Abstract
Due to their excellent properties and unique structures, transition metal sulfides play an important role in the development of efficient and stable photoelectric catalysts. In recent years, their potential applications have expanded from photoelectric catalysis to energy storage, especially as materials for key components of electrochemical energy storage. As a typical multifunctional metal sulfide catalyst, Co9S8 is highly attractive due to its high conductivity, better stability, suitable band structure, enhanced performance and wide applications. A large number of studies have shown that strategically modified Co9S8-based materials have greater advantages in various applications compared with pure Co9S8. Therefore, this review will evaluate the physicochemical properties and the preparation of different dimensions of Co9S8-based materials, and the influence of different structures on the photoelectrochemical energy of materials will be described. In addition, the research progress regarding the evolution of hydrogen photocatalytic, electrocatalytic water splitting and various electrochemical energy storage materials will be emphasized. Finally, the challenges faced by Co9S8-based materials and the research directions for their future applications will be discussed.
1. Introduction
The need for large-scale production of renewable energy is driven by the depletion of fossil fuels and serious environmental pollution problems [1,2,3]. Efficient and environmentally friendly photoelectric catalytic technology has been widely used in energy conversion. However, there are still some problems to be solved, such as the recombination of carriers and the slow kinetics of electrocatalytic reactions, which hinder the further development of photoelectric catalytic technology. Therefore, it is necessary to explore excellent photoelectric catalysts to improve the reaction efficiency.
Metal sulfide (MSx) is considered to be one of the most important materials for the preparation of highly photoelectrochemical active materials, owing to its high stability and excellent redox properties [4,5,6]. As a typical cobalt-rich sulfide, Co9S8 has high conductivity, good stability and a relatively narrow band gap. Meanwhile, Co9S8 shows great diversity in size, structure and performance when used by photoelectric catalysts and by various materials for energy storage. In order to obtain more active sites and better performance, Co9S8 modification strategies are usually implemented, including morphology control, heterostructure construction, defect engineering, etc. [7,8,9]. For example, Zhao first developed a hierarchical Co9S8@ZnAgInS heterostructure cage to achieve efficient photocatalytic hydrogen production. Li used the doping strategy to prepare an efficient all-pH electrocatalyst Co9S8/nitrogen-doped carbo@MoS2. The Co9S8-based materials after modification have a controllable high specific surface area and a reasonable pore size distribution, which is conducive to the exposure of the active site, and has made an outstanding contribution to electrocatalytic water splitting and photoelectric catalysis hydrogen evolution. In addition, as a key material for energy storage, Co9S8-based materials have been proven to improve the coulomb efficiency and cycle life of batteries, and can also provide rich surface functional groups for Faraday reactions of supercapacitors.
In this review, we first briefly introduce the physicochemical properties of Co9S8. Subsequently, the preparation of different dimensions and applications of Co9S8-based materials in photoelectric catalysis hydrogen evolution, electrocatalytic water splitting and various means of electrochemical energy storage are discussed (Figure 1). This study will provide prospects for the development of Co9S8-based materials as photoelectric catalysts and new-generation energy storage materials in the future.
Figure 1.
Properties, preparation and application (photocatalytic hydrogen evolution, electrocatalytic water splitting, batteries and supercapacitors) of Co9S8-based materials.
2. Properties
Co9S8 nanomaterials have attracted attention from various fields due to their distinct structures and surface areas, thus exhibiting excellent physical and chemical properties. Magnetism, the most fundamental property of Co9S8, is profoundly affected by the temperature of the reaction system. For example, Robert F. Heidelberg summarized and described the susceptibility of cobalt–sulfur systems, which mentioned that Co9S8 is antiferromagnetic when the Néel temperature is higher than the decomposition temperature [10]. Erika Dutková and colleagues studied the magnetic properties of Co9S8 nanoparticles synthesized by mechanical chemistry using a Superconducting Quantum Interference Device (SQUID) magnetometer. The room-temperature magnetic data supports the paramagnetic spin structure of the nanoparticle, and the temperature dependence of the magnetization at low temperatures suggests a transition from paramagnetism to weak ferromagnetism or ferromagnetism [11]. In addition, Zhou and colleagues also studied the magnetic properties of Co9S8 hollow microspheres successfully prepared and reached the same conclusion [12]. Therefore, the study of the magnetic properties of Co9S8 confirms the potential application of Co9S8 in magnetic fields.
A large number of metallic sulfides have outstanding electrical conductivity, such as Co9S8 and Ni3S2, but magnetic instability and electron interactions make them of insignificant importance [13]. To play metallic sulfides’ crucial role, the most common approach is to increase the number of free carriers by introducing different dopants, and an increase in the doping concentration leads to an increase in the conductance [14]. It is worth noting that Co9S8 also has rich redox properties, which contributes to the generation of active sites for chemical reactions, especially electrocatalytic hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), which also plays an important role in the further application of Co9S8 in the field of photoelectric catalysis [15].
3. Different Dimensions of Co9S8-Based Materials
Pokropivny and Skorokhod proposed a classification scheme for nanostructured materials (NSMs) in 2007, namely zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D) and three-dimensional (3D) [16]. It is noteworthy that the physical, chemical and electronic properties of nanomaterials with different dimensions could be quite different from one another, which is attributed to the fact that the intensity of electron motion increases with the increase of dimension [17]. So far, many technologies have been developed for controllable preparation of 0D–3D Co9S8 nanomaterials, and 0D nanoparticles, 1D nanotubes, nanorods, nanowires and needles, 2D nanosheets, nanoplates, nanolayers and nanowalls, 3D hollow spheres and flower-like structures have been obtained. Therefore, based on the above classification, this section discusses the structure and preparation method of Co9S8 nanomaterials from 0D–3D.
3.1. Zero-Dimensional (0D) Structure
In recent years, the great potential of 0D-nanostructured materials in photoelectric catalysis, solar cells and lithium ion batteries has promoted the synthesis of 0D-structured Co9S8-based materials. Feng prepared carbon-coated Co9S8 nanoparticles (Co9S8@C) from a mixture of cobalt nitrate and trithiocyanuric acid by direct heat treatment in N2 atmosphere at 700 ℃. It is noteworthy that trithiocyanuric acid is both a sulfur and a carbon source in this reaction system [18]. Wang also produced well-coated Co9S8@C nanomaterials using the vulcanization method (Figure 2a) [19]. Furthermore, unlike the above, Liu prepared Co9S8@C ultrafine nanomaterials using sucrose as the carbon source and using solid state reaction and mechanical ball milling (Figure 2b). It is certain that ball milling is not only a process of particle size reduction, but also promotes more effective binding between carbon and Co9S8 [20]. Lu synthesized carbon-decorated and graphene-fixed amorphous Co9S8 hollow nanoparticles using a “thermal injection” method [21]. A large number of experimental studies have shown that wrapping unique structurally modified nanoparticles in a doped mesoporous network with more active sites is an effective way to obtain ideal materials with high activity. Zhang, Luo, Du and Wang have reported that metal-organic framework (MOFs)-derived Co9S8 nanoparticles embedded in nitrogen and sulfur co-doped carbon composites show excellent performance [22,23,24,25]. Using inorganic salt as a template, Yu prepared a series of Co9S8 nanoparticles embedded with N and S co-doped carbon through three steps of grinding, carbonation and acid leaching (Figure 2c). It is suggested that the ratio of precursor, annealing temperature and the type of salt can adjust the porosity, heteroatomic content and crystal structure of the product [26,27,28]. Different synthesis methods have different advantages. Zhang used carbon dots (CDs) to prepare Co9S8 nanoparticles in N and S co-doped carbon materials. CDs distributed uniformly in the matrix of Co9S8 nanoparticles and played a key role in improving specific surface area and electrical conductivity (Figure 2d) [29]. Wang grew monodispersed Co9S8 nanoparticles with a particle size of about 8 nm due to the restriction of organic ligand oleamine on the surface of Reduced Graphene Oxide (RGO) by a simple thermal injection method [30]. Li prepared hollow carbon nanotubes with single cobalt atoms integrated with Co9S8 nanoparticles. The structure of the material was verified by X-ray absorption spectroscopy and aberration-corrected scanning transmission electron microscopy [31]. Zhang reported a mild ion exchange method for the preparation of carbon nanofibers modified with Co9S8 nanoparticles. Specifically, Co9S8 nanoparticles were converted from cobalt oxide precursor nanoparticles in a microwave hydrothermal process and then modified on the carbon nanofiber skeleton. The composition of nanofibers can be adjusted by changing the content of cobalt acetate in the precursor. In terms of materials, Co9S8 nanoparticles with favorable dispersion act as redox centers for energy storage, while porous carbon nanofibers provide fast electron transfer pathways [32].
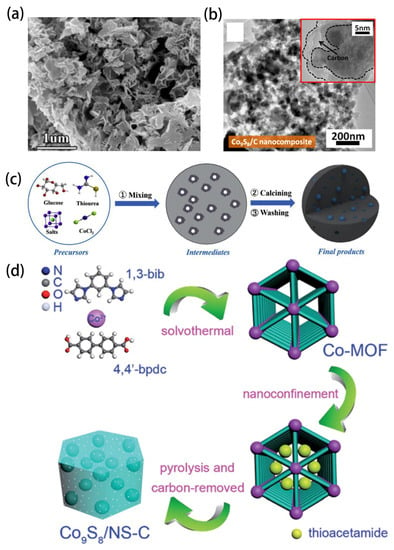
Figure 2.
(a) SEM images of Co9S8/C-T precursor. Reprinted with permission from Reference [19]. Copyright (2018) Elsevier Ltd. All rights reserved. (b) TEM images of Co9S8/C Nanocomposite. Reprinted with permission from Reference [20]. Copyright (2018) Springer Ltd. All rights reserved. (c) The synthetic strategy of the alkali metal doped Co9S8 NPs embedded, N, S co-doped carbons. Reprinted with permission from Reference [26]. Copyright (2020) Elsevier Ltd. All rights reserved. (d) The synthesis process of Co9S8/NS-C. Reprinted with permission from Reference [29]. Copyright (2017) Royal Society of Chemistry.
3.2. One-Dimensional (1D) Structure
Recently reported 1D Co9S8-based nanomaterials can be divided into nanowires, nanotubes and nanorods according to their morphology. The application of nanowire in an energy storage system is beneficial to the strong contact area between electrode and electrolyte. In addition, the high electrochemical activity is due to the abundance of active sites, rapid electron transport and gas release. Yang grew Co9S8 nanowires on carbon fiber paper by using a hydrothermal method (Figure 3a). Notably, Co9S8 nanowires were encapsulated in a fluffiness shell consisting of a continuous N, S co-doped carbon coating decorated with abundant vertically growing N, co-doped carbon nanotube materials. The material not only provides excellent voids for rapid mass transfer, but also provides a large specific surface area for loading of active sites [33]. Dong constructed a one-dimensional cobalt sulfide and vanadium sulfide heterojunction nanowire bi-functional electrocatalyst grown on carbon cloth (Figure 3b). The 1D linear Co9S8-V3S4 heterojunction has strong electron coupling, which is favorable for hydrogen evolution reaction [34]. Similar to nanowire, nanotubes and nanorods have attracted the attention of researchers because of their extreme specific surface area. Liu obtained a core-shell nanotube array with rich oxygen vacancy Co9S8@NiCo-LDH (Figure 3c). In situ growth and electrodeposition of flexible carbon cloth carrier were used [35]. Similarly, Zhu successfully fabricated Co9S8@Ni(OH)2 core-shell nanotube arrays with very large specific surface area by electrodeposition (Figure 3d) [36]. Zhang and Li assembled one-dimensional Co9S8 nanotubes and two-dimensional nanosheets to prepare materials with excellent synergistic properties (Figure 3e) [37,38]. Wu synthesized a novel hybrid material of Molybdenum sulfide laminate modified by Co9S8 nanotubes through continuous solvent heat treatment. X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy and other techniques were used to characterize the material, which not only proved that the decoration was amorphous MoSx, but also showed that there was a strong interface interaction between the two phases [39]. Du successfully synthesized a series of Co9S8 nanorod arrays with dual-function electrocatalysts doped with Mo on nickel foam for the first time by using a hydrothermal sulfidation process (Figure 3f) [40]. In addition, Du reported an in situ deposition method for adjusting the Ni(OH)2 loading capacity on a Co9S8 surface by adjusting the number of deposition cycles (Figure 3g,h). As a perfect carrier, one-dimensional Co9S8 nanorods enhanced the uniform dispersion of amorphous Ni(OH)2 nanoparticles. The electrolytic deposition of amorphous Ni(OH)2 nanoparticles coated on the surface of Co9S8 nanorods greatly improved the interface effect and provided a more effective active center for the water splitting reaction [41].
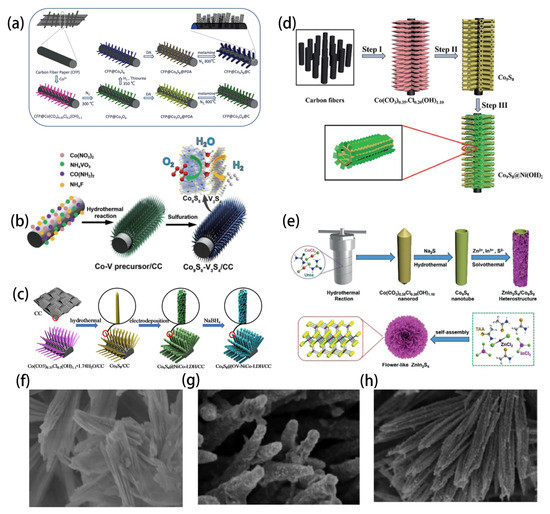
Figure 3.
(a) The synthetic process for CFP supported Co9S8@N,S-codoped carbon nanowires with surfaces. Reprinted with permission from Reference [33]. Copyright (2018) Royal Society of Chemistry. (b) Schematic diagram of the synthesis of 1D Co9S8-V3S4/CC nanowires. Reprinted with permission from Reference [34]. Copyright (2021) Springer Ltd. All rights reserved. (c) Schematic diagram of the detailed synthesis process of Co9S8@OV-NiCo-LDH core-shell nanotube arrays. Reprinted with permission from Reference [35]. Copyright (2022) Elsevier Ltd. All rights reserved. (d) Schematic fabrication process of Co9S8@Ni(OH)2 core–shell NT arrays on CFs. Reprinted with permission from Reference [36]. Copyright (2017) Royal Society of Chemistry. (e) Illustration of the fabrication process of hierarchical Co9S8/ZnIn2S4 tubular photocatalyst. Reprinted with permission from Reference [37]. Copyright (2020) Wiley-VCH. (f) SEM of Co9S8-3. Reprinted with permission from Reference [40]. Copyright (2019) Elsevier Ltd. All rights reserved. (g,h) SEM of Co9S8/NF. Reprinted with permission from Reference [41]. Copyright (2019) Elsevier Ltd. All rights reserved.
3.3. Two-Dimensional (2D) Structure
This section demonstrates the preparation of various 2D nanoplates and nanosheets made from Co9S8-based nanomaterials. Due to its elevated surface volume ratio, nanometer thickness and fascinating electrocatalytic activity, Co9S8 is considered as an ideal material for electrocatalysts. By changing the filling coefficient, Yang and colleagues synthesized cobalt sulfide precursors with different morphs by hydrothermal method and obtained ultra-thin Co9S8 nanosheets under the condition that the filling rate of hot autoclaves reached 60% (Figure 4a) [42]. Li successfully designed and synthesized dual-function electrocatalyst Co9S8/C nanosheets by reducing Co3O4/CNS precursor with sodium sulfide (Figure 4b) [43]. One of the benefits of using 2D materials is that such composites can easily interact and combine with other materials of different dimensions. For example, Zhang designed a novel two-dimensional hexagonal WS2-sided Co9S8 heterostructure nanosheet structure on the surface of a carbon nanofiber skeleton [44]. Xing reported and characterized, in detail, layered Co3O4@ Co9S8 nanowalls assembled from numerous nanosheets [45]. The material with a large number of defects can induce positive effects on the atoms in the ultrathin two-dimensional structure to improving its intrinsic activity, which is beneficial to improve the excellent properties of the material. Wang and colleagues first synthesized porous 2D Co9S8 nanosheets rich in defects and applied them to the cathode of Li-O2 batteries (Figure 4c). It is worth mentioning that the two-dimensional structure and in-plane holes of the catalyst not only facilitate mass transfer, but also provide active sites for sulfur-rich substances [46]. In addition, using cobalt dibutyl dithiocarbamate as raw material, Zhang and colleagues prepared defective Co9S8-based ultrathin nanosheets by direct pyrolysis in organic amine solvent [47]. Doping is a particularly popular method to improve material properties. Both Wang and Li successfully synthesized two-dimensional Co9S8 nanosheets uniformly distributed on the surface of two-dimensional N-doped graphene [48,49]. Li reported a sandwich structure in which Co9S8 is confined between the graphene scaffold folding layer and the N-carbon layer (Figure 4d). Due to the synergistic effect of graphene and carbon layers, the structural stability of the material is highly improved. Hao focused on Co9S8 nanosheet arrays grown on carbon cloth under optimal nickel incorporation conditions. The addition of Ni can optimize the electronic structure of Co9S8 and improve the conductivity and charge transfer efficiency. In addition, the nanosheet structure endows a two-dimensional charge transfer pathway, exposing more active sites [50]. Tang and Fu both obtained N and S co-doped Co9S8 nanoplates [51,52]. Tang prepared nitrogen-sulfur double-doped graphene and quasihexagonal Co9S8 nanoplates by a simple ion assembly method (Figure 4e). Fu used Co9S8 nanosheets and ethyl orthosilicate to directly form two-dimensional carbon precursors and disperse them into thiourea solution, and then removed them through pyrolysis and template to obtain N and S co-doped Co9S8/NSC porous carbon plates.
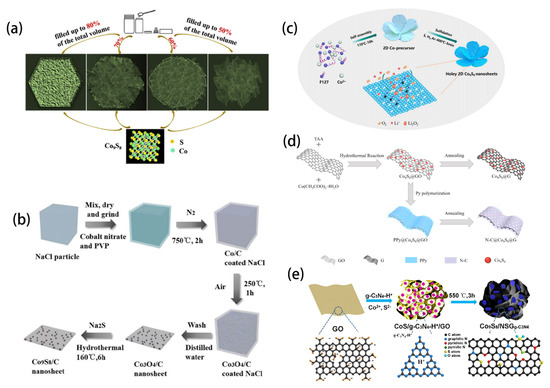
Figure 4.
(a) Schematic illustration of the controllable synthesis of Co9S8 catalysts. Reprinted with permission from Reference [42]. Copyright (2018) Elsevier Ltd. All rights reserved. (b) Schematic illustration of synthesis of Co9S8/C. Reprinted with permission from Reference [43]. Copyright (2019) Royal Society of Chemistry. (c) Schematic illustration for the synthesis of the holey Co9S8 nanosheets Reprinted with permission from Reference [46]. Copyright (2020) Elsevier Ltd. All rights reserved. (d) Schematic illustration of synthesis of N–C@Co9S8@G sandwich structure. Reprinted with permission from Reference [49]. Copyright (2020) Elsevier Ltd. All rights reserved. (e) Schematic demonstration of the preparation process for Co9S8/NSGg-C3N4. Reprinted with permission from Reference [51]. Copyright (2017) American Chemical Society.
3.4. Three-Dimensional (3D) Structure
It is well known that the properties of nanomaterials depend on their morphology and size. Therefore, compared with 2D materials, 3D materials have a very wide application potential due to their controllable structure, large specific surface area, strong absorption capacity, superior electron and ion transport performance and additional advantages. Rajith Illathvalappil synthesized Co9S8-Ni3S2 layered nanoflower arrays which were self-supporting on nickel foam with the required porosity and structural characteristics using a one-pan hydrothermal method without using any adhesives (Figure 5a). The active modulation achieved in this experiment is attributed to the existence of the open pore morphology of Co9S8-Ni3S2 nanoflowers formed on the nickel foam, which is conducive to the adsorption of reactants and the transformation of gaseous products [53]. Xia successfully prepared a Co9S8-based photocatalyst for efficient hydrogen evolution (Figure 5b). They combined CdS quantum dots with hollow dodecahedron Co9S8 to form a Co9S8/CdS heterostructure using a low-energy atmospheric single-pot strategy [54]. Similarly, Qiu designed a hollow cobalt sulfide cube embedded with cadmium sulfide quantum dots as a template using the one-pot hydrothermal method (Figure 5c). Hollow CdS/Co9S8 utilizes multi-light reflection in the cube structure to achieve enhanced photocatalytic activity [55]. Zhao synthesized CoNi2S4/Co9S8 nanostructures with high porosity, suitable pore size distribution and novel structure using simple chemical bath deposition [56]. By using a simple triethanolamine-assisted self-template strategy, Wang and colleagues prepared a unique three-dimensional honeycomb cobalt sulfide network composed of cross-linked nanosheets (Figure 5d). It was found for the first time that the introduction of triethanolamine in the reaction can effectively increase the proportion of high-priced Co3+ in the final product [57]. Li reported the atomic layer deposition of cobalt sulfide. In this process, high quality Co9S8 films with layer-by-layer growth behavior were produced using H2S as reactant. Co9S8 films can also be conformally deposited into deep and narrow grooves with an aspect ratio of 10:1, which indicates that this process has a high application prospect for Co9S8 conformal coating on 3D nanostructures with elevated aspect ratio [58].
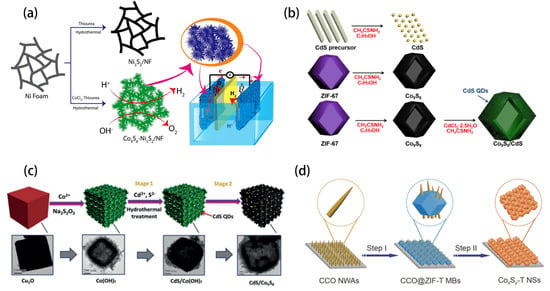
Figure 5.
(a) Schematic illustration of the synthesis of Co9S8 -Ni3S2 /NF through the hydrothermal route and the use of this system as the bi-functional electrode in water electrolysis. Reprinted with permission from Reference [53]. Copyright (2020) Wiley-VCH. (b) Preparative Process for CdS, Co9S8, and Co9S8/CdS. Reprinted with permission from Reference [54]. Copyright (2021) American Chemical Society. (c) The preparative process for CdS/Co9S8 and corresponding TEM images; scale bar (200 nm). Reprinted with permission from Reference [55]. Copyright (2017) Wiley-VCH. (d) Schematic illustration of the formation of the CoxSy-TNSs. Reprinted with permission from Reference [57]. Copyright (2021) Springer Ltd. All rights reserved.
4. Applications
As mentioned above, Co9S8-based nanomaterials are technically valuable functional materials for various applications. For photocatalytic technology, the keys to its excellent light capture and charge transfer are its higher specific surface area, more abundant active sites, shorter charge carrier migration distance and electronic structure. In addition to being the matrix material, Co9S8-based nanomaterials also promote the transformation of reaction kinetics and finally improve the electrode output. In the following sections, we will selectively review some typical examples involving Co9S8-based nanomaterials.
4.1. Photoelectric Catalysis
4.1.1. Photocatalytic Hydrogen Evolution Reaction (HER)
H2, as a high energy density zero-pollution emission energy carrier, is considered as a promising alternative to fossil fuels. In general, Pt-based materials have been shown to be the most effective HER catalysts. However, their high cost and low reserves make them difficult to realize for large-scale industrial applications. Therefore, the development of greatly active and stable earth-rich catalysts to reduce or eliminate the dependence on noble metal-based catalysts for HER remains a great challenge. Co9S8-based catalysts have been used as H2-generating catalysts for non-noble metals and great progress has been made. To further improve the catalytic performance of Co9S8-based catalysts, several modification strategies such as defect engineering, doping and heterojunction engineering have been developed. For example, Zhang fabricated Co9S8-based ultrathin sheets with a thickness of 4 to 6 nm that are rich in edge defects, cavity defects and dislocation defects. Moreover, the catalytic activity of hydrogen evolution was optimized by tuning the coordination solvent and pyrolysis parameters to control the defects of Co9S8-based nanosheets. As shown in Figure 6a,b, the apparent defects on the surface of Co9S8-based nanosheets were confirmed by HRTEM images. They also assessed the thickness of Co9S8-based nanosheets as ~4 nm by AFM analysis (Figure 6c) [47]. In addition to the defects mentioned above, appropriate heteroatomic doping can adjust the electronic structure, which is an effective way to improve the performance of the electrocatalyst for hydrogen evolution. N, S, P heteroatom-doped composites have attracted much attention due to their high HER activity [58,59,60]. Among them, the study found that phosphorous-doped metal sulfide is particularly interesting, because P atoms have a similar atomic radius and electronegativity to S atoms, which makes it easy for P atoms to be doped into sulfide by replacing S atoms to bond with metal atoms and cause lattice distortion [61,62]. Such small changes to the parent lattice structure may provide new active sites, optimize the adsorption energy of hydrogen, or regulate the electronic band structure, which may be beneficial to improve its electrocatalytic performance [63]. Gao’s team systematically studied the effect of the amount of P doping on the electrocatalytic properties of CoS2. Through theoretical and experimental studies, Jin and colleagues established ternary pyrite cobalt phosphorus sulfide (CoPS) as a HER electrocatalyst with high performance [64]. Although the above two have made great contributions to exploring the application of P-doped CoS2 and CoPS to HER, there are few studies on the catalysis of P-doped Co1−xS, Co9S8 and other cobalt sulfide compounds. Qu reported a general synthesis method for various P-doped cobalt sulfide compounds, including CoS2/Co1−xS, Co1−xS and Co9S8 phase [65] (Figure 6h,i). It is found that hydrothermal temperature has a crucial influence on the phase of final products. Therefore, different phase products are synthesized by controlling the hydrothermal temperature. Because P atoms and S atoms have a similar atomic radius and electronegativity, P atoms are easily doped into sulfide by replacing the S atoms and bonding with metal atoms, which leads to lattice distortion. Through experimental study and theoretical calculation, it is shown that the introduction of P can effectively improve the conductivity of cobalt sulfide and optimize the hydrogen adsorption energy. This study provides guidance for the design of P-doped Co9S8 and other cobalt sulfide compounds as HER electrocatalysts. In order to improve HER efficiency, the construction of heterogeneous catalysts is also a popular strategy at present. Wu successfully prepared MoS2/Co9S8 heterostructure and showed excellent catalytic performance and good stability [66]. As shown in Figure 6f,g, MoS2 is evenly distributed on the surface of Co9S8, which is conducive to MoS2 exposing more boundary active sites. In addition, the heterostructure formed between MoS2 and Co9S8 is more favorable for electron transfer. The LSV curve and Tafel slope results show that, as shown in Figure 6h,i, the heterogeneous structure of MoS2/Co9S8 greatly improves HER catalytic performance. In summary, to obtain high performance Co9S8-based HER electrocatalysts, the following points should be paid more attention. The intrinsic activity of each active site was improved by optimizing the hydrogen adsorption energy of the electrocatalyst. Moreover, improving the conductivity of electrocatalysts is equally important for accelerating the charge transfer. Furthermore, attention should be paid to improving the macroscopic structure, hydrophilic and hydrophobic characteristics, and enhancing the mass diffusion between the H2 catalyst and the electrolyte.
Photocatalysis as a green and efficient H2-generation pathway has also received extensive attention [67,68,69]. Co9S8 is an interesting visible light active photocatalyst with a special electronic structure, tunable optical properties and wide corresponding range of light. Despite the encouraging progress, the catalytic efficiency of pure Co9S8 photocatalysts is still not ideal. Therefore, coupling Co9S8 to a semiconductor with an appropriate bandgap to form a heterostructure is a successful technique to efficiently collect electrons and induce charge transfer at the interface. Taraka Prabhu Yendrapati constructed an efficient and stable Co9S8@CdIn2S4 heterostructure for the photocatalytic generation of H2 in visible light [70]. On the basis of retaining the polyhedral form of Co9S8, CdIn2S4 is uniformly decorated on the Co9S8 cage, as shown in Figure 6j,l. Two photoactive sulfide semiconductors are reasonably incorporated, and this hollow heterostructure can effectively achieve the separation and transfer of photoinduced charge, providing a broad surface area and abundant exposure sites for the photocatalytic redox reaction, so that it has stronger light-trapping ability and significant HER catalytic activity.
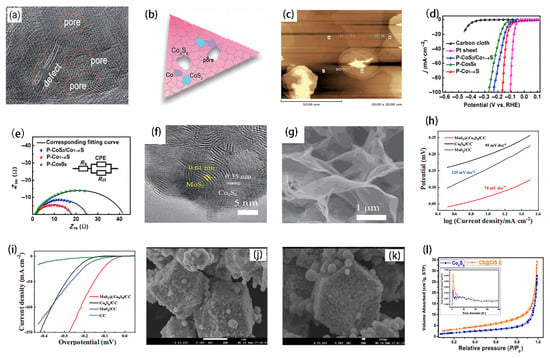
Figure 6.
(a) HRTEM images of DR-Co9S8-II with pore defects and dislocation. (b) Schematic drawing of defective structures in DR-Co9S8-II nanosheets. (c) AFM images of DR-Co9S8-II. Reprinted with permission from Reference [47]. Copyright (2018) Royal Society of Chemistry. (d) LSV curves of bare carbon cloth, P-Co9S8, P-Co1−xS, P-CoS2/Co1−xS and Pt sheet. (e) Nyquist plots of P-Co9S8, P-Co1−xS and P-CoS2/Co1−xS. Reprinted with permission from Reference [65]. Copyright (2021) Elsevier Ltd. All rights reserved. (f) SEM images of MoS2@Co9S8/CC. (g) HRTEM. The electrochemical measurements of all synthesized catalysts in 1 M KOH. (h) HER polarization curves. (i) Tafel plots. Reprinted with permission from Reference [66]. Copyright (2019) Springer Ltd. All rights reserved. (j-l) FESEM images of CS@CIS2 photocatalysts. Reprinted with permission from Reference [70]. Copyright (2021) American Chemical Society.
4.1.2. Electrocatalytic Water Splitting
With the exhaustion of non-renewable resources such as fossil fuels and the increasingly serious environmental pollution, people are focusing on renewable clean energy. Electrochemical water decomposition is an efficient method to produce H2 and O2. Electrocatalytic water separation in the reaction process consists of two half reactions: HER at the cathode and OER at the anode. There are problems of high overpotential and slow kinetics in the reaction process. As a result, many scholars try to develop noble metal-free OER/HER dual-function catalysts to improve gas production rate and reduce energy consumption, among which, Co9S8-based materials have made great progress (Table 1). For example, Dong successfully prepared 1D Co9S8-V3S4/CC heterojunction dual-function electrocatalysis for water splitting and obtained good HER and OER performance, as shown in Figure 7a,b [34]. In this study, the researchers not only tested the performance of the catalyst, but also explained its catalytic mechanism. As shown in Figure 7c, XPS results confirmed the formation of the Co9S8-V3S4 heterojunction. It is noteworthy that the Co peak of Co9S8-V3S4/CC shifted to a positive position compared with Co9S8/CC and the V peak of Co9S8-V3S4/CC shifts negatively with respect to V3S4/CC. These results indicate that there is a strong electron interaction at the interface, where V3S4 acts as an electron attractor to obtain electrons from Co9S8, resulting in the higher Co3+ and lower valence V2+ states in Co9S8-V3S4/CC. In conclusion, the electron transfer from Co9S8 to V3S4 occurs at the interface due to the strong electron coupling effect of the Co9S8-V3S4 heterojunction, where the electron attraction of V3S4 (V2+) optimizes the adsorption of H* active substance and is favorable for HER. The electron loss of Co9S8 (Co3+) responds to the enhancement of OER activity. In addition to being a dual-function electrocatalyst, Co9S8-based materials are also widely used as three-function electrocatalysts. Based on the concept of sustainable energy development, Liang designed a three-function electrocatalytic material containing Co9S8/Co in situ encapsulated in N-doped carbon nanotubes (Figure 7d), achieving efficient electrocatalytic reactions of oxygen reduction, oxygen evolution and hydrogen evolution [71]. The microstructure analysis shows that Co9S8 and Co nanoparticles are in situ integrated into N-doped graphite carbon tube (Figure 7e). In this study, they highlight the importance of the rich Co-N and Co-N-C coupling centers generated by in situ engineering of the conversion of metal sulfides to carbon–nitrogen frameworks for the multifunctional catalytic conversion of sustainable energy. As shown in Figure 7f,g, XPS confirmed the existence of abundant Co-N and Co-N-C coupled active sites at the interface. In addition, Li also reported Co9S8 as a multifunctional electrocatalytic material [31]. The theoretical calculation and experimental results showed that the synergistic effect between Co9S8 nanoparticles and single cobalt atom will reduce the reaction barrier, as shown in Figure 7h,i, thus promoting the generation of ORR, OER and HER. According to the above studies, it can be concluded that bifunctional or even trifunctional catalysts with excellent HER and OER performance reflect the diversity of their catalytic functions and may show great potential in other types of applications [41].

Table 1.
Electrocatalytic Activity of Co9S8-Based materials.
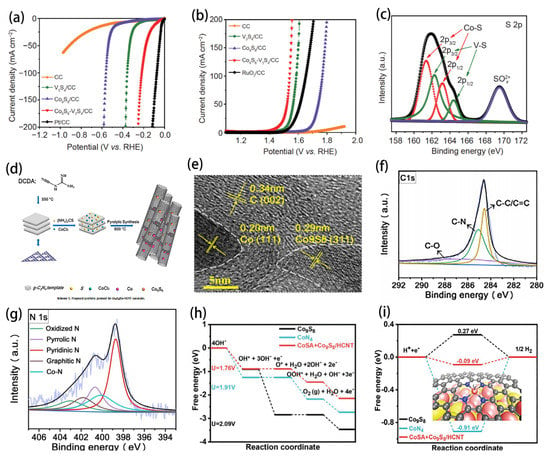
Figure 7.
(a) Polarization curves of bare CC, V3S4/CC, Co9S8/CC, Co9S8-V3S4/CC and Pt-C/CC catalysts with a scan rate of 2 mV s−1 in 1.0 mol L−1 KOH for HER. (b) Polarization curves of bare CC, V3S4/CC, Co9S8/CC, Co9S8-V3S4/CC and RuO2/CC catalysts with a scan rate of 2 mV s−1 in 1.0 mol L−1 KOH for OER. (c) XPS survey spectrum of S 2p of Co9S8-V3S4/CC. Reprinted with permission from Reference [34]. Copyright (2019) Elsevier Ltd. All rights reserved. (d) Proposed synthetic protocol for Co9S8/Co-NCNT nanotube. (e) HRTEM images of Co9S8/Co-NCNT-0.05. (f–g) High resolution XPS spectra of Co9S8/Co-NCNT-0.05. Reprinted with permission from Reference [71]. Copyright (2020) Wiley-VCH. (h) Free energy diagrams for the thermodynamic limiting potentials of OER steps. (i) Free energy diagrams of Co9S8, CoN4, and CoSA + Co9S8/HCNT for HER and inset is the model of adsorption configuration of HER on CoSA + Co9S8/HCNT. Reprinted with permission from Reference [31]. Copyright (2021) Springer Ltd. All rights reserved.
4.2. Electrochemical Energy Storage
Electrochemical energy storage devices such as supercapacitors, Li/Na ion batteries, Li-S batteries and Zn-air batteries play an important role in modern society. In order to follow the needs of social development, it is very desirable to obtain a better, new generation of batteries. Therefore, as a key component of energy storage devices, the performance of electrode materials is particularly important. Co9S8-based materials have the natural advantage of building better electrode materials. By carefully adjusting the chemical composition and microstructure of transition metal sulfides, significant electrical conductivity can be obtained, thus solving many existing problems and bringing significant development to supercapacitors and batteries.
4.2.1. Batteries
Lithium-Ion Batteries
Rechargeable lithium-ion batteries (LIBs) have been widely used in many modern electronic products, such as electric vehicles and portable electronic devices, due to their high energy density, environmental friendliness and long-term cycle stability. However, the theoretical capacity of graphite used as an anode material limits the development of LIBs. Transition metal Co9S8, which has become a high-performance LIBs electrode material, has been widely studied because of its large theoretical capacity and high safety. For example, as shown in Figure 8a, Chen prepared N and S co-doped carbon skeleton cobalt sulfide nanoparticles (Co9S8/NSC) by a one-step method [82]. As an anode for lithium-ion batteries, the composite material has good reversible capacity, high velocity performance and long-term cycling stability (Figure 8b,c). Although Co9S8-based nanomaterials have made great progress as electrode materials, their further development is still greatly hindered by their volume expansion and electrical conductivity. Therefore, many scholars adjust the structure of materials or compound Co9S8 with other materials to get better electrode materials [49,83,84]. He demonstrated an expandable electrostatic spinning method for preparing Co9S8/Ni3S2@S-CNFs materials as independent anode materials [85]. In particular, this study forms an independent 3D nanofiber network that allows lithium ions to easily access the active nanoparticles, thereby enhancing the activity and stability of the material.
Sodium-Ion Batteries
In recent years, with the increasing demand for low-cost, long-life, high-energy density storage systems, sodium ion batteries (SIBs) have been considered as a promising alternative to LIBs due to the abundant supply and low cost of sodium [86,87,88]. At present, Co9S8-based materials has good conductivity as SIBs electrode material and can be a cathode material with great potential for commercialization. Li and his colleagues demonstrated that the rich grain boundaries and three-layer exposed interfaces of the prepared Co9S8-carbon material are the main capacitors for the storage performance of high sodium ions through experiments and simulation analysis [89]. Wang and colleagues prepared Co9S8@S-CF materials by in situ pore-forming, sulfurization, sulfur doping, and carbonization [90]. As shown in Figure 8d, sulfur-doped foam carbon has abundant voids. These interstitial spaces can construct three-dimensional ion/electron paths to accelerate the transport of sodium ions/electrons to Co9S8 nanoclusters. In addition, the reversible capacity retention rate is up to 80% after 1000 cycles, and the Coulomb efficiency is over 95%, which is very close to the theoretical capacity of hybrid material, as shown in Figure 8e. The results show that the material has a unique foam-like structure and is superior to Co9S8 nanosheets in terms of reversible capacity, capacity retention and cyclic stability. According to the above research, it can be found that Co9S8-based materials pave a new way for the development of advanced electrodes for SIBs, and can, therefore, be extended to the development of various metal sulfide based electrodes for SIBs.
Lithium–Sulfur Batteries
Lithium–sulfur batteries have been widely explored because of their rich sulfur resources and high energy density, which overcomes the limitations of existing energy storage devices [91,92,93]. However, there are still many limitations that restrict the large-scale commercial development of lithium–sulfur batteries, such as large volume changes, irreversible loss of soluble lithium polysulfide in electrolytes and reduced number of electrochemical active reaction sites. To address the above technical obstacles, Co9S8-based nanomaterials can be combined with the electrode matrix to maximize the use of sulfur and inhibit the dissolution of polysulfides due to their excellent physical and chemical properties. Chen proposed that injecting Co9S8 into N-doped graphene to improve the electronic conductivity of the base. It is worth noting that the highly polar Co9S8 is considered to be an excellent chemical adsorbent and catalyst. It not only effectively immobilized the soluble lithium polysulfide through chemical binding, but also significantly catalyzed the electrochemical conversion of the soluble lithium polysulfide, and finally, inhibited its shuttle effect [94]. In addition, Li prepared Co9S8-doped graphene as the main material for a lithium–sulfur battery [95]. As shown in Figure 8f, the electron spin resonance spectrum verified the presence of sulfur vacancies in the sample. The use of UV-Vis adsorption spectrum indicates that the sulfur vacancy generated in the material effectively improves the adsorption capacity of polysulfide anions. In addition, density functional theory has calculated the adsorption energy of the material for polysulfide (Figure 8g,h). Finally, electrochemical experiments showed that the sulfur vacancy promoted the electrode kinetics of polysulfide catalytic conversion. The results showed that it is an effective way to develop high performance lithium–sulfur batteries by using sulfur vacancy to enhance sulfur conversion kinetics. Although the Co9S8-based materials play a role in promoting the performance of Li-S batteries, the shuttle effect, large amount of variation of sulfur and the formation of polysulfide have not been completely solved, and the real reality of Li-S batteries still needs a lot of exploration.
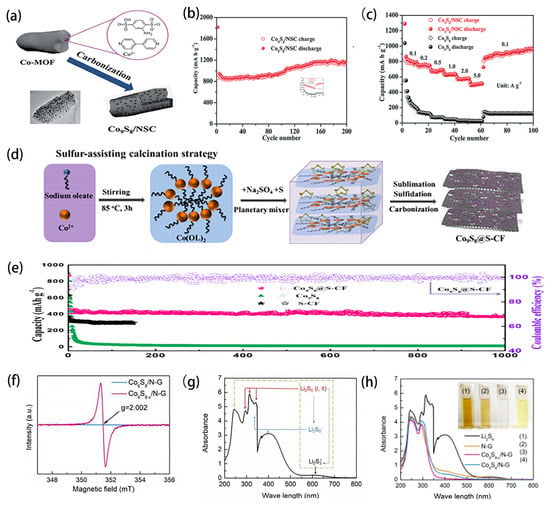
Figure 8.
(a) Schematic drawing of the formation process to obtain the hybrid composite Co9S8/NSC. (b) the cycling performance at 0.1 A g 1 of Co9S8/NSC. (c) rate capability. Reprinted with permission from Reference [82]. Copyright (2019) Royal Society of Chemistry. (d) Schematic illustration for in situ formation of Co9S8 nanoclusters confifined in sulfur-doped carbon foam (denoted as Co9S8@S-CF). (e) Cycling performance of the Co9S8@S-CF and Co9S8 nanosheets and S-CF electrodes at 0.25 C (1 C = 400 mA g−1). Reprinted with permission from Reference [90]. Copyright (2019) American Chemical Society. (f) Electron spin resonance spectra of Co9S8/N-doped graphene and Co9S8-x/N-doped graphene. (g) Assignment of absorption bands in UV-Vis spectrum of Li2S6 DOL/DME solution. (h) UV-Vis spectra of the Li2S6 solution and after static adsorption of the different host materials. Reprinted with permission from Reference [95]. Copyright (2013) Royal Society of Chemistry.
Zn-Air Batteries
Dual-function electrocatalytic materials are an important part of many renewable energy devices. For example, in the water splitting mentioned above, HER and OER are the two main reactions. In metal–air batteries, the ORR and OER activity at the side of the air electrode is a key parameter that determines the performance of the battery, and the voltage difference between the two reactions determines the efficiency and energy consumption of the device. Although a simple mixture of a single catalytically active catalyst can also achieve the effect of dual-function catalyst, the corresponding energy density and power density will decrease owing to the increase of mass load. Therefore, it is reasonable to study the corresponding dual-function catalysts, especially for demanding batteries. Co9S8-based materials are an excellent choice for metal–air batteries, especially for zinc–air batteries, due to their excellent bi-functional properties in ORR and OER [96,97]. Zheng synthesized a series of N- and S-doped carbon NSC/Co9S8 materials. Considering that the structure of the electrode material is an important factor in the optimization of the electrocatalyst, they introduced homogeneous mesopores or macropores in the carbon structure [98]. Excellent electrocatalytic activity of the oxygen reduction reaction was obtained, which was mainly due to the improvement of wettability and the interaction with O2. Although Co9S8 is a promising non-noble metal bi-functional electrocatalyst, the electrocatalytic performance of cobalt-based nanocatalysts is still unsatisfactory due to the poor electrical conductivity and easy aggregation during continuous operation. In order to solve the above problems, Cao synthesized the honeycomb structure porous carbon (Co9S8/NSC) in situ composite by a simple method inspired by honeycomb in nature [99]. Co9S8 nanoparticles embedded in the carbon matrix of a 3D interconnection network gave it high electrical conductivity, which not only promoted the electron transfer and charge transfer at the interface, but also promoted the exposure and rapid transfer of active sites of ORR/OER-related species, and the obtained composite material exhibited high cyclic stability.
4.2.2. Supercapacitors
Supercapacitors have become a research hotspot because of their advantages, such as high power density, high coulomb efficiency and long cycle span [100,101]. In the field of energy storage, supercapacitors play an important role in complementing or even replacing conventional batteries. However, capacitors still have disadvantages beyond battery devices, such as low energy density and self-discharge. Therefore, in order to improve the electrochemical performance of supercapacitors, many scholars have conducted a lot of research and gained some new understandings. For example, only specific functional groups such as pyridine- or pyrrole-type nitrogen are favorable for Faraday reactions, the transfer of electrons and ions on the surface is critical to the rate capacity, and the morphology is also related to electrochemical properties [102,103,104]. In addition, it is also crucial to find excellent electrode materials for capacitors. Co9S8-based materials can improve the conductivity by introducing a pseudocapacitance reaction, expanding specific surface area and constructing porous structure [105,106].
Based on the above, Co9S8-based materials provide a good opportunity to construct capacitor electrodes with excellent performance. In order to further improve the conductivity of Co9S8-based materials in supercapacitor electrodes, Emad S. Goda successfully designed a novel structure encapsulated by nitrogen-doped graphene (Al-doped Co9S8@NG) as the positive electrode of the capacitor [107]. As shown in Figure 9a,b, the composite developed has a maximum capacity value of about 736 C/g at a current density of 1 A/g and has an ultra-long cycle stability after 10,000 cycles. These results indicate that carbon substrate, as a fast charge transfer agent, can balance the change of electrode volume and enhance the conductivity of nanoparticles, thus obtaining electrodes with good electrochemical characteristics. However, in addition to electrical conductivity, the structural instability of Co9S8-based materials often reduces their electrocatalytic activity during the long-term cycle, and reasonable improvement of their structural stability is still a challenge for energy conversion and storage applications. Wang used density functional theory to predict the stability of Co9S8, as shown in Figure 9c–f [108]. The calculation results show that compared with pure Co9S8, Fe that is doped in an octahedral position can improve its stability. In order to verify the theoretical results, Co9S8 hollow spheres with different Co/Fe molar ratios were prepared by one-step hydrothermal experiment and showed excellent electrocatalytic performance. As shown in Figure 9g–i, the Tafel slope is 63.9 mV dec−1 and its overpotential is 268 mV at the current density of 10 mA cm−2. To be sure, these impressive findings open the door to the use of Co9S8-based materials as electrodes for high-efficiency energy storage capacitors.
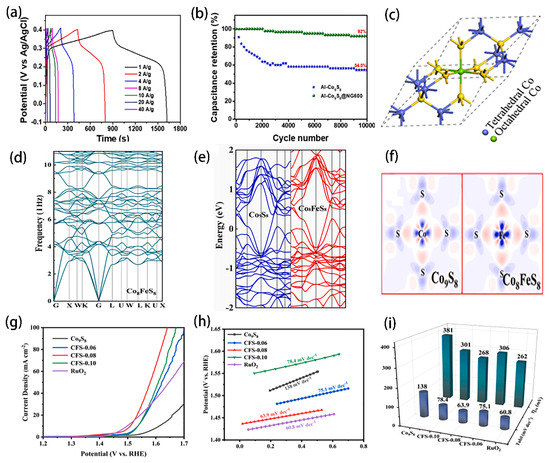
Figure 9.
(a) GCD patterns performed at various applied current densities from 1 to 40 A/g for core-shell Al-Co9S8@NG600. (b) Cyclic life analysis for Al-Co9S8, Al- Co9S8@NG600 electrodes at an applied current density value of 20 A/g. Reprinted with permission from Reference [107]. Copyright (2022) Elsevier Ltd. All rights reserved. (c) The primitive unit cell of Co9S8, Green is octahedral metal, Blue is tetrahedral metal, and Yellow is sulfurs. (d) The phonon dispersions of Co8FeS8. (e) Calculated band structures. (f) charge density difference. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article). (g) The LSV curves for different catalysts in 1.0 M KOH at 5 mV s−1. (h) Tafel slopes. (i) Corresponding overpotential at 10 mA cm−2 and Tafel slopes of different catalysts, respectively. Reprinted with permission from Reference [108]. Copyright (2022) Elsevier Ltd. All rights reserved.
5. Conclusions and Outlooks
This paper reviews the theoretical and experimental results of non-noble metal Co9S8-based materials in photoelectric catalysis and electrochemical energy storage. These results provide valuable reference for further elucidating the reaction mechanism of catalyst or electrode materials. In addition, the physicochemical properties and synthesis strategies of Co9S8-based materials with different dimensions are discussed in detail. It should be noted that there are still some key issues that need to be resolved and explored, even though the excellent properties of Co9S8-based materials facilitate their application in many fields.
- (i)
- The research regarding their reaction mechanism was not thorough, which restricts the further development of Co9S8-based materials as excellent catalyst and electrode materials.
- (ii)
- As an ideal catalyst, excellent results in different pH must be shown to make the water splitting process more efficient. However, experimental studies have shown that the proton concentration is bound to change during the HER process, which leads to different degrees of influence on Co9S8-based materials working in acidic or alkaline media.
- (iii)
- Some renewable energy installations typically require excellent bi-functional catalytic performance, such as the Zn-air batteries mentioned above. However, the role of bi-functional catalytic activity in different electrolytes is not the same, which hinders its practical application.
- (iv)
- We review the applications of Co9S8-based materials as photocatalysts and energy storage materials in recent years, but not limited to this, we can also explore its applications in biosensors, organic catalytic synthesis and other new fields, which are of great value for facilitating multi-field applications.
In summary, Co9S8-based materials have shown great advantages as catalysts and electrode materials, but their applications in other fields remain a challenge. Therefore, this paper mainly aims to introduce the research progress of properties, preparation strategies and applications of Co9S8-based materials in different dimensions. It provides reference for better design of Co9S8-based materials for wider applications to alleviate these challenges. We believe that the extraordinary physical and chemical properties of Co9S8-based materials will enable exciting advances in high performance energy storage devices and energy applications in the future.
Author Contributions
Conceptualization and writing, C.W. and X.P.; investigation and supervision, G.W. and L.G.; validation, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Training Program of Innovation and Entrepreneurship for Undergraduates (202110719055).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Long, J.; Gong, Y.; Lin, J. Metal-organic framework-derived Co9S8@CoS@CoO@C nanoparticles as efficient electro-and photo-catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 10495–10509. [Google Scholar] [CrossRef]
- Riaz, M.S.; Yuan, X.; Zhao, Y.; Dong, C.; Nong, S.; Ali, Z.; Huang, F. Porous NiCo2S4/Co9S8 microcubes templated by sacrificial ZnO spheres as an efficient bifunctional oxygen electrocatalyst. Adv. Sustain. Syst. 2019, 3, 1800167. [Google Scholar] [CrossRef]
- Chen, T.-T.; Wang, R.; Li, L.-K.; Li, Z.-J.; Zang, S.-Q. MOF-derived Co9S8/MoS2 embedded in tri-doped carbon hybrids for efficient electrocatalytic hydrogen evolution. J. Energy Chem. 2020, 44, 90–96. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Sun, X.; Yang, C.; Zheng, Y.-Z.; Yang, L.; Li, S.; Tao, X. Edge-oriented, high-percentage 1T′-phase MoS2 nanosheets stabilize Ti3C2 MXene for efficient electrocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 284, 119708. [Google Scholar] [CrossRef]
- Hu, C.; Ma, K.; Hu, Y.; Chen, A.; Saha, P.; Jiang, H.; Li, C. Confining MoS2 nanocrystals in MOF-derived carbon for high performance lithium and potassium storage. Green Energy Environ. 2021, 6, 75–82. [Google Scholar] [CrossRef]
- Ma, G.; Xu, X.; Feng, Z.; Hu, C.; Zhu, Y.; Yang, X.; Yang, J.; Qian, Y. Carbon-coated mesoporous Co9S8 nanoparticles on reduced graphene oxide as a long-life and high-rate anode material for potassium-ion batteries. Nano Res. 2020, 13, 802–809. [Google Scholar] [CrossRef]
- Xing, Y.; Li, D.; Li, L.; Tong, H.; Jiang, D.; Shi, W. Accelerating water dissociation kinetic in Co9S8 electrocatalyst by mn/N Co-doping toward efficient alkaline hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 7989–8001. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Hu, H.; Pan, Y.; Zhao, W.; Liu, J.; Zhao, X.; Wang, J.; Yang, Z.; Zhao, Q. Robust NiCoP/CoP heterostructures for highly efficient hydrogen evolution electrocatalysis in alkaline solution. ACS Appl. Mater. Inter. 2019, 11, 15528–15536. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Yanzhang, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B. When cubic cobalt sulfide meets layered molybdenum disulfide: A core–shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef]
- Heidelberg, R.F.; Luxem, A.H.; Talhouk, S.; Banewicz, J.J. The magnetic susceptibilities of the cobalt-sulfur system. Inorg. Chem. 1966, 5, 194–197. [Google Scholar] [CrossRef]
- Dutková, E.; Čaplovičová, M.; Škorvánek, I.; Baláž, M.; Zorkovská, A.; Baláž, P.; Čaplovič, Ľ. Structural, surface and magnetic properties of chalcogenide Co9S8 nanoparticles prepared by mechanochemical synthesis. Inorg. Chem. 2018, 745, 863–867. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Yao, H.B.; Wang, Y.; Liu, H.L.; Gao, M.R.; Shen, P.K.; Yu, S.H. Hierarchical hollow Co9S8 microspheres: Solvothermal synthesis, magnetic, electrochemical, and electrocatalytic properties. Chem. Eur. J. 2010, 16, 12000–12007. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lu, M.-Y.; Chen, L.-J. Metal sulfide nanostructures: Synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 2012, 22, 19–30. [Google Scholar] [CrossRef]
- Li, H.; Qian, X.; Xu, C.; Huang, S.; Zhu, C.; Jiang, X.; Shao, L.; Hou, L. Hierarchical porous Co9S8/nitrogen-doped carbon@MoS2 polyhedrons as pH universal electrocatalysts for highly efficient hydrogen evolution reaction. ACS Appl. Mater. Inter. 2017, 9, 28394–28405. [Google Scholar] [CrossRef]
- Dong, D.; Wu, Z.; Wang, J.; Fu, G.; Tang, Y. Recent progress in Co9S8 -based materials for hydrogen and oxygen electrocatalysis. J. Mater. Chem. A 2019, 7, 16068–16088. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Nurpeissova, A.; Adylkhanova, A.; Adair, D.; Taniguchi, I.; Bakenov, Z. Morphology and dimension variations of copper sulfide for high-performance electrode in rechargeable batteries: A review. ACS Appl. Enegr. Mater. 2020, 3, 11480–11499. [Google Scholar] [CrossRef]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Feng, L.-L.; Li, G.-D.; Liu, Y.; Wu, Y.; Chen, H.; Wang, Y.; Zou, Y.-C.; Wang, D.; Zou, X. Carbon-armored Co9S8 nanoparticles as all-pH efficient and durable H2-evolving electrocatalysts. ACS Appl. Mater. Inter. 2015, 7, 980–988. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Cheng, S.; Pan, Q.; Yao, M.; Zhu, Y.; Wu, P.; Luo, H.; Yang, L.; Liu, M. Template synthesis of carbon-coated Co9S8 composite with largely improved capacity for lithium ion batteries. Mater. Res. Lett. 2018, 217, 163–166. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhao, Y.; Dong, Y.; Fan, Q.; Kuang, Q. A promising mechanical ball-milling method to synthesize carbon-coated Co9S8 nanoparticles as high-performance electrode for supercapacitor. J. Mater. Sci. 2017, 52, 13552–13560. [Google Scholar] [CrossRef]
- Lu, M.; Liao, C.; Jiang, C.; Du, Y.; Zhang, Z.; Wu, S. Remarkable high-temperature performance of hollow Co9S8 nanoparticles integrated with carbon materials for lithium-ion batteries. Electrochim. Acta 2017, 250, 196–202. [Google Scholar] [CrossRef]
- Zhang, S.; Li, D.; Chen, S.; Yang, X.; Zhao, X.; Zhao, Q.; Komarneni, S.; Yang, D. Highly stable supercapacitors with MOF-derived Co9S8/carbon electrodes for high rate electrochemical energy storage. J. Mater. Chem. A 2017, 5, 12453–12461. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Q.; Du, S.; Li, J.; Zhong, J.; Deng, X.; Liu, Y. Porous Co9S8/nitrogen, sulfur-doped carbon@ Mo2C dual catalyst for efficient water splitting. ACS Appl. Mater. Inter. 2018, 10, 22291–22302. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, R.; Lv, Y.-R.; Wei, Y.-L.; Zang, S.-Q. One-step MOF-derived Co/Co9S8 nanoparticles embedded in nitrogen, sulfur and oxygen ternary-doped porous carbon: An efficient electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 3203–3206. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, G.; Lv, J.; Ma, Y.; Wang, Y.; Hu, C.; Zhang, J.; Yang, J.; He, G.; Zhang, M. Co9S8/CoS@S, N co-doped porous carbon derived from MOFs as an efficient catalyst for the oxygen evolution reaction. J. Phys. Chem. Solids 2021, 148, 109696. [Google Scholar] [CrossRef]
- Yu, H.; Sun, X.; Tang, D.; Huang, Y.; Zhang, W.; Miao, S.; Qiao, Z.-A.; Wang, J.; Zhao, Z. Molten salt strategy to synthesize alkali metal-doped Co9S8 nanoparticles embedded, N, S co-doped mesoporous carbon as hydrogen evolution electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 6006–6014. [Google Scholar] [CrossRef]
- Yu, H.; Tang, D.; Huang, Y.; Zhang, W.; Sun, X.; Yang, X.; Qiao, Z.A.; Wang, J.; Zhao, Z. Nitrogen and Sulfur Co-Doped Mesoporous Carbon Embedded with Co9S8 Nanoparticles: Efficient Electrocatalysts for Hydrogen Evolution. Chempluschem 2019, 84, 1604–1609. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Miao, S.; Du, Y.; Huang, Y.; Tang, D.; Qiao, Z.-A.; Wang, J.; Zhao, Z. Synthesis of Co9S8 nanoparticle embedded, N, S Co-doped mesoporous carbon with salts as templates for electrocatalytic hydrogen evolution. Micropor. Mesopor. Mat 2020, 302, 110235. [Google Scholar] [CrossRef]
- Zhang, P.; Bin, D.; Wei, J.-S.; Niu, X.-Q.; Chen, X.-B.; Xia, Y.-Y.; Xiong, H.-M. Efficient oxygen electrocatalyst for Zn–air batteries: Carbon dots and Co9S8 nanoparticles in a N, S-codoped carbon matrix. ACS Appl. Mater. Inter. 2019, 11, 14085–14094. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Xie, Y.; Jiang, W.; Wang, X.; Jiang, Y.; Zhou, W.; Pan, K. In situ growth of Co9S8 nanocrystals on reduced graphene oxide for the enhanced catalytic performance of dye-sensitized solar cell. J. Alloys Compd. 2019, 803, 216–223. [Google Scholar] [CrossRef]
- Li, Y.; Cao, R.; Li, L.; Tang, X.; Chu, T.; Huang, B.; Yuan, K.; Chen, Y. Simultaneously integrating single atomic cobalt sites and Co9S8 nanoparticles into hollow carbon nanotubes as trifunctional electrocatalysts for Zn–air batteries to drive water splitting. Small 2020, 16, 1906735. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Teng, C.; Wu, Z.; Ye, Z.; Zhi, M.; Hong, Z. Co9S8 nanoparticle-decorated carbon nanofibers as high-performance supercapacitor electrodes. RSC Adv. 2018, 8, 27574–27579. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, L.; Xiao, L.; Zhang, L.; Wang, M.J.; Li, J.; Wei, Z. Co9S8@N, S-codoped carbon core–shell structured nanowires: Constructing a fluffy surface for high-density active sites. J. Mater. Chem. A 2018, 6, 14752–14760. [Google Scholar] [CrossRef]
- Dong, X.; Jiao, Y.; Yang, G.; Yan, H.; Wu, A.; Guo, D.; Wang, Y.; Tian, C.; Fu, H. One-dimensional CO9S8-V3S4 heterojunctions as bifunctional electrocatalysts for highly efficient overall water splitting. Sci. China Mater. 2021, 64, 1396–1407. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Yu, X.; Zhang, H.; Zhang, H.; Li, S.; Huang, F. Introducing oxygen vacancies for improving the electrochemical performance of Co9S8@NiCo-LDH nanotube arrays in flexible all-solid battery-capacitor hybrid supercapacitors. Energy 2022, 238, 121767. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, M.; Liu, Y.; Shen, H.; Lei, Y.; Shi, W. Hexagonal prism-like hierarchical Co9S8@Ni(OH)2 core–shell nanotubes on carbon fibers for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 22782–22789. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.; Zhou, R.; Li, R.; Qiu, L.; Yuan, D. Co9S8-Ni3S2 heterointerfaced nanotubes on Ni foam as highly efficient and flexible bifunctional electrodes for water splitting. Electrochim. Acta 2019, 299, 152–162. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Construction of hierarchical hollow Co9S8/ZnIn2S4 tubular heterostructures for highly efficient solar energy conversion and environmental remediation. Angew. Chem. 2020, 132, 8332–8338. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, K.; Wang, T.; Xu, X.; Zhao, Y.; Sun, Y.; Zhong, W.; Du, Y. Cobalt Sulfide Nanotubes (Co9S8) Decorated with Amorphous MoSx as Highly Efficient Hydrogen Evolution Electrocatalyst. ACS Appl. Nano Mater. 2018, 1, 1083–1093. [Google Scholar] [CrossRef]
- Du, X.; Ma, G.; Zhang, X. Mo-doped Co9S8 nanorod array as a high performance electrochemical water splitting catalyst in alkaline solution. Int. J. Hydrogen Energy 2019, 44, 27765–27771. [Google Scholar] [CrossRef]
- Du, X.; Huang, C.; Zhang, X. Surface modification of a Co9S8 nanorods with Ni(OH)2 on nickel foam for high water splitting performance. Int. J. Hydrogen Energy 2019, 44, 19953–19966. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, M.; Li, H.; Sun, G.; Ma, S. Controllable synthesis of ultrathin Co9S8 nanosheets as a highly efficient electrocatalyst for overall water splitting. Electrochim. Acta 2018, 281, 198–207. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Guo, H.; Xia, W.; Jiang, C.; Gao, B.; Wu, C.; Wang, T.; He, J. N-Doped porous carbon nanosheets decorated with graphitized carbon layer encapsulated Co9S8 nanoparticles: An efficient bifunctional electrocatalyst for the OER and ORR. Nanoscale 2019, 11, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Lu, Z.W.; Wang, Y.H.; Dai, Z.; Zhao, H.; Sun, G.Z.; Lan, W.; Pan, X.J.; Zhou, J.Y.; Xie, E.Q. Cooperative chemisorption of polysulfides via 2D hexagonal WS2-rimmed Co9S8 heterostructures for lithium–sulfur batteries. Chem. Eng. J. 2020, 392, 123734. [Google Scholar] [CrossRef]
- Xing, L.; Dong, Y.; Wu, X. Hierarchical Co3O4@Co9S8 nanowall structures assembled by many nanosheets for high performance asymmetric supercapacitors. RSC. Adv. 2018, 8, 28172–28178. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Shi, L.; Qian, R.; Wen, Z. Realizing the growth of nano-network Li2O2 film on defect-rich holey Co9S8 nanosheets for Li-O2 battery. Chem. Eng. J. 2020, 396, 125228. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Gao, J.; Han, G.; Hu, M.; Wu, X.; Cao, H.; Wang, X.; Li, B. Defect-rich (Co–CoS2)x@Co9S8 nanosheets derived from monomolecular precursor pyrolysis with excellent catalytic activity for hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 7977–7987. [Google Scholar] [CrossRef]
- Wang, X.; Su, D.; Xiao, Y.; Xu, S.; Fang, S.; Cao, S. Ultra-dispersed island-like Co9S8 nanoparticles composed of nanosheets in-situ grown on nitrogen-doped graphene for asymmetric supercapacitor. Electrochim. Acta 2019, 293, 419–425. [Google Scholar] [CrossRef]
- Li, N.; Sun, L.; Wang, K.; Xu, S.; Zhang, J.; Guo, X.; Liu, X. Sandwiched N-carbon@Co9S8@Graphene nanosheets as high capacity anode for both half and full lithium-ion batteries. J. Energy Chem. 2020, 51, 62–71. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, W.; Li, L.; Tian, J.; Xie, J.; Lei, F.; Cui, G.; Zhang, Y.; Tang, B. Nickel incorporated Co9S8 nanosheet arrays on carbon cloth boosting overall urea electrolysis. Electrochim. Acta 2020, 338, 135883. [Google Scholar] [CrossRef]
- Tang, Y.; Jing, F.; Xu, Z.; Zhang, F.; Mai, Y.; Wu, D. Highly crumpled hybrids of nitrogen/sulfur dual-doped graphene and Co9S8 nanoplates as efficient bifunctional oxygen electrocatalysts. ACS Appl. Mater. Inter. 2017, 9, 12340–12347. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Feng, S.; Du, D.; Engelhard, M.H.; Xiao, D.; Li, D.; Lin, Y. Two-dimensional N, S-codoped carbon/Co9S8 catalysts derived from Co(OH)2 nanosheets for oxygen reduction reaction. ACS Appl. Mater. Inter. 2017, 9, 36755–36761. [Google Scholar] [CrossRef]
- Illathvalappil, R.; Walko, P.S.; Kanheerampockil, F.; Bhat, S.K.; Devi, R.N.; Kurungot, S. Hierarchical Nanoflower Arrays of Co9S8-Ni3S2 on Nickel Foam: A Highly Efficient Binder-Free Electrocatalyst for Overall Water Splitting. Chem. Eur. J. 2020, 26, 7900–7911. [Google Scholar] [CrossRef]
- Xia, C.; Xue, C.; Bian, W.; Liu, J.; Wang, J.; Wei, Y.; Zhang, J. Hollow Co9S8/CdS nanocages as efficient photocatalysts for hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 2743–2751. [Google Scholar] [CrossRef]
- Qiu, B.; Zhu, Q.; Du, M.; Fan, L.; Xing, M.; Zhang, J. Efficient solar light harvesting CdS/Co9S8 hollow cubes for Z-scheme photocatalytic water splitting. Angew. Chem. Int. Ed. 2017, 56, 2684–2688. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, W.; Zhang, H.; Zhou, D. Facile synthesis of CoNi2S4/Co9S8 composites as advanced electrode materials for supercapacitors. Appl. Surf. Sci. 2017, 426, 1206–1212. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Li, Q.; Lu, W.; Ning, J.; Zhong, Y.; Zhang, Z.; Hu, Y. Molecule-assisted modulation of the high-valence Co3+ in 3D honeycomb-like CoxSy networks for high-performance solid-state asymmetric supercapacitors. Sci. China Mater. 2021, 64, 840–851. [Google Scholar] [CrossRef]
- Li, Y.-W.; Wu, Q.; Ma, R.-C.; Sun, X.-Q.; Li, D.-D.; Du, H.-M.; Ma, H.-Y.; Li, D.-C.; Wang, S.-N.; Dou, J.-M. A Co-MOF-derived Co9S8@NS-C electrocatalyst for efficient hydrogen evolution reaction. RSC Adv. 2021, 11, 5947–5957. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, W.; Chen, L.; Huang, Z.; Zhang, N.; Fu, C.; Kuang, Y. Co9S8 nanoparticles embedded in a N, S co-doped graphene-unzipped carbon nanotube composite as a high performance electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 1014–1021. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Zhang, X.; Wu, T.; Zhao, H.; Wang, G. Co9S8@N, P-doped porous carbon electrocatalyst using biomass-derived carbon nanodots as a precursor for overall water splitting in alkaline media. RSC Adv. 2017, 7, 19181–19188. [Google Scholar] [CrossRef]
- Brumme, T.; Calandra, M.; Mauri, F. First-principles theory of field-effect doping in transition-metal dichalcogenides: Structural properties, electronic structure, Hall coefficient, and electrical conductivity. Phys. Rev. B 2015, 91, 155436. [Google Scholar] [CrossRef]
- Liu, W.; Hu, E.; Jiang, H.; Xiang, Y.; Weng, Z.; Li, M.; Fan, Q.; Yu, X.; Altman, E.I.; Wang, H. A highly active and stable hydrogen evolution catalyst based on pyrite-structured cobalt phosphosulfide. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Xia, B.; Sun, C.; Liu, Y.; Liu, P.; Gao, D. Facile one-step synthesis of phosphorus-doped CoS2 as efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 259, 955–961. [Google Scholar] [CrossRef]
- Cabán-Acevedo, M.; Stone, M.L.; Schmidt, J.; Thomas, J.G.; Ding, Q.; Chang, H.-C.; Tsai, M.-L.; He, J.-H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Matem. 2015, 14, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Wu, T.; Yu, Y.; Wang, Z.; Zhou, Y.; Tang, Z.; Yue, Q. Rational design of phosphorus-doped cobalt sulfides electrocatalysts for hydrogen evolution. Nano Res. 2019, 12, 2960–2965. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Jiang, J.; Lin, W.; Zhu, S.; Sha, J.; Ma, L.; Zhao, N. In-situ synthesis of MoS2/Co9S8 heterostructure for efficient HER electrocatalyst. Mater. Lett. 2021, 292, 129621. [Google Scholar] [CrossRef]
- Su, P.; Liu, H.; Ma, Q.; Jin, Z. Mn0.05Cd0.95S decorated MOF-derived Co9S8 hollow polyhedron for efficient photocatalytic hydrogen evolution. Int. J. Energy Res. 2021, 45, 13040–13054. [Google Scholar] [CrossRef]
- Li, X.-l.; He, R.-b.; Dai, Y.-j.; Li, S.-s.; Xiao, N.; Wang, A.-x.; Gao, Y.-q.; Li, N.; Gao, J.-f.; Zhang, L.-h. Design and fabrication of Co9S8/Zn0.5Cd0.5S hollow nanocages with significantly enhanced photocatalytic hydrogen production activity. Chem. Eng. J. 2020, 400, 125474. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Liu, X.; Huo, P.; Yan, Y.; Wang, L.; Liao, G.; Liu, C. Interface engineering of Co9S8/CdIn2S4 ohmic junction for efficient photocatalytic H2 evolution under visible light. J. Colloid Interf. Sci. 2021, 600, 794–803. [Google Scholar] [CrossRef]
- Yendrapati, T.P.; Soumya, J.; Bojja, S.; Pal, U. Robust Co9S8@CdIn2S4 cage for efficient photocatalytic H2 evolution. J. Phys. Chem. C 2021, 125, 5099–5109. [Google Scholar] [CrossRef]
- Liang, D.; Mao, J.; Liu, P.; Yan, J.; Song, W. In-situ growth of NCNT and encapsulation of Co9S8/Co as a sustainable multifunctional electrocatalyst. J. Colloid Interf. Sci. 2019, 557, 291–300. [Google Scholar] [CrossRef]
- Si, F.; Tang, C.; Gao, Q.; Peng, F.; Zhang, S.; Fang, Y.; Yang, S. Bifunctional CdS@Co9S8/Ni3S2 catalyst for efficient electrocatalytic and photo-assisted electrocatalytic overall water splitting. J. Mater. Chem. A 2020, 8, 3083–3096. [Google Scholar] [CrossRef]
- Bai, J.; Meng, T.; Guo, D.; Wang, S.; Mao, B.; Cao, M. Co9S8@MoS2 core-shell heterostructures as trifunctional electrocatalysts for overall water splitting and Zn-air batteries. ACS Appl. Mater. Inter. 2018, 10, 1678–1689. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Cui, M.; Xiong, J.; Mi, L.; Chen, S. Heterostructured MoO2@MoS2@Co9S8 nanorods as high efficiency bifunctional electrocatalyst for overall water splitting. Appl. Surf. Sci. 2021, 543, 148804. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, H.; Yu, D.; Zhang, S.; Chen, J.; Wang, J.; Wan, M.; Zhang, M.; Du, M. A facile strategy to synthesize cobalt-based self-supported material for electrocatalytic water splitting. Part. Part. Syst. Char. 2017, 34, 1700189. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, J.; Liu, J.; Zhang, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical amorphous carbon-coated Co/Co9S8 nanoparticles on MoS2 toward synergetic electrocatalytic water splitting. Ind. Eng. Chem. Res. 2019, 58, 23093–23098. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Liang, X. Co9S8 nanowires@NiCo LDH nanosheets arrays on nickel foams towards efficient overall water splitting. Sci. Bull. 2019, 64, 158–165. [Google Scholar] [CrossRef]
- Zang, Z.; Wang, X.; Li, X.; Zhao, Q.; Li, L.; Yang, X.; Yu, X.; Zhang, X.; Lu, Z. Co9S8 nanosheet coupled Cu2S nanorod heterostructure as efficient catalyst for overall water splitting. ACS Appl. Mater. Inter. 2021, 13, 9865–9874. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, B.; Wu, J.; Huang, K.; Cao, X.; Lu, Y.; Zhang, Y.; Li, C.; Huang, Y. Growth of Lattice Coherent Co9S8/Co3O4 Nano-Heterostructure for Maximizing the Catalysis of Co-Based Composites. Chemcatchem 2020, 12, 2431–2435. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Zang, Y.; Liu, R.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Co/Co9S8@S, N-doped porous graphene sheets derived from S, N dual organic ligands assembled Co-MOFs as superior electrocatalysts for full water splitting in alkaline media. Nano Energy 2016, 30, 93–102. [Google Scholar] [CrossRef]
- Kim, M.; Seok, H.; Selvam, N.C.S.; Cho, J.; Choi, G.H.; Nam, M.G.; Kang, S.; Kim, T.; Yoo, P.J. Kirkendall effect induced bifunctional hybrid electrocatalyst (Co9S8@MoS2/N-doped hollow carbon) for high performance overall water splitting. J. Power Source 2021, 493, 229688. [Google Scholar] [CrossRef]
- Chen, L.; Yang, W.; Li, X.; Han, L.; Wei, M. Co9S8 embedded into N/S doped carbon composites: In situ derivation from a sulfonate-based metal-organic framework and its electrochemical properties. J. Mater. Chem. A 2019, 7, 10331–10337. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Z.; Yang, K.; Mei, T.; Yan, D.; Wang, X. Design and synthesize hollow spindle Ni-doped Co9S8@ZnS composites and their enhanced cycle performance. J. Alloys Compd. 2021, 853, 157118. [Google Scholar] [CrossRef]
- Zhao, S.; Tian, X.; Zhou, Y.; Ma, B.; Natarajan, A. Three-dimensionally interconnected Co9S8/MWCNTs composite cathode host for lithium-sulfur batteries. J. Energy Chem. 2020, 46, 22–29. [Google Scholar] [CrossRef]
- He, Z.; Guo, H.; LaCoste, J.D.; Cook, R.A.; Hussey, B.; Zhang, X.; Gang, D.D.; Hao, J.; Chen, L.; Cooke, P. Directly embedded Ni3S2/Co9S8@S-doped carbon nanofiber networks as a free-standing anode for lithium-ion batteries. Sustain. Energy Fuels 2021, 5, 166–174. [Google Scholar] [CrossRef]
- Yin, M.; Zhao, D.; Feng, C.; Zhou, W.; Jiao, Q.; Feng, X.; Wang, S.; Zhao, Y.; Li, H.; Feng, T. Construction of porous Co9S8 hollow boxes with double open ends toward high-performance half/full sodium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 6305–6314. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Sun, F.; Yu, X.; Kang, W.; Zhang, J. One-step synthesis of Co9S8@Ni3S2 heterostructure for enhanced electrochemical performance as sodium ion battery anode material and hydrogen evolution electrocatalyst. J. Solid State Chem. 2020, 285, 121230. [Google Scholar] [CrossRef]
- Yin, M.; Feng, X.; Zhao, D.; Zhao, Y.; Li, H.; Zhou, W.; Liu, H.; Bai, X.; Wang, H.; Feng, C. Hierarchical Co9S8@carbon hollow microspheres as an anode for sodium ion batteries with ultralong cycling stability. ACS Sustain. Chem. Eng. 2019, 7, 6122–6130. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Zhu, S.; Fan, K.; Lyu, L.; Yao, H.; Li, Y.; Hu, J.; Huang, H.; Mai, Y.W. Fiber-in-Tube Design of Co9S8-Carbon/Co9S8: Enabling Efficient Sodium Storage. Angew. Chem. 2019, 131, 6305–6309. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.-x.; Feng, X.; Chen, W.; Qian, J.; Ai, X.; Yang, H.; Cao, Y. In situ formation of Co9S8 nanoclusters in sulfur-doped carbon foam as a sustainable and high-rate sodium-ion anode. ACS Appl. Mater. Inter. 2019, 11, 19218–19226. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Yang, X.; Zhao, Z.; Yang, H.; Feng, M.; Zhang, W.; Zheng, W. Synergistic dual-confinement effect: Merit of hollowly metallic Co9S8 in packaging enhancement of electrochemical performance of Li-S batteries. ACS Appl. Energy Mater. 2019, 2, 1428–1435. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.; Huang, M.; Shang, H.; Jiang, J.; Qiao, Y.; Liu, W.; Zhou, H.; Li, T.; Zhou, X. MOF-derived Co9S8/C hollow polyhedra grown on 3D graphene aerogel as efficient polysulfide mediator for long-life Li-S batteries. Mater. Lett. 2020, 277, 128331. [Google Scholar] [CrossRef]
- Chen, T.; Ma, L.; Cheng, B.; Chen, R.; Hu, Y.; Zhu, G.; Wang, Y.; Liang, J.; Tie, Z.; Liu, J. Metallic and polar Co9S8 inlaid carbon hollow nanopolyhedra as efficient polysulfide mediator for lithium-sulfur batteries. Nano Energy 2017, 38, 239–248. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Ren, Y.; Zeng, W. N-doped graphene embellished with Co9S8 enable advanced sulfur cathode for high-performance lithium-sulfur batteries. Int. J. Energy Res. 2020, 44, 4961–4968. [Google Scholar] [CrossRef]
- Li, H.-J.; Song, Y.-H.; Xi, K.; Wang, W.; Liu, S.; Li, G.-R.; Gao, X.-P. Sulfur vacancies in Co9S8−x/N-doped graphene enhancing the electrochemical kinetics for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 10704–10713. [Google Scholar] [CrossRef]
- Huang, L.; Zuo, L.; Yu, T.; Wang, H.; He, Z.; Zhou, H.; Su, S.; Bian, T. Two-dimensional Co/Co9S8 nanoparticles decorated N, S dual-doped carbon composite as an efficient electrocatalyst for zinc-air battery. J. Alloys Compd. 2022, 897, 163108. [Google Scholar] [CrossRef]
- Peng, W.; Wang, Y.; Yang, X.; Mao, L.; Jin, J.; Yang, S.; Fu, K.; Li, G. Co9S8 nanoparticles embedded in multiple doped and electrospun hollow carbon nanofibers as bifunctional oxygen electrocatalysts for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 268, 118437. [Google Scholar] [CrossRef]
- Zheng, Q.; Xiong, Y.; Tang, K.; Wu, M.; Hu, H.; Zhou, T.; Wu, Y.; Cao, Z.; Sun, J.; Yu, X. Modulation of pore-size in N, S-codoped carbon/Co9S8 hybrid for a stronger O2 affinity toward rechargable zinc-air battery. Nano Energy 2022, 92, 106750. [Google Scholar] [CrossRef]
- Cao, Z.-Q.; Wu, M.-Z.; Hu, H.-B.; Liang, G.-J.; Zhi, C.-Y. Monodisperse Co9S8 nanoparticles in situ embedded within N, S-codoped honeycomb-structured porous carbon for bifunctional oxygen electrocatalyst in a rechargeable Zn–air battery. NPG Asia Mater. 2018, 10, 670–684. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- Ito, Y.; Christodoulou, C.; Nardi, M.V.; Koch, N.; Kläui, M.; Sachdev, H.; Müllen, K. Tuning the magnetic properties of carbon by nitrogen doping of its graphene domains. J. Am. Chem. Soc. 2015, 137, 7678–7685. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lin, D.; Feng, H.; Zeng, Y.; Tong, Y.; Lu, X. Boosting the energy density of carbon-based aqueous supercapacitors by optimizing the surface charge. Angew. Chem. 2017, 129, 5546–5551. [Google Scholar] [CrossRef]
- Sun, S.; Luo, J.; Qian, Y.; Jin, Y.; Liu, Y.; Qiu, Y.; Li, X.; Fang, C.; Han, J.; Huang, Y. Metal–Organic Framework Derived Honeycomb Co9S8@C Composites for High-Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1801080. [Google Scholar] [CrossRef]
- Liu, S.; Tong, M.; Liu, G.; Zhang, X.; Wang, Z.; Wang, G.; Cai, W.; Zhang, H.; Zhao, H. S, N-containing Co-MOF derived Co9S8@S, N-doped carbon materials as efficient oxygen electrocatalysts and supercapacitor electrode materials. Inorg. Chem. Front. 2017, 4, 491–498. [Google Scholar] [CrossRef]
- Goda, E.S.; ur Rehman, A.; Pandit, B.; Eissa, A.A.-S.; Hong, S.E.; Yoon, K.R. Al-doped Co9S8 encapsulated by nitrogen-doped graphene for solid-state asymmetric supercapacitors. Chem. Eng. J. 2022, 428, 132470. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Z.; Gong, X.; Jiang, C.; Zhang, C.; Xu, J.; Li, Y.; Bao, J.; Cui, Y.; Wang, H. Enhancing stability of Co9S8 by iron incorporation for oxygen evolution reaction and supercapacitor electrodes. Chem. Eng. J. 2022, 431, 133980. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).