Solution and Solid-State Optical Properties of Trifluoromethylated 5-(Alkyl/aryl/heteroaryl)-2-methyl-pyrazolo[1,5-a]pyrimidine System
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthetic Procedures
2.2.1. 2,5-Dimethyl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3a)
2.2.2. 2-Methyl-5-phenyl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3b)
2.2.3. 5-(4-Methoxyphenyl)-2-methyl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3c)
2.2.4. 5-(4-Fluorophenyl)-2-methyl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3d)
2.2.5. 5-(4-Bromophenyl)-2-methyl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3e)
2.2.6. 2-Methyl-5-(4-nitrophenyl)-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3f)
2.2.7. 2-Methyl-5-(2-thienyl)-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine (3g)
2.3. Photophysical Measurements
2.3.1. Photophysical Measurements in Solution
2.3.2. Photophysical Measurements in the Solid State
2.4. Thermogravimetric Analysis
3. Results
3.1. Synthesis and Structural Characterization
3.2. Photophysical Properties of Pyrazolo[1,5-a]pyrimidines (3a–g)
3.2.1. Solution Analysis
3.2.2. Aggregation-Induced Emission Behavior
3.2.3. Solid-State Analysis—First Evidences
3.3. Thermal Stability in the Solid State
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Searched as a Topic the Words Organic Compounds and Fluorescent Properties in the Last 5 Years. Available online: https://www.webofscience.com/wos/woscc/citation-report/f531ee1e-c0ea-42bf-90a4-e37ad40ddb7c-2901a49f (accessed on 8 March 2022).
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigreros, A.; Ortiz, A.; Insuasty, B. Effect of π-conjugated linkage on photophysical properties: Acetylene linker as the better connection group for highly solvatochromic probes. Dye. Pigm. 2014, 111, 45–51. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, H.; Qian, L.; Han, M.; Wu, X.; Guo, Z.; Zhan, H. Diversified AIE and mechanochromic luminescence based on carbazole derivative decorated dicyanovinyl groups: Effects of substitution sites and molecular packing. CrystEngComm 2020, 22, 2166–2172. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Han, Q.; Gao, A.; Wang, B.; Chang, X.; Hou, J.T. Design of large π-conjugated α-cyanostilbene derivatives as colorimetric sensors for volatile acids and organic amine gases. J. Mater. Chem. C 2020, 8, 4058–4064. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, Y.; Wang, C.; Wei, Y.; Liao, Q.; Li, Q.; Li, Z. Phenanthroimidazole derivatives with minor structural differences: Crystalline polymorphisms, different molecular packing, and totally different mechanoluminescence. J. Mater. Chem. C 2019, 7, 13759–13763. [Google Scholar] [CrossRef]
- Li, G.Y.; Han, K.L. The sensing mechanism studies of the fluorescent probes with electronically excited state calculations. WIREs Comput. Mol. Sci. 2018, 8, e1351. [Google Scholar] [CrossRef]
- Kournoutas, F.; Fihey, A.; Malval, J.P.; Spangenberg, A.; Fecková, M.; Le Poul, P.; Katan, C.; Robin-Le Guen, F.; Bureš, F.; Achelle, S.; et al. Branching effect on the linear and nonlinear optical properties of styrylpyrimidines. Phys. Chem. Chem. Phys. 2020, 22, 4165–4176. [Google Scholar] [CrossRef]
- Gherasim, C.; Airinei, A.; Tigoianu, R.; Craciun, A.M.; Danac, R.; Nicolescu, A.; Lucian, D.; Mangalagiu, I.I. Synthesis and photophysical insights of new fused N-heterocyclic derivatives with isoquinoline skeleton. J. Mol. Liq. 2020, 310, 113196. [Google Scholar] [CrossRef]
- Kappenberg, Y.G.; Stefanello, F.S.; Zanatta, N.; Martins, M.A.P.; Nogara, P.A.; Rocha, J.B.T.; Tisoco, I.; Iglesias, B.A.; Bonacorso, H.G. Hybridized 4-trifluoromethyl-(1,2,3-triazol-1-yl)quinoline system: Synthesis, photophysics, selective DNA/HSA bio- interactions and molecular docking. ChemBioChem 2022, 23, e202100649. [Google Scholar] [CrossRef]
- Rocha, I.O.; Kappenberg, Y.G.; Rosa, W.C.; Frizzo, C.P.; Zanatta, N.; Martins, M.A.P.; Tisoco, I.; Iglesias, B.A.; Bonacorso, H.G. Photophysical, photostability, and ROS generation properties of new trifluoromethylated quinoline-phenol Schiff bases. Beilstein J. Org. Chem. 2021, 17, 2799–2811. [Google Scholar] [CrossRef]
- Silva, B.; Stefanello, F.S.; Feitosa, S.C.; Frizzo, C.P.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. Novel 7-(1H-pyrrol-1-yl)spiro[chromeno[4,3-b]quinoline-6,10-cycloalkanes]: Synthesis, cross-coupling reactions, and photophysical properties. New J. Chem. 2021, 45, 4061–4070. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Martins, M.A.P.; Marzari, M.R.B.; Campos, P.T.; Claramunt, R.M.; Garcıa, M.A.; Sanz, D.; Alkorta, I.; Elguero, J. Structural Studies of 2-Methyl-7-substituted Pyrazolo[1,5-a]pyrimidines. J. Heterocycl. Chem. 2010, 47, 1259–1268. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Kappenberg, Y.G.; Araujo, J.N.; Zorzin, S.F.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. Trifluoromethyl-substituted aryldiazenyl-pyrazolo[1,5-a]pyrimidin-2-amines: Regioselective synthesis, structure, and optical properties. J. Fluor. Chem. 2022, 255–256, 109967. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.; Bravo, N.; Zapata-rivera, J.; Portilla, J. Pyrazolo[1,5-a]pyrimidines-based fluorophores: A comprehensive theoretical-experimental study. RSC Adv. 2020, 10, 39542–39552. [Google Scholar] [CrossRef] [PubMed]
- Tigreros, A.; Macías, M.; Portilla, J. Photophysical and crystallographic study of three integrated pyrazolo[1,5-a]pyrimidine—Triphenylamine systems. Dye. Pigm. 2021, 184, 108730. [Google Scholar] [CrossRef]
- Castillo, J.C.; Tigreros, A.; Portilla, J. 3-Formylpyrazolo[1,5-a]pyrimidines as key intermediates for the preparation of functional fluorophores. J. Org. Chem. 2018, 83, 10887–10897. [Google Scholar] [CrossRef]
- Tigreros, A.; Castillo, J.; Portilla, J. Cyanide chemosensors based on 3-dicyanovinylpyrazolo[1,5- a]pyrimidines: Effects of peripheral 4-anisyl group substitution on the photophysical properties. Talanta 2020, 215, 120905. [Google Scholar] [CrossRef]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo[1,5-a]pyrimidines: Current Approaches in Synthetic Transformations and Uses As an Antitumor Scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef]
- Buriol, L.; München, T.S.; Frizzo, C.P.; Marzari, M.R.B.; Zanatta, N.; Bonacorso, H.G.; Martins, M.A.P. Resourceful synthesis of pyrazolo[1,5-a]pyrimidines under ultrasound irradiation. Ultrason. Sonochem. 2013, 20, 1139–1143. [Google Scholar] [CrossRef]
- Goldfarb, D.S. Method for Altering the Lifespan of Eukaryotic Organisms. US Patent 2009/0163545 A1, 25 June 2009. [Google Scholar]
- Rosa, W.C.; Rocha, I.O.; Schmitz, B.F.; Rodrigues, M.B.; Martins, M.A.P.; Zanatta, N.; Acunha, T.V.; Iglesias, B.A.; Bonacorso, H.G. Synthesis and photophysical properties of trichloro(fluoro)-substituted 6-(3-oxo-1-(alk-1-en-1-yl)amino)coumarins and their 2,2-Difluoro-2H-1,3,2-oxazaborinin-3-ium-2-uide heterocycles. J. Fluor. Chem. 2020, 238, 109614. [Google Scholar] [CrossRef]
- Santos, G.C.; Rocha, I.O.; Stefanello, F.S.; Copetti, J.P.P.; Tisoco, I.; Martins, M.A.P.; Zanatta, N.; Frizzo, C.P.; Iglesias, B.A.; Bonacorso, H.G. Investigating ESIPT and donor-acceptor substituent effects on the photophysical and electrochemical properties of fluorescent 3,5-diaryl-substituted 1-phenyl-2-pyrazolines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120768. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.S.; Kappenberg, Y.G.; Ketzer, A.; Franceschini, S.Z.; Salbego, P.R.S.; Acunha, T.V.; Nogara, P.A.; Rocha, J.B.T.; Martins, M.A.P.; Zanatta, N.; et al. New 1-(Spiro[chroman-2,1′-cycloalkan]-4-yl)-1H-1,2,3-Triazoles: Synthesis, QTAIM/MEP analyses, and DNA/HSA-binding assays. J. Mol. Liq. 2021, 324, 114729. [Google Scholar] [CrossRef]
- Effenberger, F. Synthese und Reaktionen von 1,4-Bis-athoxymethylen-butandion-(2.3). Chem. Ber. 1965, 98, 2260–2265. [Google Scholar] [CrossRef]
- Effenberger, F.; Maier, R.; Schönwälder, K.-H.; Ziegler, T. Die Acylierung von Enolethern mit reaktiven Carbonsäure-chloriden. Chem. Ber. 1982, 115, 2766–2782. [Google Scholar] [CrossRef]
- Hojo, M.; Masauda, R.; Sakaguchi, S.; Takagawa, M. A convenient synthetic method for β-alkoxy-and β-phenoxyacrylic acids and 3, 4-dihydro-2H-pyran-5-and 2, 3-dihydrofuran-4-carboxylic acids. Synthesis 1986, 12, 1016–1017. [Google Scholar] [CrossRef]
- Bonacorso, H.G.; Martins, M.A.P.; Bittencourt, S.R.T.; Lourega, R.V.; Zanatta, N.; Flores, A.F.C. Trifluoroacetylation of unsymmetrical ketone acetals. A convenient route to obtain alkyl side chain trifluoromethylated heterocycles. J. Fluor. Chem. 1999, 99, 177–182. [Google Scholar] [CrossRef]
- Hojo, M.; Masuda, R.; Kokuryo, Y.; Shioda, H.; Matsuo, S. Electrophilic substitutions of olefinichydrogens II. Acylation of vinyl ethers and N-vinyl amides. Chem. Lett. 1976, 5, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Colla, A.; Martins, M.A.P.; Clar, G.; Krimmer, S.; Fischer, P. Trihaloacetylated enol ethers-general synthetic procedure and heterocyclic ring closure reactions with hydroxylamine. Synthesis 1991, 6, 483–486. [Google Scholar] [CrossRef]
- Flores, A.F.C.; Brondani, S.; Zanatta, N.; Rosa, A.; Martins, M.A.P. Synthesis of 1,1,1-trihalo-4-methoxy-4-[2-heteroaryl]-3-buten-2-ones, the corresponding Butan-1,3-dione and azole derivatives. Tetrahedron Lett. 2002, 43, 8701–8705. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Balenkova, E.S. Preparation of α,β-unsaturated trifluromethylketones and their application in the synthesis of heterocycles. ARKIVOC 2011, i, 246–328. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, G.M.; Flores, A.F.C.; Clar, G.; Zanatta, N.; Martins, M.A.P. Sintese de β-aril-β-metóxiviniltrialometilcetonas: Acilação de cetais. Quim. Nova 1994, 17, 24–26. [Google Scholar]
- Rosa, W.C.; Rocha, I.O.; Schmitz, B.F.; Martins, M.A.P.; Zanatta, N.; Tisoco, I.; Iglesias, B.A.; Bonacorso, H.G. 4-(Trifluoromethyl) coumarin-fused pyridines: Regioselective synthesis and photophysics, electrochemical, and antioxidative activity. J. Fluor. Chem. 2021, 248, 109822. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chemie Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Bristow, W.T.; Webb, K.S. Intercomparison study on accurate mass measurement of small molecules in mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1086–1098. [Google Scholar] [CrossRef] [Green Version]



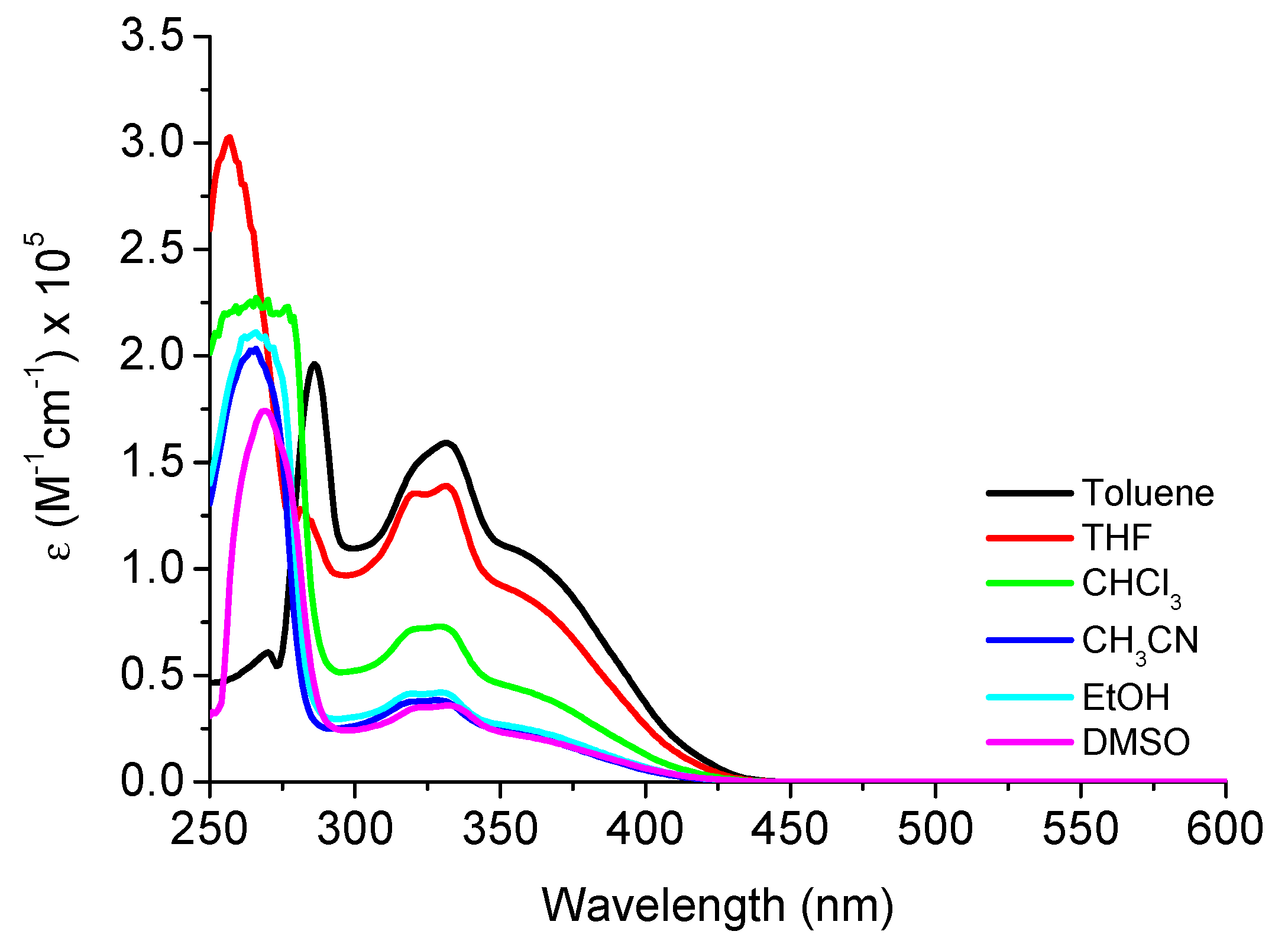

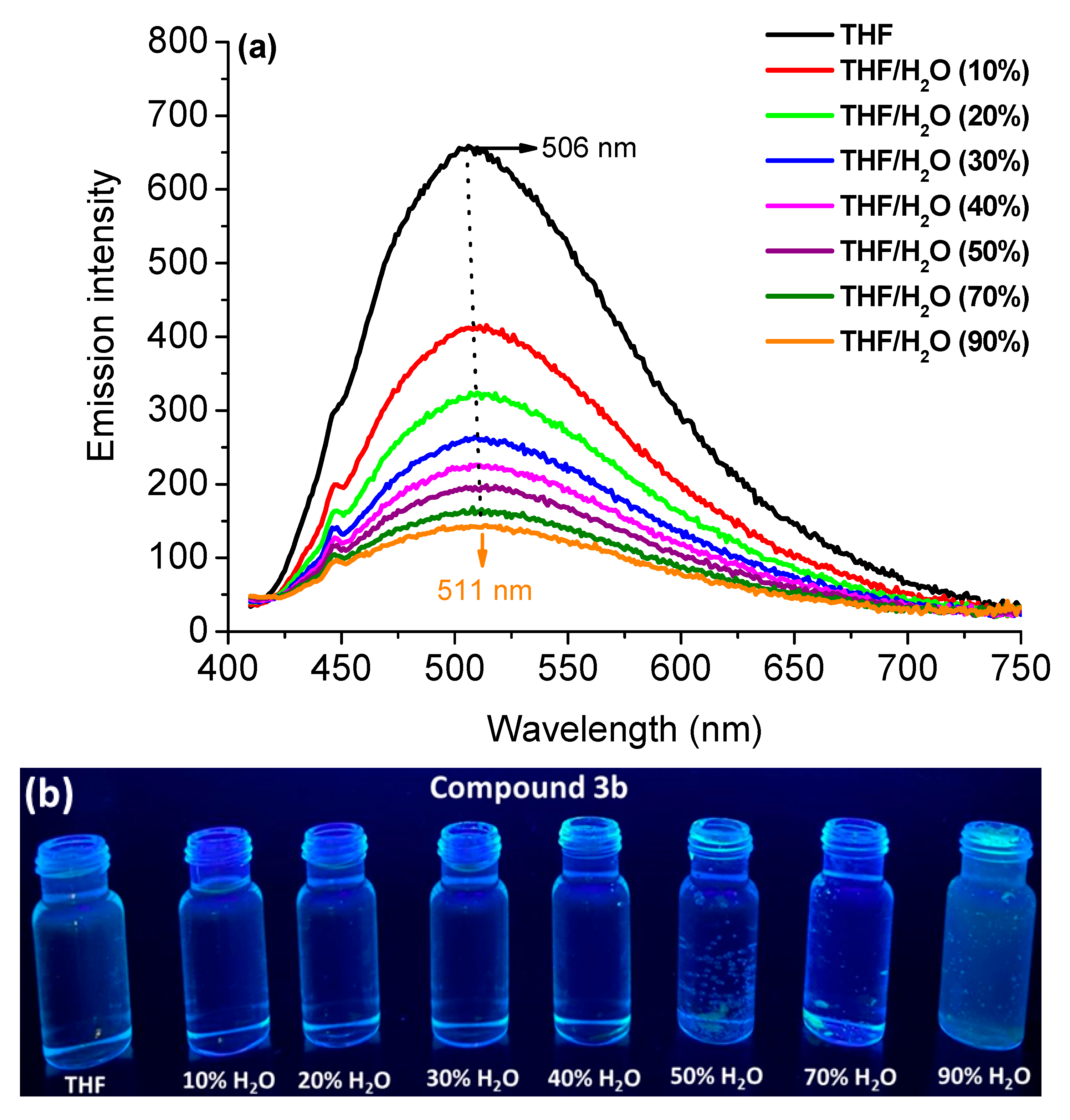


| Compound | Solvent a | λabs, nm (ε; M−1cm−1) | λem, nm (QY, %) b | SS (nm/cm−1) c |
|---|---|---|---|---|
| 3a | CHCl3 | 274 (15670); 309 (4850) | 501 (67.0) | 192/12,400 |
| THF | 273 (19870); 308 (4505) | 473 (87.0) | 165/11,325 | |
| Toluene | 283 (16120); 309 (8870) | 492 (92.0) | 183/12,040 | |
| CH3CN | 306 (12550); 337 (7150) | 511 (67.0) | 174/10,105 | |
| EtOH | 305 (17500); 338 (9690) | 456 (55.0) | 118/7655 | |
| DMSO | 307 (9995); 342 (5120) | 441 (86.0) | 99/6565 | |
| 3b | CHCl3 | 266 (22520); 325 (7320); 357 (sh) | 504 (79.0) | 147/8170 |
| THF | 256 (30195); 324 (13535); 362 (sh) | 507 (78.0) | 145/7900 | |
| Toluene | 286 (19550); 331 (15950); 362 (sh) | 508 (88.0) | 146/7940 | |
| CH3CN | 265 (20210); 324 (3800); 360 (sh) | 506 (71.0) | 146/8015 | |
| EtOH | 265 (21035); 325 (4175); 365 (sh) | 514 (71.0) | 149/7940 | |
| DMSO | 269 (17410); 328 (3570); 362 (sh) | 521 (91.0) | 159/8430 | |
| 3c | CHCl3 | 290 (13420); 338 (5785); 367 (sh) | 500 (77.0) | 133/7250 |
| THF | 256 (18080); 295 (23610); 339 (12985); 368 (sh) | 500 (75.0) | 132/7170 | |
| Toluene | 294 (22040); 338 (19095); 365 (sh) | 501 (84.0) | 136/7435 | |
| CH3CN | 287 (14085); 324 (3800); 360 (sh) | 554 (63.0) | 194/9730 | |
| EtOH | 289 (11740); 337 (5285); 367 (sh) | 487 (56.0) | 120/6715 | |
| DMSO | 269 (10020); 327 (2010); 364 (sh) | 509 (88.0) | 145/7825 | |
| 3d | CHCl3 | 269 (15780); 331 (2870); 370 (sh) | 504 (79.0) | 134/7185 |
| THF | 256 (17380); 288 (8800); 326 (9640); 362 (sh) | 509 (77.0) | 147/7980 | |
| Toluene | 289 (18920); 336 (6020); 369 (sh) | 510 (87.0) | 141/7490 | |
| CH3CN | 266 (20720); 325 (4070); 359 (sh) | 516 (73.0) | 157/8475 | |
| EtOH | 267 (17185); 328 (3030); 360 (sh) | 514 (73.0) | 154/8320 | |
| DMSO | 269 (15720); 327 (3125); 359 (sh) | 521 (93.0) | 162/8660 | |
| 3e | CHCl3 | 276 (17465); 330 (3800); 364 (sh) | 507 (79.0) | 143/7750 |
| THF | 257 (9915); 291 (9720); 329 (8275); 365 (sh) | 511 (78.0) | 146/7830 | |
| Toluene | 290 (22170); 334 (15485); 362 (sh) | 510 (87.0) | 148/8015 | |
| CH3CN | 272 (10370); 327 (2195); 364 (sh) | 516 (73.0) | 152/8090 | |
| EtOH | 274 (20375); 327 (4695); 366 (sh) | 514 (73.0) | 148/7865 | |
| DMSO | 275 (17760); 330 (4285); 366 (sh) | 524 (90.0) | 158/8240 | |
| 3f | CHCl3 | 299 (10955); 344 (sh) | 496 (64.0) | 152/8910 |
| THF | 271 (10545); 296 (12640); 342 (sh) | 524 (73.0) | 182/10,155 | |
| Toluene | 297 (11850); 346 (sh); 382 (sh) | 520 (89.0) | 138/6950 | |
| CH3CN | 295 (11625); 341 (sh); 373 (sh) | 545 (70.0) | 172/8460 | |
| EtOH | 292 (17630); 340 (sh); 373 (sh) | 419 (24.0) | 46/2940 | |
| DMSO | 257 (14555); 301 (16145); 347 (sh) | 558 (95.0) | 211/10,900 | |
| 3g | CHCl3 | 280 (19115); 337 (8370); 375 (sh) | 481 (63.0) | 106/5875 |
| THF | 257 (15280); 304 (20765); 336 (19370); 378 (sh) | 511 (75.0) | 133/6885 | |
| Toluene | 290 (17520); 345 (8170); 375 (sh) | 510 (86.0) | 135/7060 | |
| CH3CN | 276 (11520); 343 (5715); 372 (sh) | 517 (73.0) | 145/7540 | |
| EtOH | 278 (18050); 343 (8620); 371 (sh) | 496 (56.0) | 125/6790 | |
| DMSO | 281 (21175); 346 (11215); 375 (7770) | 520 (89.0) | 145/7435 |
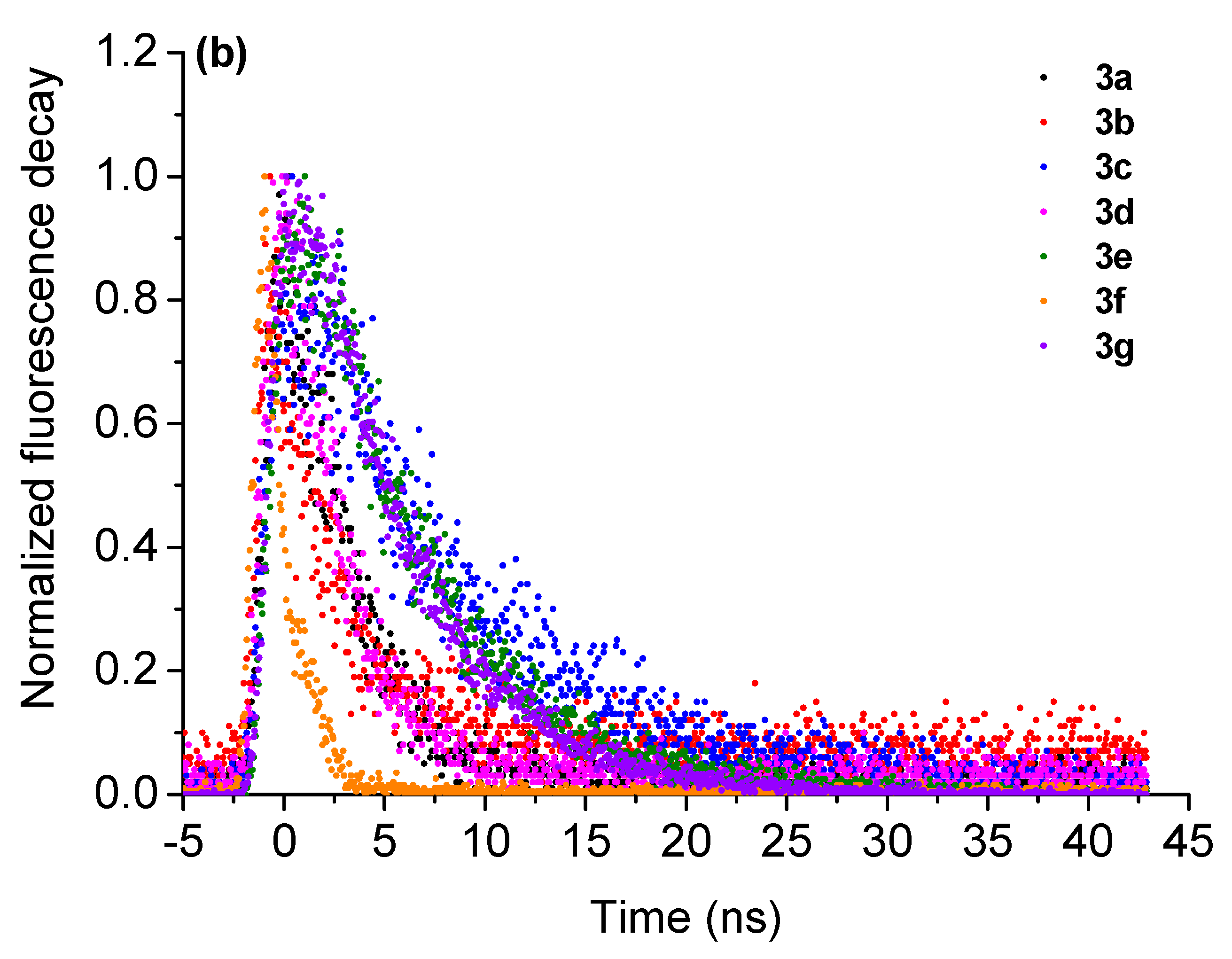
| Compound | λabs, nm | λem nm (QY,%) a | SS (nm/cm−1) b | τf, ns (χ2) c | kr (108 s−1) d | knr (108 s−1) e |
|---|---|---|---|---|---|---|
| 3a | 261, 338, 417 | 493 (29.0) | 76/3700 | 3.50 ± 0.44 (1.131901) | 0.83 | 2.03 |
| 3b | 286, 338, 425 | 493 (23.0) | 68/3245 | 3.03 ± 0.59 (1.143012) | 0.76 | 2.55 |
| 3c | 283, 335, 425 | 485 (21.0) | 60/2910 | 8.62 ± 0.37 (1.091558) | 0.24 | 0.92 |
| 3d | 294, 340, 427 | 509 (28.0) | 82/3770 | 3.00 ± 0.45 (1.051683) | 0.93 | 2.40 |
| 3e | 290, 428 | 483 (24.0) | 55/2660 | 6.62 ± 0.48 (1.131901) | 0.36 | 1.15 |
| 3f | 283, 335, 427 | 542 (29.0) | 115/4970 | 1.36 ± 0.82 (1.151343) | 2.13 | 5.22 |
| 3g | 268, 331, 433 | 507 (24.0) | 74/3370 | 6.14 ± 0.52 (0.919048) | 0.39 | 1.23 |
| Compound | T0.05 (°C) | Td (°C) | Mass Loss (%) |
|---|---|---|---|
| 3a | 72 | 110 | 99 |
| 3b | 117 | 161 | 97 |
| 3c | 171 | 200 | 99 |
| 3d | 134 | 169 | 96 |
| 3e | 147 | 187 | 97 |
| 3f | 187 | 230 | 99 |
| 3g | 151 | 187 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanello, F.S.; Vieira, J.C.B.; Araújo, J.N.; Souza, V.B.; Frizzo, C.P.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. Solution and Solid-State Optical Properties of Trifluoromethylated 5-(Alkyl/aryl/heteroaryl)-2-methyl-pyrazolo[1,5-a]pyrimidine System. Photochem 2022, 2, 345-357. https://doi.org/10.3390/photochem2020024
Stefanello FS, Vieira JCB, Araújo JN, Souza VB, Frizzo CP, Martins MAP, Zanatta N, Iglesias BA, Bonacorso HG. Solution and Solid-State Optical Properties of Trifluoromethylated 5-(Alkyl/aryl/heteroaryl)-2-methyl-pyrazolo[1,5-a]pyrimidine System. Photochem. 2022; 2(2):345-357. https://doi.org/10.3390/photochem2020024
Chicago/Turabian StyleStefanello, Felipe S., Jean C. B. Vieira, Juliane N. Araújo, Vitória B. Souza, Clarissa P. Frizzo, Marcos A. P. Martins, Nilo Zanatta, Bernardo A. Iglesias, and Helio G. Bonacorso. 2022. "Solution and Solid-State Optical Properties of Trifluoromethylated 5-(Alkyl/aryl/heteroaryl)-2-methyl-pyrazolo[1,5-a]pyrimidine System" Photochem 2, no. 2: 345-357. https://doi.org/10.3390/photochem2020024
APA StyleStefanello, F. S., Vieira, J. C. B., Araújo, J. N., Souza, V. B., Frizzo, C. P., Martins, M. A. P., Zanatta, N., Iglesias, B. A., & Bonacorso, H. G. (2022). Solution and Solid-State Optical Properties of Trifluoromethylated 5-(Alkyl/aryl/heteroaryl)-2-methyl-pyrazolo[1,5-a]pyrimidine System. Photochem, 2(2), 345-357. https://doi.org/10.3390/photochem2020024







