Photooxidation of 2,2′-(Ethyne-1,2-diyl)dianilines: An Enhanced Photocatalytic Properties of New Salophen-Based Zn(II) Complexes
Abstract
:1. Introduction
2. Materials and Methods
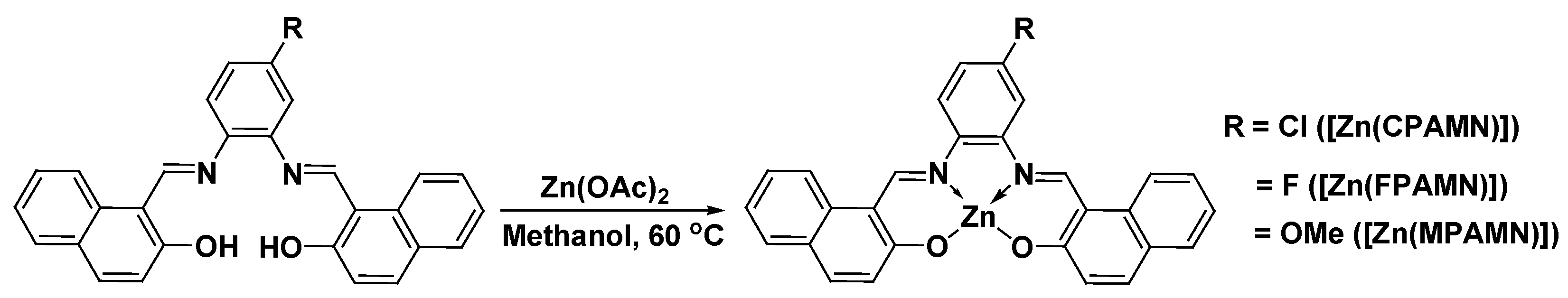
2.1. Synthesis of N2, O2—Donor-Based Schiff’s Base Ligands
2.2. Synthesis of Zn(II) Complexes
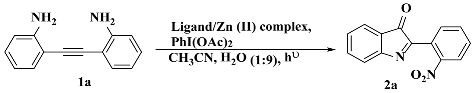
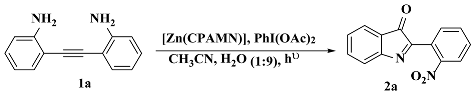
3. Results and Discussion
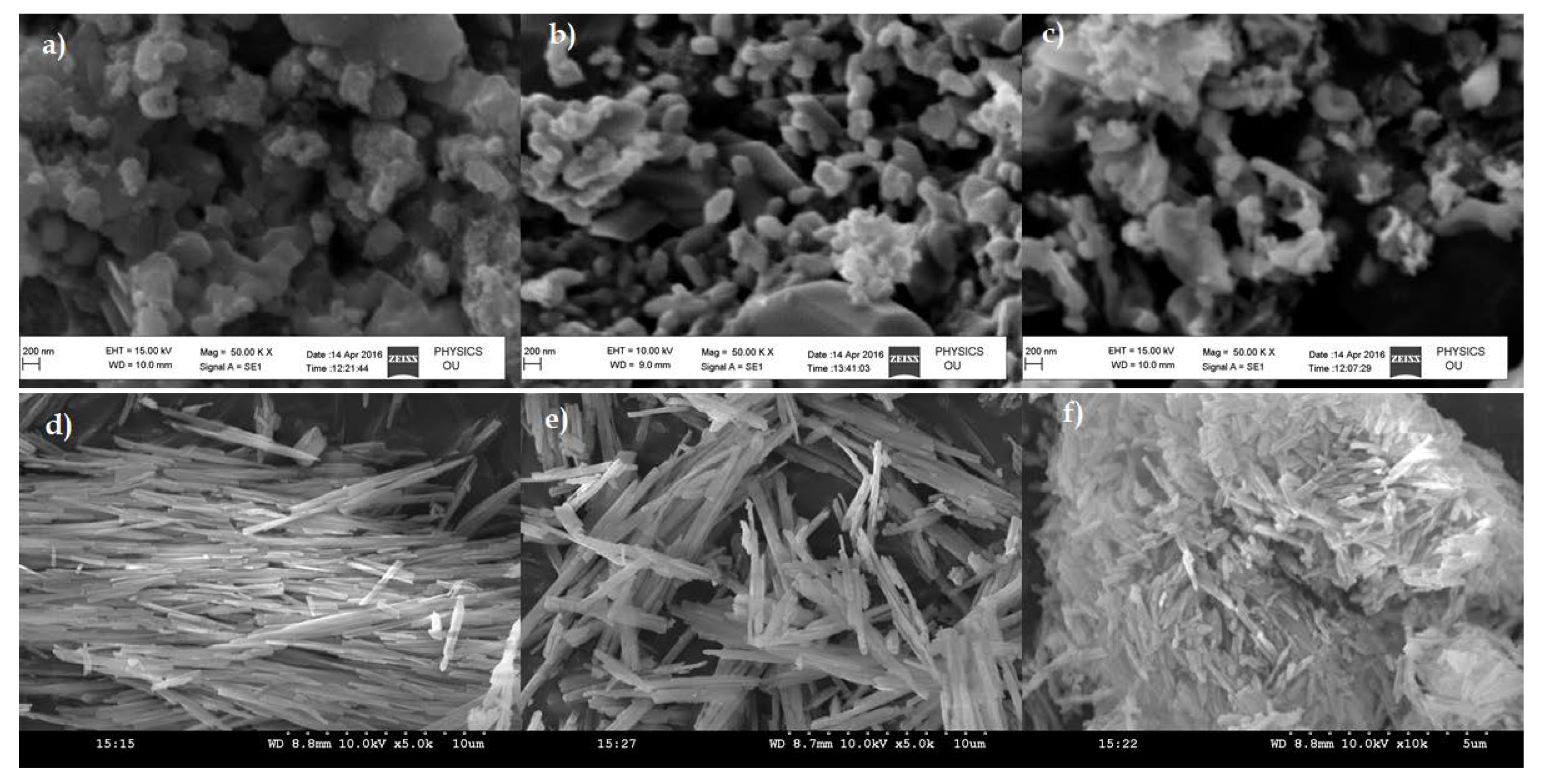
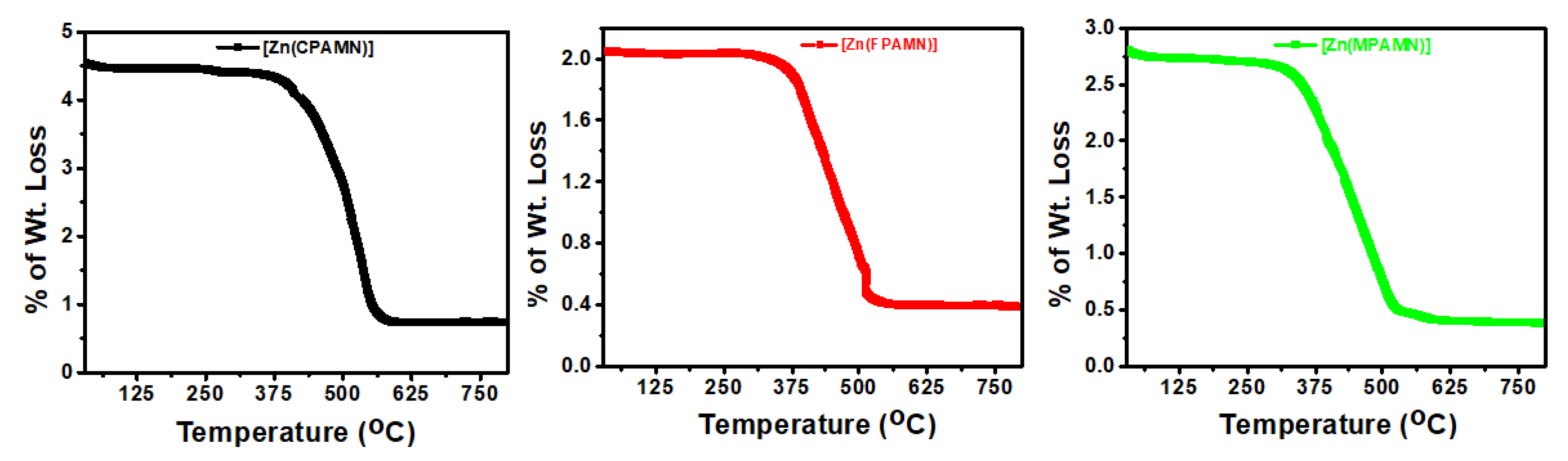
3.1. Molar Conductance and Thermal Analysis
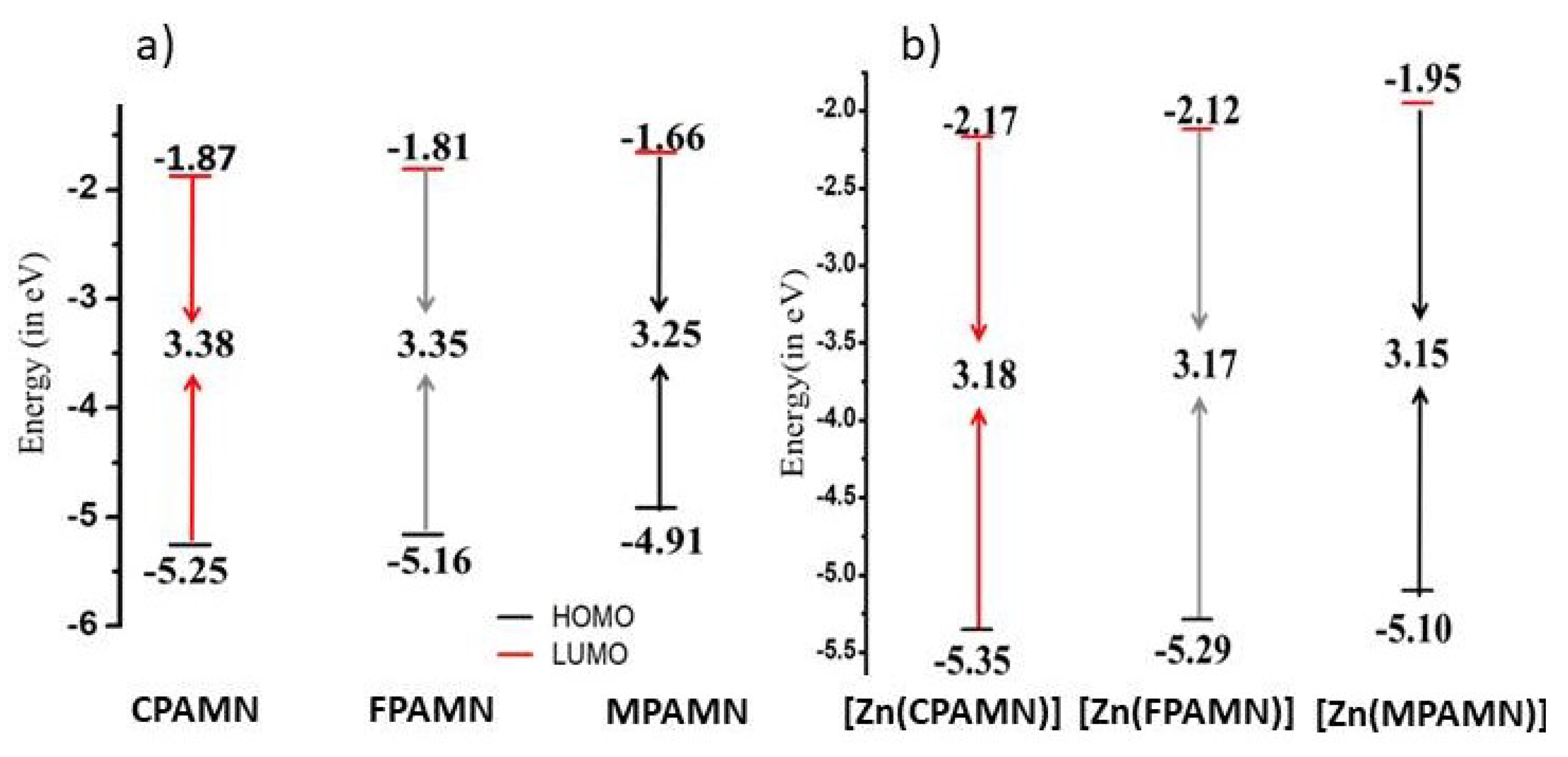
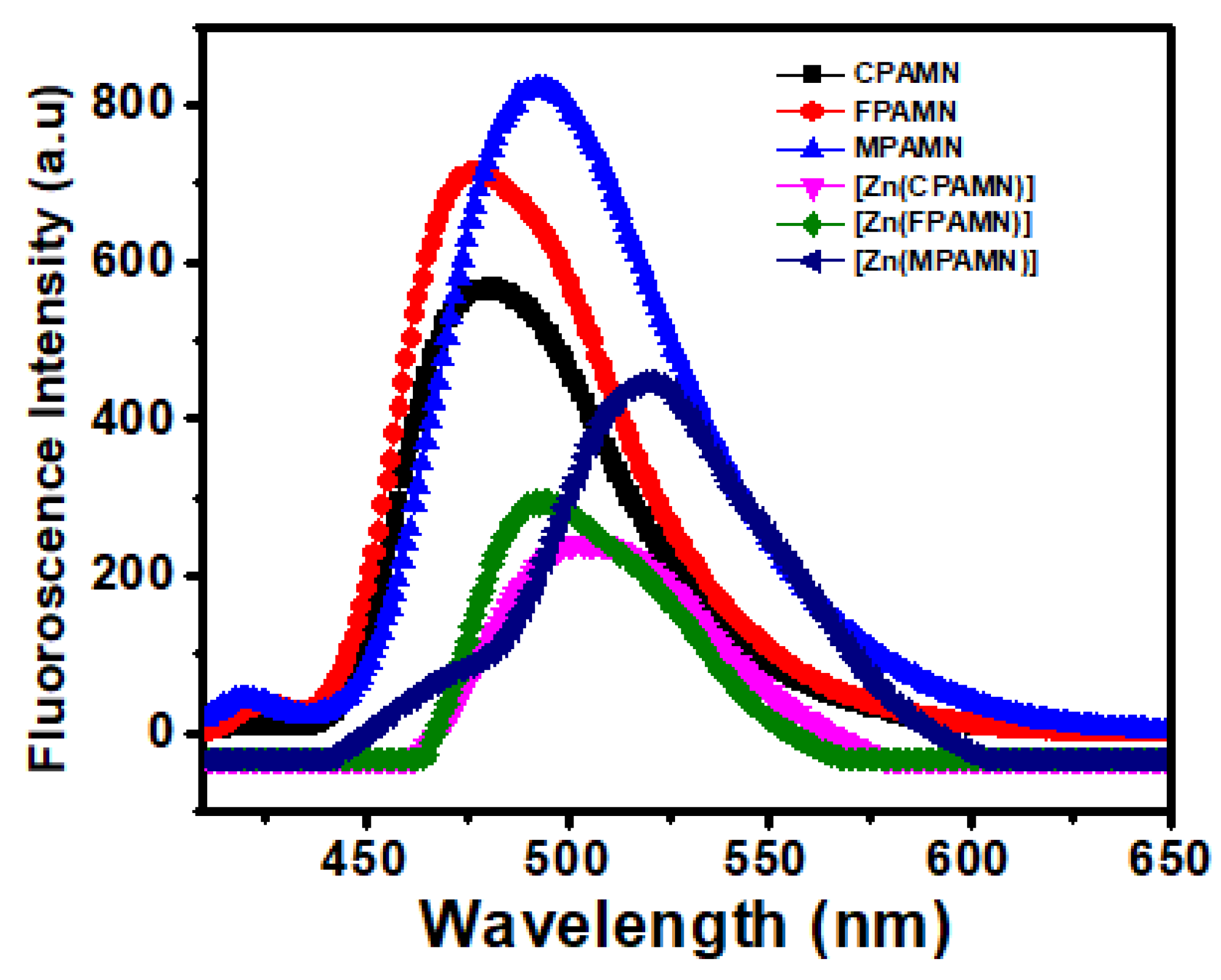
3.2. Absorption and Emission Studies
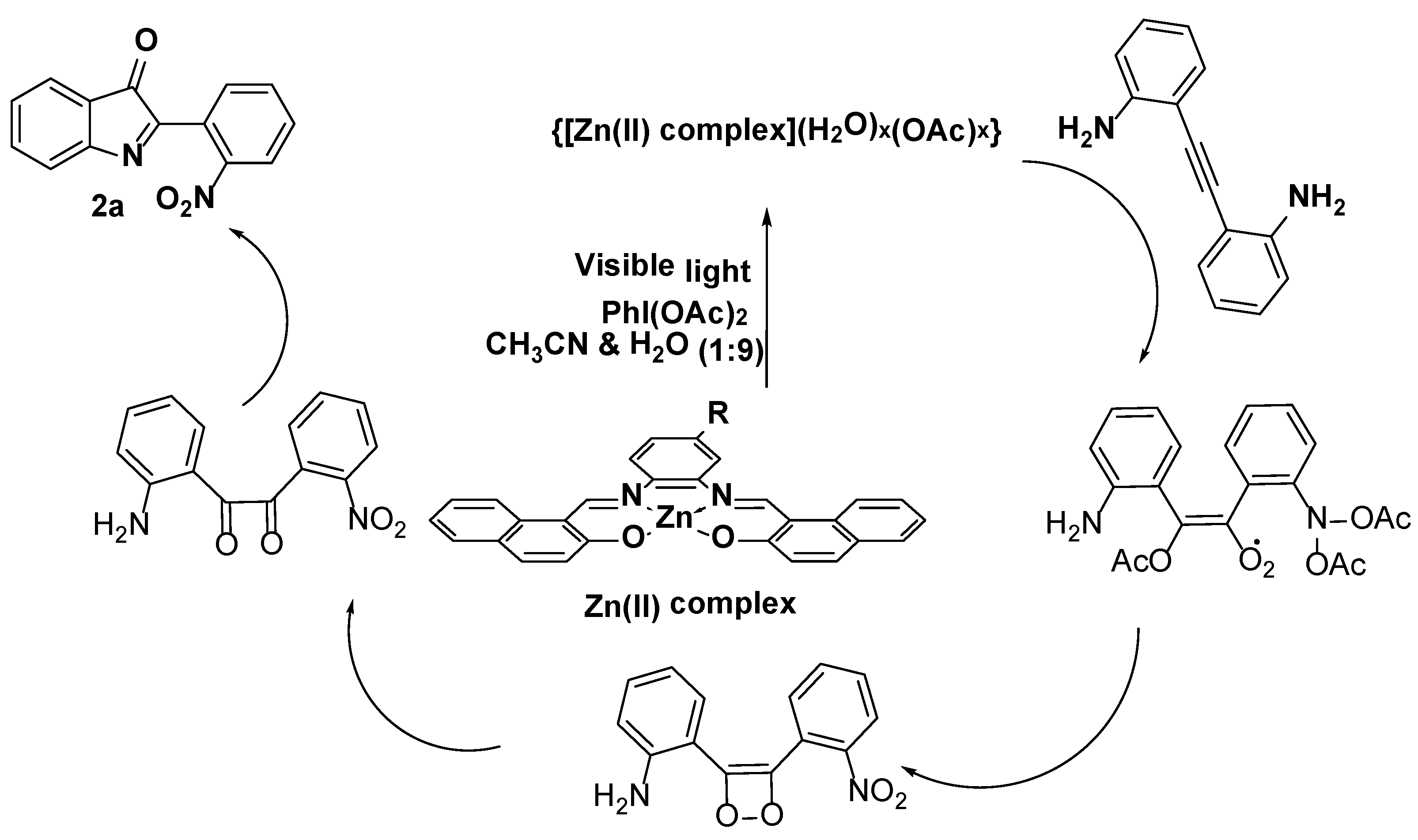
3.3. Photocatalysis
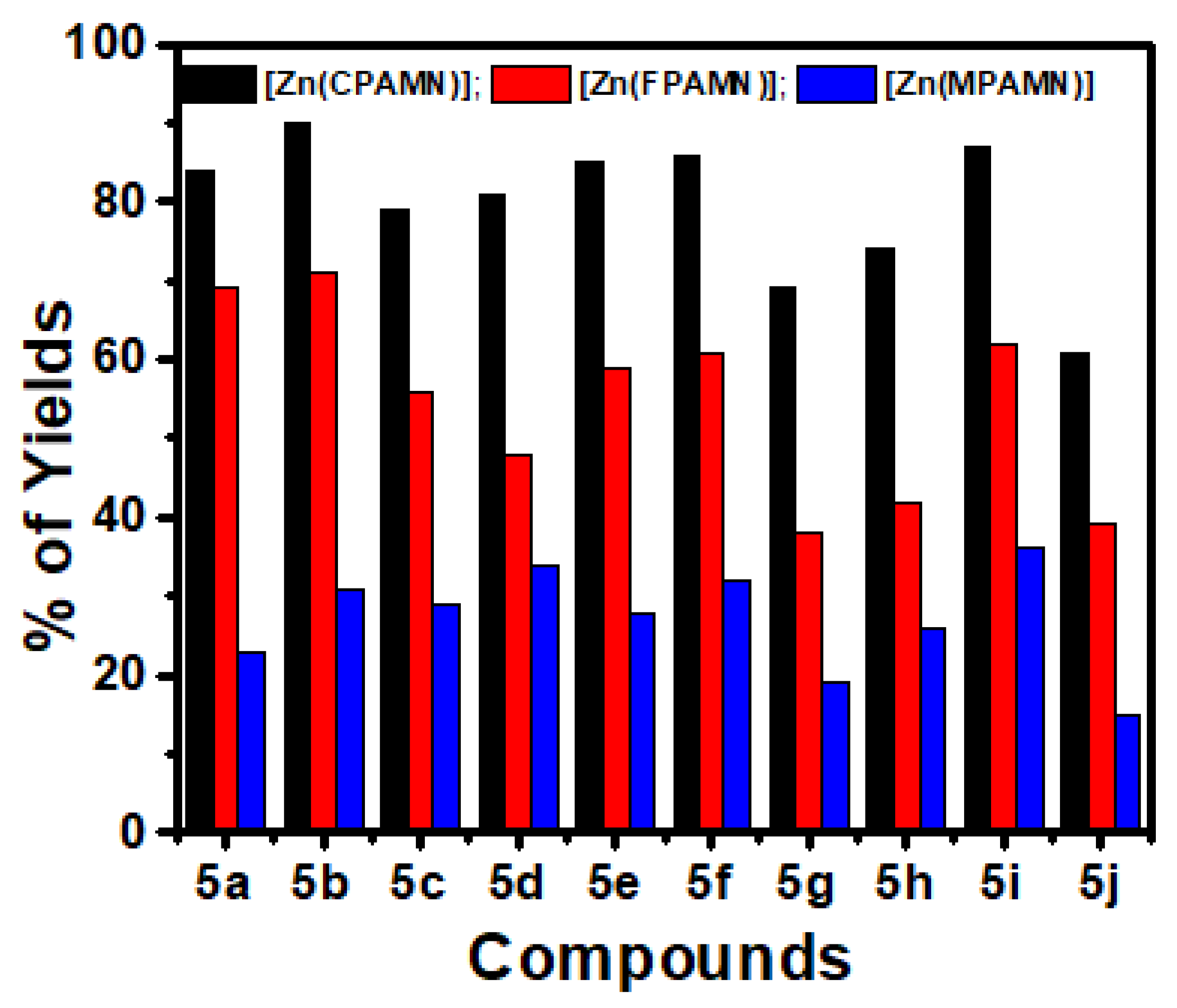
3.3.1. Optimization of Catalysis (Ligands and Zn(II) Complexes
3.3.2. Identifying the Active Species and Optimization of Oxidant for Photooxidation Process
Effect of Visible Light Intensity
Effect of Solvent
Recyclability and Stability of the Catalytic System
3.3.3. The Efficiency of the Photocatalyst System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauscher, F.J., III.; Morris, J.F.; Tournay, O.E.; Cook, D.M.; Curran, T. Binding of the Wilms’ tumor locus zinc finger protein to the EGR-1 consensus sequence. Science 1990, 250, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Wieghardt, K. The active sites in manganese-containing metalloproteins and inorganic model complexes. Angew. Chem. Int. Ed. Engl. 1989, 28, 1153–1172. [Google Scholar] [CrossRef]
- Deeney, F.A.; Harding, C.J.; Morgan, G.G.; McKee, V.; Nelson, J.; Teat, S.J.; Clegg, W. Response to steric constraint in azacryptate and related complexes of iron-(II) and-(III). J. Chem. Soc. Dalton Trans. 1998, 11, 1837–1844. [Google Scholar] [CrossRef]
- Srinivasan, K.; Michaud, P.; Kochi, J.K. Epoxidation of olefins with cationic (salophen) manganese (III) complexes. The modulation of catalytic activity by substituents. J. Am. Chem. Soc. 1986, 108, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Mukherjee, R.; Richardson, J.F.; Buchanan, R.M. Spin-state regulation of iron (III) centres by axial ligands with tetradentate bis (picolinamide) in-plane ligands. J. Chem. Soc. Dalton Trans. 1993, 16, 2451–2457. [Google Scholar] [CrossRef]
- Goodson, P.A.; Oki, A.R.; Glerup, J.; Hodgson, D.J. Design, synthesis, and characterization of bis (.mu.-oxo) dimanganese (III, III) complexes. Steric and electronic control of redox potentials. J. Am. Chem. Soc. 1990, 112, 6248–6254. [Google Scholar] [CrossRef]
- Devereux, M.; McCann, M.; Leon, V.; Geraghty, M.; McKee, V.; Wikaira, J. Synthesis and fungitoxic activity of manganese (II) complexes of fumaric acid: X-ray crystal structures of [Mn(fum)(bipy)(H2O)] and [Mn(Phen)2(H2O)2](fum)·4H2O (fumH2=fumaric acid; bipy=2,2′-bipyridine; phen=1,10-phenanthroline). Polyhedron 2000, 19, 1205–1211. [Google Scholar] [CrossRef]
- Gultneh, Y.; Yisgedu, T.B.; Tesema, Y.T.; Butcher, R.J. Dioxo-bridged dinuclear manganese (III) and-(IV) complexes of pyridyl donor tripod ligands: Combined effects of steric substitution and chelate ring size variations on structural, spectroscopic, and electrochemical properties. Inorg. Chem. 2003, 42, 1857–1867. [Google Scholar] [CrossRef]
- Biswas, S.; Mitra, K.; Chattopadhyay, S.K.; Adhikary, B.; Lucas, C.R. Mononuclear manganese (II) and manganese (III) complexes of N2O donors involving amine and phenolate ligands: Absorption spectra, electrochemistry and crystal structure of [Mn (L3)2](ClO4). Transit. Met. Chem. 2005, 30, 393–398. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Maryam, M.; Masoud, T.M.; Jim, S.; Joel, T.M.; Iran, S. Synthesis and structure elucidation of novel salophen-based dioxo-uranium (VI) complexes: In-vitro and in-silico studies of their DNA/BSA-binding properties and anticancer activity. Eur. J. Med. Chem. 2017, 140, 172–186. [Google Scholar] [CrossRef]
- Atkins, R.; Brewer, G.; Kokot, E.; Mockler, G.M.; Sinn, E. Copper (II) and nickel (II) complexes of unsymmetrical tetradentate Schiff base ligands. Inorg. Chem. 1985, 24, 127–134. [Google Scholar] [CrossRef]
- Kushwah, N.P.; Pal, M.K.; Wadawale, A.P.; Jain, V.K. Diorgano-gallium and-indium complexes with salophen ligands: Synthesis, characterization, crystal structure and C–C coupling reactions. J. Organomet. Chem. 2009, 694, 2375–2379. [Google Scholar] [CrossRef]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salophen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Rong, M.; Wang, J.; Shen, Y.; Han, J. Catalytic oxidation of alcohols by a novel manganese Schiff base ligand derived from salicylaldehyd and l-Phenylalanine in ionic liquids. Catal. Commun. 2012, 20, 51–53. [Google Scholar] [CrossRef]
- Kervinen, K.; Korpi, H.; Leskelä, M.; Repo, T. Oxidation of veratryl alcohol by molecular oxygen in aqueous solution catalyzed by cobalt salophen-type complexes: The effect of reaction conditions. J. Mol. Catal. A Chem. 2003, 203, 9–19. [Google Scholar] [CrossRef]
- Soroceanu, A.; Cazacu, M.; Shova, S.; Turta, C.; Kožíšek, J.; Gall, M.; Breza, M.; Rapta, P.; Mac Leod, T.C.; Pombeiro, A.J. Copper (II) complexes with Schiff bases containing a disiloxane unit: Synthesis, structure, bonding features and catalytic activity for aerobic oxidation of benzyl alcohol. Eur. J. Inorg. Chem. 2013, 2013, 1458–1474. [Google Scholar] [CrossRef]
- Huang, S.; Zou, L.-Y.; Ren, A.-M.; Guo, J.-F.; Liu, X.-T.; Feng, J.-K.; Yang, B.-Z. Computational design of two-photon fluorescent probes for a zinc ion based on a salophen ligand. Inorg. Chem. 2013, 52, 5702–5713. [Google Scholar] [CrossRef]
- Tarade, K.; Shinde, S.; Sakate, S.; Rode, C. Pyridine immobilised on magnetic silica as an efficient solid base catalyst for Knoevenagel condensation of furfural with acetyl acetone. Catal. Commun. 2019, 124, 81–85. [Google Scholar] [CrossRef]
- Hu, J.; Li, K.; Li, W.; Ma, F.; Guo, Y. Selective oxidation of styrene to benzaldehyde catalyzed by Schiff base-modified ordered mesoporous silica materials impregnated with the transition metal-monosubstituted Keggin-type polyoxometalates. Appl. Catal. A Gen. 2009, 364, 211–220. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Zhang, W.; Muci, A.R.; Ecker, J.R.; Deng, L. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar] [CrossRef]
- Irie, R.; Noda, K.; Ito, Y.; Matsumoto, N.; Katsuki, T. Catalytic asymmetric epoxidation of unfunctionalized olefins. Tetrahedron Lett. 1990, 31, 7345–7348. [Google Scholar] [CrossRef]
- McGarrigle, E.M.; Gilheany, D.G. Chromium− and manganese−salophen promoted epoxidation of alkenes. Chem. Rev. 2005, 105, 1563–1602. [Google Scholar] [CrossRef] [PubMed]
- Irie, R.; Noda, K.; Ito, Y.; Matsumoto, N.; Katsuki, T. Catalytic asymmetric epoxidation of unfunctionalized olefins using chiral (salophen) manganese (III) complexes. Tetrahedron Asymmetry 1991, 2, 481–494. [Google Scholar] [CrossRef]
- Dalla Cort, A.; Mandolini, L.; Schiaffino, L. Exclusive transition state stabilization in the supramolecular catalysis of Diels–Alder reaction by a uranyl salophen complex. Chem. Commun. 2005, 30, 3867–3869. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Lu, C. Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chem. Eng. J. 2008, 144, 509–513. [Google Scholar] [CrossRef]
- Patel, U.; Parmar, B.; Dadhania, A.; Suresh, E. Zn(II)/Cd(II)-Based Metal–Organic Frameworks as Bifunctional Materials for Dye Scavenging and Catalysis of Fructose/Glucose to 5-Hydroxymethylfurfural. Inorg. Chem. 2021, 60, 9181–9191. [Google Scholar] [CrossRef]
- Chimupala, Y.; Kaeosamut, N.; Yimklan, S. Octahedral to Tetrahedral Conversion upon a Ligand-Substitution-Induced Single-Crystal to Single-Crystal Transformation in a Rectangular Zn(II) Metal–Organic Framework and Its Photocatalysis. Cryst. Growth Des. 2021, 21, 5373–5382. [Google Scholar] [CrossRef]
- Bhattacharjee, C.H.; Das, G.; Mondal, P.; Rao, N.V.S. Novel photoluminescent hemi-disclike liquid crystalline Zn(II) complexes of [N2O2] donor 4-alkoxy substituted salicyldimine Schiff base with aromatic spacer. Polyhedron 2010, 29, 3089–3096. [Google Scholar] [CrossRef]
- Song, X.; Yu, H.; Yan, X.; Zhang, Y.; Miao, Y.; Ye, K.; Wang, Y. A luminescent benzothiadiazole-bridging bis(salicylaldiminato)zinc(II) complex with mechanochromic and organogelation properties. Dalton Trans. 2018, 47, 6146–6155. [Google Scholar] [CrossRef]
- Germain, M.E.; Vargo, T.R.; Khalifah, P.G.; Knapp, M.J. Fluorescent detection of nitroaromatics and 2,3-dimethyl-2,3-dinitrobutane (DMNB) by a Zinc complex: (salophen)Zn. Inorg. Chem. 2007, 46, 4422–4429. [Google Scholar] [CrossRef]
- Dalla Cort, A.; Mandolini, L.; Pasquini, C.; Rissanen, K.; Russo, L.; Schiaffino, L. Zinc–salophen complexes as selective receptors for tertiary amines. New J. Chem. 2007, 31, 1633–1638. [Google Scholar] [CrossRef]
- Swamy, S.J.; Pola, S. Spectroscopic studies on Co(II), Ni(II), Cu(II) and Zn(II) complexes with a N4-macrocylic ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Mishra, A.; Mishra, S.; Mamba, B.; Maji, B.; Bhattacharya, S. Synthesis and DNA binding studies of Ni (II), Co (II), Cu (II) and Zn(II) metal complexes of N1, N5-bis [pyridine-2-methylene]-thiocarbohydrazone Schiff-base ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Asadi, Z.; Torabi, S.; Lotfi, N. Synthesis, characterization and thermodynamics of complex formation of some new Schiff base ligands with some transition metal ions and the adduct formation of zinc Schiff base complexes with some organotin chlorides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Nawar, N.; Hosny, N.M. Synthesis, spectral and antimicrobial activity studies of o-aminoacetophenone o-hydroxybenzoylhydrazone complexes. Transit. Met. Chem. 2000, 25, 1–8. [Google Scholar] [CrossRef]
- Dharnipathi, V.R.; Subburu, M.; Gade, R.; Basude, M.; Chetti, P.; Simhachalam, N.B.; Nagababu, P.; Bhongiri, Y.; Pola, S. A new Zn(II) complex-composite material: Piezoenhanced photomineralization of organic pollutants and wastewater from the lubricant industry. Environ. Sci. Water Res. Technol. 2021, 7, 1737–1747. [Google Scholar]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Ahemed, J.; Pasha, J.; Kore, R.; Gade, R.; Bhongiri, Y.; Chetti, P.; Pola, S. Synthesis of new Zn(II) complexes for photo decomposition of organic dye pollutants, industrial wastewater and photo-oxidation of methyl arenes under visible-light. J. Photochem. Photobiol. A Chem. 2021, 419, 113455. [Google Scholar] [CrossRef]
- Manoj Kr., P.; Singh, Y.D.; Singh, N.B.; Sarkar, U. Emissive bis-salicylaldiminato schiff base ligands and their zinc(II)complexes: Synthesis, photophysical properties, mesomorphism and DFT studies. J. Mol. Struct. 2015, 1081, 316–328. [Google Scholar]
- Subburu, M.; Gade, R.; Guguloth, V.; Chetti, P.; Ravulapelly, K.R.; Pola, S. Effective photodegradation of organic pollutantsin the presence of mono and bi-metallic complexes under visible-light irradiation. J. Photochem. Photobiol. A Chem. 2021, 406, 112996. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yang, X.-J.; Chen, B.; Li, X.-B.; Zheng, L.-Q.; Wu, L.-Z.; Tung, C.-H. Photocatalytic organic transformation by layered double hydroxides: Highly efficient and selective oxidation of primary aromatic amines to their imines under ambient aerobic conditions. Chem. Commun. 2014, 50, 6664–6667. [Google Scholar] [CrossRef] [PubMed]
- Luís, M.T.F.; Amin, I.; Maria, L.S.C. Photochemical Transformations of Tetrazole Derivatives: Applications in Organic Synthesis. Molecules 2010, 15, 3757–3774. [Google Scholar]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.; Sun, J. Enhanced photocatalytic activity over the Ag2O–gC3N4 composite under visible light. Catal. Sci. Technol. 2014, 4, 758–765. [Google Scholar] [CrossRef]
- Zhu, X.; Li, P.; Shi, Q.; Wang, L. Thiyl radical catalyzed oxidation of diarylalkynes to α-diketones by molecular oxygen under visible-light irradiation. Green Chem. 2016, 18, 6373–6379. [Google Scholar] [CrossRef]
- Guguloth, V.; Ahemed, J.; Subburu, M.; Guguloth, V.C.; Chetti, P.; Pola, S. A very fast photodegradation of dyes in the presence of new Schiff’s base N4-macrocyclic Ag-doped Pd (II) complexes under visible-light irradiation. J. Photochem. Photobiol. A Chem. 2019, 382, 111975. [Google Scholar] [CrossRef]
- Li, Y.; Lee, T.B.; Wang, T.; Gamble, A.V.; Gorden, A.E. Allylic C–H Activations Using Cu(II) 2-Quinoxalinol Salophen and tert-Butyl Hydroperoxide. J. Org. Chem. 2012, 77, 4628–4633. [Google Scholar] [CrossRef]
- Pola, S.; Subburu, M.; Guja, R.; Muga, V.; Tao, Y.-T. New photocatalyst for allylic aliphatic C–H bond activation and degradation of organic pollutants: Schiff base Ti(IV) complexes. RSC Adv. 2015, 5, 58504–58513. [Google Scholar] [CrossRef]





| Complex | ν (Ar-OH) | ν (-C=N-) | ν (Zn-O) | ν (Zn-N) | λonset (nm) | Eg (eV) | Eg (eV) (Solid State) | Surface Area (m2/g) |
|---|---|---|---|---|---|---|---|---|
| CPAMN) | 3295 | 1645 | - | - | 503.66 | 2.46 | 2.75 | 65 |
| FPAMN | 3306 | 1652 | - | - | 512.39 | 2.42 | 2.72 | 59 |
| MPAMN | 3345 | 1660 | - | - | 514.52 | 2.41 | 2.87 | 57 |
| [Zn(CPAMN)] | - | 1628 | 516 | 428 | 596.15 | 2.08 | 2.18 | 208 |
| [Zn(FPAMN)] | - | 1634 | 522 | 436 | 553.57 | 2.24 | 2.15 | 184 |
| [Zn(MPAMN)] | - | 1642 | 518 | 419 | 568.80 | 2.18 | 2.24 | 169 |
 | |||
|---|---|---|---|
| S.No. | Intensity of the Visible Light (Tungsten) | Time (h) | % Yield Compound 5a |
| 1 | CPAMN | 24 | -- |
| 2 | FPAMN | 24 | -- |
| 3 | MPAMN | 24 | -- |
| 4 | [Zn(CPAMN)] | 24 | 84 |
| 5 | [Zn(FPAMN)] | 24 | 62 |
| 6 | [Zn(MPAMN)] | 24 | 40 |
| Oxidant | Scavenger | Time (min) | Conversion Rate |
|---|---|---|---|
| Tert-butyl peroxide | t-butyl alcohol | 24 | 10 |
| Pyridine N-oxide | t-butyl alcohol | 24 | 16 |
| 4-Methylpyridine N-oxide | t-butyl alcohol | 24 | 22 |
| (Diacetoxyiodo)benzene | t-butyl alcohol | 24 | 84 |
| Bis(tert-butylcarbonyloxy) iodobenzene | t-butyl alcohol | 24 | 69 |
| [Bis(trifluoroacetoxy)iodo]benzene | t-butyl alcohol | 24 | 74 |
| Tert-butyl peroxide | Benzoquinone | 24 | 5 |
| Pyridine N-oxide | Benzoquinone | 24 | 2 |
| 4-Methylpyridine N-oxide | Benzoquinone | 24 | trace |
| (Diacetoxyiodo)benzene | Benzoquinone | 24 | 15 |
| Bis(tert-butylcarbonyloxy) iodobenzene | Benzoquinone | 24 | 8 |
| [Bis(trifluoroacetoxy)iodo]benzene | Benzoquinone | 24 | 5 |
 | |||
|---|---|---|---|
| S.No. | Intensity of the Visible Light (Tungsten) | Time (h) | % Yield Compound 5 a |
| 1 | 300 Watts | 46 | 40 |
| 2 | 400 Watts | 38 | 62 |
| 3 | 500 Watts | 24 | 84 |
| S.No. | Solvent and H2O | Time (h) | Yield (%) Compound 2a |
|---|---|---|---|
| 1 | Toluene | 24 | 32 |
| 2 | Dichloroethane | 24 | 58 |
| 3 | THF | 24 | 35 |
| 4 | 1,4-Dioxane | 24 | 18 |
| 5 | Acetone | 24 | 21 |
| 6 | Methanol | 24 | 30 |
| 7 | Ethanol | 24 | 42 |
| 8 | CH3CN | 24 | 84 |
| 9 | DMF | 24 | 13 |
| 10 | DMSO | 24 | 9 |
| 11 | Pure H2O | 24 | 65 |
 | |||
|---|---|---|---|
| Diamine Compound | Product | Complexes | Yield % |
 1a | 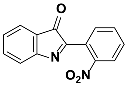 2a | [Zn(CPAMN)] | 84 |
| [Zn(FPAMN)] | 59 | ||
| [Zn(MPAMN)] | 48 | ||
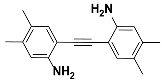 1b | 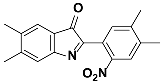 2b | Zn(CPAMN)] | 90 |
| [Zn(FPAMN)] | 61 | ||
| [Zn(MPAMN)] | 39 | ||
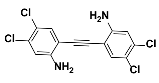 1c |  2c | Zn(CPAMN)] | 79 |
| [Zn(FPAMN)] | 55 | ||
| [Zn(MPAMN)] | 42 | ||
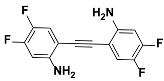 1d | 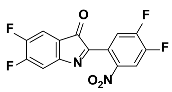 2d | Zn(CPAMN)] | 81 |
| [Zn(FPAMN)] | 49 | ||
| [Zn(MPAMN)] | 31 | ||
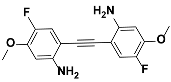 1e |  2e | Zn(CPAMN)] | 85 |
| [Zn(FPAMN)] | 51 | ||
| [Zn(MPAMN)] | 43 | ||
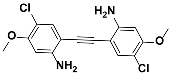 1f | 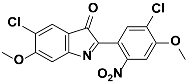 2f | Zn(CPAMN)] | 86 |
| [Zn(FPAMN)] | 49 | ||
| [Zn(MPAMN)] | 37 | ||
 1g | 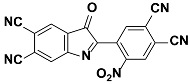 2g | Zn(CPAMN)] | 69 |
| [Zn(FPAMN)] | 50 | ||
| [Zn(MPAMN)] | 41 | ||
 1h | 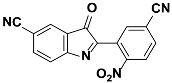 2h | Zn(CPAMN)] | 74 |
| [Zn(FPAMN)] | 38 | ||
| [Zn(MPAMN)] | 21 | ||
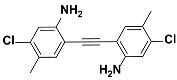 1i | 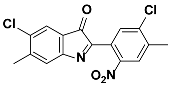 2i | Zn(CPAMN)] | 87 |
| [Zn(FPAMN)] | 56 | ||
| [Zn(MPAMN)] | 32 | ||
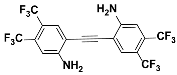 1j | 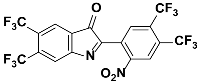 2j | Zn(CPAMN)] | 64 |
| [Zn(FPAMN)] | 35 | ||
| [Zn(MPAMN)] | 19 | ||
| S.No. | Photocatalyst | Time (h) | Yield (%) a |
|---|---|---|---|
| PC-1 | Dichloro(N,N,N′,N′-tetramethylethylenediamine)zinc (28308-00-1) | 24 | -- |
| PC-2 | Zinc bis[bis(trimethylsilyl)amide] (14760-26-0) | 24 | 5 |
| PC-3 | Zinc di[bis(trifluoromethylsulfonyl)imide] (168106-25-0) | 24 | 8 |
| PC-4 | Ziram (CAS No. 137-30-4) | 24 | 10 |
| PC-5 | Zinc phthalocyanine (14320-04-8) | 24 | -- |
| PC-6 | [Zn(CPAMN)] | 24 | 84 |
| PC-7 | [Zn(FPAMN)] | 24 | 59 |
| PC-8 | [Zn(MPAMN)] | 24 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subburu, M.; Gade, R.; Chetti, P.; Pola, S. Photooxidation of 2,2′-(Ethyne-1,2-diyl)dianilines: An Enhanced Photocatalytic Properties of New Salophen-Based Zn(II) Complexes. Photochem 2022, 2, 358-375. https://doi.org/10.3390/photochem2020025
Subburu M, Gade R, Chetti P, Pola S. Photooxidation of 2,2′-(Ethyne-1,2-diyl)dianilines: An Enhanced Photocatalytic Properties of New Salophen-Based Zn(II) Complexes. Photochem. 2022; 2(2):358-375. https://doi.org/10.3390/photochem2020025
Chicago/Turabian StyleSubburu, Mahesh, Ramesh Gade, Prabhakar Chetti, and Someshwar Pola. 2022. "Photooxidation of 2,2′-(Ethyne-1,2-diyl)dianilines: An Enhanced Photocatalytic Properties of New Salophen-Based Zn(II) Complexes" Photochem 2, no. 2: 358-375. https://doi.org/10.3390/photochem2020025






