Decoding Breast Cancer Metabolism: Hunting BRCA Mutations by Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Spectroscopy
2.3. Statistical Analysis
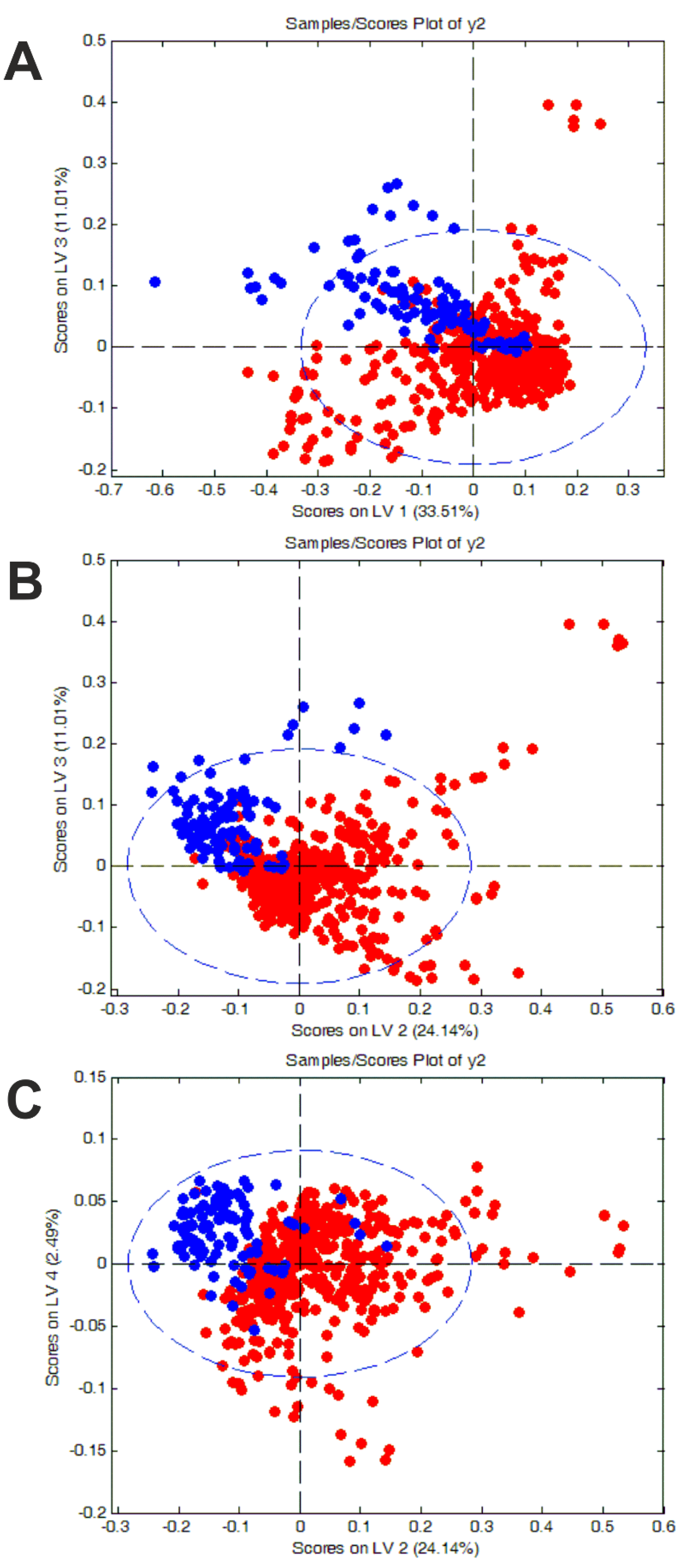
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, D.; Kulkarni, R. Overview of blood components and their preparation. Indian J. Anaesth. 2014, 58, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Jelkmann, W. Functions of the Blood. In Human Physiology; Schmidt, R.F., Thews, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 402–438. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, M.; Enawgaw, B.; Melku, M. Human blood platelets and viruses: Defense mechanism and role in the removal of viral pathogens. Thromb. J. 2018, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.S.; Mahmud, H.; Korytkowski, M.T. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes Mellitus. Endocrinol. Metab. Clin. North Am. 2018, 47, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yu, M.; Kiviat, N.B. Molecular Biomarkers for Cancer Detection in Blood and Bodily Fluids. Crit. Rev. Clin. Lab. Sci. 2006, 43, 497–560. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- El-Khoury, V.; Schritz, A.; Kim, S.-Y.; Lesur, A.; Sertamo, K.; Bernardin, F.; Petritis, K.; Pirrotte, P.; Selinsky, C.; Whiteaker, J.R.; et al. Identification of a Blood-Based Protein Biomarker Panel for Lung Cancer Detection. Cancers 2020, 12, 1629. [Google Scholar] [CrossRef]
- Berghuis, A.M.S.; Koffijberg, H.; Prakash, J.; Terstappen, L.W.M.M.; Ijzerman, M.J. Detecting Blood-Based Biomarkers in Metastatic Breast Cancer: A Systematic Review of Their Current Status and Clinical Utility. Int. J. Mol. Sci. 2017, 18, 363. [Google Scholar] [CrossRef]
- Kazarian, A.; Blyuss, O.; Metodieva, G.; Gentry-Maharaj, A.; Ryan, A.; Kiseleva, E.M.; Prytomanova, O.M.; Jacobs, I.J.; Widschwendter, M.; Menon, U.; et al. Testing breast cancer serum biomarkers for early detection and prognosis in pre-diagnosis samples. Br. J. Cancer 2017, 116, 501–508. [Google Scholar] [CrossRef]
- Hampel, H.; O’Bryant, S.E.; Molinuevo, J.L.; Zetterberg, H.; Masters, C.L.; Lista, S.; Kiddle, S.J.; Batrla, R.; Blennow, K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018, 14, 639–652. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Atchley, D.P.; Albarracin, C.T.; Lopez, A.; Valero, V.; Amos, C.I.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Arun, B.K. Clinical and Pathologic Characteristics of Patients with BRCA-Positive and BRCA-Negative Breast Cancer. J. Clin. Oncol. 2008, 26, 4282–4288. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T.; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 652–665. [Google Scholar] [CrossRef]
- Narod, S.A. BRCA mutations in the management of breast cancer: The state of the art. Nat. Rev. Clin. Oncol. 2010, 7, 702–707. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation–Positive Women with Ovarian Cancer: A Report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Hedau, S.; Batra, M.; Singh, U.R.; Bharti, A.C.; Ray, A.; Das, B.C. Expression of BRCA1 and BRCA2 proteins and their correlation with clinical staging in breast cancer. J. Cancer Res. Ther. 2015, 11, 158–163. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sheikh, S.E.; Kandil, M.A.; El-Sayed, M.E.; Green, A.R.; Ellis, I.O. Expression of BRCA1 protein in breast cancer and its prognostic significance. Hum. Pathol. 2008, 39, 857–865. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhang, Y.; Deng, Q.; Liang, H. Expression and mutations of BRCA in breast cancer and ovarian cancer: Evidence from bioinformatics analyses. Int. J. Mol. Med. 2018, 42, 3542–3550. [Google Scholar] [CrossRef]
- Al-Mulla, F.; Abdulrahman, M.; Varadharaj, G.; Akhter, N.; Anim, J.T. BRCA1 Gene Expression in Breast Cancer: A Correlative Study between Real-time RT-PCR and Immunohistochemistry. J. Histochem. Cytochem. 2005, 53, 621–629. [Google Scholar] [CrossRef]
- Andres, J.L.; Fan, S.; Turkel, G.J.; Wang, J.-A.; Twu, N.-F.; Yuan, R.-Q.; Lamszus, K.; Goldberg, I.D.; Rosen, E.M. Regulation of BRCA1 and BRCA2 expression in human breast cancer cells by DNA-damaging agents. Oncogene 1998, 16, 2229–2241. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Wu, J.; Zhang, Z.; Tang, Y.; Li, X.; Liu, S.; Cao, S.; Li, X. Association Between BRCA Status and Triple-Negative Breast Cancer: A Meta-Analysis. Front. Pharmacol. 2018, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Welcsh, P.L. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001, 10, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Stoppa-Lyonnet, D.; Fleischmann, E.; Muhr, D.; Pagès, S.; Sandberg, T.; Caux, V.; Moeslinger, R.; Langbauer, G.; Borg, A.; et al. Denaturing High-Performance Liquid Chromatography Detects Reliably BRCA1 and BRCA2 Mutations. Genomics 1999, 62, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Peer, C.J.; Mannargudi, B.; Swaisland, H.; Lee, J.-M.; Kohn, E.C.; Figg, W.D. A Sensitive and Robust Ultra HPLC Assay with Tandem Mass Spectrometric Detection for the Quantitation of the PARP Inhibitor Olaparib (AZD2281) in Human Plasma for Pharmacokinetic Application. Chromatography 2014, 1, 82–95. [Google Scholar] [CrossRef]
- Gross, E.; Arnold, N.; Goette, J.; Schwarz-Boeger, U.; Kiechle, M. A comparison of BRCA1 mutation analysis by direct sequencing, SSCP and DHPLC. Hum Genet. 1999, 105, 72–78. [Google Scholar] [CrossRef]
- D’Argenio, V.; Esposito, M.V.; Telese, A.; Precone, V.; Starnone, F.; Nunziato, M.; Cantiello, P.; Iorio, M.; Evangelista, E.; D’Aiuto, M.; et al. The molecular analysis of BRCA1 and BRCA2: Next-generation sequencing supersedes conventional approaches. Clin. Chim. Acta 2015, 446, 221–225. [Google Scholar] [CrossRef]
- Wallace, A.J. New challenges for BRCA testing: A view from the diagnostic laboratory. Eur. J. Hum. Genet. 2016, 24 (Suppl. S1), S10–S18. [Google Scholar] [CrossRef]
- Szczerba, E.; Kamińska, K.; Mierzwa, T.; Misiek, M.; Kowalewski, J.; Lewandowska, M.A. BRCA1/2 Mutation Detection in the Tumor Tissue from Selected Polish Patients with Breast Cancer Using Next Generation Sequencing. Genes 2021, 12, 519. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child.-Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Jones, M.A.; Rhodenizer, D.; da Silva, C.; Huff, I.J.; Keong, L.; Bean, L.J.; Coffee, B.; Collins, C.; Tanner, A.K.; He, M.; et al. Molecular diagnostic testing for congenital disorders of glycosylation (CDG): Detection rate for single gene testing and next generation sequencing panel testing. Mol. Genet. Metab. 2013, 110, 78–85. [Google Scholar] [CrossRef]
- Dahui, Q. Next-generation sequencing and its clinical application. Cancer Biol. Med. 2019, 16, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Konstantinos, K.V.; Panagiotis, P.; Antonios, V.T.; Agelos, P.; Argiris, N.V. PCR–SSCP: A Method for the Molecular Analysis of Genetic Diseases. Mol. Biotechnol. 2008, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.R.; Eccles, D.M.; Rahman, N.; Young, K.; Bulman, M.; Amir, E.; Shenton, A.; Howell, A.; Lalloo, F. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J. Med Genet. 2004, 41, 474–480. [Google Scholar] [CrossRef]
- Mahdieh, N.; Rabbani, B. An Overview of Mutation Detection Methods in Genetic Disorders. Iran. J. Pediatr. 2013, 23, 375–388. [Google Scholar] [PubMed]
- Xue, Y.; Ankala, A.; Wilcox, W.R.; Hegde, M.R. Solving the molecular diagnostic testing conundrum for Mendelian disorders in the era of next-generation sequencing: Single-gene, gene panel, or exome/genome sequencing. Genet. Med. 2015, 17, 444–451. [Google Scholar] [CrossRef]
- Shashi, V.; McConkie-Rosell, A.; Rosell, B.; Schoch, K.; Vellore, K.; McDonald, M.; Jiang, Y.-H.; Xie, P.; Need, A.; Goldstein, D.B. The utility of the traditional medical genetics diagnostic evaluation in the context of next-generation sequencing for undiagnosed genetic disorders. Genet. Med. 2014, 16, 176–182. [Google Scholar] [CrossRef]
- Crossley, B.M.; Bai, J.; Glaser, A.; Maes, R.; Porter, E.; Killian, M.L.; Clement, T.; Toohey-Kurth, K. Guidelines for Sanger sequencing and molecular assay monitoring. J. Veter-Diagn. Investig. 2020, 32, 767–775. [Google Scholar] [CrossRef]
- Sikkema-Raddatz, B.; Johansson, L.F.; de Boer, E.N.; Almomani, R.; Boven, L.G.; van den Berg, M.P.; van Spaendonck-Zwarts, K.Y.; van Tintelen, J.P.; Sijmons, R.H.; Jongbloed, J.D.H.; et al. Targeted Next-Generation Sequencing can Replace Sanger Sequencing in Clinical Diagnostics. Hum. Mutat. 2013, 34, 1035–1042. [Google Scholar] [CrossRef]
- Kluska, A.; Balabas, A.; Paziewska, A.; Kulecka, M.; Nowakowska, D.; Mikula, M.; Ostrowski, J. New recurrent BRCA1/2 mutations in Polish patients with familial breast/ovarian cancer detected by next generation sequencing. BMC Med Genom. 2015, 8, 19. [Google Scholar] [CrossRef]
- Tuffaha, H.W.; Mitchell, A.; Ward, R.L.; Connelly, L.; Butler, J.R.; Norris, S.; Scuffham, P.A. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet. Med. 2018, 20, 985–994. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Hömig-Hölzel, C.; Savola, S. Multiplex Ligation-dependent Probe Amplification (MLPA) in Tumor Diagnostics and Prognostics. Diagn. Mol. Pathol. 2012, 21, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Bozsik, A.; Pócza, T.; Papp, J.; Vaszkó, T.; Butz, H.; Patócs, A.; Oláh, E. Complex Characterization of Germline Large Genomic Rearrangements of the BRCA1 and BRCA2 Genes in High-Risk Breast Cancer Patients—Novel Variants from a Large National Center. Int. J. Mol. Sci. 2020, 21, 4650. [Google Scholar] [CrossRef] [PubMed]
- Engert, S.; Wappenschmidt, B.; Betz, B.; Kast, K.; Kutsche, M.; Hellebrand, H.; Goecke, T.O.; Kiechle, M.; Niederacher, D.; Schmutzler, R.K.; et al. MLPA screening in theBRCA1gene from 1506 German hereditary breast cancer cases: Novel deletions, frequent involvement of exon 17, and occurrence in single early-onset cases. Hum. Mutat. 2008, 29, 948–958. [Google Scholar] [CrossRef]
- Durmaz, A.A.; Karaca, E.; Demkow, U.; Toruner, G.; Schoumans, J.; Cogulu, O. Evolution of Genetic Techniques: Past, Present, and Beyond. BioMed Res. Int. 2015, 2015, 461524. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopeć, M. Double face of cytochrome c in cancers by Raman imaging. Sci. Rep. 2022, 12, 2120. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Jarota, A.; Surmacki, J.; Imiela, A.; Kopec, M. A look into the use of Raman spectroscopy for brain and breast cancer diagnostics: Linear and non-linear optics in cancer research as a gateway to tumor cell identity. Expert Rev. Mol. Diagn. 2020, 20, 99–115. [Google Scholar] [CrossRef]
- Nargis, H.F.; Nawaz, H.; Ditta, A.; Mahmood, T.; Majeed, M.I.; Rashid, N.; Muddassar, M.; Bhatti, H.N.; Saleem, M.; Jilani, K.; et al. Raman spectroscopy of blood plasma samples from breast cancer patients at different stages. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117210. [Google Scholar] [CrossRef]
- Giamougiannis, P.; Silva, R.V.O.; Freitas, D.L.D.; Lima, K.M.G.; Anagnostopoulos, A.; Angelopoulos, G.; Naik, R.; Wood, N.J.; Martin-Hirsch, P.L.; Martin, F.L. Raman spectroscopy of blood and urine liquid biopsies for ovarian cancer diagnosis: Identification of chemotherapy effects. J. Biophotonics 2021, 14, e202100195. [Google Scholar] [CrossRef]
- Giamougiannis, P.; Morais, C.L.M.; Grabowska, R.; Ashton, K.M.; Wood, N.J.; Martin-Hirsch, P.L.; Martin, F.L. A comparative analysis of different biofluids towards ovarian cancer diagnosis using Raman microspectroscopy. Anal. Bioanal. Chem. 2021, 413, 911–922. [Google Scholar] [CrossRef]
- Allain, L.R.; Vo-Dinh, T. Surface-enhanced Raman scattering detection of the breast cancer susceptibility gene BRCA1 using a silver-coated microarray platform. Anal. Chim. Acta 2002, 469, 149–154. [Google Scholar] [CrossRef]
- Culha, M.; Stokes, D.; Allain, L.R.; Vo-Dinh, T. Surface-Enhanced Raman Scattering Substrate Based on a Self-Assembled Monolayer for Use in Gene Diagnostics. Anal. Chem. 2003, 75, 6196–6201. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Isola, N.R.; Alarie, J.P.; Stokes, D.L.; Vo-Dinh, T. Synthesis and characterization of SERS gene probe for BRCA-1 (breast cancer). Faraday Discuss. 2006, 132, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Allain, L.R.; Stokes, D.L. Cancer gene detection using surface-enhanced Raman scattering (SERS). J. Raman Spectrosc. 2002, 33, 511–516. [Google Scholar] [CrossRef]
- Coluccio, M.L.; Gentile, F.; Das, G.; Nicastri, A.; Perri, A.M.; Candeloro, P.; Perozziello, G.; Zaccaria, R.P.; Gongora, J.S.T.; Alrasheed, S.; et al. Detection of single amino acid mutation in human breast cancer by disordered plasmonic self-similar chain. Sci. Adv. 2015, 1, e1500487. [Google Scholar] [CrossRef]
- Surmacki, J.; Brozek-Pluska, B.; Kordek, R.; Abramczyk, H. The lipid-reactive oxygen species phenotype of breast cancer. Raman spectroscopy and mapping, PCA and PLSDA for invasive ductal carcinoma and invasive lobular carcinoma. Molecular tumorigenic mechanisms beyond Warburg effect. Analyst 2015, 140, 2121–2133. [Google Scholar] [CrossRef]
- Brozek-Pluska, B. Statistics assisted analysis of Raman spectra and imaging of human colon cell lines—Label free, spectroscopic diagnostics of colorectal cancer. J. Mol. Struct. 2020, 1218, 128524. [Google Scholar] [CrossRef]
- Beton, K.; Wysocki, P.; Brozek-Pluska, B. Mevastatin in colon cancer by spectroscopic and microscopic methods—Raman imaging and AFM studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120726. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Kopec, M.; Błaszczyk, M.; Radek, M.; Abramczyk, H. Raman imaging and statistical methods for analysis various type of human brain tumors and breast cancers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120091. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B. Raman Imaging in Biochemical and Biomedical Applications. Diagnosis and Treatment of Breast Cancer. Chem. Rev. 2013, 113, 5766–5781. [Google Scholar] [CrossRef] [PubMed]
- Toland, A.E.; on behalf of the BIC Steering Committee; Forman, A.; Couch, F.J.; Culver, J.O.; Eccles, D.M.; Foulkes, W.D.; Hogervorst, F.B.L.; Houdayer, C.; Levy-Lahad, E.; et al. Clinical testing of BRCA1 and BRCA2: A worldwide snapshot of technological practices. NPJ Genom. Med. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.H.; Machado, P.M.; Brandão, R.D.; Laranjeira, C.T.; Eugénio, J.S.; Fernandes, A.H.; André, S.P. Familial Breast/Ovarian Cancer and BRCA1/2 Genetic Screening: The Role of Immunohistochemistry as an Additional Method in the Selection of Patients. J. Histochem. Cytochem. 2007, 55, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
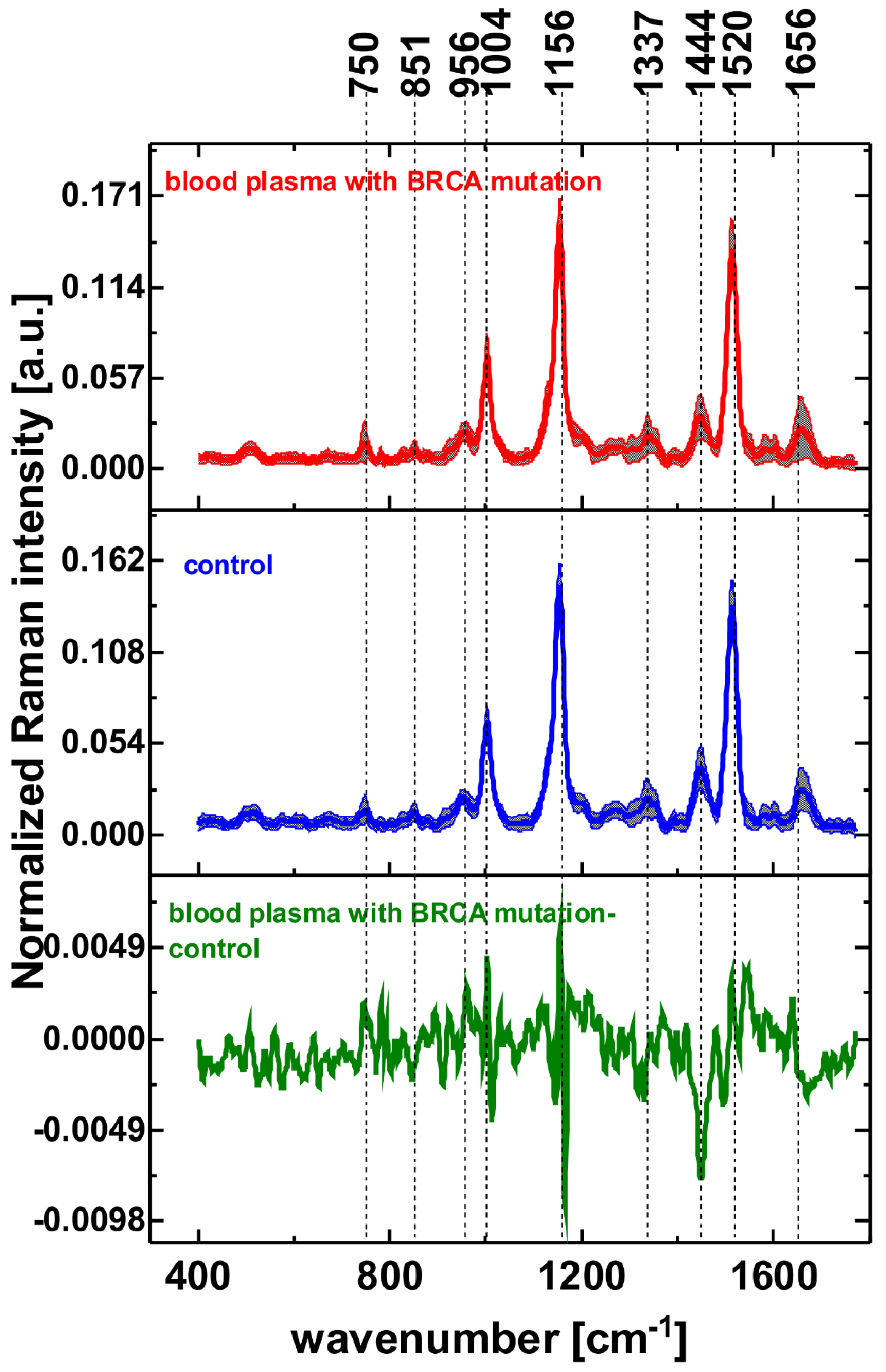

| Wavenumber [cm−1] | Tentative Assignments |
|---|---|
| 750 | tryptophan, cytochrome c, hemoglobine |
| 851 | tyrosine |
| 956 | hydroxyproline/collagen backbone |
| 1004 | phenylalanine |
| 1156 | carotenoids |
| 1337 | cytochrome b |
| 1444 | fatty acids, triglycerides |
| 1520 | carotenoids |
| 1656 | amide I, lipids |
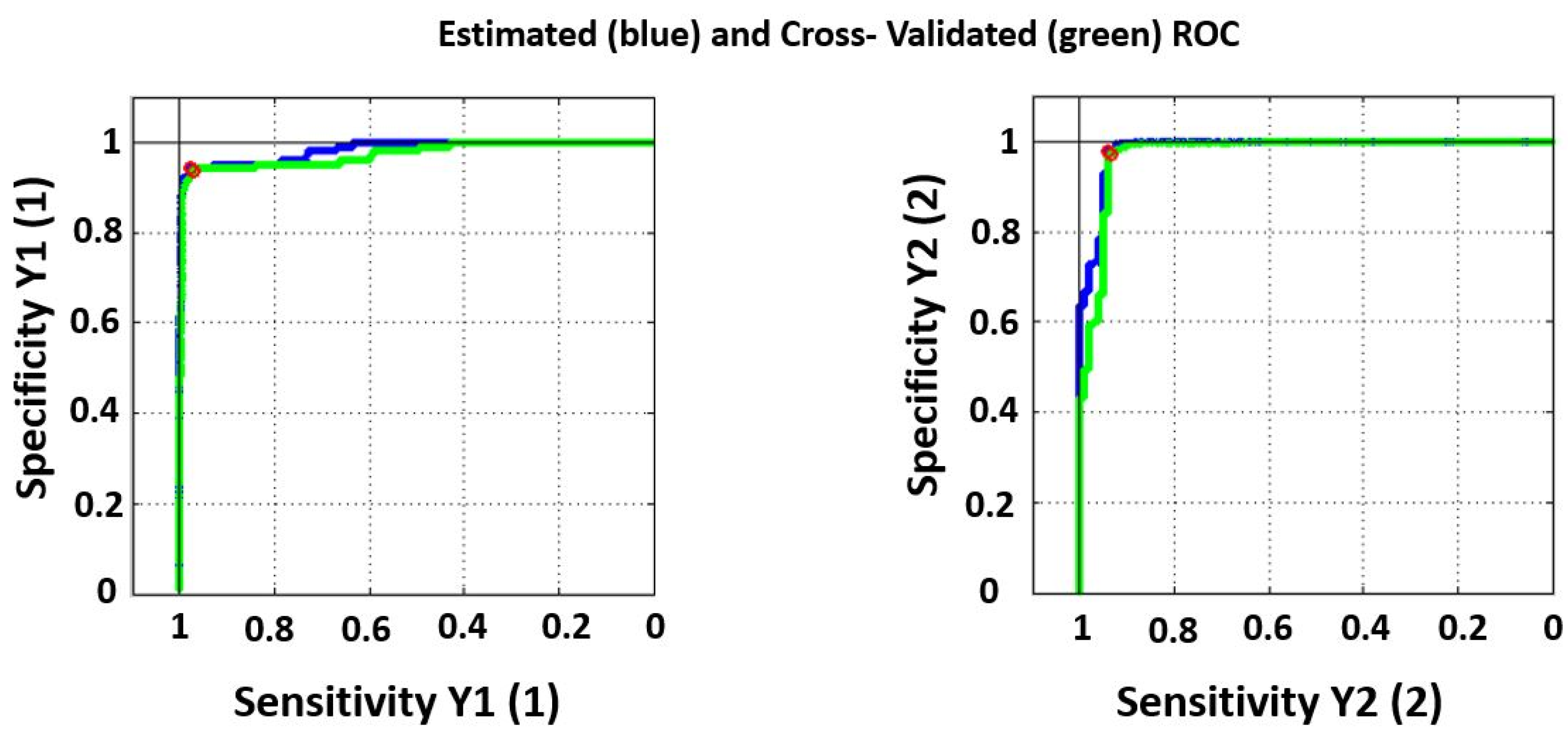
| Healthy Blood Plasma | Blood Plasma with BRCA Mutation | |
|---|---|---|
| Sensitivity (calibration) | 0.942 | 0.976 |
| Specificity (calibration) | 0.976 | 0.942 |
| Sensitivity (cross validation) | 0.933 | 0.970 |
| Specificity (cross validation) | 0.970 | 0.933 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopec, M.; Romanowska-Pietrasiak, B.; Abramczyk, H. Decoding Breast Cancer Metabolism: Hunting BRCA Mutations by Raman Spectroscopy. Photochem 2022, 2, 752-764. https://doi.org/10.3390/photochem2030048
Kopec M, Romanowska-Pietrasiak B, Abramczyk H. Decoding Breast Cancer Metabolism: Hunting BRCA Mutations by Raman Spectroscopy. Photochem. 2022; 2(3):752-764. https://doi.org/10.3390/photochem2030048
Chicago/Turabian StyleKopec, Monika, Beata Romanowska-Pietrasiak, and Halina Abramczyk. 2022. "Decoding Breast Cancer Metabolism: Hunting BRCA Mutations by Raman Spectroscopy" Photochem 2, no. 3: 752-764. https://doi.org/10.3390/photochem2030048
APA StyleKopec, M., Romanowska-Pietrasiak, B., & Abramczyk, H. (2022). Decoding Breast Cancer Metabolism: Hunting BRCA Mutations by Raman Spectroscopy. Photochem, 2(3), 752-764. https://doi.org/10.3390/photochem2030048







