Abstract
Luminescent micelles are extensively studied molecular scaffolds used in applied supramolecular chemistry. These are particularly important due to their uniquely organized supramolecular structure and chemically responsive physical and optical features. Various luminescent tags can be incorporated with these amphiphilic micelles to create efficient luminescent probes that can be utilized as “chemical noses” (sensors) for toxic and hazardous materials, bioimaging, drug delivery and transport, etc. Due to their amphiphilic nature and well-defined reorganized self-assembled geometry, these nano-constructs are desirable candidates for size and shape complementary guest binding or sensing a specific analyte. A large number of articles describing micellar fluorogenic probes are reported, which are used for cation/anion sensing, amino acid and protein sensing, drug delivery, and chemo-sensing. However, this particular review article critically summarizes the sensing application of nitroaromatic (e.g., trinitrotoluene (TNT), trinitrobenzene (TNB), trinitrophenol (TNP), dinitrobenzene (DNB), etc.) and nitramine explosives (e.g., 1,3,5-trinitro-1,3,5-triazinane, trivially named as “research department explosive” (RDX), 1,3,5,7-tetranitro-1,3,5,7-tetrazocane, commonly known as “high melting explosive” (HMX) etc.). A deeper understanding on these self-assembled luminescent “functional materials” and the physicochemical behavior in the presence of explosive analytes might be helpful to design the next generation of smart nanomaterials for forensic applications. This review article will also provide a “state-of-the-art” coverage of research involving micellar–explosive adducts demonstrating the intermolecular charge/electron transfer (CT/ET) process operating within the host–guest systems.
1. Introduction
In the last decades, significant advancement has been made to construct the luminescent micellar systems for sensing target analytes, ion transport, and bioimaging. These luminophoric materials are used to demonstrate the rudimentary models for various CT/ET processes in an aqueous solution in the presence of various chemical entities [1,2,3,4,5,6,7]. Based on the fundamental idea of CT/ET transition, a considerable number of elegant luminous micellar systems are developed for sensing metal ions [8,9,10,11,12] and anions [13,14,15], biomolecules [16,17,18,19,20], detection of explosive [21,22,23,24,25], etc. to name a few. Moreover, these micellar systems can be typically considered as the simplest biological mimic of cellular membranes having both hydrophobic and hydrophilic parts in a single molecular platform. Therefore, they might be useful materials considering the artificial synthetic models compatible for biosensing. In addition to that, augmenting particular luminophoric moieties to the micellar systems may provide specific hybrid nano-composites selective for target analytes. It is noteworthy that compared to the conventional luminescent/optical sensors, these luminescent micellar sensors are a new category that require more exposure to unfurl their potential applications in sensing activity.
The ever-increasing use of explosive chemicals for homeland security, armed military forces, mining, road construction, demolition of old buildings and civil structures, and more importantly by separatist groups, terrorist organizations, and criminals has raised the necessity of their trace level detection by various sensitive techniques. Depending on the chemical structures, these explosives can be categorized as nitroaromatics, nitrate esters, nitramines, and peroxides. Among them, nitroaromatic and nitramine explosives are the most commonly used high energy explosives (secondary explosives), widely used by armed forces and also by separatist and terrorist organizations. Nitroaromatic explosives are aromatic compounds containing single or multiple electron poor nitro (–NO2) groups. The most common examples of nitroaromatic compounds are DNT, TNT, TNP (or PA), etc. On the other hand, the representative examples of chemical explosives fall under the category of nitramine, which includes RDX, HMX, and Tetryl, etc. Sensing of these explosive analytes for forensic applications by using various “chemical noses” in different medium, is therefore, an important topic in applied supramolecular chemistry research, which is growing rapidly in the present era. In current literature, explosive sensing and their materials applications by various purely organic or composite materials have been described in a few review articles [26,27]. However, to the best of our knowledge, a detailed review article based on supramolecular micellar systems exclusively meant for forensic applications has yet to appear in the current literature. Considering the applied supramolecular chemistry, materials chemistry research, sensor development, and timeliness of this topic, we therefore, strongly believe that a comprehensive review article is urgently necessary to summarize the recent development of the luminescent micellar sensors. Particularly, in this review article we will highlight the progress on various luminescent micellar systems which are effective in an aqueous medium. The efforts have also been made on the working principles of the sensing behavior of each micellar–analyte supramolecular system. We also discuss the role of explosive analytes to control the CT phenomena, which has tremendous influence on both ground and excited state dynamics. In general, the luminescence quenching processes are involved (with few exceptions of enhanced emission) in the sensing of explosive analytes depending on their acceptor strengths and geometries.
Therefore, the present review article is designed to address a brief coverage on metal ion sensing, anion sensing, and biosensing followed by exclusive highlight on explosive sensing. Given its breadth and scope, we anticipate that this review will provide a general glimpse on what has been done in the area of micellar sensors, thus allowing for further advances in this research field. Consequently, in this review we provide a “state-of-the-art” coverage of research involving various micellar systems used for explosive detection and sensing in an aqueous medium by means of a donor–acceptor (D–A) type of supramolecular interaction along with their design strategy and working principle. A deeper understanding of supramolecular chemistry involved with these luminous micellar systems along with the structure-property relationship of micelle–analyte adducts, and their physicochemical properties might provide a general platform to researchers to design the next generation “functional materials” of this kind.
2. Luminescent Micellar Systems
The chemistry of luminescent micellar systems originally developed decades ago, however, a short discussion might be relevant here for neophytes. Figure 1a shows a typical model structure of a micelle that is structurally different from reverse micelle liposome and lipid bilayer (Figure 1b–d). Typically, a micellar system consists of a hydrophobic core resulted from self-assembled organic synthons having a hydrophilic head and hydrophobic tail groups (cf. Figure 1, Inset) [28,29,30]. To construct a luminescent micellar system, either polyaromatic hydrocarbon (PAH)-based fluorophore(s) or a luminescent metal complex tag should be attached or grafted to the skeleton. While the hydrophobic and hydrophilic part self-assembled to form a micellar structure, the luminophores too stacked either on the surface or within the core. The specific arrangement of luminophores in the core/surface/intermediate layer of micelle results in monomer/excimer emission with much enhanced quantum yield compared to that in organic solvents under identical conditions. In the presence of explosive materials (which are generally electron poor in nature due to the presence of multiple NO2 groups present in a single unit), a D–A type of host–guest complex can be produced with micellar systems where the explosive molecules are intercalated within the lyophilic part of the micelles. Normally, the sensing behavior resulted from either by quenching of initial luminescence of the existing luminophore or quenching of the excimer emission by intermolecular D–A charge transfer transitions. Few cases are also reported where the luminescence sensing was achieved by the fluorescence “Turn ON” mechanism. The efficacy of such a micellar system as a sensor can be easily determined by the Stern–Volmer constant (KSV) using the Stern–Volmer equation given below:
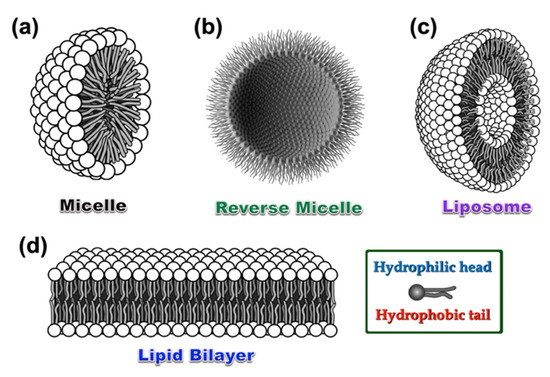
Figure 1.
Graphical representation of a standard micelle (a), reverse micelle (b), liposome (c), and lipid bilayer (d), respectively. Inset: A single unit consists of hydrophobic aliphatic tail and hydrophilic head, which takes part to construct all the above-mentioned self-assembled structures with various geometries under different environments.
Where, I0 is the initial emission intensity of a fluorophoric unit, Ifinal is the final emission intensity, Iq is the fluorescence intensity in the presence of quencher, [F] is the concentration of the fluorophore, and [Q] is the concentration of added quencher Q, respectively. The nonlinear nature of the Stern–Volmer plot indicates a combined static and dynamic quenching process involved in a particular system or an energy transfer process within the host–guest system under consideration.
The sensing mechanism to detect the explosive traces are mainly of four different types, viz. (1) by emission quenching phenomenon due to intermolecular D–A interactions with photo-induced electron transfer (PET) transition; (2) the emission enhancement by counter-ion displacement process; (3) the luminescence quenching of an aggregated sample exhibiting aggregation-induced emission (AIE), by CT/ET to the explosive analytes; and (4) by the change of a ratiometric emission of a double luminophoric system (vide infra). The graphical illustration of sensing mechanisms by the luminescence “Turn OFF” pathway involved within micellar systems and explosive analytes as the representative examples are given in Figure 2a,b. The absence of π–π interactions between two neighboring aggregated units and the activation of intermolecular D–A interactions within micelle and explosive analytes mainly contributed to the quenched emission intensity in these micellar systems. It is noteworthy that both the classic polyaromatic hydrocarbons (PAHs), (viz. anthracene, pyrene, perylene, etc.) and some specific cyclometallated Pt(II)/Ir(III) luminophoric materials can be used to construct these micellar sensors that are effective in an aqueous medium. In addition to that presence of charge, hydrophilic and hydrophobic groups existing in micellar systems also play an important role towards sensing property.
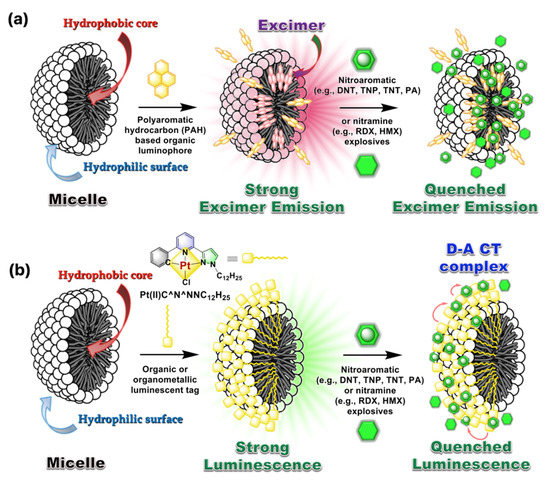
Figure 2.
The proposed mechanism involved in the “Turn OFF” luminescent sensing of explosive analytes. (a) Luminescence quenching of excimer-based emission in the presence of explosive analytes. (b) Luminescence quenching of a surface-modified micellar system by supramolecular D–A CT transition within the cyclometallated luminophore (D) and explosive analytes (A). The models are redrawn based on the concept used in references [22,23].
2.1. Luminescent Micellar Systems for Metal Ion Sensing
A wide variety of luminescent micellar systems have been reported so far in the current literature demonstrating metal ion sensing [8,9,10,11,12,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. However, as our main focus is on explosive sensing in a micellar medium, we will cover the metal ion sensing in this section in brief. Most well-known cases that have fused heterocyclic organic fluorophores or traditional polyaromatic hydrocarbons (PAH) have been utilized (see Chart 1 for details) to construct luminescent micellar sensors. The major advantages of these micellar organic luminophores include: (i) Enhanced emission intensity of the monomer or excimer through vibrational rigidity, (ii) selective site binding with metal ions as compared to bulk phase, (iii) mimicking biological membrane-like systems and in vivo sensing feasibility, (iv) selective and sensitive sensing of metal ions through enhanced emission of organic luminophore via controlled binding, and (v) the use of lipophilic fluorophores in aqueous medium solubilized by micelles, etc. to name a few.
Chart 1.
Chemical structures of few representative fluorophores and receptors (both chelated and macrobicyclic) used for metal ion sensing in an aqueous micellar medium.
In addition to that, depending on the charge state of micellar systems (cationic, anionic, or neutral) and the nature of coordinating atoms, the sensing efficacy is directly related to the pH of the medium. Based on these ideas, various luminescent probes to detect transition metal ions (viz. Cu2+, Fe3+, Hg2+, Cd2+, etc.) have been successfully demonstrated, which are able to work in an aqueous medium. For example, the fluorescent “Turn ON” metal ion sensors 10a,b are reported by Nakatsuji and Akashi et al. which are constructed by using laterally unsymmetric bicyclic oxa-aza cryptands [34]. These photosensitive monoazacryptand derivatives are used to sense the alkali and alkaline earth metal ions in the presence of various surfactants, depending on the size of the inner core. Typically, the sensing mechanism involves the deactivation pathway of the PET process between cryptands and fluorophore (pyrene), which results in the “Turn ON” emission switching upon metal binding. It was noticed that the 15-crown-5 -based fluorophoric system (10a) is selective for K+, however, the corresponding larger analogue 10b (18-crown-6 -based system) is selective for Ba2+ in an aqueous medium at ambient condition. It is speculated that a bigger core diameter of oxa-aza cryptand 10b provides a suitable platform to encapsulate Ba2+ that make the receptor 10b selective for Ba2+ ion.
Taking advantage of the aggregated pyrene-based excimer emission, Ding and co-workers used a series of synthetic luminescent molecular probes for constructing the surfactant ensembled metal ion sensors, which are nicely summarized in a recent article [12]. Among the series, the neutral bispyrene derivative shown in Figure 3 inset provides substantial sensing behavior to various di- and tri-valent metal ions. Interestingly, when the bispyrene/SDS assemblies are titrated against Fe3+, Cu2+, Hg2+, emission quenching was observed (cf. Figure 3a,b in case of Fe3+, Cu2+ respectively), however, incremental addition of Mg2+, Ca2+, Co2+, Ni2+, Zn2+, Cd2+, and Pb2+ exhibit ratiometric response (cf. Figure 3c,d in case of Co2+ and Zn2+ as representative examples). A prominent optical change in the presence of these metal ions under exposure of UV light reflects their potential applications in metal ion sensing. The suggested mechanism involving the sensing behavior of bispyrene/SDS adduct against these di- and tri-valent metal ions by emission quenching and ratiometric pathway is shown in Figure 3e. The flexibility of the long aliphatic bridge of bispyrene derivative plays an important role during the sensing process.
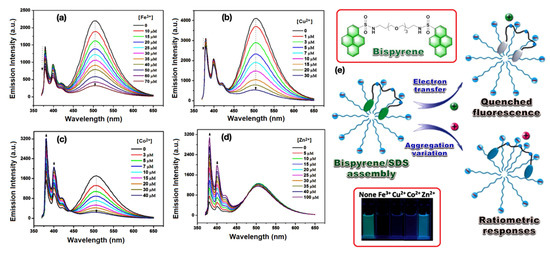
Figure 3.
(a–c) Emission quenching of bispyrene/SDS assemblies with the incremental addition of Fe3+, Cu2+, and Co2+ respectively, in an aqueous medium at ambient condition. (d) The ratiometric change of bispyrene/SDS assemblies when titration was performed against Zn2+ ion. (e) The schematic representation of a bispyrene/SDS self-assembled sensor towards various metal ions. Inset: Top, the Chemdraw representation of bispyrene derivative; bottom, the photograph of bispyrene/SDS sensor in the presence of Fe3+, Cu2+, Co2+, and Zn2+ respectively, under exposure of UV light. This figure is reproduced with permission from reference [12], Copyright © 2022, American Chemical Society.
Apart from these organic fluorophores, a DANSYL appended macromolecular system is reported by Zhao et al. to detect Hg2+ ion selectively in an aqueous medium. This lyophilic organic molecular probe is solubilized in an aqueous medium by the help of surfactant-based micellar systems. A more detailed account on luminescent micellar systems for metal ion sensing can be found in the following recent review article [33].
2.2. Luminescent Micellar Systems for Anion Sensing
Among various anionic species, sensing of halides (e.g., F−, Cl−, Br−, and I−) and cyanide (CN−) by luminescent micellar probes have been extensively studied [13,14,15,51,52,53,54,55]. Although a large number of model sensors are reported in current literature, however, as mentioned earlier, our primary focus in this current review is to highlight the most compelling applications of micellar systems for explosive sensing. Therefore, exclusive discussion on anion sensing with micellar systems in this part is intentionally precluded to avoid repetition. Nevertheless, emphasis is placed on some quintessential micellar systems as the representative examples which can be treated as model micellar systems for anion sensing in an aqueous medium. The sensing strategies mostly relied on using the receptors containing the metal complexes which selectively binds specific anions to give a substantial luminescence signal. In addition to that, another interesting class of anion sensor is also observed in current literature where the anion reacts with the receptor/fluorophore to produce a substituted/addition product with changed luminescence output. Few representative examples of such fluorophoric systems reported so far in the current literature for anion sensing in aqueous micellar medium are shown in Chart 2. Considering CN− sensing in water, the bis-indolyl fluorophores, 11–13, undergo Michael addition reaction with CN− which give rise to the new luminophores. Nevertheless, the probes exhibit very weak detection limit due to poor solubility as a result of aggregation. However, in micellar media, the probes behave effectively with a much lower detection limit (8 ppb) through improved solubilization of the probes. The other fluorophores used for CN− sensing includes 2,4,6- triarylthiopyrylium (14) encapsulated with a neutral surfactant (Triton X-100)-based micelle reported by Manez et al. [13]. In a similar fashion, the probes 15–20 has been used for sensing the halides (Cl−, F−) and cyanide (CN−) ions after being solubilized through micellar media.
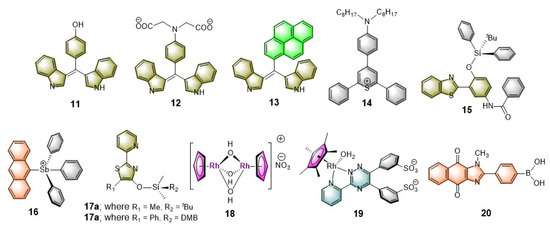
Chart 2.
Chemical structures of various luminophores used for sensing specific anions in the aqueous micellar media.
To demonstrate Cl− sensing by metal complexes, the work of Severin and coworkers is noteworthy [15]. Their aqueous buffered solution was loaded with a Rh(II)-complex (19) as a coordinating site and hydroxypyrenesulfonate as fluorophore, which is mixed with a supplementary surfactant. The resulting adduct remarkably increases Cl− affinity. It was described that Cl− binds to the metal center, which in turn changes the phase behavior of the whole system. The metal complex now in turn becomes amphiphilic in nature as compared to its initial ionic state, which can easily move towards the lyophilic core of the micelle. The resulting species causes significant modulation of the luminescence property. A fluorescence quenching was observed, which is directly related to the added Cl− concentration (Figure 4a,b). This in situ generated anion sensor showcased a model system which exhibits high sensitivity and selectivity, by signal transduction mechanism, which can effectively work in a neutral aqueous solution (i.e., at pH = 7).
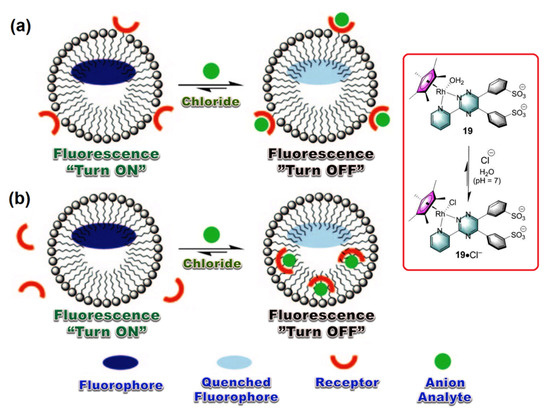
Figure 4.
(a,b) The proposed mechanism involved in “Turn OFF” luminescent sensing of Cl− anion as analyte by using a Rh(II)-metal complex. Inset: The working principle of activation of metal complex by chloride anion complexation. This graphic is reproduced with permission from reference [15], Copyright © 2022 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
Bhattacharya et al. reported a “Turn-ON” luminescence sensor composed by the surface modification of cationic micellar system (CTAB) with naphthalimide derivative [53]. Due to the partial CT from Br− to the naphthalimide moieties, the CTAB micellar adduct is non-emissive in nature. However, upon addition of strong anions (e.g., PO43−, SO42−, MoO42−, WO42−, H2PO4− etc.) this adduct exhibits significant enhancement of emission under identical experimental condition. It was speculated that these strong anions can effectively replace the bromide counter anions from the periphery of CTAB. Therefore, partial CT transition operating within the Br− anions to the naphthalimide moieties is restricted, that results a substantial fluorescence enhancement of the adduct. Moreover, these strong anions rearrange the initial spherical micellar structure to an elongated core-modified geometry as shown in Figure 5.
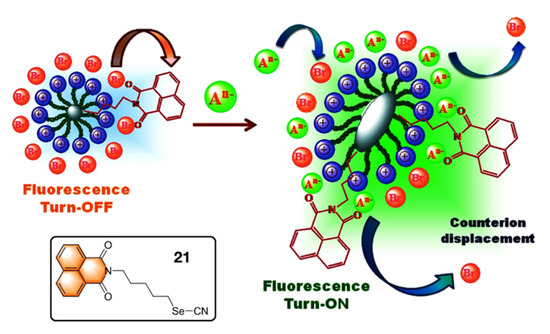
Figure 5.
The proposed mechanism involved in “Turn ON” luminescent sensing of anions as analytes. Inset: The chemical structure of the naphthalimide-based fluorophoric unit 21. This figure is reproduced with permission from reference [53], Copyright © 2022 Elsevier B.V.
Sessler and Khashab et al. reported an interesting thermo-responsive amphiphilic polymer, P1 {poly(N-isopropylacrylamide)-b-poly(calix[4]pyrrole-co-methyl methacrylate} [56] where hydrophobic calix[4]pyrrole (C4P) are attached as the anion receptor (Figure 6, Inset). This polymer was designed to capture the contaminated anions within pendent C4Ps in its micellar form which effectively works under an extraction-free condition. This is a typical demonstration of a water purification process by a synthetic C4P-based polymeric system where the target anions can be effectively “captured” and “removed” from contaminated water under easy experimental conditions. Owing to the thermo-responsive characteristics of the hydrophilic block chain, the anion incorporated micelles that can be precipitated out from the aqueous phase upon heating the aqueous solution at 50 °C. Then, a simple filtration technique can be employed to remove the generated precipitate from the anion contaminated aqueous solution. Finally, the pristine polymer (P1) can be easily recovered by treating the anion-trapped micelles with a dilute nitric acid (0.2 M) solution at room temperature. The whole reversible process of anion removal and regeneration of the polymer (P1) is provided in Figure 6.
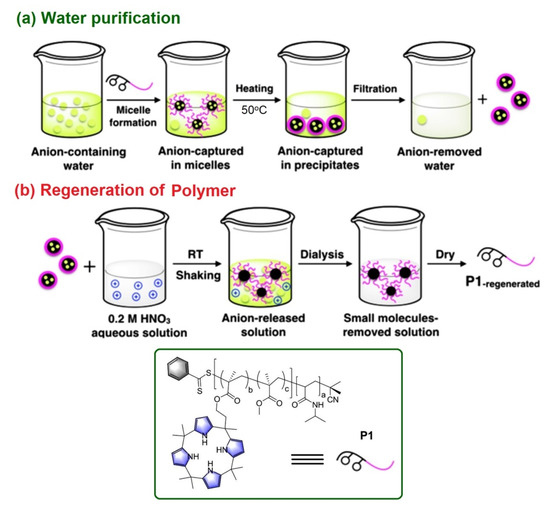
Figure 6.
(a) The proposed mechanism involved in targeted anion “capture” and “remove” by the use of polymer P1 with pendent C4P receptors. (b) The sequence used to regenerate the pristine polymer P1 under ambient condition. Inset: The chemical structure and cartoon representation of P1. Reproduced with permission from reference [56], 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
2.3. Luminescent Micellar Systems for Bio Sensing
Biosensors are the integrated receptor-transducer devices that emerge as a core research topic for many researchers in recent time. This is mainly due to their wide range of applications in the area of health care and diagnosis, environmental monitoring, etc. to name a few [57,58]. Among them, much attention has been devoted in designing the luminescent biosensors as they offer outstanding advantages, such as high sensitivity, low background noise, and facile sample preparation [59,60,61]. Considering the advancement of nanotechnology and nanoscience, nanostructured micellar probes have been considered as a promising candidate for bio-sensing owing to their structural flexibility and versatility [62]. However, only a handful of luminescent biosensor probes based on micellar systems appeared in the recent literature [16,17,18,19,20,63,64,65,66,67,68,69,70,71,72,73,74]. We will provide a very brief glimpse on these luminescent biosensors, which are used to create self-assembled nanostructure materials showing promise for use as next generation potential as “functional materials”.
Pioneering work by Li and Yang et al. on poly β-Cyclodextrin/TPdye nanomicelle-based two-photon nanoprobe is reported describing two-photon-induced fluorescent microscopic detection of enzymatic activities in living cells and tissues [63]. In this nanoprobe, a two-photon active (TPA) donor molecule trans-4-[p-(N,N-diethylamino)styryl]-N-methylpyridinium iodide (DEASPI) was used as a complementary combination with β-CDP nanomicelle. This combined adduct serves as a potential TPA fluorophore and also behaves like a carrier vehicle to deliver a specific peptide sequence to the living cell through fast endocytosis. For example, an adamantine GRRRDEVDK-BHQ2 peptide (Ad-DEVD-BHQ2) can be easily mounted to construct a robust inclusion complex, DEASPI/βCDP@Ad-DEVD-BHQ2 onto the nanomicellar surface by various noncovalent interactions (cf. Figure 7). The adduct DEASPI/βCDP@Ad-DEVD-BHQ2 was then used to demonstrate both in vitro and in vivo enzymatic activities assay of caspase-3 in the complex biological environment. From their experiments, it is evident that this “Turn-ON” fluorescent biosensor offers a new platform for high-contrast imaging of enzymatic activities within live cells and tissues. (Figure 7, Inset) Thus, DEASPI/βCDP@Ad-DEVD-BHQ2 nanoconjugate also offers the opportunity to screen enzyme inhibitors, which are also able to evaluate the apoptosis-associated disease progression.
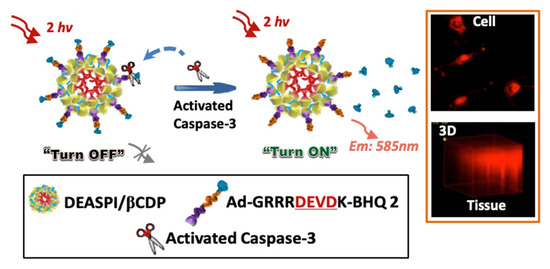
Figure 7.
Schematic illustration of the DEASPI/βCDP@Ad-DEVD-BHQ2 nanoconjugate for caspase-3 activity assay. Orange Inset: The TPM image of HeLa cells treated with 4 μM STS for 3 h after incubating with DEASPI/βCDP@Ad-DEVD-BHQ2 nanoconjugate (top); and TPM image of the 1.0 mm thick cervical tumor tissue slice pretreated with doxorubicin after incubating with DEASPI/βCDP@Ad-DEVD-BHQ2 nanoconjugate (0.15 mg/mL, concentration of the nanoconjugate refers to the concentration of βCDP). Black Inset: Cartoon representations of few components. This figure is reproduced with permission from reference [63], Copyright © 2022, American Chemical Society.
Another fluorescent micellar nanoprobe (NanoDPA-NMP-tyr) is reported describing sensing of tyrosinase (TYR) in a living B16 cellular medium [16]. NanoDPA-NMP-tyr is constructed with a hydrophobic interior of the amphiphilic copolymer mPEG-DSPE that shows a very fast response towards TYR with high sensitivity and selectivity with a detection limit of 0.057 U/mL. The sensing behavior involved in this nanoprobe is thought to be due to the Förster Resonance Energy Transfer (FRET) from 9,10-diphenylanthracene (DPA) (λem = ∼445 nm) donor to the naphthalimide-tyrosene (NMP-tyr) (λabs = ∼450 nm) acceptor.
Recently Lee et al. reported an interesting model sensor [17] to sense heparin (HP) with amplified fluorescence response resulted from inter-fluorophore excitation migration within a self-assembled micellar medium. For this purpose, a coumarin-based amphiphilic fluorescent molecule (see Figure 8, Inset) is designed which forms a micellar structure consisting of a hydrophobic core and hydrophilic surface, where the aggregated coumarins promote the energy and electron transport under bioanalytical conditions. The sensing response of micellized 22 (5.0 × 10−6 M) via ionic (cationic–anionic) interactions is also highly selective for HP among the other tested analytes e.g., dextrose, sucrose, glucose, mannitol, ATP, sodium citrate, Na2SO4, Na3PO4, sodium hyaluronate (HA), and chondroitin sulfate sodium salt (ChS) in a 10-mM HEPES buffer solution at pH 7.4 at ambient condition (Figure 8a). High selectivity of the micellized 22 (5.0 × 10−6 M) for HP via fluorescence suppression, reaches a quenching maximum of ~78.4% with an incubation of HP of concentration 2.0 × 10−6 M (Figure 8b). The sensing mechanism for the detection of heparin by the conjugated micellar system of coumarin derivative 22 is shown in Figure 8c.
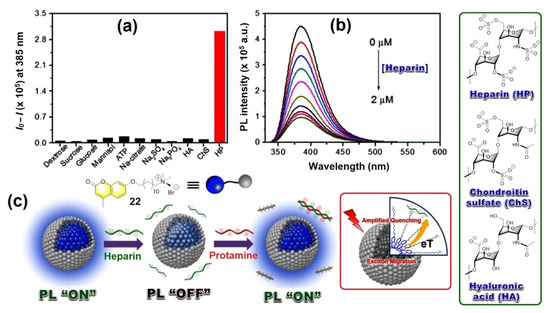
Figure 8.
(a) Fluorescence response of micellized 22 (5.0 × 10−6 M) towards various analytes (1.7 × 10−6 M), and (b) Fluorescence intensity changes of micellized 22 upon gradual addition of HP (0 ~ 2.0 × 10−6 M) in 10 mM HEPES buffer solution at pH 7.4. λexc = 320 nm and fluorescence intensity were monitored at a 385 nm wavelength. (c) The proposed mechanism involving “ON”/”OFF” fluorescence switching by heparin. Green Inset: Structure of various biomolecules. Red Inset: The schematic diagram of amplified quenching mechanism involved with micellized 22.
Fan et al. demonstrated discriminative luminescence sensors [18] for metal and nonmetal proteins by employing a pyrene/bispyrene-based fluorophoric system which are encapsulated in a cationic micellar structure. In case of a metal-free protein system, comparative luminescence intensity of monomer and excimer emission of pyrenes displayed quenching of an excimer emission whereas the monomer emission remain unchanged. On the other hand, metal containing proteins showed substantial quenching of both monomer and excimer emission under identical experimental conditions.
Selective detection of ATP is another important case of bio-sensing in recent times. Pang et al. have demonstrated a cationic squaraine dye encapsulated by CTAB based micelles, which can selectively detect ATP [19]. It is speculated that the ionic interactions between cationic CTAB head groups and anionic ATP along with the π−π staking of ATP and the dye molecules enormously contributed towards sensing behavior.
Likewise, Jiang et al. recently reported a luminescent micellar system [20] for ATP, comprised of a cationic surfactant, dodecyltrimethylammonium bromide (DTAB), and a near infrared (NIR) emissive cyanine dye. As mentioned above, a similar π−π staking within ATP and dye molecule triggered an effective energy transfer (ET) from the dye to ATP that resulted in significant quenching of emission intensity. A more detailed account on luminescent micellar sensors for sensing bioanalytes can be found in reference [4,5].
2.4. Luminescent Micellar Systems for Explosive Sensing
Trace level detection of explosive molecules is very important and timely in forensic investigations. Particularly, effective on-the-spot testing of suspected explosive analytes is in high demand. Micellar luminescent assembly for sensitive and discriminative detection of explosive samples is a topic of current interest which is gaining popularity in the last two decades. This is due to their simple design strategy and the use of cost-effective organic fluorophores (both amphiphilic and lipophilic) in an aqueous medium. Various nitroaromatic and nitramine explosives used so far to demonstrate sensing behavior are provided in Chart 3. We briefly described the reported micellar assemblies used for explosive detection in following four major categories.
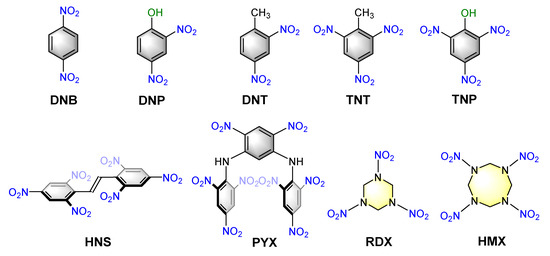
Chart 3.
Chemical structures of various nitroaromatic and nitramine explosives considered in this review article.
2.4.1. Hydrophobic Organic Fluorophore Encapsulated Micellar Systems
The strategy has been most widely studied and has proven as very cost effective but highly sensitive and selective for trace level detection of explosives. Here the hydrophobic organic fluorophore is added to an aqueous micellar solution and depending on the structural motif of the fluorophore, it occupies the core or periphery of the micellar host in the solution. The presence of explosive traces in the solution will result in a fluorescence response (typically either “Turn-OFF” or “Turn-ON” manner) through interaction with the fluorophore encapsulated in a micellar structure. The hydrophobic nature of the majority of explosive molecules also favors the strategy as those are extracted very efficiently from the bulk phase to micellar core, which ensures their ultralow detection limit. Taking advantage of the hydrophobic extraction, this new strategy has also been used for extraction and chromatographic/mass spectrometric analysis of such analytes under investigations.
Anslyn et al. used a powerful but cost-effective micellar sensor comprised by commercially available, nonionic, polysorbate surfactant-based (viz. Tween 80) to detect nitroaromatic and nitramine explosives [21] with high (96%) accuracy with a detection limit of 19 μM. The sensor design is encouraged by the fundamental concept of ratiometric luminescence quenching of pyrene monomer and excimer emission; and by the quenching of a pyrene–perylene FRET pair based dual emission.
When pyrene and perylene are co-dissolved in Tween 80, excitation of pyrene (λex = 336 nm) results in slightly relaxed pyrene monomer emission along with a significant perylene emission via FRET. In the presence of nitrated analytes (e.g., TNT as a representative example shown in Figure 9), this FRET-pair sensor did not display any ratiometric luminescence quenching in emission spectroscopic titration, however, both the pyrene and perylene-based emissions quenched substantially by these electron poor nitroaromatics and nitramines.
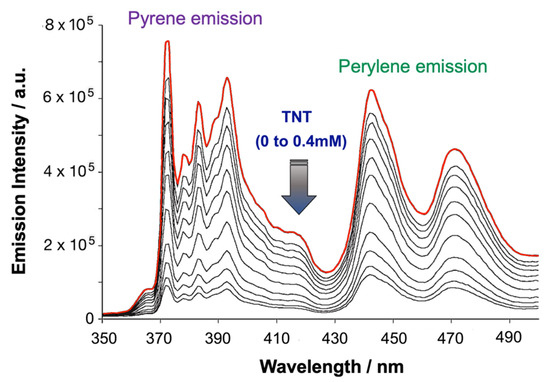
Figure 9.
Emission spectroscopic titration of pyrene−perylene (20 μM/20 μM) in Tween 80 (2 mM aqueous medium) micellar probe against the incremental addition of TNT (0 mM to 0.4 mM). This figure is reproduced with permission from reference [21], Copyright © 2022 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany).
Another interesting example of a dimeric pyrene-based luminophoric system (Py-diIm-Py) was reported by Ding et al. demonstrating explosive sensing [22] by the quenching of excimer emission. This dicationic luminophore effectively encapsulated the surface of the anionic sodium dodecyl sulfate (SDS) micelle as a result of electrostatic interactions from a highly luminescent micellar probe. However, in cationic dodecyl trimethyl ammonium bromide (DTAB) or in neutral Triton X-100 (TX100) micellar medium, py-diIm-Py mostly dispersed in solvent without significant interactions with surfactants. Such variation of the fluorophore location in the micellar medium drastically influences the sensing property of the adduct to the analytes. As noted by the authors, this system effectively sense explosives by means of presumed quenching of excimer emission with a favorable geometrical and electronic (D–A) complementarity with analytes. The results shown in Figure 10 revealed that the electron poor NACs are sandwiched by two pyrene moieties that results in the disintegration of excimers.
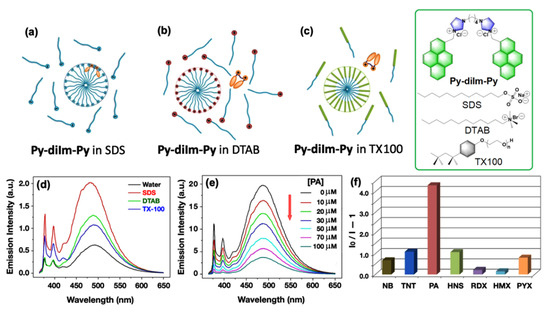
Figure 10.
(a–c) Proposed fluorophoric distribution in 8 mM SDS, 14 mM DTAB, and 0.24 mM TX100 at ambient condition. (d) Emission spectra of 1.0 μM Py-diIm-Py solution in water, and various micellar media. (e) The emission spectroscopic titration result of Py-diIm-Py@SDS micellar adduct with incremental addition of PA as analyte. (f) The bar diagram depicting emission quenching efficacy of 100 μM explosive analytes to the Py-diIm-Py@SDS micellar sensor. [Py-diIm-Py] = 1.0 μM; [SDS] = 8 mM; [DTAB] = 14 mM; [TX100] = 0.24 mM; λex = 345 nm in each case. Inset: The chemical structures of Py-diIm-Py, SDS, DTAB, and TX100, respectively. This figure is reproduced with permission from reference [22], Copyright © 2022, American Chemical Society.
A similar type of monomeric and dimeric pyrene-derivatives were reported by Cho et al. to detect the ppb level of TNT and RDX in the CTAB micellar medium [24]. Like the cationic Py-diIm-Py-based micellar systems discussed earlier, these neutral monomers and dimeric probes show the sensing behavior by exhibiting excimer quenching phenomenon in the presence of explosive analytes.
Amphiphilic pyrene-based luminophores can be self-assembled to form micellar structures in an aqueous phase and can be used for sensing explosive traces. This technique eliminates the need of using additional surfactants-based micelles. Although this strategy looks attractive and promising, very little exposure has been given in this topic. Chupakhin et al. synthesized a series of amphiphilic pyrene derivatives [25] and investigated their structural dynamics, luminescence behavior for sensing performance in aqueous phase. Their studies showed that by introducing various hydrophilic tail groups to the hydrophobic pyrene head results in the formation of micellar structures in an aqueous solution at a concentration of ≤10−5 M. Interestingly, in aqueous micellar structures, the system showed exclusively monomer emission without exhibiting excimer emission. The monomeric pyrene emission peaks (at 384 and 402 nm) with high quantum yield (up to 0.76) were obtained in an aqueous micellar form, which is much higher than the emission quantum yield of pyrene in an organic phase. Taking advantage of π-electron rich pyrene-based micellar probes, another series of chemosensors is also reported [26], demonstrating sensing of ultra-trace nitramine explosive in an aqueous medium at a 12-ppb level. The sensing mechanism involves photo-induced electron transfer (PET) from the LUMO (−1.64 eV) of pyrene-based micellar adduct to the LUMO of RDX (−2.96 eV) or other nitroaromatic compounds as inferred from DFT calculations.
A perylene monoamide-based fluorescent micellar probe (PMI-OH) was reported [27] by Zhou and Yu et al. The ability of this system to detect PA was investigated in a solution phase using UV–vis and emission spectroscopic techniques. The PMI-OH sensor (cf. Figure 11, Inset) exhibits enhanced excimer emission at λmax at 630 nm with a nonionic surfactant Triton X-100 (TX100) which significantly quenched upon incremental addition of PA as a result of electron transfer (ET) from the PMI-OH donor to the PA acceptor.
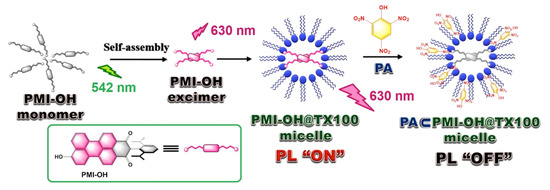
Figure 11.
Schematic representation of the “ON” and “OFF” states of the PMI-OH@TX100 micellar probe in the presence of PA.
A significant quenching efficacy of 91.53% was reported in presence of PA, whereas, DNP and NP show 62.26% and 53.13%, respectively under identical experimental conditions. This observation is directly related to the lowest reduction potential of PA among the tested analytes which therefore, serve as the most efficient electron acceptor among this series. The proposed mechanism (see Figure 11) is associated with the reverse micellar system wherein PMI-OH excimers formed at the hydrophilic core resulted in luminescence switched “ON”. However, in the presence of PA, an “OFF” state resulted due to the electron transfer transition from the PMI-OH excimer (−3.73 eV) to the LUMO of PA (−3.89 eV). It was speculated that the hydroxyl groups play an important role on forming such a luminous micellar core by proper geometrical arrangement within the hydrophilic pocket.
Apart from pyrene- or perylene-based fluorogenic systems, star-shaped truxene-based hyperbranched π-conjugated polymeric (HCP) chemosensors [75] were also used for sensing the explosive materials in an aqueous medium. Inspired by the pioneering works of Swager [76,77] on poly(p-phenyleneethynylene) (PPE)-based fluorogenic polymers, these hyperbranched truxene derivatives were introduced which exhibit high efficacy towards explosive sensing due to a “molecular wire effect” owing to the presence of multiple acetylene moieties. When the hydrophobic HCPs are mixed with amphiphilic block copolymer, e.g., F127 (a polyethyleneglycol-polypropyleneglycol polymer) highly luminophoric nanomicellar systems can be produced that works more efficiently in aqueous medium compared to organic solvent (e.g., in THF). Upon addition of the explosive analytes in an aqueous medium, substantial quenching of HCP-based luminescence was seen (Figure 12). It is speculated that the presence of alkyl and alkyloxy side chains might play an important role on sensing performance of these HCPs. Moreover, the pluronic F-127 assists to solubilize the hydrophobic HCP polymers into the aqueous phase which triggered effective sensing of explosive analytes in the aqueous medium. The luminescence quenching phenomena in presence of explosives resulted from the Förster energy transfer (FRET) mechanism operating within the HCP polymer and the added explosive analytes as indicated by the non-linear nature of Stern–Volmer plots. It is reported that the luminescence quenching efficiency and the Ksv values follows the trend 2NA > PA > NP > TNT > DNB > DNT > 3NA > NB. The limit of detection (LOD) of HCPs and their micelles for the analytes are provided in Table 1.
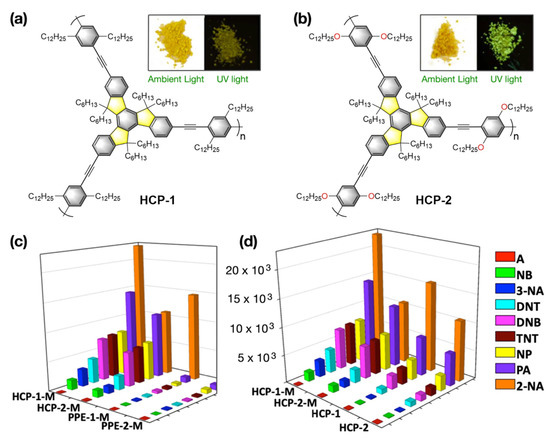
Figure 12.
(a,b) The chemical structures of truxene-based polymeric luminophores HCP-1 and HCP-2. Inset: The photographs of HCP-1 and HCP-2 under ambient light and upon irradiation provided by a UV lamp. (c,d) Fluorescence quenching efficiencies of HCPs-M and PPEs-M for different analytes (left) and fluorescence quenching efficiencies of HCP-1, HCP-2, HCP-1-M, and HCP-2-M respectively, for different analytes (right). The z-axis denotes the Stern–Volmer constant KSV. This figure is reproduced with permission from reference [75], Copyright © 2022, American Chemical Society.

Table 1.
LOD for HCPs and HCPs/Micellar adducts (viz. HCP-1-M and HCP-2-M) towards various analytes.
Wang and Sun et al. reported a more simplified version of conjugated fluorescent polymers, viz. poly(9,9′-dioctylfluorene (PFO), and poly(2,7-(9,9-hexylfluorene)-alt-4,4′-phenylether (PFPE), which can effectively sense the trace amount of NACs in an aqueous medium. In the presence of Pluronic F127, these hydrophobic polymeric probes self-assembled in the form of nanoaggregates [78] or nanomicelles [79] in water. Like other polymeric systems, in the case of these hybrid polymeric probes, the sensing mechanism follows a similar deactivation pathway in the presence of NAC analytes.
Bhattacharya et al. synthesized two p-phenylenevinylene-based inexpensive micellar chromogenic probes, 24 [80], (Figure 13, inset) with various conjugation to demonstrate naked eye sensing of TNT in an aqueous medium. Significant fluorescence quenching (4.5 to 5 fold) in the presence of TNP was demonstrated with the help of both the probes.
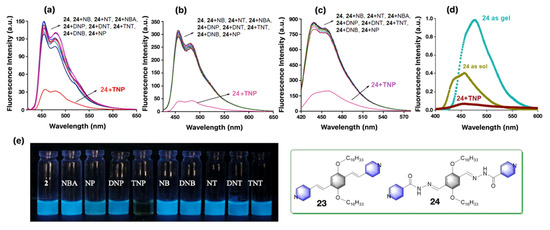
Figure 13.
Fluorescence response of molecular probe 24 (10 μM) against various explosive analytes in water (pH 7.0), (a); in 1mM Brij-58 micellar adduct, (b); in THF, (c); respectively, at 298 K (λex = 390 nm). (d) The emission spectroscopic changes of 14 mM of probe 24 (λex = 390 nm) in sol and gel in THF and in the presence of 2 equiv TNP at 298 K. (e) The photographs of the luminescent solution of 24 as seen in the absence and presence of indicated explosive analytes with irradiation provided by 365-nm UV lamp at ambient condition. Inset: The chemical structures of the chromophoric units with various conjugations used for sensing nitroaromatic explosives in these multiple media. This figure is reproduced with permission from reference [80], Copyright © 2022, American Chemical Society.
Interestingly, the probes are equally effective in micellar, organogel, and solid-state strip as shown in Figure 13, which can be used for on-site detection of the explosive materials in the nanomolar range. The visual optical change under 365-nm UV lamp of 20 μM solution of 24 in the presence of Brij-58 micellar (1mM) adduct in the presence of 2 equiv of TNP as analyte exhibits a significant emission quenching (cf. Figure 13e). A similar type of D–A supramolecular ET transition from the electron rich chromophoric probes (23 and 24) to the electron poor nitroaromatic (NACs) analytes was thought to be operational in a sensing event.
Zhang et al. also reported an amphiphilic cellulose-based micelle encapsulated TPE based fluorophore for trace level detection of nitroaromatic explosives in water [81]. Their strategy used an amphiphilic cationic cellulose derivative where hydrophobic long alkyl chains were introduced. The cellulose molecule forms micellar structures in aqueous medium and the hydrophobic TPE fluorophore gets encapsulated in its core through hydrophobic interaction with alkyl chains. An enhanced orange emission (λmax = 533 nm) of the micelle encapsulated TPE fluorophore was observed which gets quenched in the presence of electron-deficient nitroaromatic explosive molecules (TNT, TNP, DNP, DNT, NT, and NP). The reported sensor showed the highest detection efficiency for TNP with a detection limit of 50 nM.
In addition to these fully organic classic luminophoric systems, cyclometallated metal complexes can be used as an alternate source to construct potential luminophoric adducts. For example, few of the current authors reported [23] an interesting luminophoric micellar adduct for sensing the nitroaromatic explosives in a solution phase, vapor phase, as well as a solid-state paper strip at ambient condition by using the cyclometalated Pt(II)C^N^N-based luminophoric units. As the hydrophobic alkyl chains (C12H25) are immersed into the lyophilic micelle core, the PtC^N^N head-groups might have been positioned on the surface of micelles to create a highly luminophoric platform which can effectively detect these explosives in an aqueous medium by quenching the Pt(II)C^N^N-based intense luminescence. It was speculated that the quenching mechanism involves an intermolecular supramolecular charge transfer (CT) transition originating from Pt(II)C^N^N-antenna moiety to the electron deficient explosives (cf. Figure 2b). Photoluminescence titrations of various Pt(II)C^N^N/micellar adducts (e.g., Pt(II)C^N^N-NC12H25/Triton X-100, Pt(II)C^N^N-NC12H25/SDS, and Pt(II)C^N^N-NC12H25/CTAB) against incremental addition of TNP (upto 200 μM) as shown in Figure 14 a–c, indicate significant luminescence quenching phenomena as the result of excited state photo-induced electron transfer (PET) process. Lifetime measurements were performed to understand the effect of nitroaromatic analytes on excited state behavior of Pt(II)C^N^N-NC12H25/micellar adduct. Moderate reduction of lifetime values from 49 ns to 35 ns (in presence of 2 equiv of TNT) and 31 ns (in presence of 2 equiv of TNP) were seen which support the luminescence quenching by PET from Pt(II)C^N^N-NC12H25/micelle donor to the electron poor explosive acceptors.
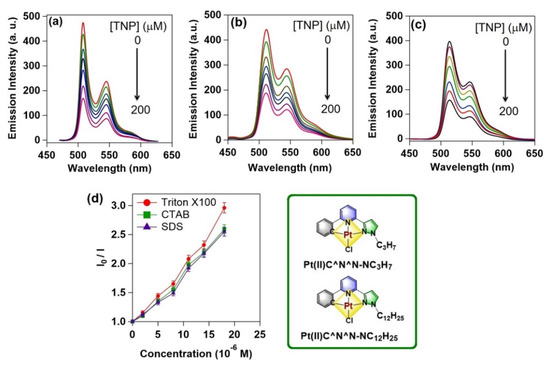
Figure 14.
(a–c) Emission spectroscopic titration results of Pt(II)-C^N^N-NC12H25 impregnated micelles (Triton X-100, SDS, CTAB, respectively) with incremental addition of 0 μM to 200 μM TNP as explosive analyte in aqueous medium at 298 K. (d) Stern-Volmer plots obtained from each individual emission spectroscopic titration experiments. Inset: The chemical structures of Pt(II)C^N^N-based luminophoric materials with two different alkyl chains (e.g., C3H7 and C12H25) used for making the micellar adducts. This figure is reproduced with permission from reference [23], Copyright © 2022, American Chemical Society.
2.4.2. Aggregation-Induced Emission (AIE)-Based Luminescent Micellar Systems for Explosive Sensing
Aggregation-induced emission is a distinctive photophysical phenomenon where structurally flexible weak luminescent probes exhibit strong emission upon aggregation due to the induced vibrational rigidity [82]. The most common strategy to realize AIE is to add a nonsolvent in the solution of such molecules to stimulate aggregation by decreasing the solubility or making solid state material of such molecules through polymerization. Micellar structures with AIE active molecules also result in strong emission through aggregation of the monomer units in the micellar core and are the potential candidates for detection of explosive traces in the aqueous phase. Moreover, extraction of hydrophobic explosives from aqueous phase to the micellar core is an added advantage. The most widely used AIE compound used in present days is tetraphenylethylene (TPE). Liang et al. successfully anchored TPE to an amphiphilic triblock polymer, which self-assembled to form micellar structures in an aqueous medium (cf. Figure 15) [83]. As described, the hydrophobic TPE chromophore of the block polymer is self-organized in the micellar core-shell interface region, resulting in significant aggregation. Consequently, around 60-fold luminescence enhancement was noticed. The hydrophobic aromatic pollutants (e.g., benzene, toluene, xylene, ehtylbenzene, etc.) present in the aqueous medium can be efficiently extracted to the micellar core. This causes swelling of micelles as evidenced by their increased hydrodynamic diameter. However, the swelling process reduces aggregation among TPE chromophores, that exhibit quenched fluorescence intensity. The process is ultrafast and a nice concentration dependent fluorescence quenching phenomenon was observed with ultralow detection limit of 1 μg/L.
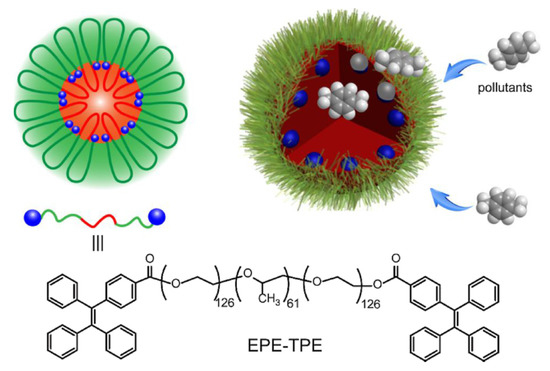
Figure 15.
Schematic illustration of swellable micelles of fluorescent polymers for sensing pollutants in water. This figure is reproduced with permission from reference [83], Copyright © 2022 Elsevier B.V.
The excited state lifetime of TPE micelle was found to be 3.95 ns, which gradually decreases upon gradual addition of aromatic compounds and hence it was confirmed that a dynamic collisional quenching mechanism is operative within the micelle–pollutant host–guest supramolecular system.
A very similar TPE-based amphiphilic molecule was synthesized by Nabeel et al., where arboxyl functionalized hyperbranched polymer, poly(3-ethyl-3-oxetanemethanol)-star-poly(ethylene oxide) was linked with TPE (Figure 16) [84]. The AIE chromophore anchored amphiphilic polymer forms spherical aggregates in aqueous THF and the resulted construct showed highly enhanced TPE-based aggregation induced emission. This aggregated chromophore was utilized for sensitive detection of PA in aqueous phase by fluorescence quenching process. PA being highly electron poor analyte readily forms donor–acceptor adduct with aggregated TPE moieties by π–π interactions that resulted in fluorescence quenching through electron transfer process. Ultralow detection limit (up to 20 ppb) was achieved and hence the strategy is very effective for trace level detection of PA in an aqueous phase.
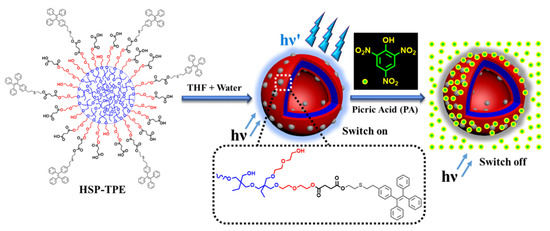
Figure 16.
Illustration of self-assembly of the copolymer (HSP-TPE) containing fluorescent TPE probe and sensing behavior of HSP-TPE aggregates towards picric acid (PA). In HSP-TPE. the blue, red, and grey color present hydrophobic core, hydrophilic arms, and probe molecules resp. The PL intensity of HSP-TPE aggregates is quenched upon addition of picric acid. This figure is reproduced with permission from reference [84], Copyright © 2022 Elsevier B.V.
Metal complex as AIE chromophore have also been successfully demonstrated by Rajagopal et al. for trace level detection of explosives [85]. Two alkoxy-bridged binuclear Re(I) complexes 25a,b were used (see Figure 17, Inset), which showed poor luminescence in organic solvent (CH2Cl2) where the complexes are highly soluble. However, upon addition of a nonsolvent (e.g., acetonitrile, in which 25a,b is sparingly soluble) or in common surfactant (CTAB, SDS, Triton X-100, where the solubility of 25a,b is less)-based micellar environment show highly enhanced emission (~500 times) which results through AIE.
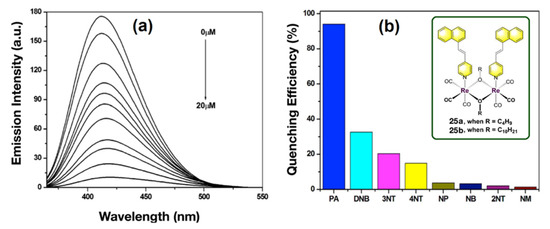
Figure 17.
(a) Emission change of nanoaggregates of complexes 25b (20 μM) in CH2Cl2/CH3CN mixture (10:90 v/v) with incremental addition of PA. (b) The bar diagram representing the relative emission quenching of complex 25b after adding 20 equiv of various added analytes. Inset: The structure of the alkoxy-bridged binuclear Re(I) complexes. This figure is reproduced with permission from reference [85], Copyright © 2022, American Chemical Society.
The long hydrophobic alkyl chain of these complexes enables their incorporation in the hydrophobic region of the micelle or in reverse micelle. Among various NAE, PA showed the highest quenching of the emission spectra with a very high Stern–Volmer constant of 1 × 105 M−1 and the authors demonstrated selective sensing of PA among other NAE.
2.4.3. Ratiometric Luminescence Sensing of Explosives by Micellar Structure Containing Two Luminophores
Sensing of target analytes by means of ratiometric luminescence probes is an effective technique compared to that of single luminophoric “Turn ON/OFF” molecular sensors. This is mainly due to the high sensitivity and reliability of a ratiometric probe which is reflected by their self-calibration resulted from two (or more) distinguished spectral signatures [86]. Likewise, ratiometric sensing of explosives by luminophoric micellar adducts could be realized by introducing two different luminophores in the same medium. The design strategy could be made by considering selective interactions of explosives with one of the luminophores while the other luminophore remains innocent towards analytes. Recently, Maity et al. successfully demonstrated a ratiometric luminescence probe for the detection of nitroaromatic explosives by employing two different luminescent metal complexes (viz. red luminescent Eu(III)-complex and green luminescent Pt(II)C^N^N-complex) in micellar host [87]. In a nonionic surfactant-based aqueous micellar structure (e.g., in TX100), both the luminophores were added simultaneously wherein the hydrophobic Eu(III)-complex occupies the hydrophobic micellar core, and partially ionic Pt(II)C^N^N-complexes remain attached on a micellar surface. The addition of PA to the micellar adduct resulted in significant quenching of Pt(II)C^N^N-based emission (at 508–545 nm) as a result of supramolecular D–A interactions with Pt(II)C^N^N-luminophore grafted on the surface. However, the red emission (614 nm) contributed by Eu(III)-complex remains slightly increased (Figure 18). In contrary, when the titration was performed against PA in CH2Cl2, quenching of both emission peaks was observed and hence confirms the very similar D–A supramolecular interactions between PA with both luminophores (Figure 19).
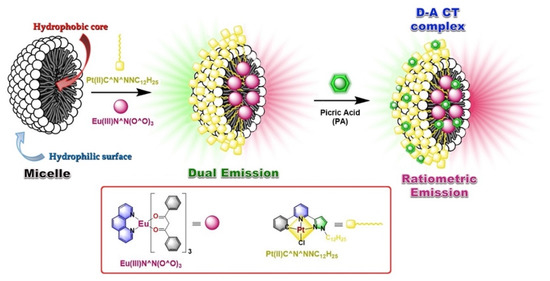
Figure 18.
The proposed model of ratiometric probe showing ratiometric sensing of PA at ambient condition in an aqueous medium. The model also depicts preferential entrapment of luminophores and analytes within the micelle adduct. Inset: The chemical structures of luminophores Eu(III)N^N(O^O)3 and Pt(II)C^N^NNC12H25.
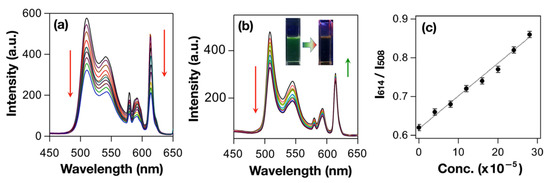
Figure 19.
(a) PL titration spectra of Eu(III)N^N(O^O)3+Pt(II)C^N^NNC12H25 composite in DCM at 298 K with various amounts of TNP, (b) PL titration spectra of Triton X-100@ Eu(III)N^N(O^O)3+Pt(II)C^N^NNC12H25 composite in water at 298 K with various amount of TNP, and (c) the ratiometric emission intensity plot of I614/I508 vs. concentrations of TNP obtained from the luminescence titration plot. This figure is reproduced with permission from reference [87].
3. Conclusions and Future Scope
In summary, we reviewed various luminescent micellar probes used as chemosensors for different analytes (e.g., metal ions, anions and biomolecules, in brief) with major focus on explosive sensing in an aqueous medium. Overall, these micellar systems offer several advantages including simple operation techniques, and production of low-cost sensing materials with synthetically less-challenging steps. Moreover, these functional materials are highly sensitive with fast response time, which also provide available options of using hydrophobic, hydrophilic, or amphiphilic luminophoric adducts on desired. Likewise, efficient extraction of hydrophobic explosive analytes to micellar core were demonstrated for sensitive detection of target analytes. The luminophores comprising small organic or inorganic metal complexes, large organic macromolecules/polymers, and AIE systems, used for effective sensing of explosives are exclusively described. Although the micellar luminescence sensing of explosives is quite encouraging for analytical chemists in terms of simplicity, cost effectiveness, and sensitivity, but there are some real challenges that remain unsolved and need to be explored in coming years. Firstly, among various categories of explosive analytes, nitroaromatic explosives have been explored exclusively, however, only a handful of model sensor systems are reported so far which showcased selectivity and sensitivity for non-aromatic nitramine explosives like RDX, PETN, and HMX. Secondly, in terms of mechanistic understanding, these complex supramolecular systems are still not fully understood, which required more in-depth photophysical studies coupled with theoretical modeling. Moreover, the existing sensing strategies are mainly relied on spherical micellar systems, although other micellar structures, like for example liposome, niosome, vesicles, and planar bilayers may open new sensing opportunities considering selectivity and sensitivity. Furthermore, properly designed fluorescent surfactants capable of forming micellar structures in an aqueous medium in the absence of additional surfactant with effective sensing performance is another opportunity to explore. Finally, as discussed in this current review article we believe that the luminescent nanomaterials constructed by metal clusters while encapsulated within a self-assembled micellar medium may provide effective sensor systems.
Author Contributions
Conceptualization, P.M., R.P. and A.J.; software, P.M. and A.J.; writing—original draft preparation, S.P., R.S., S.B.M., P.M., R.P. and A.J.; writing—review and editing, P.M. and A.J.; visualization, A.J.; supervision, A.J.; project administration, A.J.; All authors have read and agreed to the published version of the manuscript.
Funding
P.M. thanks the Council of Scientific and Industrial Research (CSIR), India (project no. 01(2873)/17/EMR-II) for financial support. R.P. is thankful to the Science and Engineering Research Board (SERB), Government of India, (file no CRG/2018/1377)) for funding. A.J. is grateful to the Science and Engineering Research Board (SERB), Government of India, for a core research grant (CRG/2021/000674).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mancin, F.; Scrimin, P.; Tecilla, P.; Tonellato, U. Amphiphilic metalloaggregates: Catalysis, transport, and sensing. Coord. Chem. Rev. 2009, 253, 2150–2165. [Google Scholar] [CrossRef]
- Mancin, F.; Rampazzo, E.; Tecilla, P.; Tonellato, U. Self-assembled fluorescent chemosensors. Chem. Eur. J. 2006, 12, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Diaz-Fernandez, Y.A.; Pasotti, L. Micelles as nanosized containers for the self-assembly of multicomponent fluorescent sensors. Coord. Chem. Rev. 2009, 253, 2226–2240. [Google Scholar] [CrossRef]
- Dey, N.; Bhattacharya, S. A Glimpse of Our Journey into the Design of Optical Probes in Self-assembled Surfactant Aggregates. Chem. Rec. 2016, 16, 1934–1949. [Google Scholar] [CrossRef]
- Fan, J.; Ding, L.; Fang, Y. Surfactant aggregates encapsulating and modulating: An effective way to generate selective and discriminative fluorescent sensors. Langmuir 2019, 35, 326–341. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Grandini, P.; Mancin, F.; Tecilla, P.; Scrimin, P.; Tonellato, U. Exploiting the Self-Assembly Strategy for the Design of Selective CuII Ion Chemosensors. Angew. Chem. Int. Ed. 1999, 38, 3061–3064. [Google Scholar] [CrossRef]
- Berton, M.; Mancin, F.; Stocchero, G.; Tecilla, P.; Tonellato, U. Self-Assembling in Surfactant Aggregates: An Alternative Way to the Realization of Fluorescence Chemosensors for Cu(II) Ions. Langmuir 2001, 17, 7521–7528. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gulyani, A. First report of Zn2+ sensing exclusively at mesoscopic interfaces. Chem. Commun. 2003, 39, 1158–1159. [Google Scholar] [CrossRef]
- Gujar, V.; Sangale, V.; Ottoor, D. A Selective Turn off Fluorescence Sensor Based on Propranolol-SDS Assemblies for Fe3+ Detection. J. Fluoresc. 2019, 29, 91–100. [Google Scholar] [CrossRef]
- Qiao, M.; Fan, J.; Ding, L.; Fang, Y. Fluorescent Ensemble Sensors and Arrays Based on Surfactant Aggregates Encapsulating Pyrene-Derived Fluorophores for Differentiation Applications. ACS Appl. Mater. Interfaces 2021, 13, 18395–18412. [Google Scholar] [CrossRef] [PubMed]
- Ábalos, T.; Royo, S.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Costero, A.M.; Gil, S.; Parra, M. Surfactant-assisted chromogenic sensing of cyanide in water. New J. Chem. 2009, 33, 1641–1645. [Google Scholar] [CrossRef]
- Hu, R.; Feng, J.; Hu, D.; Wang, S.; Li, S.; Li, Y.; Yang, G. A Rapid Aqueous Fluoride Ion Sensor with Dual Output Modes. Angew. Chem. Int. Ed. 2010, 49, 4915–4918. [Google Scholar] [CrossRef] [PubMed]
- Riis-Johannessen, T.; Severin, K. A Micelle-Based Chemosensing Ensemble for the Fluorimetric Detection of Chloride in Water. Chem. Eur. J. 2010, 16, 8291–8295. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, J.; Teng, Z.; Cao, T.; Gong, D.; Liu, W.; Cao, Y.; Qin, W.; Guo, H.; Iqbal, A. Self-Assembling Ratiometric Fluorescent Micelle Nanoprobe for Tyrosinase Detection in Living Cells. ACS Appl. Nano Mater. 2019, 2, 3819–3827. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, B.; Roh, E.; Kim, H.; Lee, S.H. Micellization-induced amplified fluorescence response for highly sensitive detection of heparin in serum. Sci. Rep. 2020, 10, 9438. [Google Scholar] [CrossRef]
- Fan, J.; Ding, L.; Bo, Y.; Fang, Y. Fluorescent Ensemble Based on Bispyrene Fluorophore and Surfactant Assemblies: Sensing and Discriminating Proteins in Aqueous Solution. ACS Appl. Mater. Interfaces 2015, 7, 22487–22496. [Google Scholar] [CrossRef]
- Xu, Y.; Malkovskiy, A.; Wang, Q.; Pang, Y. Molecular assembly of a squaraine dye with cationic surfactant and nucleotides: Its impact on aggregation and fluorescence response. Org. Biomol. Chem. 2011, 9, 2878–2884. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, M.; Luo, H.; Zhang, Q.; Guo, L.; Li, Z.; Jiang, Y. Aggregation-Switching Strategy for Promoting Fluorescent Sensing of Biologically Relevant Species: A Simple Near-Infrared Cyanine Dye Highly Sensitive and Selective for ATP. Anal. Chem. 2017, 89, 6210–6215. [Google Scholar] [CrossRef]
- Hughes, A.D.; Glenn, I.C.; Patrick, A.D.; Ellington, A.; Anslyn, E.V. A Pattern Recognition Based Fluorescence Quenching Assay for the Detection and Identification of Nitrated Explosive Analytes. Chem. Eur. J. 2008, 14, 1822–1827. [Google Scholar] [CrossRef]
- Ding, L.; Bai, Y.; Cao, Y.; Ren, G.; Blanchard, G.J.; Fang, Y. Micelle-Induced Versatile Sensing Behavior of Bispyrene-Based Fluorescent Molecular Sensor for Picric Acid and PYX explosives. Langmuir 2014, 30, 7645–7653. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Bhatt, A.; Agrawal, B.; Jana, A. Pt(II)C^N^N-Based Luminophore−Micelle Adducts for Sensing Nitroaromatic Explosives. Langmuir 2017, 33, 4291–4300. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Choi, J.-H.; Cho, D.-G. Simple Pyrene Derivatives as Fluorescence Sensors for TNT and RDX in Micelles. Bull. Korean Chem. Soc. 2014, 35, 3158–3162. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Taniya, O.S.; Slovesnova, N.V.; Kim, G.A.; Santra, S.; Zyryanov, G.V.; Kopchuk, D.S.; Majee, A.; Charushin, V.N.; Chupakhin, O.N. Fluorescent Detection of 2,4-DNT and 2,4,6-TNT in Aqueous Media by Using Simple Water-Soluble Pyrene Derivatives. Chem. Asian. J. 2016, 11, 775–781. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Taniya, O.S.; Kopchuk, D.S.; Giri, K.; Mukherjee, A.; Santra, S.; Majee, A.; Rahman, M.; Zyryanov, G.V.; Bakulev, V.A.; et al. 1-Hydroxypyrene-based micelle-forming sensors for the visual detection of RDX/TNG/PETN-based bomb plots in water. New J. Chem. 2018, 42, 19864–19871. [Google Scholar] [CrossRef]
- Li, W.; Zhou, H.; Nawaz, M.A.H.; Niu, N.; Yang, N.; Ren, J.; Yu, C. A perylene monoimide probe based fluorescent micelle sensor for the selective and sensitive detection of picric acid. Anal. Methods 2020, 12, 5353–5359. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Mukherjee, P.S. π-electron rich small molecule sensors for the recognition of nitroaromatics. Chem. Commun. 2015, 51, 16014–16032. [Google Scholar] [CrossRef] [PubMed]
- To, K.C.; Ben-Jaber, S.; Parkin, I.P. Recent Developments in the Field of Explosive Trace Detection. ACS Nano. 2020, 14, 10804–10833. [Google Scholar] [CrossRef]
- Clarke, S. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd edition (Charles, T.J.). J. Chem. Educ. 1981, 58, A246. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile Nanoarchitectonics: From Basic Physical Chemistry to Advanced Applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef]
- Peng, H.Q.; Niu, L.Y.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Biological Applications of Supramolecular Assemblies Designed for Excitation Energy Transfer. Chem. Rev. 2015, 115, 7502–7542. [Google Scholar] [CrossRef]
- Fernandez, Y.D.; Gramatges, A.P.; Amendola, V.; Foti, F.; Mangano, C.; Pallavicini, P.; Patroni, S. Using micelles for a new approach to fluorescent sensors for metal cations. Chem. Commun. 2004, 40, 1650–1651. [Google Scholar] [CrossRef]
- Nakahara, Y.; Kida, T.; Nakatsuji, Y.; Akashi, M. Fluorometric sensing of alkali metal and alkaline earth metal cations by novel photosensitive monoazacryptand derivatives in aqueous micellar solutions. Org. Biomol. Chem. 2005, 3, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhong, Z. Detection of Hg2+ in Aqueous Solutions with a Foldamer-Based Fluorescent Sensor Modulated by Surfactant Micelles. Org. Lett. 2006, 8, 4715–4717. [Google Scholar] [CrossRef][Green Version]
- Mallick, A.; Mandal, M.C.; Haldar, B.; Chakrabarty, A.; Das, P.; Chattopadhyay, N. Surfactant-Induced Modulation of Fluorosensor Activity: A Simple Way to Maximize the Sensor Efficiency. J. Am. Chem. Soc. 2006, 128, 3126–3127. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Diaz-Fernandez, Y.A.; Foti, F.; Mangano, C.; Patroni, S. Fluorescent Sensors for Hg2+ in Micelles: A New Approach that Transforms an ON–OFF into an OFF–ON Response as a Function of the Lipophilicity of the Receptor. Chem. Eur. J. 2007, 13, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, X.; Qian, J.; Xu, Y. Micelle-Induced Versatile Performance of Amphiphilic Intramolecular Charge-Transfer Fluorescent Molecular Sensors. Chem. Eur. J. 2007, 13, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Avirah, R.R.; Jyothish, K.; Ramaiah, D. Dual-Mode Semisquaraine-Based Sensor for Selective Detection of Hg2+ in a Micellar Medium. Org. Lett. 2007, 9, 121–124. [Google Scholar] [CrossRef]
- Pallavicini, P.; Pasotti, L.; Patroni, S. Residual and exploitable fluorescence in micellar self-assembled ON-OFF sensors for copper(II). Dalton Trans. 2007, 36, 5670–5677. [Google Scholar] [CrossRef]
- Das, P.; Mallick, A.; Sarkar, D.; Chattopadhyay, N. Application of anionic micelle for dramatic enhancement in the quenching-based metal ion fluorosensing. J. Collo. Inter. Sci. 2008, 320, 9–14. [Google Scholar] [CrossRef]
- Ding, L.; Wang, S.; Liu, Y.; Cao, J.; Fang, Y. Bispyrene/surfactant assemblies as fluorescent sensor platform: Detection and identification of Cu2+ and Co2+ in aqueous solution. J. Mater. Chem. A 2013, 1, 8866–8875. [Google Scholar] [CrossRef]
- Kumari, N.; Dey, N.; Jha, S.; Bhattacharya, S. Ratiometric, Reversible, and Parts per Billion Level Detection of Multiple Toxic Transition Metal Ions Using a Single Probe in Micellar Media. ACS Appl. Mater. Interfaces 2013, 5, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Mishra, A.; Krishnamoorthy, G. Specific site binding of metal ions on the intramolecular charge transfer fluorophore in micelles. Analyst 2013, 138, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Jha, S.; Misra, S.K.; Bhattacharya, S. A Probe for the Selective and Parts-per-Billion-Level Detection of Copper(II) and Mercury(II) using a Micellar Medium and Its Utility in Cell Imaging. ChemPlusChem 2014, 79, 1059–1064. [Google Scholar] [CrossRef]
- Cao, Y.; Ding, L.; Hu, W.; Peng, J.; Fang, Y. A surfactant-modulated fluorescent sensor with pattern recognition capability: Sensing and discriminating multiple heavy metal ions in aqueous solution. J. Mater. Chem. A 2014, 2, 18488–18496. [Google Scholar] [CrossRef]
- Wang, S.; Ding, L.; Fan, J.; Wang, Z.; Fang, Y. Bispyrene/Surfactant-Assembly-Based Fluorescent Sensor Array for Discriminating Lanthanide Ions in Aqueous Solution. ACS Appl. Mater. Interfaces 2014, 6, 16156–16165. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.K.; Pandeeswar, M.; Govindaraju, T. Assembly Modulation of PDI Derivative as a Supramolecular Fluorescence Switching Probe for Detection of Cationic Surfactant and Metal Ions in Aqueous Media. ACS Appl. Mater. Interfaces 2014, 6, 21369–21379. [Google Scholar] [CrossRef]
- Bhowmick, R.; Alam, R.; Mistri, T.; Bhattacharya, D.; Karmakar, P.; Ali, M. Morphology-Directing Synthesis of Rhodamine-Based Fluorophore Microstructures and Application toward Extra- and Intracellular Detection of Hg2+. ACS Appl. Mater. Interfaces 2015, 7, 7476–7485. [Google Scholar] [CrossRef]
- Niikura, K.; Anslyn, E.V. Triton X-100 Enhances Ion-Pair-Driven Molecular Recognition in Aqueous Media. Further Work on a Chemosensor for Inositol Trisphosphate. J. Org. Chem. 2003, 68, 10156–10157. [Google Scholar]
- Jamkratoke, M.; Tumcharern, G.; Tuntulani, T.; Tomapatanaget, B. A Selective Spectrofluorometric Determination of Micromolar Level of Cyanide in Water Using Naphthoquinone Imidazole Boronic-Based Sensors and a Surfactant Cationic CTAB Micellar System. J. Fluoresc. 2011, 21, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Ortiz, L.K.; Täuscher, E.; Leite Bastos, E.; Görls, H.; Weiß, D.; Beckert, R. Hydroxythiazole-Based Fluorescent Probes for Fluoride Ion Detection. Eur. J. Org. Chem. 2012, 2535–2541. [Google Scholar] [CrossRef]
- Kumari, N.; Jha, S.; Bhattacharya, S. A Chemodosimetric Probe Based on a Conjugated Oxidized Bis-Indolyl System for Selective Naked-Eye Sensing of Cyanide Ions in Water. Chem. Asian J. 2012, 7, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Rana, D.K.; Bhattacharya, S.C. Fluorescence Turn-on of a Naphthalimide Derivative by Anions in Cationic Micellar Network: An Overture towards a Simple Chemosensing Platform. Sens. Actuators B 2013, 176, 467–474. [Google Scholar] [CrossRef]
- Kumari, N.; Jha, S.; Bhattacharya, S. An Efficient Probe for Rapid Detection of Cyanide in Water at Parts per Billion Levels and Naked-Eye Detection of Endogenous Cyanide. Chem. Asian J. 2014, 9, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, C.; Chen, W.; Long, L.; Zhang, G.; Khashab, N.M.; Sessler, J.L. Removal of Anions from Aqueous Media by Means of a Thermoresponsive Calix[4]pyrrole Amphiphilic Polymer. Chem. Eur. J. 2018, 24, 15791–15795. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Jiang, H.; Li, Z.; Wang, X. Advances in nano-scaled biosensors for biomedical applications. Analyst 2013, 138, 4427–4435. [Google Scholar] [CrossRef]
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yokoyama, K. Design and Synthesis of Intramolecular Charge Transfer-Based Fluorescent Reagents for the Highly-Sensitive Detection of Proteins. J. Am. Chem. Soc. 2005, 127, 17799–17802. [Google Scholar] [CrossRef]
- Royer, C.A. Probing Protein Folding and Conformational Transitions with Fluorescence. Chem. Rev. 2006, 106, 1769–1784. [Google Scholar] [CrossRef]
- Maiti, S.; Fortunati, I.; Ferrante, C.; Scrimin, P.; Prins, L.J. Dissipative self-assembly of vesicular nanoreactors. Nat. Chem. 2016, 8, 725–731. [Google Scholar] [CrossRef]
- Yan, H.; He, L.; Zhao, W.; Li, J.; Xiao, Y.; Yang, R.; Tan, W. Poly β-Cyclodextrin/TPdye Nanomicelle-based Two-Photon Nanoprobe for Caspase-3 Activation Imaging in Live Cells and Tissues. Anal. Chem. 2014, 86, 11440–11450. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Bandyopadhyay, P. A simple strategy for charge selective biopolymer sensing. Chem. Commun. 2011, 47, 8937–8939. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Abelt, C.J. Dual-Sensor Fluorescent Probes of Surfactant-Induced Unfolding of Human Serum Albumin. J. Phys. Chem. B 2015, 119, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ding, L.; Hu, W.; Chen, X.; Chen, X.; Fang, Y. Ternary System Based on Fluorophore–Surfactant Assemblies—Cu2+ for Highly Sensitive and Selective Detection of Arginine in Aqueous Solution. Langmuir 2014, 30, 15364–15372. [Google Scholar] [CrossRef]
- Jiang, B.P.; Guo, D.S.; Liu, Y. Self-Assembly of Amphiphilic Perylene−Cyclodextrin Conjugate and Vapor Sensing for Organic Amines. J. Org. Chem. 2010, 75, 7258–7264. [Google Scholar] [CrossRef] [PubMed]
- Köstereli, Z.; Scopelliti, R.; Severin, K. Pattern-based sensing of aminoglycosides with fluorescent amphiphiles. Chem. Sci. 2014, 5, 2456–2460. [Google Scholar] [CrossRef]
- Lopez, F.; Cuomo, F.; Ceglie, A.; Ambrosone, L.; Palazzo, G. Quenching and Dequenching of Pyrene Fluorescence by Nucleotide Monophosphates in Cationic Micelles. J. Phys. Chem. B 2008, 112, 7338–7344. [Google Scholar] [CrossRef]
- Xu, Y.; Li, B.; Xiao, L.; Li, W.; Zhang, C.; Sun, S.; Pang, Y. The sphere-to-rod transition of squaraine-embedded micelles: A self-assembly platform displays a distinct response to cysteine and homocysteine. Chem. Commun. 2013, 49, 7732–7734. [Google Scholar] [CrossRef]
- Hu, W.; Ding, L.; Cao, J.; Liu, L.; Wei, Y.; Fang, Y. Protein Binding-Induced Surfactant Aggregation Variation: A New Strategy of Developing Fluorescent Aqueous Sensor for Proteins. ACS Appl. Mater. Interfaces 2015, 7, 4728–4736. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zheng, D.; Huang, X.; Ding, L.; Xin, Y.; Fang, Y. A Single, Discriminative Sensor Based on Supramolecular Self-Assemblies of an Amphiphilic Cholic Acid-Modified Fluorophore for Identifying Multiple Proteins. Sens. Actuators B 2018, 263, 336–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Ding, L. Fluorescent Ensemble Based on Dansyl derivative/SDS Assemblies as Selective Sensor for Asp and Glu in Aqueous Solution. J. Photochem. Photobiol. A 2017, 333, 56–62. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, J.; Raghupathi, K.R.; Thayumanavan, S. A Supramolecular Dissociation Strategy for Protein Sensing. Chem. Commun. 2015, 51, 17265–17268. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Smarsly, E.; Han, J.; Bender, M.; Seehafer, K.; Wacker, I.; Schröder, R.R.; Bunz, U.H.F. Truxene-Based Hyperbranched Conjugated Polymers: Fluorescent Micelles Detect Explosives in Water. ACS Appl. Mater. Interfaces 2017, 9, 3068–3074. [Google Scholar] [CrossRef]
- Yang, J.-S.; Swager, T.M. Porous Shape Persistent Fluorescent Polymer Films: An Approach to TNT Sensory Materials. J. Am. Chem. Soc. 1998, 120, 5321–5322. [Google Scholar] [CrossRef]
- Yang, J.-S.; Swager, T.M. Fluorescent Porous Polymer Films as TNT Chemosensors: Electronic and Structural Effects. J. Am. Chem. Soc. 1998, 120, 11864–11873. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Li, D.; Chen, H.; Sun, R. Fluorescent amphiphilic cellulose nanoaggregates for sensing trace explosives in aqueous solution. Chem. Commun. 2012, 48, 5569–5571. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, H.; Wang, X.; Sun, R. F127/conjugated polymers fluorescent micelles for trace detection of nitroaromatic explosives. Dye. Pigment. 2016, 125, 367–374. [Google Scholar] [CrossRef]
- Dey, N.; Samanta, S.K.; Bhattacharya, S. Selective and Efficient Detection of Nitro-Aromatic Explosives in Multiple Media including Water, Micelles, Organogel and Solid Support. ACS Appl. Mater. Interfaces 2013, 5, 8394–8400. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Zhou, J.; Kondo, T. Fluorescent micelles based on hydrophobically modified cationic cellulose for sensing trace explosives in aqueous solutions. J. Mater. Chem. C 2013, 1, 5756–5764. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Gao, H.; Zhu, F.; Ge, M.; Liang, G. Rapid detection of aromatic pollutants in water using swellable micelles of fluorescent polymers. Sens. Actuators B Chem. 2019, 283, 415–425. [Google Scholar] [CrossRef]
- Nabeel, F.; Rasheed, T.; Mahmood, M.F.; Khan, S.U.D. Hyperbranched copolymer based photoluminescent vesicular probe conjugated with tetraphenylethene: Synthesis, aggregation-induced emission and explosive detection. J. Mol. Liq. 2020, 308, 113034. [Google Scholar] [CrossRef]
- Sathish, V.; Ramdass, A.; Lu, Z.Z.; Velayudham, M.; Thanasekaran, P.; Lu, K.L.; Rajagopal, S. Aggregation-Induced Emission Enhancement in Alkoxy-Bridged Binuclear Rhenium(I) Complexes: Application as Sensor for Explosives and Interaction with Microheterogeneous Media. J. Phys. Chem. B 2013, 117, 14358–14366. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Dave, P.; Bhagat, B.; Agrawal, B.; Maity, P. A supramolecular strategy for ratiometric luminescence sensing of nitroaromatic explosives in water. Indian J. Chem. Sec. A 2020, 59, 1814–1821. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).