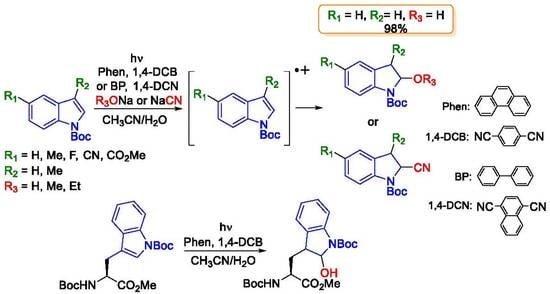
Transition-Metal-Free Access to 2-Subsituted Indolines from Indoles via Dearomative Nucleophilic Addition Using Two-Molecule Organic Photoredox Catalysts
Abstract
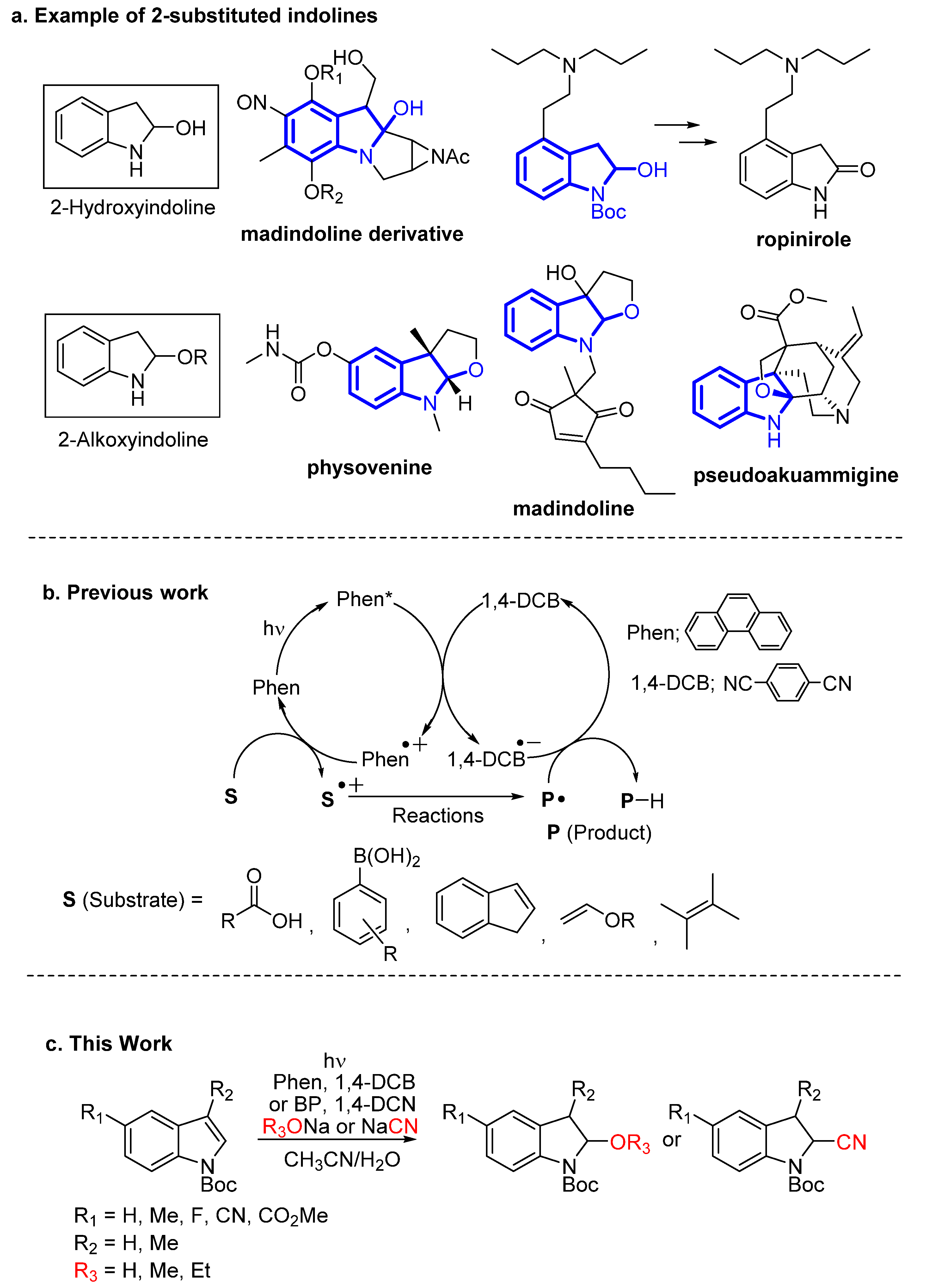
1. Introduction
2. Materials and Methods
General Information
- General Procedure for the Preparation of N-Boc Indoles 1:
- General Procedure for the Photoreaction of 1:
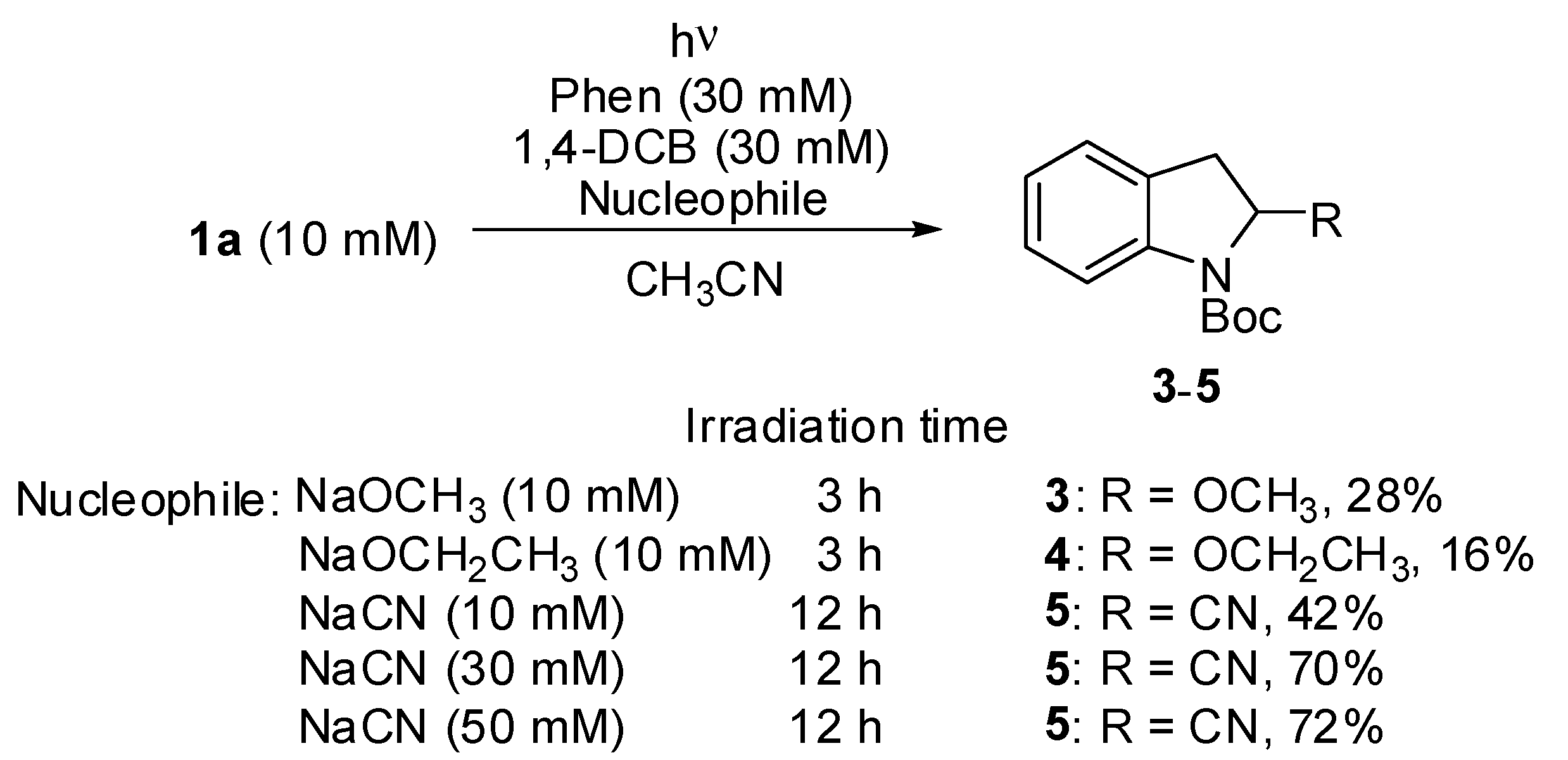
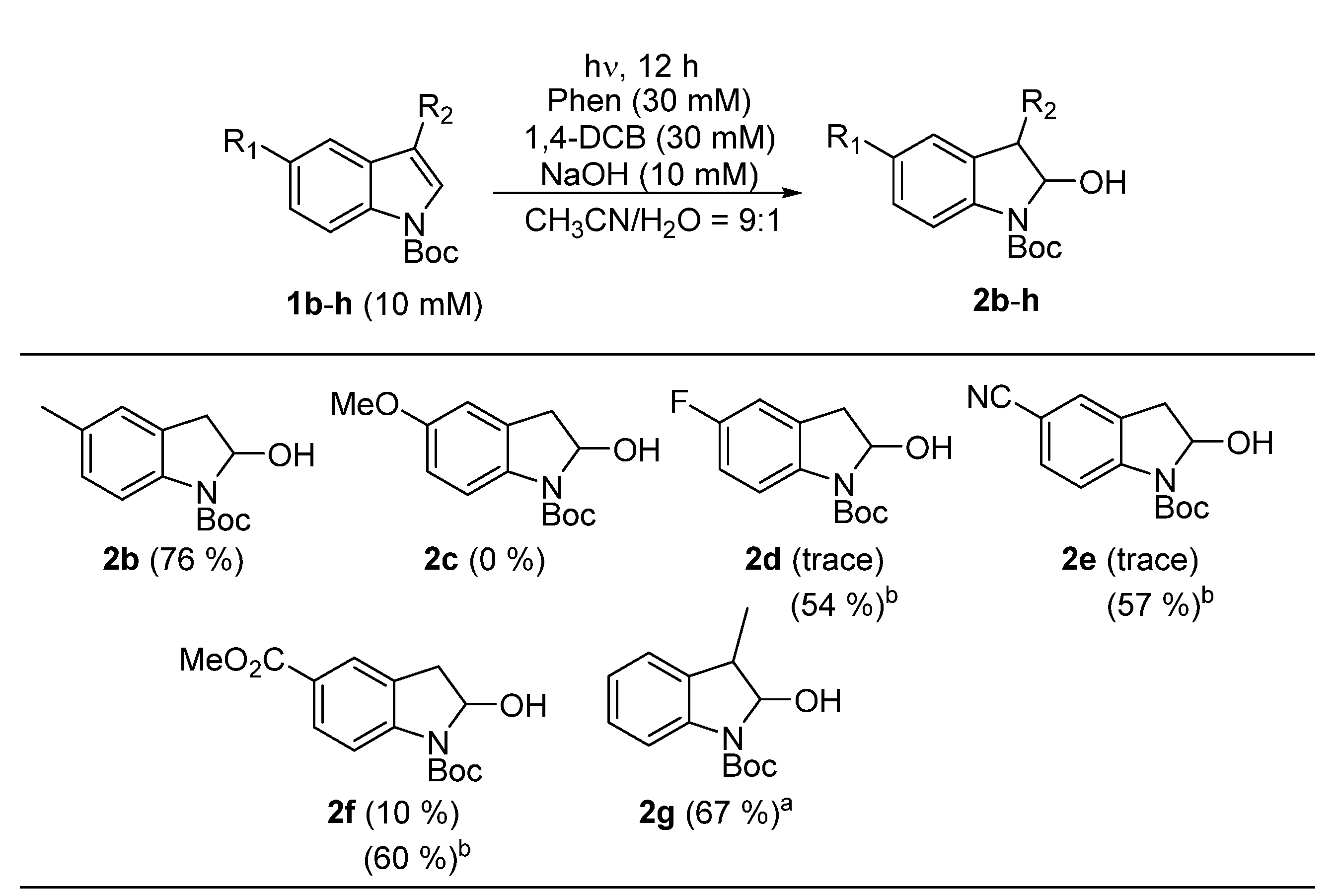
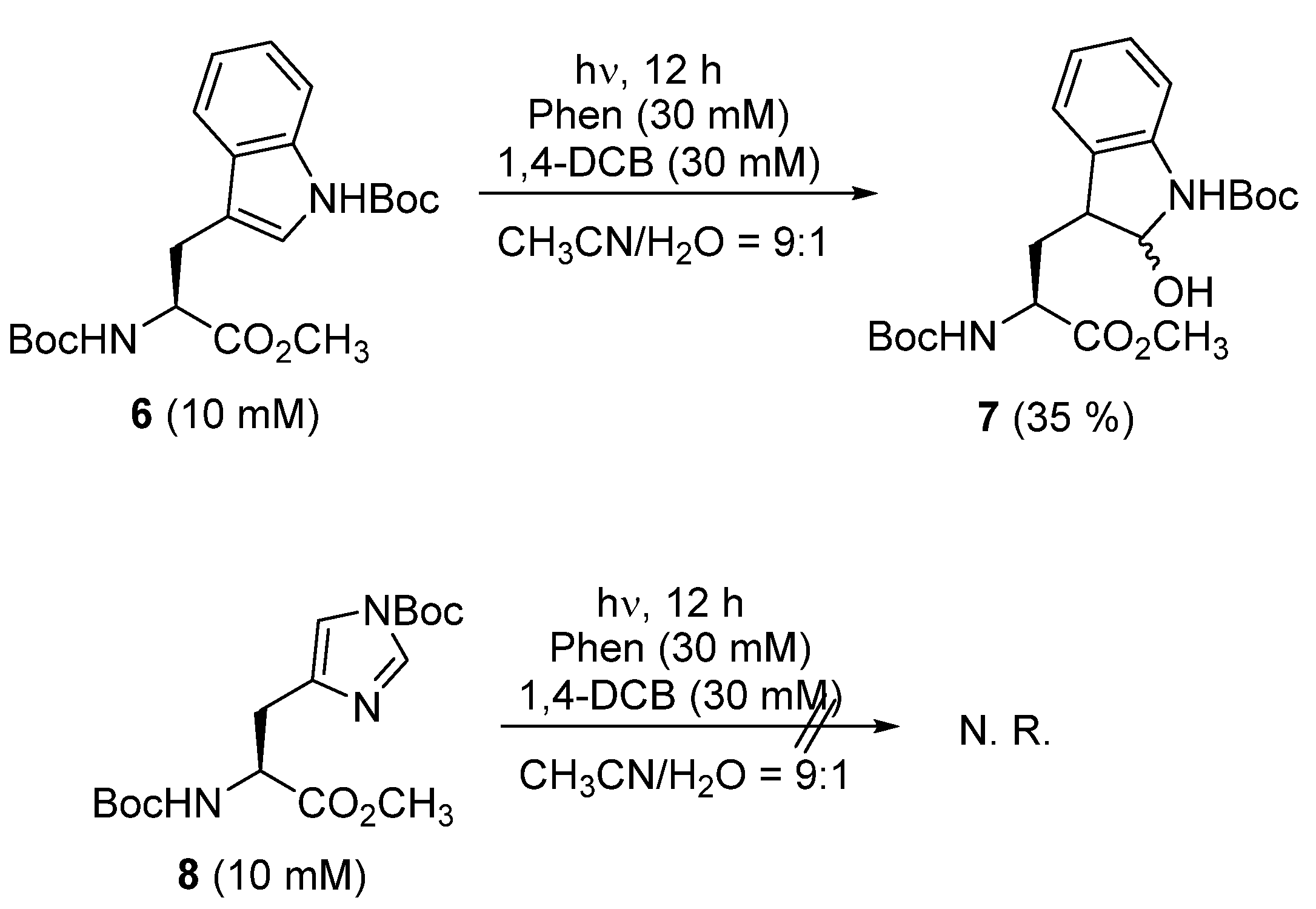
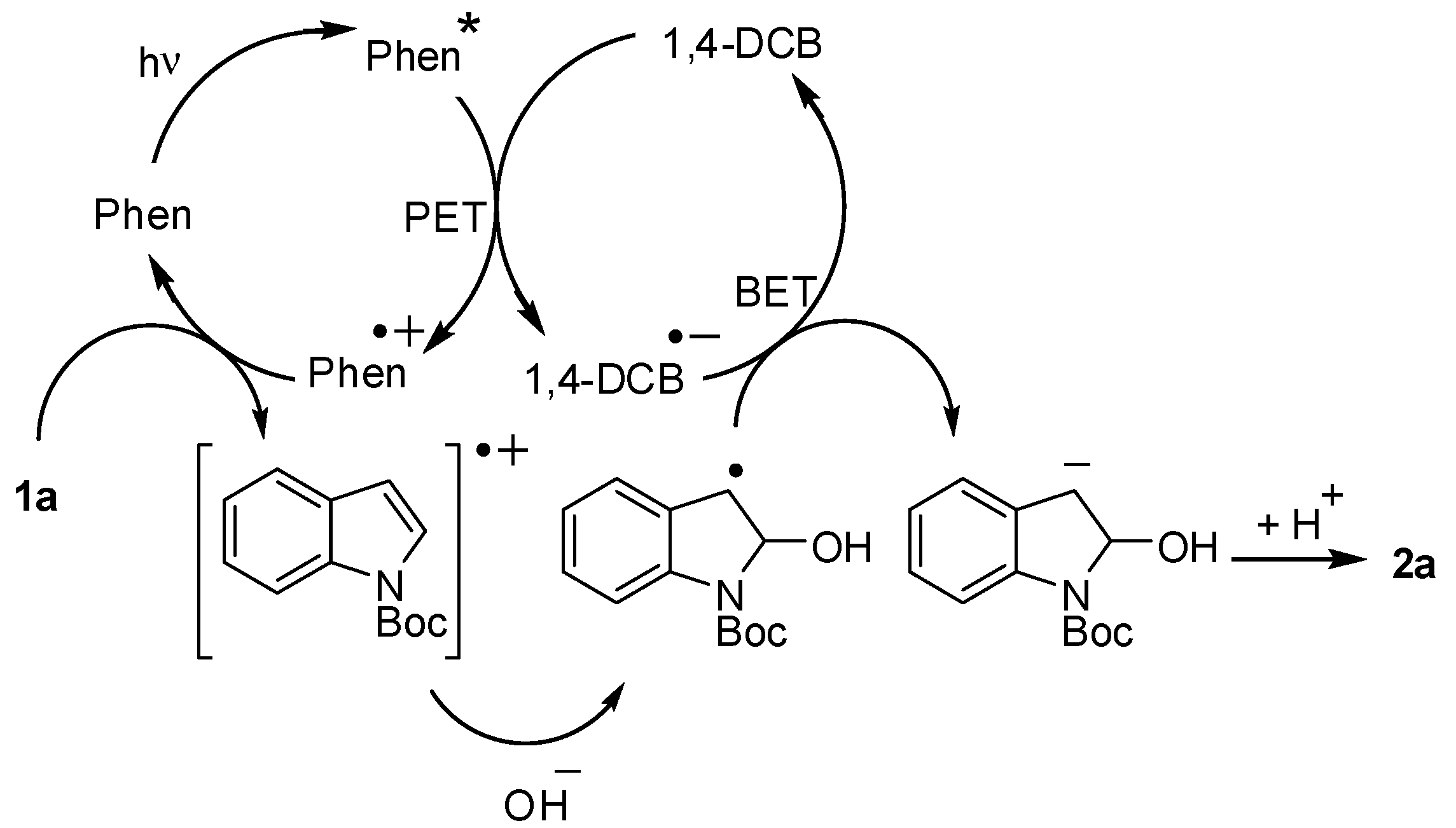
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayashi, M.; Kim, Y.-P.; Takamatsu, S.; Enomoto, A.; Shinose, M.; Takahashi, Y.; Tanaka, H.; Komiyama, K.; Ohmura, S.J. Madindoline, a novel inhibitor of IL-6 activity from Streptomyces sp. K93-0711. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1996, 49, 1091–1095. [Google Scholar] [CrossRef]
- Hirose, T.; Sunazuka, T.; Yamamoto, D.; Kojima, N.; Shirahata, T.; Harigaya, Y.; Kuwajima, I.; Ohmura, S. Determination of the absolute stereochemistry and asymmetric total synthesis of madindolines A and B: A practical improvement to a second-generation approach from the first-generation. Tetrahedron 2005, 61, 6015–6039. [Google Scholar] [CrossRef]
- Sunazuka, T.; Hirose, T.; Ohmura, S. Total synthesis of madindolines, potent selective inhibitors, novel bioactive microbial metabolites. J. Synth. Org. Chem. Jpn. 2005, 63, 1090–1101. [Google Scholar] [CrossRef]
- Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama, K.; Kam, T.-S. Biologically active aspidofractinine alkaloids from Kopsia singapurensis. J. Nat. Prod. 2007, 70, 1783–1789. [Google Scholar] [CrossRef]
- Yousuf, Z.; Richards, A.K.; Dwyer, A.N.; Linclau, B.; Harrowven, D.C. The development of a short route to the API ropinirole hydrochloride. Org. Biomol. Chem. 2015, 13, 10532–10539. [Google Scholar] [CrossRef]
- Roche, S.P.; Tendoung, J.-J.Y.; Tréguier, B. Advances in dearomatization strategies of indoles. Tetrahedron 2015, 71, 3549–3591. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.L. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 2019, 36, 1589–1605. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-B.; Jia, Y.-X. Recent progress in transition-metal-catalyzed enantioselective indole functionalizations. Org. Biomol. Chem. 2017, 15, 3550–3567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Song, H.; Qin, Y. Total synthesis of indoline alkaloids: A cyclopropanation strategy. Acc. Chem. Res. 2011, 44, 447–457. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; You, S.-L. Copper(I)-catalyzed cascade dearomatization of 2-substituted tryptophols with iodonium Salts. Org. Lett. 2012, 14, 4525–4527. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Dai, L.-X.; You, S.-L. Cascade dearomatization of N-substituted tryptophols via Lewis acid-catalyzed Michael reactions. Org. Biomol. Chem. 2012, 10, 7177–7183. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.-P.; Liu, C.; You, S.-L. Ru-catalyzed intermolecular dearomatization reaction of indoles with allylic alcohols. Chem. Sci. 2013, 4, 3239–3243. [Google Scholar] [CrossRef]
- Ye, Z.; Rawal, V.H. Palladium-catalyzed C3-benzylation of indoles. J. Am. Chem. Soc. 2012, 134, 111–114. [Google Scholar]
- Liu, H.; Jiang, G.; Pan, X.; Wan, X.; Lai, Y.; Ma, D.; Weiqing, X. Highly asymmetric bromocyclization of tryptophol: Unexpected accelerating effect of DABCO-derived bromine complex. Org. Lett. 2014, 16, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-Z.; Zeng, X.-Y.; Liang, X.; Lei, T.; Wei, K.; Yang, Y.-R. Iridium-catalyzed enantioselective indole cyclization: Application to the total synthesis and absolute stereochemical assignment of (−)-Aspidophylline A. Angew. Chem. Int. Ed. 2016, 55, 4044–4048. [Google Scholar] [CrossRef]
- Boal, B.W.; Schammel, A.W.; Grag, N.K. An interrupted Fischer indolization approach toward fused indoline-containing natural products. Org. Lett. 2009, 11, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Schammel, A.W.; Boal, B.W.; Zu, L.; Mesganaw, T.; Grag, N.K. Copper(I)-catalyzed cascade dearomatization of 2-substituted tryptophols with arylidonium salts. Tetrahedron 2010, 66, 4687–4695. [Google Scholar] [CrossRef]
- Clark, R.D.; Muchowski, J.M.; Fisher, L.E.; Flippin, L.A.; Repke, D.B.; Souchet, M. Preparation of indoles and oxindoles from N-(tert-butoxycarbonyl)-2-alkylanilines. Synthesis 1991, 1991, 871–878. [Google Scholar] [CrossRef]
- Chiou, W.-H.; Kao, C.-L.; Tsai, J.-C.; Chang, Y.-M. Domino Rh-catalyzed hydroformylation–double cyclization of o-amino cinnamyl derivatives: Applications to the formal total syntheses of physostigmine and physovenine. Chem. Commun. 2013, 49, 8232–8234. [Google Scholar] [CrossRef]
- Nambu, H.; Hirota, W.; Fukumoto, M.; Tamura, T.; Yakura, T. An efficient route to highly substituted indoles via tetrahydroindol-4(5H)-one intermediates produced by ring-opening cyclization of spirocyclopropanes with amines. Chem. Eur. J. 2017, 23, 16799–16805. [Google Scholar] [CrossRef]
- Review, S.; Yoshimi, Y. Photoinduced electron transfer-promoted decarboxylative radical reactions of aliphatic carboxylic acids by organic photoredox system. J. Photochem. Photobiol. A 2017, 342, 116–130. [Google Scholar]
- Yoshimi, Y.; Itou, T.; Hatanaka, M. Decarboxylative reduction of free aliphatic carboxylic acid by photogenerated cation radical. Chem. Commun. 2007, 48, 5244–5246. [Google Scholar] [CrossRef]
- Itou, T.; Yoshimi, Y.; Morita, T.; Tokunaga, Y.; Hatanaka, M. Decarboxylative photosubstitution of dicyanobenzenes with aliphatic carboxylate ions. Tetrahedron 2009, 65, 263–269. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Masuda, M.; Mizunashi, T.; Nishikawa, K.; Maeda, K.; Koshida, N.; Itou, T.; Morita, T.; Hatanaka, M. Inter- and intramolecular addition reactions of electron-deficient alkenes with alkyl radicals, generated by SET-photochemical decarboxylation of carboxylic acids, serve as a mild and efficient method for the preparation of γ-amino acids and macrocyclic lactones. Org. Lett. 2009, 11, 4652–4655. [Google Scholar] [PubMed]
- Nishikawa, K.; Yoshimi, Y.; Maeda, K.; Morita, T.; Takahashi, I.; Itou, T.; Inagaki, S.; Hatanaka, M. Radical photocyclization route for macrocyclic lactone ring expansion and conversion to macrocyclic lactams and ketones. J. Org. Chem. 2013, 78, 582–589. [Google Scholar] [CrossRef]
- Yamawaki, M.; Ukai, A.; Kamiya, Y.; Sugihara, S.; Sakai, M.; Yoshimi, Y. Metal-Free Photoinduced decarboxylative radical polymerization using carboxylic acids as benign radical initiators: Introduction of complex molecules into polymer chain ends. ACS Macro Lett. 2017, 6, 381–385. [Google Scholar] [CrossRef]
- Kubosaki, S.; Takeuchi, H.; Iwata, Y.; Tanaka, Y.; Osaka, K.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Visible and UV-light-induced decarboxylative radical reactions of benzoic acids using organic photoredox catalysts. J. Org. Chem. 2020, 85, 5362–5369. [Google Scholar] [CrossRef]
- Iwata, Y.; Tanaka, Y.; Kubosaki, S.; Morita, T.; Yoshimi, Y. A strategy for generating aryl radicals from arylborates through organic photoredox catalysis: Photo-Meerwein type arylation of electron-deficient alkenes. Chem. Commun. 2018, 54, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Itou, T.; Hatanaka, M. Redox-photosensitized reaction of indene using photosensitive surfactant in emulsion: Dependence on oil droplet size and surfactant charge. Tetrahedron Lett. 2006, 47, 3257–3260. [Google Scholar] [CrossRef]
- Yamawaki, M.; Asano, A.; Furutani, T.; Izumi, Y.; Tanaka, Y.; Osaka, K.; Morita, T.; Yoshimi, Y. Photoinduced electron transfer-promoted reactions using exciplex-type organic photoredox catalyst directly linking donor and acceptor arenes. Molecules 2019, 24, 4453. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kubosaki, S.; Osaka, K.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Two types of cross-coupling reactions between electron-rich and electron–deficient alkenes assisted by nucleophilic addition using organic photoredox catalyst. J. Org. Chem. 2018, 83, 13625–13635. [Google Scholar] [CrossRef]
- Maeda, K.; Saito, H.; Osaka, K.; Nishikawa, K.; Sugie, M.; Morita, T.; Takahashi, I.; Yoshimi, Y. Direct modification of tripeptides using photoinduced decarboxylative radical reactions. Tetrahedron 2015, 71, 1117–1123. [Google Scholar] [CrossRef]
- Osaka, K.; Sugie, M.; Yamawaki, M.; Morita, T.; Yoshimi, Y. N-Acryloyl amino acid esters and peptides as radical acceptors in photoinduced decarboxylative radical reaction. J. Photochem. Photobiol. A 2016, 317, 50–55. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwasaki, T.; Morita, T.; Yoshimi, Y. A strategy for O-alkylation of serine and threonine from serinyl and threoninyl acetic acids by photoinduced decarboxylative radical reactions: Connection between serine/threonine and carbohydrates/amino acids at the side chain. J. Org. Chem. 2018, 83, 3702–3709. [Google Scholar] [CrossRef]
- Osaka, K.; Usami, A.; Iwasaki, T.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Sequential intermolecular radical addition and reductive radical cyclization of tyrosine and phenylalanine derivatives with alkenes via photoinduced decarboxylation: Access to ring-constrained γ-amino acids. J. Org. Chem. 2019, 84, 9480–9488. [Google Scholar] [CrossRef] [PubMed]
- Gieseler, A.; Steckhan, E.; Wiest, O.; Knoch, F. Photochemically induced radical-cation Diels-Alder reaction of indole and electron-rich dienes. J. Org. Chem. 1991, 56, 1405–1411. [Google Scholar] [CrossRef]
- Wiest, O.; Steckhan, E. Radical cation Diels-Alder reaction of indoles and exocyclic dienes. Tetrahedron Lett. 1993, 34, 6391. [Google Scholar] [CrossRef]
- González-Béjar, M.; Stiriba, S.-E.; Miranda, M.A.; Pérez-Prietoa, J. Diels-Alder reaction between indoles and cyclohexadienes photocatalyzed by a (thia)pyrylium salt. ARKIVOC 2007, 4, 344–355. [Google Scholar] [CrossRef]
- Muneer, M.; Saquib, M.; Qamar, M.; Bahnemann, D. Photocatalyzed reaction of indole in an aqueous suspension of titanium dioxide. Res. Chem. Intermed. 2010, 36, 121–125. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Bureš, F.; Lee, R.; Ye, X.; Jiang, Z. Visible light photocatalytic aerobic oxygenation of indoles and pH as a chemoselective switch. ACS Catal. 2016, 6, 6853–6860. [Google Scholar] [CrossRef]
- Wu, K.; Fang, C.; Kaur, S.; Liu, P.; Wang, T. Methylene blue-catalyzed oxidative cleavage of N-carbonylated indoles. Synthesis 2018, 50, 2897–2907. [Google Scholar]
- Festa, A.A.; Voskressensky, L.G.; Van der Eycken, E.V. Visible lightmediated chemistry of indoles and related heterocycles. Chem. Soc. Rev. 2019, 48, 4401–4423. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, P.; Gao, F.; Dong, Y.; Huang, H.; Wang, C.; Zhou, Z.; Wang, W. Organophotocatalytic dearomatization of indoles, pyrroles and benzo(thio)furans via a Giese-type transformation. Commun. Chem. 2021, 4, 20. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Y.; Li, W.; Cheng, Y.; Zhu, C. Visible-light-induced aerobic dearomative reaction of indole derivatives: Access to heterocycle fused or spirocyclo indolones. Chem. Commun. 2016, 52, 4761–4763. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Y.; Xia, Q.; Song, H.; Song, H.; Liu, Y.; Wang, Q. Visible-light-mediated dearomatization/cyanation cascade reaction of indoles: Access to highly functionalized spiro-γ-lactam indolines with two contiguous sterically congested quaternary carbon stereocenters. Adv. Synth. Catal. 2018, 360, 2879–2884. [Google Scholar] [CrossRef]
- Guirado, G.; Fleming, C.N.; Lingenfelter, T.G.; Williams, M.L.; Zuihof, H.; Dinnocenzo, J.P. Nanosecond redox equilibrium method for determining oxidation potentials in organic media. J. Am. Chem. Soc. 2004, 126, 14086–14094. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Hayashi, S.; Nishikawa, K.; Haga, Y.; Maeda, K.; Morita, T.; Itou, T.; Okada, Y.; Ichinose, N.; Hatanaka, M. Influence of solvent, electron acceptors, and arenes on photochemical decarboxylation of free carboxylic acid via single electron transfer (SET). Molecules 2010, 15, 2623–2630. [Google Scholar] [CrossRef]
- Lee, S.; Yi, K.Y.; Yoo, S. Introduction of heterocycles at the 2-position of indoline as ester bioisosteres. Bull. Korean Chem. Soc. 2004, 25, 207–212. [Google Scholar] [CrossRef][Green Version]
- Yamawaki, M.; Okita, Y.; Yamamoto, T.; Morita, T.; Yoshimi, Y. Photoinduced electron transfer-promoted debenzylation of phenylalanine and tyrosine derivatives using dicyanoarene. Tetrahedron 2017, 73, 7239–7244. [Google Scholar] [CrossRef]
- Roth, H.G.; Romero, N.A.; Nicewicz, D.A. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 2016, 27, 714–723. [Google Scholar]
 | ||||
|---|---|---|---|---|
| Entry | Photocatalysts and NaOH | Irradiation Time/h | Yield of 2a/% | Recovery of 1a/% |
| 1 | Phen (20 mM), 1,4-DCB (20 mM) | 6 | 25 | 60 |
| 2 a | Phen (20 mM), 1,4-DCB (20 mM) | 6 | 33 | 50 |
| 3 a | Phen (30 mM), 1,4-DCB (30 mM) | 6 | 47 | 44 |
| 4 | Phen (20 mM), 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 73 | 8 |
| 5 b | Phen (20 mM), 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 0 | 99 |
| 6 | 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 0 | 91 |
| 7 | Phen (20 mM), NaOH (20 mM) | 12 | 0 | 93 |
| 8 | NP (20 mM), 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 51 | 26 |
| 9 | 1-MNP (20 mM), 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 33 | 39 |
| 10 | Phen (20 mM), 1,3-DCB (20 mM), NaOH (20 mM) | 12 | 60 | 14 |
| 11 | Phen (20 mM), 1,2-DCB (20 mM), NaOH (20 mM) | 12 | 62 | 10 |
| 12 | BP (20 mM), 1,4-DCN (20 mM), NaOH (20 mM) | 12 | 40 | 0 |
| 13 | BP (20 mM), 1,4-DCN (20 mM), NaOH (20 mM) | 8 | 70 | 0 |
| 14 c | Phen (20 mM), 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 10 | 66 |
| 15 d | Phen (20 mM), 1,4-DCB (20 mM), NaOH (20 mM) | 12 | 55 | 39 |
| 16 | Phen (5 mM), 1,4-DCB (5 mM), NaOH (20 mM) | 12 | 43 | 33 |
| 17 a | Phen (30 mM), 1,4-DCB (30 mM), NaOH (10 mM) | 12 | 98 | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Ikeda, T.; Nachi, Y.; Mizuno, T.; Maeda, K.; Sakamoto, C.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Transition-Metal-Free Access to 2-Subsituted Indolines from Indoles via Dearomative Nucleophilic Addition Using Two-Molecule Organic Photoredox Catalysts. Photochem 2021, 1, 448-457. https://doi.org/10.3390/photochem1030027
Tanaka Y, Ikeda T, Nachi Y, Mizuno T, Maeda K, Sakamoto C, Yamawaki M, Morita T, Yoshimi Y. Transition-Metal-Free Access to 2-Subsituted Indolines from Indoles via Dearomative Nucleophilic Addition Using Two-Molecule Organic Photoredox Catalysts. Photochem. 2021; 1(3):448-457. https://doi.org/10.3390/photochem1030027
Chicago/Turabian StyleTanaka, Yosuke, Takumi Ikeda, Yasuhiro Nachi, Taisei Mizuno, Kousuke Maeda, Chisato Sakamoto, Mugen Yamawaki, Toshio Morita, and Yasuharu Yoshimi. 2021. "Transition-Metal-Free Access to 2-Subsituted Indolines from Indoles via Dearomative Nucleophilic Addition Using Two-Molecule Organic Photoredox Catalysts" Photochem 1, no. 3: 448-457. https://doi.org/10.3390/photochem1030027
APA StyleTanaka, Y., Ikeda, T., Nachi, Y., Mizuno, T., Maeda, K., Sakamoto, C., Yamawaki, M., Morita, T., & Yoshimi, Y. (2021). Transition-Metal-Free Access to 2-Subsituted Indolines from Indoles via Dearomative Nucleophilic Addition Using Two-Molecule Organic Photoredox Catalysts. Photochem, 1(3), 448-457. https://doi.org/10.3390/photochem1030027