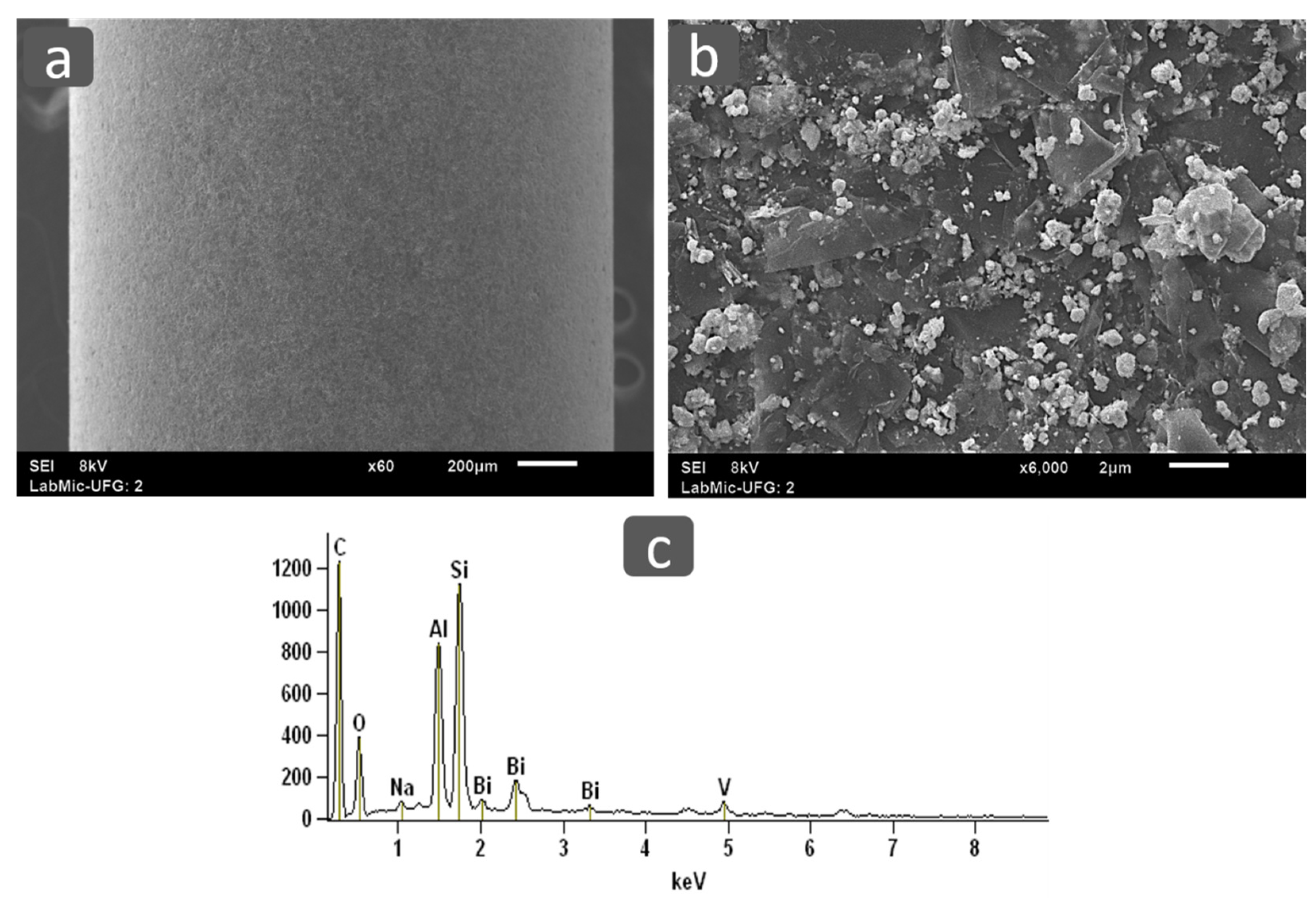
3.2.2. Scanning Electron Microscope and Energy Dispersive Spectroscopy (SEM/EDS)
3.2.3. DLS and Zeta Potential Measurements
3.3. Electrochemical Characterization
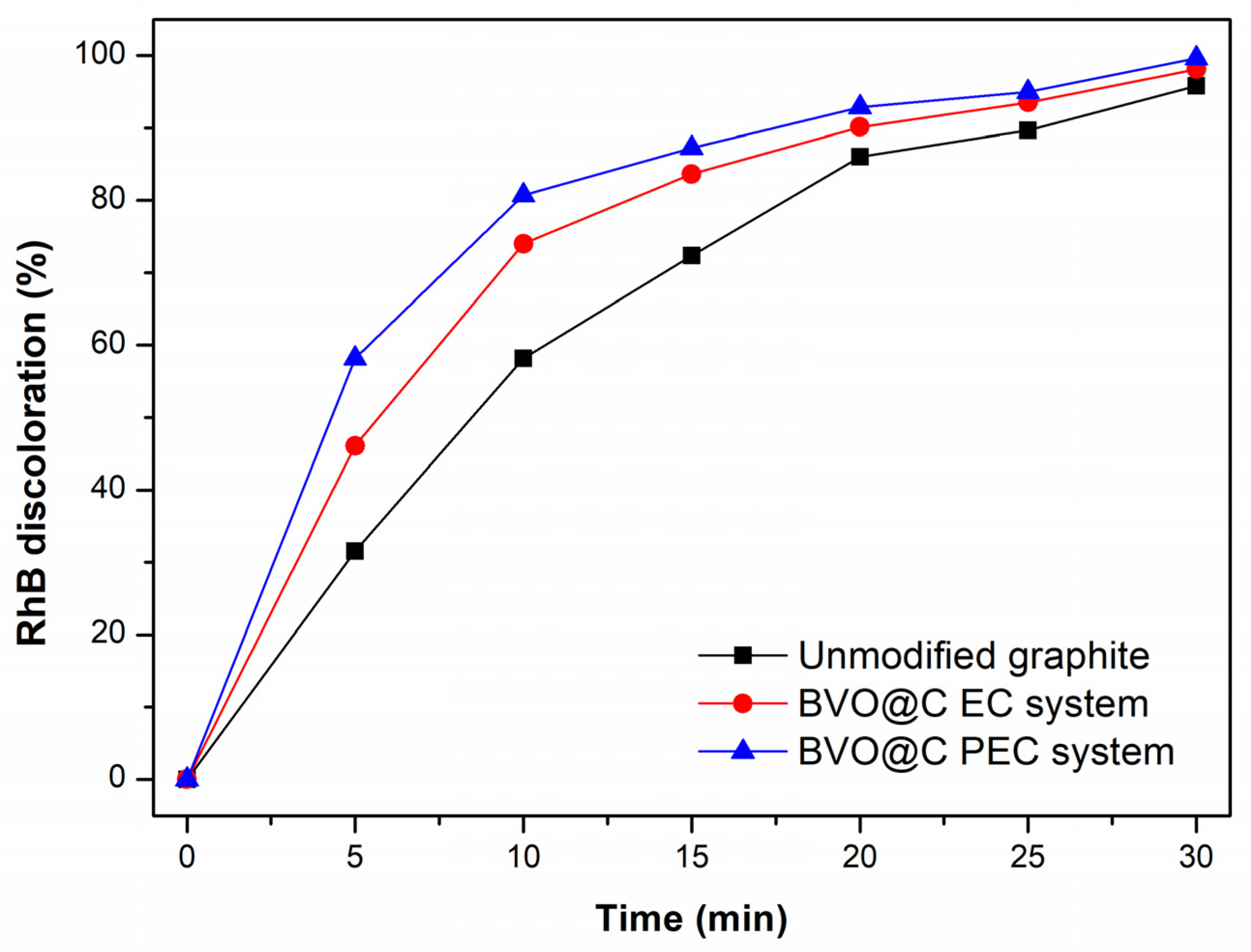
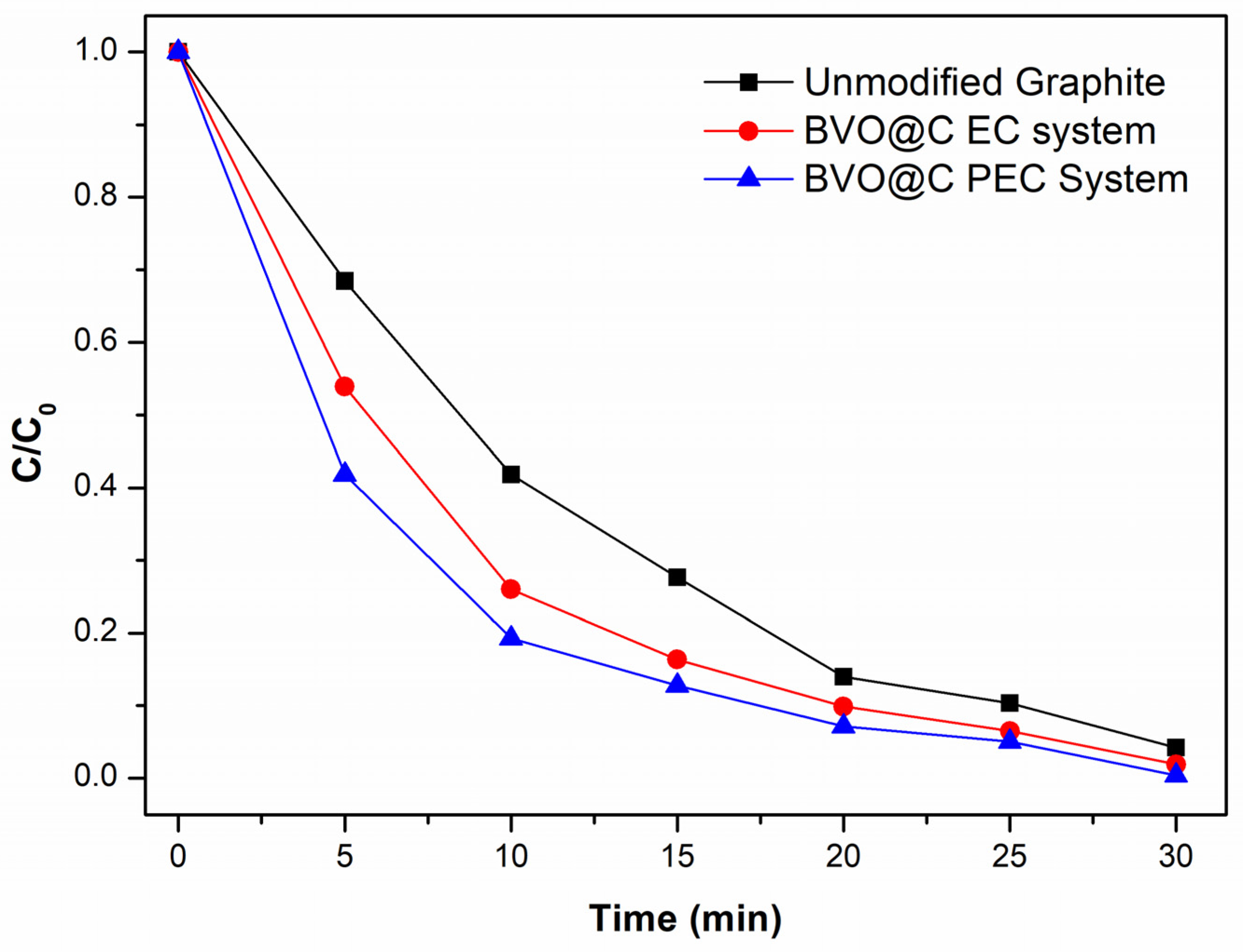
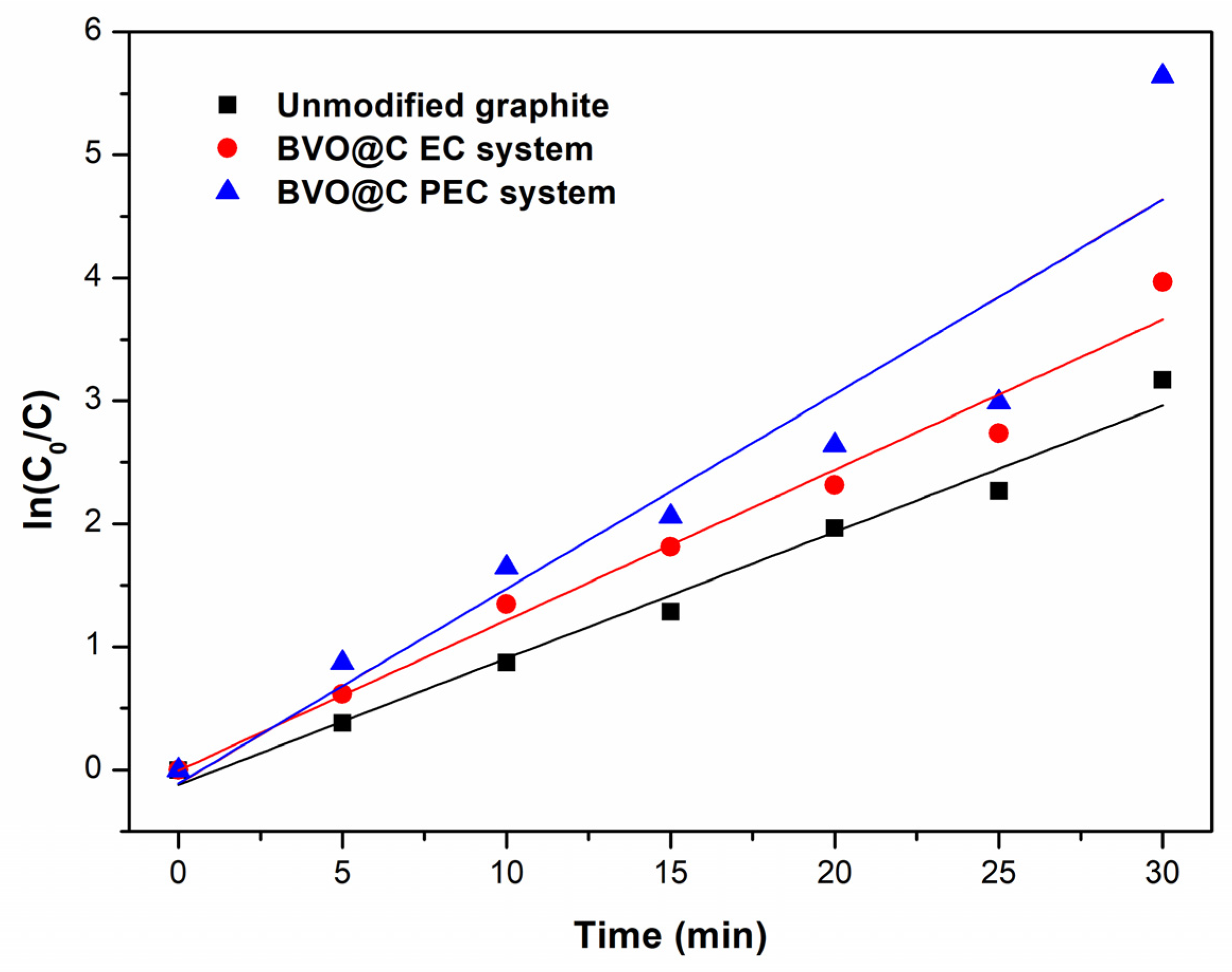
3.4. Photoelectrocatalytic (PEC) and Elecrocatalytic (EC) Tests
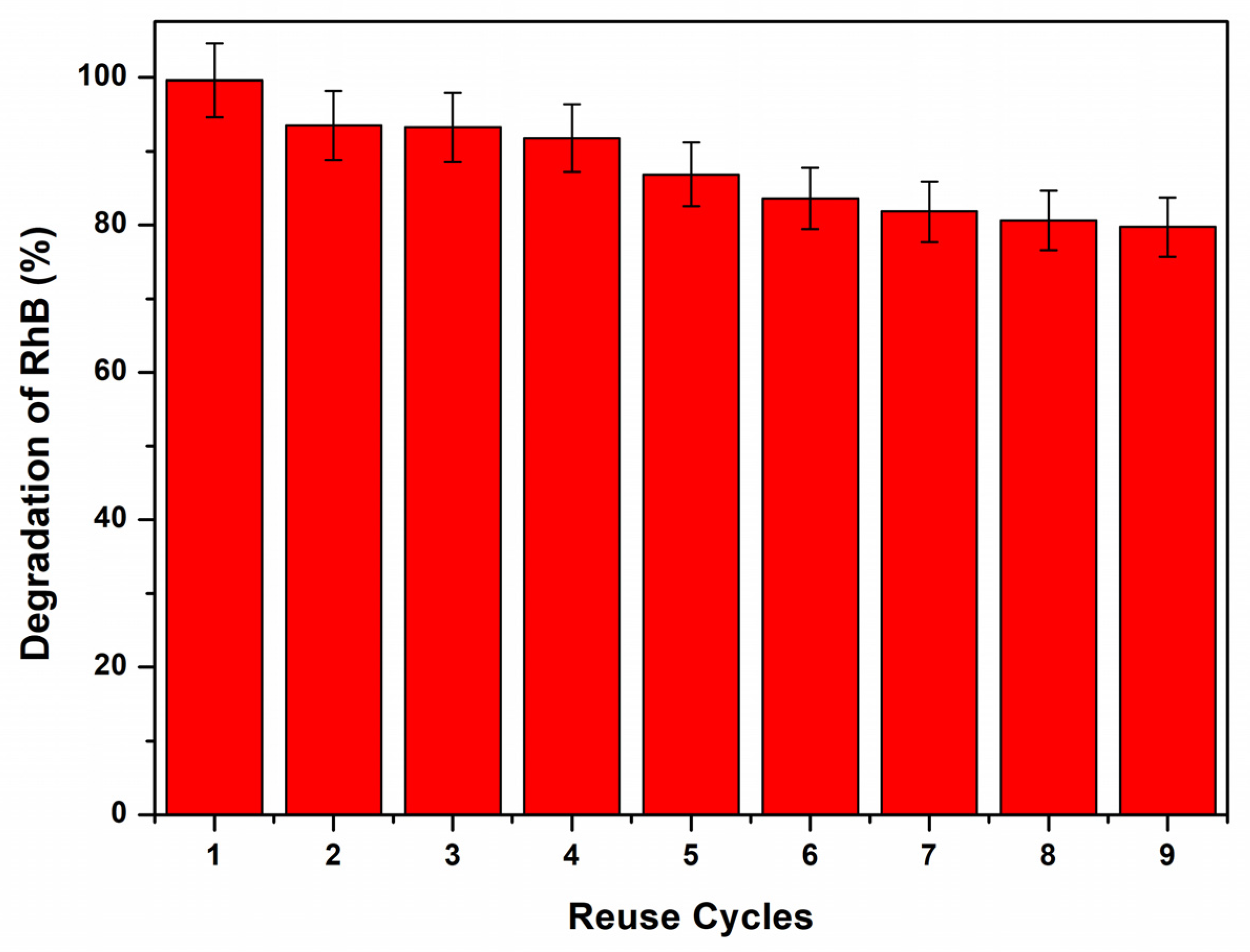
3.5. Reuse Cycles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode Materials for Aqueous Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Liu, Q. N-eicosane/expanded graphite as composite phase change materials for electro-driven thermal energy storage. J. Energy Storage 2020, 29, 101339. [Google Scholar] [CrossRef]
- Lv, Y.; Han, C.; Zhu, Y.; Zhang, T.; Yao, S.; He, Z.X.; Dai, L.; Wang, L. Nanostructured N-doped carbon materials for vanadium redox flow battery: Properties, structures, and perspectives. J. Mater. Sci. Technol. 2021, 75, 96–109. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water: An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Ruifeng, R.; Pengfei, T.; Lu, Y.; Huanhuan, Z.; Mingyuan, Z.; Jiaoyang, C.; Hele, L.; Jun, P. Controlled fabrication of bismuth vanadate nanotubes for visible light photocatalysis. Mater. Lett. 2022, 324, 132742. [Google Scholar]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non-Metal-Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Agrawal, A.; Siddiqui, S.A.; Soni, A.; Sharma, G.D. Advancements, frontiers and analysis of metal oxide semiconductor, dye, electrolyte and counter electrode of dye sensitized solar cell. Sol. Energy 2022, 233, 378–407. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Vaya, D.; Kaushik, B.; Surolia, P.K. Recent advances in graphitic carbon nitride semiconductor: Structure, synthesis and applications. Mater. Sci. Semicond. Process. 2021, 137, 106181. [Google Scholar] [CrossRef]
- Moharam, M.M.; El Shazly, A.N.; Anand, K.V.; Rayan, D.E.-R.A.; Mohammed, M.K.A.; Rashad, M.M.; Shalan, A.E. Semiconductors as Effective Electrodes for Dye Sensitized Solar Cell Applications. Top. Curr. Chem. 2021, 379, 20. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Recent Progress in LDH@Graphene and Analogous Heterostructures for Highly Active and Stable Photocatalytic and Photoelectrochemical Water Splitting. Chem. Asian J. 2021, 16, 2211–2248. [Google Scholar] [CrossRef]
- Jana, S.; Konar, S.; Mitra, B.C.; Mondal, A.; Mukhopadhyay, S. Fabrication of a new heterostructure Au/Pt/SnO2: An excellent catalyst for fast reduction of para-nitrophenol and visible light assisted photodegradation of dyes. Mater. Res. Bull. 2021, 141, 111351. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon SZ, N.; Mohamad, I.S. Heterojunctions of rGO/Metal Oxide Nanocomposites as Promising Gas-Sensing Materials—A Review. Nanomaterials 2022, 12, 2278. [Google Scholar] [CrossRef]
- Kouvelis, K.; Kampioti, A.A.; Petala, A.; Frontistis, Z. Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System. Catalysts 2022, 12, 882. [Google Scholar] [CrossRef]
- Sun, H.; Zou, C.; Tang, W. Designing double Z-scheme heterojunction of g-C3N4/Bi2MoO6/Bi2WO6 for efficient visible-light photocatalysis of organic pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130105. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Sun, J.; Wu, X.; Liang, H.; Qu, Y.; Jing, L. BiFeO3/Bi2Fe4O9 S-scheme heterojunction hollow nanospheres for high-efficiency photocatalytic o-chlorophenol degradation. Appl. Catal. 2022, 319, 121893. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Hu, T.; Liu, X. Controlled synthesis of palygorskite/Bi5O7I hybrid microspheres with high efficient photodegradation of antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126225. [Google Scholar] [CrossRef]
- Wu, L.; Yue, X.; Chang, Y.; Wang, K.; Zhang, J.; Sun, J.; Wei, Z.; Kowalska, E. Photocatalytic Degradation of Tetracycline under Visible Light Irradiation on BiVO4 Microballs Modified with Noble Metals. Catalysts 2022, 12, 1293. [Google Scholar] [CrossRef]
- Fan, H.; Yi, G.; Zhang, Z.; Zhang, X.; Li, P.; Zhang, C.; Chen, L.; Zhang, Y.; Sun, Q. Fabrication of Ag particles deposited BiVO4 photoanode for significantly efficient visible-light driven photoelectrocatalytic degradation of β-naphthol. J. Environ. Chem. Eng. 2022, 10, 107221. [Google Scholar] [CrossRef]
- Lotfi, S.; Ouardi, M.E.; Ahsaine, H.A.; Assani, A. Recent progress on the synthesis, morphology and photocatalytic dye degradation of BiVO4 photocatalysts: A review. Catal. Rev. 2022, 1–45. [Google Scholar]
- Zhang, M.; Shao, C.; Li, X.; Zhang, P.; Sun, Y.; Su, C.; Zhang, X.; Ren, J.; Liu, Y. Carbon-modified BiVO4 microtubes embedded with Ag nanoparticles have high photocatalytic activity under visible light. Nanoscale 2012, 4, 7501–7508. [Google Scholar] [CrossRef] [PubMed]
- Ghazkoob, N.; Shoushtari, M.Z.; Kazeminezhad, I.; Baghal, S.L. Investigation of structural, magnetic, optical and photocatalytic properties of zinc ferrite nanowires/bismuth vanadate composite. J. Alloys Compd. 2022, 900, 163467. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Nandi, D.; Parameswaranpillai, J.; Lee, J.; Shivanna, J.M.; Nithya, R.; Siengchin, S. Adsorption of Cationic Dye onto ZSM-5 Zeolite-Based Bio Membrane: Characterizations, Kinetics and Adsorption Isotherm. J. Polym. Environ. 2022, 30, 3279–3292. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.; Khan, S.B.; Akhtar, K.; Asiri, A.M. Catalytic reduction of picric acid, nitrophenols and organic azo dyes via green synthesized plant supported Ag nanoparticles. J. Mol. Liq. 2018, 268, 87–101. [Google Scholar] [CrossRef]
- Kadam, A.N.; Babu, B.; Lee, S.W.; Kim, J.; Yoo, K. Morphological guided sphere to dendrite BiVO4 for highly efficient organic pollutant removal and photoelectrochemical performance under solar light. Chemosphere 2022, 305, 135461. [Google Scholar] [CrossRef]
- Gomes, L.E.; Plaça, L.F.; Rosa, W.S.; Gonçalves, R.V.; Ullah, S.; Wender, H. Increasing the Photocatalytic Activity of BiVO4 by Naked Co(OH)2 Nanoparticle Cocatalysts. Photochem 2022, 2, 866–879. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, L.; Fan, W.; Ren, J.; Wang, Q.; Wang, A.; Shang, D.; Zhu, W. Efficient photoelectrochemical water oxidation of cobalt phthalocyanine decorated BiVO4 photoanode by improving kinetics. Appl. Surf. Sci. 2021, 564, 150463. [Google Scholar] [CrossRef]
- Fard, S.G.; Haghighi, M.; Shabani, M. Facile one-pot ultrasound-assisted solvothermal fabrication of ball-flowerlike nanostructured (BiOBr)x(Bi7O9I3)1−x solid-solution for high active photodegradation of antibiotic levofloxacin under sun-light. Appl. Catal. B Environ. 2019, 248, 320–331. [Google Scholar] [CrossRef]
- Aguilera-Ruiz, E.; Zambrano-Robledo, P.; Vazquez-Arenas, J.; Cruz-Ortiz, B.; Peral, J.; García-Pérez, U.M. Photoactivity of nanostructured spheres of BiVO4 synthesized by ultrasonic spray pyrolysis at low temperature. Mater. Res. Bull. 2021, 143, 111447. [Google Scholar] [CrossRef]
- Rendevski, S.J.; Dyussenbekov, A.M.; Nurlanov, F.N. A practical lab on composite materials and sensors, enhanced with electrical percolation threshold theory. Eur. J. Phys. 2020, 41, 055802. [Google Scholar] [CrossRef]
- Hartmann, L.A. Strategic minerals from southern Brazil: Geology of amethyst and agate geodes. In Technology for the Gem, Jewelry and Mining Sector; Hartmann, L.A., da Silva, J.T., Eds.; Federal University of Rio Grande do Sul, Institute of Geosciences: Porto Alegre, Brazil, 2010; pp. 30–39. [Google Scholar]
- Internacional Centre for Difraction Data. Available online: https://www.icdd.com/pdfsearch/ (accessed on 22 September 2021).
- Malashchonak, M.V.; Streltsov, E.A.; Kuliomin, D.A.; Kulak, A.I.; Mazanik, A.V. Monoclinic bismuth vanadate band gap determination by photoelectrochemical spectroscopy. Mater. Chem. Phys. 2017, 201, 189–193. [Google Scholar] [CrossRef]
- Wei, T.; Jin, Z.; Li, F.; Sun, Z.; Xu, L. Solar water oxidation using TaON–BiVO4 photoanodes functionalized with WO3. J. Chem. Soc. 2021, 50, 1780–1787. [Google Scholar] [CrossRef]
- Neto, J.S.O.; Lima, A.F.; Limeira, Y.F.X.; Cruz, C.P.T. Characterization of historical mortar sin the 18th century house landmark: A case study. Braz. J. Dev. 2021, 7, 28270–28286. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.; Bae, T.; Yuan, J.; Yu, J. Fabrication of binder-free pencil trace electrode for lithium-ion battery: Simplicity and high performance. Langmuir 2016, 18, 4415–4423. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, K.S.; Pathak, A.D.; Sharma, C.S. Pyrolyzed pencil graphite coated cellulose paper as an interlayer: An effective approach for high-performance lithium-sulfur battery. Appl. Surf. Sci. 2020, 533, 147483. [Google Scholar] [CrossRef]
- El-Katori, E.E.; Kasim, E.A.; Ali, D.A. Sol–gel synthesis of mesoporous NiO/ZnO heterostructure nanocomposite for photocatalytic and anticorrosive applications in aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 2021, 636, 128153. [Google Scholar] [CrossRef]
- Jana, A.K. Solar cells based on dyes. J. Photochem. Photobiol. A Chem. 2000, 132, 1–17. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. Ag-doped BiVO4/BiFeO3 photoanode for highly efficient and stable photocatalytic and photoelectrochemical water splitting. Sci. Total Environ. 2020, 736, 138640. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Guan, J.; Catlow CR, A.; Walsh, A.; Sokol, A.A.; Buckeridge, J. Insight into the Fergusonite–Scheelite Phase Transition of ABO4-Type Oxides by Density Functional Theory: A Case Study of the Subtleties of the Ground State of BiVO4. Chem. Mater. 2022, 34, 5334–5343. [Google Scholar] [CrossRef]
- Hebert, J.; Wang, L.; Wang, X.; Baker, J.; Rivera, N.; Troedel, M.; Li, Z. Mechanisms of safranin O interaction with 1:1 layered clay minerals. Sep. Sci. Technol. 2020, 56, 1985–1995. [Google Scholar] [CrossRef]
- Mascaro, L.H.; Pockett, A.; Mitchels, J.M.; Peter, L.M.; Cameron, P.J.; Celorrio, V.; Fermin, D.J.; Sagu, J.S.; Wijayantha, K.G.U.; Kociok-Kohn, G.; et al. One-step preparation of the BiVO4 film photoelectrode. J. Solid State Electrochem. 2014, 19, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Rullens, F.; Laschewsky, A.; Devillers, M. Bulk and Thin Films of Bismuth Vanadates Prepared from Hybrid Materials Made from an Organic Polymer and Inorganic Salts. Chem. Mater. 2006, 18, 771–777. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wu, J.; Hu, C. Electrochemical oxidation of rhodamine B on RuO2–PdO–TiO2/Ti electrode. Electrochim. Acta. 2012, 68, 69–73. [Google Scholar] [CrossRef]
- Baddouh, A.; Bessegato, G.G.; Rguiti, M.M.; El Ibrahimi, B.; Bazzi, L.; Hilali, M.; Zanoni, M.V.B. Electrochemical decolorization of Rhodamine B dye: Influence of anode material, chloride concentration and current density. J. Environ. Chem. Eng. 2018, 6, 2041–2047. [Google Scholar] [CrossRef] [Green Version]
- Honeychurch, K.C. Voltammetric Behaviour of Rhodamine B at a Screen-Printed Carbon Electrode and Its Trace Determination in Environmental Water Samples. Sensors 2022, 22, 4631. [Google Scholar] [CrossRef]
- Lee, J.; Liu, X.; Kumar, A.; Hwang, Y.; Lee, E.; Yu, J.; Kim, Y.D.; Lee, H. Phase-selective active sites on ordered/disordered titanium dioxide enable exceptional photocatalytic ammonia synthesis. Chem. Sci. 2021, 12, 9619–9629. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, X.; Chen, Y.; Li, H.; Li, R.; Dong, X.; Lee, H.; Ma, H. Highly Enhanced Photoelectrocatalytic Oxidation via Cooperative Effect of Neighboring Two Different Metal Oxides for Water Purification. J. Phys. Chem. C 2020, 124, 11525–11535. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Kannan, V.R.; Alsalhi, M.S.; Rajasekar, A.; Devanesan, S.; Narenkumar, J.; Kim, W.; Liu, X. Intimately coupled gC3N4 photocatalysis and mixed culture biofilm enhanced detoxification of sulfamethoxazole: Elucidating degradation mechanism and toxicity assessment. Environ. Res. 2022, 214, 113824. [Google Scholar] [CrossRef]
- Zhou, B.; Qu, J.; Zhao, X.; Liu, H. Fabrication and photoelectrocatalytic properties of nanocrystalline monoclinic BiVO4 thin-film electrode. J. Environ. Sci. 2011, 23, 151–159. [Google Scholar] [CrossRef]
- Pelegrini, R.; Peralta-Zamora, P.; de Andrade, A.R.; Reyes, J.; Durán, N. Electrochemically assisted photocatalytic degradation of reactive dyes. Appl. Catal. 1999, 22, 83–90. [Google Scholar] [CrossRef]
- Wang, N.; Hu, Q.; Du, X.; Xu, H.; Hao, L. Study on decolorization of Rhodamine B by raw coal fly ash catalyzed Fenton-like process under microwave irradiation. Adv. Powder Technol. 2019, 30, 2369–2378. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zou, G.; Chen, Q.; Guo, Y.; Liang, S.; Hu, L.; North, M.; Xie, H. Carboxylcellulose hydrogel confined-Fe3O4 nanoparticles catalyst for Fenton-like degradation of Rhodamine B. Int. J. Biol. Macromol. 2021, 180, 792–803. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Van, H.T.; Ngo, Q.N.; Thai, V.N.; Hoang, V.H.; Hai, N.T.T. Improving Fenton-like oxidation of Rhodamin B using a new catalyst based on magnetic/iron-containing waste slag composite. Environ. Technol. Innov. 2021, 23, 101582. [Google Scholar] [CrossRef]
- Sun, X.-F.; Wang, S.-G.; Liu, X.-W.; Gong, W.-X.; Bao, N.; Gao, B.-Y.; Zhang, H.-Y. Biosorption of Malachite Green from aqueous solutions onto aerobic granules: Kinetic and equilibrium studies. Bioresour. Technol. 2008, 99, 3475–3483. [Google Scholar] [CrossRef]
- Geraldino, H.C.; Freitas, T.K.; Manholer, D.D.; França, F.; Oliveira, J.H.; Volnistem, E.A.; Garcia, J.C. Electrochemical generation of H2O2 using gas diffusion electrode improved with rGO intensified with the Fe3O4/GO catalyst for degradation of textile wastewater. J. Water Process. Eng. 2020, 36, 101377. [Google Scholar] [CrossRef]
- Kumar, P.S.; Selvakumar, M.; Babu, S.G.; Induja, S.; Karuthapandian, S. CuO/ZnO nanorods: An affordable efficient p-n heterojunction and morphology dependent photocatalytic activity against organic contaminants. J. Alloys Compd. 2017, 701, 562–573. [Google Scholar] [CrossRef]
- Rao, M.P.; Wu, J.J.; Asiri, A.M.; Anandan, S.; Ashokkumar, M. Photocatalytic properties of hierarchical CuO nanosheets synthesized by a solution phase method. J. Environ. Sci. 2018, 69, 115–124. [Google Scholar] [CrossRef]
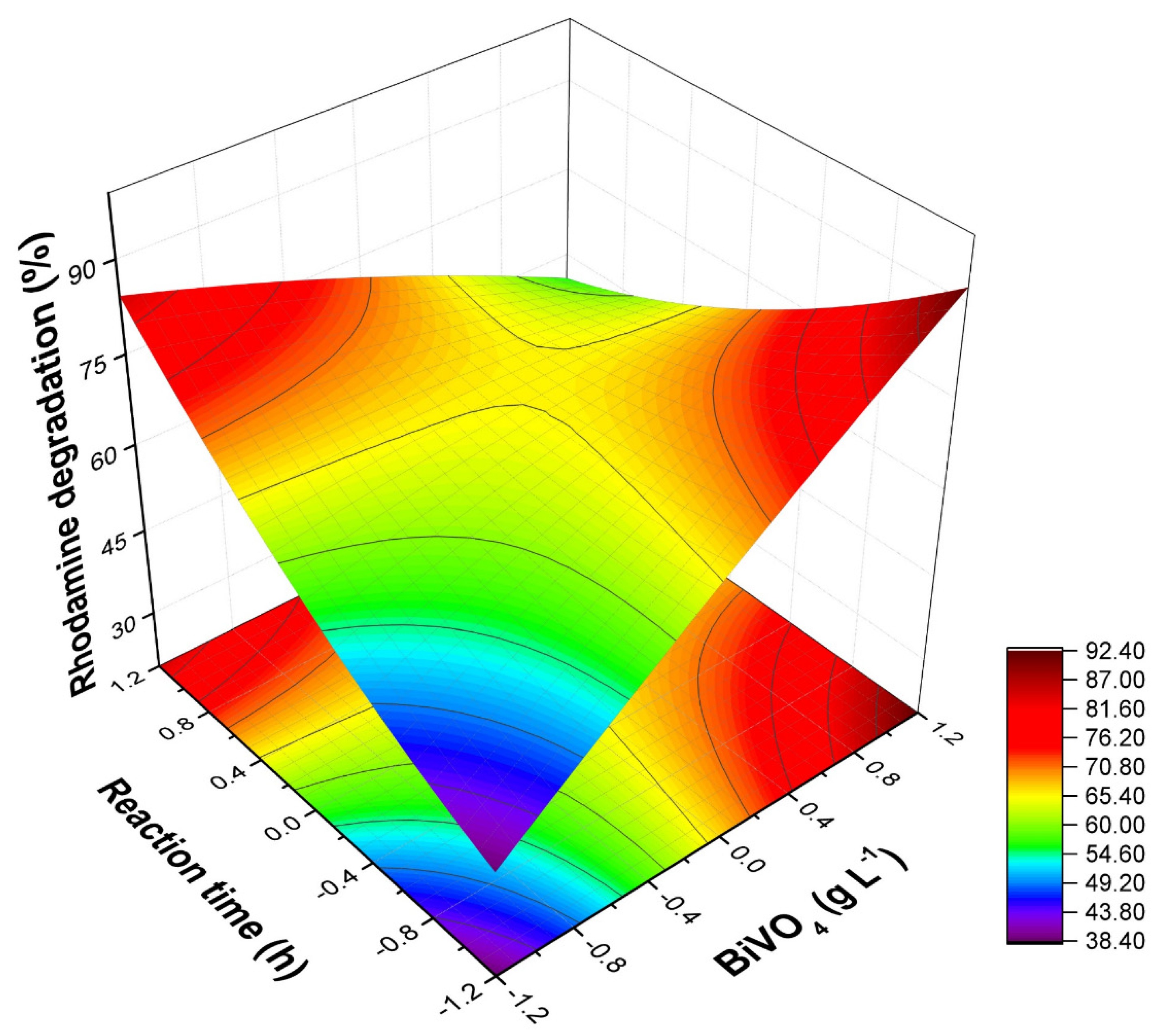

| DF | Sum Sq | Mean Sq | Fcalc | Ftab (95%) | |
|---|---|---|---|---|---|
| R | 5 | 1026.51 | 205.30 | 1.27 | 5.05 |
| r | 5 | 810.90 | 162,18 | 0 | 0 |
| T | 10 | 1837.40 | 0 | 0 | 0 |
| EP | 2 | 146.13 | 73.06 | 3.03 | 19.16 |
| Faj | 3 | 664.77 | 221.59 | 0 | 0 |
| Electrodes | CPE | RP | Chi-Square (X2) |
|---|---|---|---|
| Unmodified | 1680 Ω | 1.71 µF | 0.047513 |
| BVO@C | 41.1 Ω | 1.04 µF | 0.0069898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isecke, B.G.; Guimarães, A.S.; Teixeira, G.F.; Colmati, F.; Ribeiro de Souza, A.; de Macêdo, I.Y.L.; Duarte, L.M.; de Oliveira, S.B.; Costa, A.G.C.; Somerset, V.S.; et al. Bismuth Vanadate-Nanostructured Graphite Electrodes for Rhodamine B Photoelectrochemical Degradation. Photochem 2023, 3, 38-58. https://doi.org/10.3390/photochem3010003
Isecke BG, Guimarães AS, Teixeira GF, Colmati F, Ribeiro de Souza A, de Macêdo IYL, Duarte LM, de Oliveira SB, Costa AGC, Somerset VS, et al. Bismuth Vanadate-Nanostructured Graphite Electrodes for Rhodamine B Photoelectrochemical Degradation. Photochem. 2023; 3(1):38-58. https://doi.org/10.3390/photochem3010003
Chicago/Turabian StyleIsecke, Bruna Guimarães, Arthur Saldanha Guimarães, Guilhermina Ferreira Teixeira, Flavio Colmati, Aparecido Ribeiro de Souza, Isaac Yves Lopes de Macêdo, Lucas Mattos Duarte, Sergio Botelho de Oliveira, André Gabriel Carmo Costa, Vernon Sydwill Somerset, and et al. 2023. "Bismuth Vanadate-Nanostructured Graphite Electrodes for Rhodamine B Photoelectrochemical Degradation" Photochem 3, no. 1: 38-58. https://doi.org/10.3390/photochem3010003
APA StyleIsecke, B. G., Guimarães, A. S., Teixeira, G. F., Colmati, F., Ribeiro de Souza, A., de Macêdo, I. Y. L., Duarte, L. M., de Oliveira, S. B., Costa, A. G. C., Somerset, V. S., & Gil, E. d. S. (2023). Bismuth Vanadate-Nanostructured Graphite Electrodes for Rhodamine B Photoelectrochemical Degradation. Photochem, 3(1), 38-58. https://doi.org/10.3390/photochem3010003







